Abstract
Objective
The molecular mechanisms underlying sex differences in dyslipidemia are poorly understood. We aimed to distinguish genetic and hormonal regulators of sex differences in plasma lipid levels.
Approach and Results
We assessed the role of gonadal hormones and sex chromosome complement on lipid levels using the Four Core Genotypes mouse model (XX females, XX males, XY females, and XY males). In gonadally intact mice fed a chow diet, lipid levels were influenced by both male–female gonadal sex and XX–XY chromosome complement. Gonadectomy of adult mice revealed that the male–female differences are dependent on acute effects of gonadal hormones. In both intact and gonadectomized animals, XX mice had higher HDL cholesterol (HDL-C) levels than XY mice, regardless of male–female sex. Feeding a cholesterol-enriched diet produced distinct patterns of sex differences in lipid levels compared to a chow diet, revealing the interaction of gonadal and chromosomal sex with diet. Notably, under all dietary and gonadal conditions, HDL-C levels were higher in mice with two X chromosomes compared to mice with an X and Y chromosome. By generating mice with XX, XY and XXY chromosome complements, we determined that the presence of two X chromosomes, and not the absence of the Y chromosome, influences HDL-C concentration.
Conclusions
We demonstrate that having two X chromosomes versus an X and Y chromosome complement drives sex differences in HDL-C. It is conceivable that increased expression of genes escaping X-inactivation in XX mice regulates downstream processes to establish sexual dimorphism in plasma lipid levels.
Keywords: sex differences, sex chromosome complement, plasma lipids, HDL cholesterol
Introduction
Plasma lipid levels are used as both clinical predictors and as therapeutic targets for cardiovascular disease. As such, substantial effort has been expended to identify genetic factors that influence plasma lipid levels.1–4 A key genetic determinant of lipid levels is male–female sex. Inherent sex differences in lipid levels have led to distinct standards for the diagnosis of hyperlipidemia in men and women, but the underlying mechanisms that contribute to differences in lipid levels are not well understood. Men tend to have higher low density lipoprotein (LDL) and triglyceride (TG) levels, and lower high density lipoprotein (HDL) levels, than premenopausal women.5,6 After menopause, women often have proatherogenic lipid levels that reach or exceed those in men.5–7 These observations support a role for gonadal hormones as a key determinant of sexual dimorphism in lipid levels.7 But the relationship appears to be complex, with postmenopausal hormone therapy affording greatest cardioprotection in women when provided within 10 years of menopause.8–12 Other studies suggest that the basis for sex differences in lipid metabolism are multi-factorial, with gonadal hormones acting together with direct or indirect contributions from other sex-specific factors (reviewed in [6]).
Besides gonadal secretions, another fundamental difference between males and females is the presence of an XX or XY sex chromosome complement. Differences due to gonadal sex vs. sex chromosome complement have been difficult to discriminate because, typically, female gonads occur together with XX chromosomes, and male gonads with XY chromosomes. In the current study, we used the Four Core Genotypes (FCG) mouse model to identify independent effects on plasma lipid levels of gonadal sex (testes vs. ovaries) and sex chromosome complement (XX vs. XY). The FCG model consists of four types—or “sexes”—of mice: gonadal male mice with either XX or XY sex chromosomes, and gonadal female mice with XX or XY sex chromosomes.13–15 In the FCG model, the Y chromosome is deleted for the testis-determining Sry gene, which is provided instead by an Sry transgene inserted into an autosome. As a result, gonadal sex segregates independently from the sex chromosome complement. Sex differences observed between gonadal males and females can be attributed to the action of gonadal hormones, whereas differences between XX and XY mice can be ascribed to the number of X or Y chromosomes. Additionally, by comparing intact and gonadectomized mice, further distinction can be made between the effects of gonadal hormones during development and those resulting from acute effects of hormones in adulthood.
We recently used the C57BL/6 FCG mouse model to determine how sex chromosome complement contributes to sex differences in metabolic traits, such as body weight, adiposity, and hepatic lipid content. Specifically, when gonadectomized as adults to remove acute gonadal effects, XX mice have increased obesity and fatty liver compared to XY mice, regardless of whether they originally had male or female gonads.16 We hypothesized that sex chromosome complement may also contribute to sex differences in plasma lipid profiles. Here we report that gonadal hormones and sex chromosome complement have independent effects on plasma lipoprotein levels. These results have implications for understanding the basis for sex differences in plasma lipid levels, and may inform about key risk factors in the metabolic syndrome.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Acute gonadal hormones and the sex chromosome complement influence plasma lipid levels
To analyze sex differences, we measured fasting lipid levels (total cholesterol, HDL cholesterol (HDL-C), TG, and free fatty acids (FFA)) in the four genotypes of FCG mice. We defined HDL-C levels as the cholesterol present in particles that lack apoB, and LDL/VLDL cholesterol levels as that from all non-HDL particles (see Materials and Methods). Statistical analyses were performed by two-way ANOVA, with gonadal sex (male or female) and sex chromosome complement (XX or XY) as covariates.
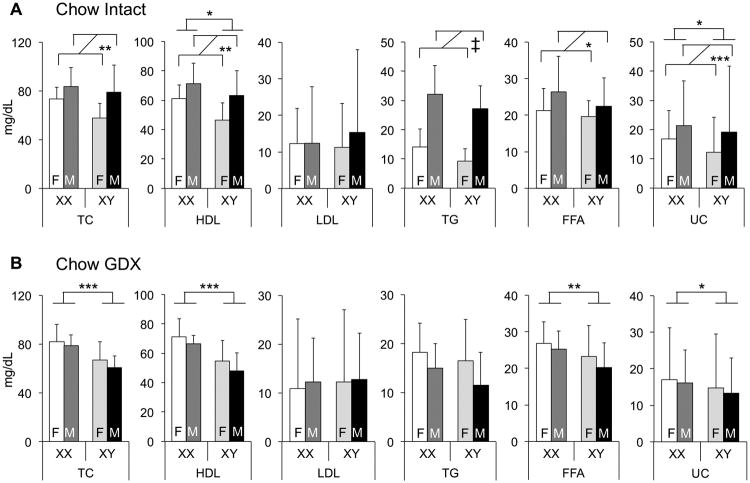
We first assessed plasma lipid levels in gonadally intact mice fed a chow diet. Total cholesterol levels were similar to those reported previously for C57BL/6 mice, with HDL-C accounting for the majority of plasma cholesterol, as is typical in mice.17,18 Compared to females, male mice had higher levels of total and HDL-C, as well as TG and FFA (Fig. 1A). Males also had slightly higher amounts of unesterified cholesterol (UC in Fig. 1). Notably, however, animals of both gonadal sexes with XX chromosomes had 20% higher HDL-C levels than XY mice (p<0.02). These results indicate that male–female gonads are a determinant of sex differences in plasma lipid levels, but also reveal that the sex chromosome complement influences HDL-C levels, even in the presence of normal gonadal hormone levels.
Figure 1.
Plasma lipid levels are regulated by both gonadal sex and sex chromosome complement. A and B, Concentrations of total cholesterol (TC), unesterified cholesterol (UC), high-density lipoprotein (HDL) cholesterol, triglycerides (TG), and free fatty acids (FFA) were measured in 7.5-month-old gonadally intact (A) and gonadectomized (GDX, B) Four Core Genotypes mice fed a standard chow diet (n = 8). Low-density lipoprotein (LDL) cholesterol values were calculated by subtracting HDL from TC. Values represent the mean ± standard deviation. Significant comparisons for sex chromosome complement and for gonadal sex are denoted by brackets. A significant interaction of sex chromosome complement and gonadal sex is denoted by “Int.” *, P≤0.05; **, P≤0.01; ***,P≤0.001; ‡, P≤0.000001. F, gonadal female; M, gonadal male.
The sex differences in lipid levels that were observed between males and females could result from either long-term or short-term effects of gonadal secretions.19 To distinguish between these, we gonadectomized mice after they reached adulthood (75 days of age) and determined lipid levels 5 months later, at which point acute effects of gonadal hormones should be absent, but long-term effects might persist. Gonadectomized mice did not exhibit the male–female differences in lipid levels that were present in gonadally intact mice indicating that much of the male–female dimorphism in plasma lipid levels is related to acute effects of gonadal secretions. However, as observed in intact mice, HDL and unesterified cholesterol levels were higher in XX compared to XY mice (p<0.0003 and p<0.04), regardless of original gonad type (Fig. 1B). After gonadectomy, total cholesterol levels and FFA levels were also higher in XX than XY mice (p<0.007 and p<0.003). Thus, the effects of XX chromosome complement on HDL and unesterified cholesterol levels are robust, occurring in both the presence and absence of gonadal secretions, and gonadectomy exposes underlying effects of chromosome complement on total cholesterol and FFA levels.
Lipoprotein composition differs in XX and XY mice
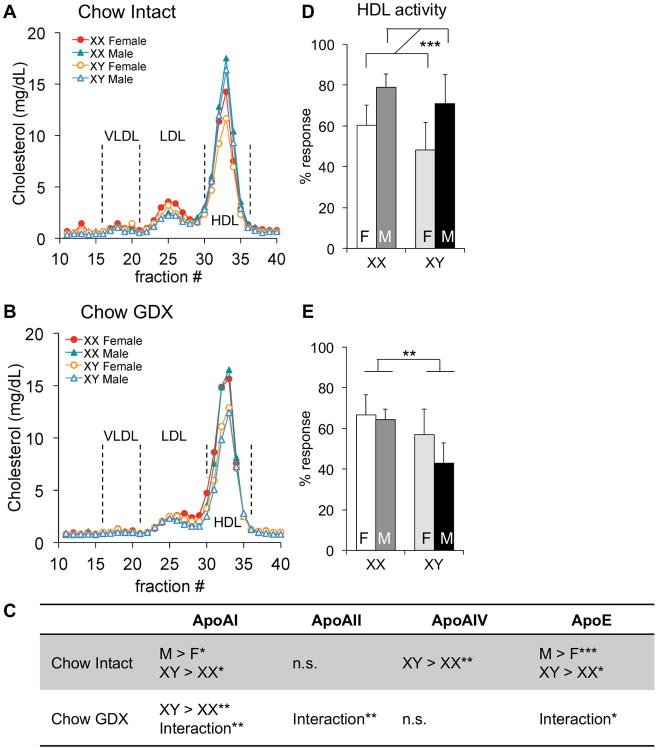
As described above, HDL-C values (determined after fractionation of apoB-containing lipids) are higher in mice with XX compared to XY chromosome complement. We wondered if sex chromosome complement influences HDL characteristics such as particle size, apolipoprotein content, or HDL-apolipoprotein (apo)A-I exchange activity (a measure of HDL function). To assess whether HDL size distribution differs among the FCG genotypes, we fractionated plasma samples by fast protein liquid chromatography (FPLC) and quantified cholesterol content of the resulting fractions. HDL-C peaks directly mirrored the results of biochemical fractionation, with highest HDL-C levels in the XX mice within each sex (XX males > XY males and XX females > XY females), and higher levels in males than females in gonadally intact mice (Fig. 2A). In mice that had been gonadectomized as adults, the HDL-sized particles were more abundant in XX compared to XY mice, and male–female differences were not detectable (Fig. 2B). Minor sex differences were observed in cholesterol levels in LDL-sized particles, with slightly higher levels in females than males in both intact and gonadectomized mice (Fig. 2A and B).
Figure 2.
HDL lipoprotein composition and apoAI exchange activity is influenced by sex. Plasma was collected from 7.5-month-old gonadally intact (A, D) and gonadectomized (GDX, B, E) chow-fed mice. A and B, Three representative plasma samples from each genotype were pooled and assayed using fast protein liquid chromatography. C, Plasma levels of apolipoproteins were quantified by immunoblot densitometry. Direction of statistically significant comparisons for gonadal sex and for sex chromosomes are shown. A significant interaction of sex chromosome complement and gonadal sex is denoted by “Interaction.” D and E, HDL apoAI dissociation activity was measured by electron paramagnetic resonance and represented as % response. Values represent the mean ± standard deviation. *, P≤0.05; **, P≤0.01; ***,P≤0.001; n.s., not significant. F, gonadal female; M, gonadal male.
We assessed the relative plasma apolipoprotein levels in plasma from the FCG mice. Consistent with the higher HDL-C levels in intact male mice, levels of the major HDL apolipoprotein, apoA-I, were slightly higher in males than females (p<0.02; Fig. 2C; Suppl. Table I; Suppl. Fig. I). A similar male–female difference was observed for apoE, a component of multiple lipoprotein classes (p<0.0005). Apolipoprotein levels were also influenced by sex chromosome complement. The levels of apoA-I, apoA-IV and apoE were higher in XY compared to XX mice of both gonadal sexes (p<0.02, p<0.03 and p<0.01, respectively). This was unexpected, given the higher HDL-C levels in XX compared to XY mice. Gonadectomy abolished the male–female differences in apolipoprotein levels, but maintained the higher apoA-I levels in XY compared to XX mice (p<0.006). Removal of the gonads also uncovered interactions between sex chromosome complement and the original gonadal sex (Fig. 2C; Suppl. Table 1). ApoB levels were very low in all chow-fed mice, and neither apoB nor apoA-II levels differed among the four genotypes (Suppl. Table I and Suppl Fig. I). Overall, our results reveal complex effects of sex chromosome complement on plasma lipoprotein composition, with XX chromosome complement favoring higher HDL-C content, but lower total levels of apolipoproteins that are often associated with HDL including apoA-I and apoA-IV.
The lower apolipoprotein-to-cholesterol ratio of HDL from XX compared to XY mice could influence HDL function. One assessment of HDL function is the degree to which apoA-I present on HDL can be dissociated from the lipoprotein particle (HDL–apoA-I exchange).20 A reduced HDL–apoA-I exchange rate correlates with metabolic syndrome and acute coronary syndrome in humans, and with increased atherosclerotic plaque burden in rabbits.21 We assessed the HDL–apoA-I exchange rate using site-directed spin-label electron paramagnetic resonance.21 In both intact and gonadectomized mice, the HDL-apoA-I exchange activity in plasma mirrored HDL-C concentrations, with male > female in intact mice (p=0.001) and XX > XY in gonadectomized mice (p<0.003) (Fig. 2D and E). Thus, sex differences in HDL–apoA-I exchange rates parallel those in HDL-C levels, and are influenced by both gonadal and chromosomal sex determinants.
Sex chromosome–diet interactions influence cholesterol levels and HDL activity
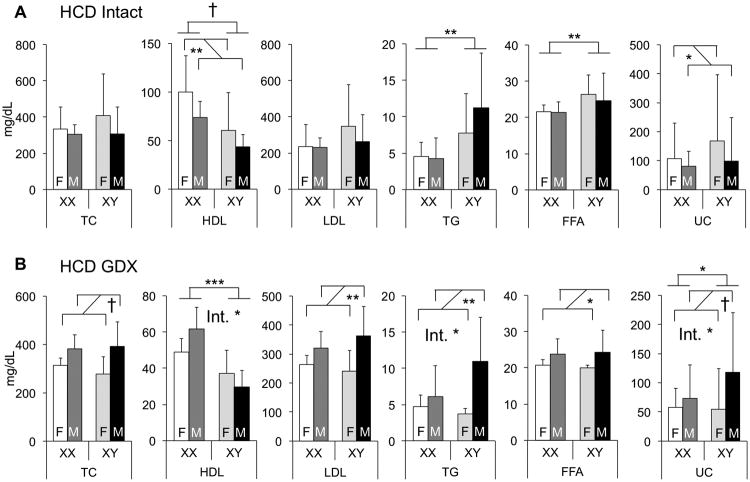
Lipid levels are highly responsive to diet. We investigated the factors underlying sexual dimorphism in lipid levels in response to dietary cholesterol by feeding FCG mice a diet containing 1.25% cholesterol (in contrast to 0.02% in chow). As expected, the cholesterol-enriched diet caused substantial increases in the absolute levels of total and LDL cholesterol (LDL-C) in all genotypes compared to levels in mice fed the chow diet. While both sex chromosomes and gonadal sex influenced lipid levels on a high cholesterol diet, specific effects differed from chow diet. As we observed on chow diet, sex chromosome complement remained an important determinant of HDL-C levels, with XX > XY (Fig. 3A). Unlike chow diet, however, sex chromosome complement also influenced TG and FFA levels, with XY > XX. HDL and UC levels were both influenced by gonadal sex in intact mice fed a cholesterol-enriched diet, with female > male. Thus, in some cases, the determinants of sexual dimorphism in lipid traits are responsive to diet.
Figure 3.
Diet interacts with gonadal sex and sex chromosome complement to modulate plasma lipid levels. A and B, Concentrations of total cholesterol (TC), unesterified cholesterol (UC), high-density lipoprotein (HDL) cholesterol, triglycerides (TG), and free fatty acids (FFA) were determined in 7.5-month-old gonadally intact (A) and GDX (B) FCG mice fed a high cholesterol diet (HCD, n = 4-10). Low-density lipoprotein (LDL) cholesterol values were calculated by subtracting HDL from TC. Values represent the mean ± standard deviation. Significant comparisons for sex chromosome complement and for gonadal sex are denoted by brackets. A significant interaction of sex chromosome complement and gonadal sex is denoted by “Int.” *, P≤0.05; **, P≤0.01; ***,P≤0.001; †, P≤0.0001. F, gonadal female; M, gonadal male.
Removal of the acute effects of gonadal secretions by gonadectomy of adult mice produced unique patterns of lipid levels among the four genotypes compared to chow diet or intact mice fed cholesterol diet. HDL-C and UC levels were higher in XX compared to XY mice (Fig. 3B). Thus, HDL and UC cholesterol levels were consistently influenced by sex chromosome complement across diets (chow and high cholesterol) and gonadal state (intact and gonadectomized). Unexpectedly, in gonadectomized mice, the cholesterol diet uncovered male–female differences in several lipid traits that were not apparent in gonadally intact mice. Thus, males had higher levels than females of total cholesterol, LDL-C, UC, TG and FFA (Fig. 3B). Interestingly, the only condition examined in which LDL-C levels exhibited sexual dimorphism was in mice gonadectomized and fed a cholesterol-enriched diet. Detection of male–female lipid level differences in gonadectomized mice suggests a role for either long-lasting (organizational) effects of gonadal hormones present in early life, or an effect of Sry acting outside of the gonads. Furthermore, the emergence of male–female dimorphism in cholesterol traits exclusively in gonadectomized animals suggests that acute effects of gonadal secretions in intact mice may counteract these organizational hormone effects.
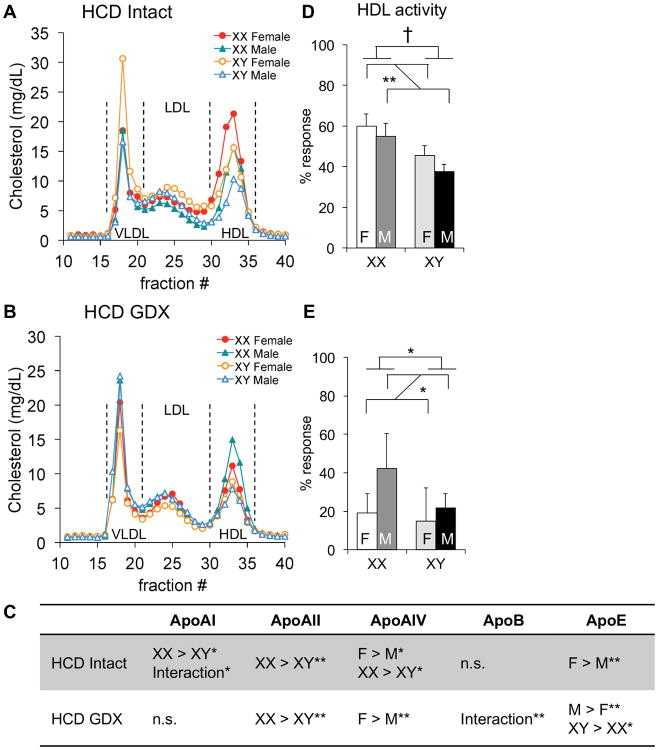
As described above, the presence of XX sex chromosomes was associated with higher HDL-C levels than XY chromosome complement. On the cholesterol-enriched diet, HDL-C levels were ∼60% higher in XX than XY mice in both intact and gonadectomized states (Fig. 3). In the intact mice on both diets, the sex chromosome effect was overlaid with male–female sex differences. The XX > XY differences in HDL-C levels of mice fed a cholesterol-enriched diet were recapitulated when HDL particles were defined by size via FPLC fractionation (Fig. 4A, B). Analysis of apolipoprotein content on the cholesterol-enriched diet showed XX > XY for several HDL apolipoproteins (apoA-I, apoA-II, and apoA-IV) in intact mice; female > male effects were also evident for apoA-IV and apoE (Fig. 4C; Suppl. Table I; Suppl. Fig. I). In gonadectomized mice fed a cholesterol-enriched diet, the sex chromosome effects on apolipoprotein content were less pronounced, but still apparent for apoA-II and apoE.
Figure 4.
Diet interacts with sex to influence HDL lipoprotein composition and apoAI exchange activity. Plasma was collected from 7.5-month-old gonadally intact (A, D) and gonadectomized (GDX, B, E) mice fed the high cholesterol diet (HCD). A and B, Three representative plasma samples from each genotype were pooled and assayed using fast protein liquid chromatography. C, Plasma levels of apolipoproteins were quantified by immunoblot densitometry. Direction of statistically significant comparisons for gonadal sex and for sex chromosomes are shown. A significant interaction of sex chromosome complement and gonadal sex is denoted by “Interaction.” D and E, HDL apoAI dissociation activity was measured by electron paramagnetic resonance and represented as % response and as activity per unit of HDL cholesterol. Values represent the mean ± standard deviation. *, P≤0.05; **, P≤0.01; †, P≤0.0001; n.s., not significant. F, gonadal female; M, gonadal male.
Assessment of HDL–apoA-I exchange activity revealed a strong effect of diet. On a chow diet, HDL activity in gonadally intact mice was higher in males than females (Fig. 2D); after cholesterol feeding, XX mice had higher HDL–apoA-I exchange activity than XY mice, and female mice had higher activity than males (Fig. 4D). Gonadectomy in combination with dietary cholesterol reduced the absolute levels of HDL–apoA-I exchange activity compared to all other dietary–gonadal hormone conditions, particularly in females (Fig. 4E); in chow fed mice, gonadectomy reduced HDL–apoA-I exchange activity only in males (Fig. 2E). These results suggest that acute effects of gonadal hormones are a determinant of HDL–apoA-I exchange capacity, with distinct sexually dimorphic effects that respond to diet.
Gene expression levels for components of cholesterol synthesis and metabolism do not explain sex differences in plasma cholesterol levels
In all dietary and gonadal conditions examined here, HDL-C levels were higher in mice with XX compared to XY chromosome complement. To investigate potential mechanisms, we examined hepatic gene expression levels for key players in cholesterol synthesis and metabolism. These included determinants of cholesterol biosynthesis (Hmgcr, Mvk), cellular lipoprotein uptake (Ldlr, Scarb1), cholesterol conversion to bile acids (Cyp7a1, Cyp8b1, Cyp27a1), and HDL lipid accumulation (Lcat, Pltp, Abca1, Abcg1). We searched for patterns of gene expression that mirrored the elevated HDL-C levels in XX compared to XY genotypes across the four cohorts of mice, all of which had higher HDL-C levels in XX compared to XY mice. In some cases (e.g., Pltp, Abcg1), male gonadal secretions appeared to inhibit gene expression, as increased expression of these genes was observed in chow-fed males after gonadectomy. And although we identified some instances of XX vs. XY differences in gene expression, we did not detect patterns that are likely to explain the elevated levels of HDL in XX compared to XY mice (Table 1 and Suppl. Figs. II-IV).
Table 1.
Sex differences in hepatic gene expression.
| Chow Intact | Chow GDX | HCD Intact | HCD GDX | |
|---|---|---|---|---|
| Cholesterol synthesis and transport | ||||
| Abca1 | M > F** | n.s. | F > M** | XY > XX* Int.** |
|
| ||||
| Abcg1 | F > M† XY > XX*** Int.** |
XY > XX† Int.* |
F > M** XY > XX** |
XY > XX** |
|
| ||||
| ApoB | XX > XY** | M > F* | M > F† | n.s. |
|
| ||||
| Hmgcr | n.s. | n.s. | M > F** XY > XX** |
n.s. |
|
| ||||
| Lcat | F > M* | M > F* | M > F† | F > M* XX > XY*** |
|
| ||||
| Ldlr | XX > XY* | n.s. | M > F† | XX > XY* |
|
| ||||
| Lipc | F > M* Int.** |
XX > XY** | M > F† | n.s. |
|
| ||||
| Mvk | n.s. | n.s. | Int.* | n.s. |
|
| ||||
| Pltp | F > M*** XY > XX* |
n.s. | F > M† XY > XX** |
XY > XX** |
|
| ||||
| Scarb1 | n.s. | n.s. | M > F** | XX > XY* |
|
| ||||
| Bile acid synthesis | ||||
| Cyp7a1 | F > M*** XX > XY** |
n.s. | F > M* Int.* |
n.s. |
|
| ||||
| Cyp8b1 | M > F** XX > XY** |
F > M** | n.s. | n.s. |
|
| ||||
| Cyp27a1 | XX > XY*** | XX > XY* Int.* |
M > F** | n.s. |
| X-inactivation escape | ||||
| Ddx3x | XX > XY*** | M > F* | M > F** XX > XY** Int.*** |
XX > XY*** |
|
| ||||
| Eif2s3x | M > F** XX > XY ‡ Int.** |
M > F* XX > XY** |
XX > XY† | XX > XY† Int.* |
|
| ||||
| Kdm5c | M > F** XX > XY ‡ |
M > F* | M > F** XX > XY ‡ Int.* |
XX > XY† Int.** |
|
| ||||
| Kdm6a | XX > XY** | F > M* XX > XY*** |
XX > XY ‡ | M > F** XX > XY** |
Hepatic gene expression was measured by quantitative PCR. P-values are represented by direction of sex difference and magnitude of significance.
P≤0.05;
P≤0.01;
P≤0.001;
P≤0.0001;
P≤0.000001; n.s., not significant.
F, gonadal female; M, gonadal male; HCD, high cholesterol diet.
Differences in X chromosome gene dosage associate with plasma HDL-C levels
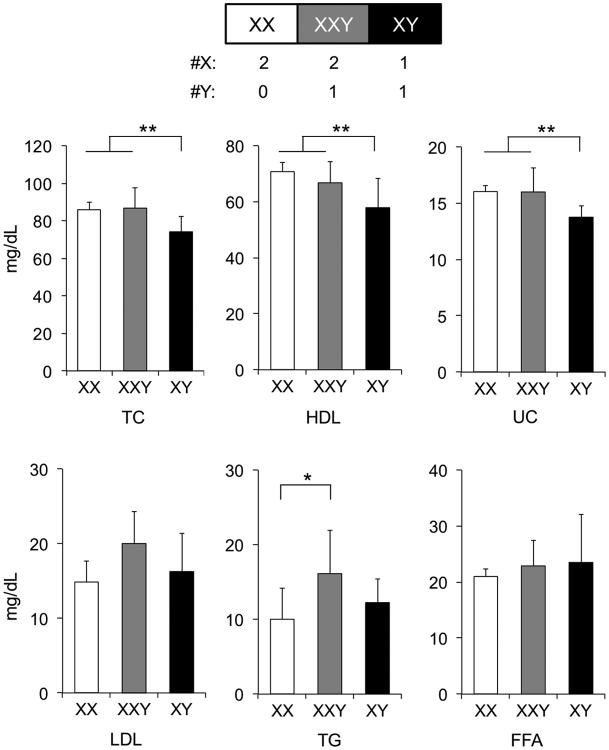
The association of HDL-C levels with XX chromosome complement suggests a mechanism that is directly related to the presence of a second X chromosome or the absence of a Y chromosome. To distinguish between these two possibilities, we measured plasma lipid levels in a mouse model differing in the number of sex chromosomes.14,22 The abnormal Y* chromosome undergoes recombination with the X chromosome to produce XX, XXY* (similar to XXY), and XY* (similar to XY) mice. Chow-fed, gonadectomized mice with two X chromosomes (XX and XXY) had higher levels of total, unesterified, and HDL-C than mice with a single X chromosome (XY); p<0.007, p<0.006 and p<0.04, respectively). The presence of the Y chromosome did not affect HDL-C levels (compare XX with XXY; Fig. 5). By contrast, mice with a Y chromosome (XXY and XY) had higher LDL-C and TG levels than mice without a Y (p<0.03 and p<0.02, respectively), regardless of the number of X chromosomes. These data indicate that the presence of X and Y chromosomes have distinct effects on lipid species, with HDL-C influenced by the number of X chromosomes, and LDL-C influenced by the presence of the Y chromosome.
Figure 5.
Elevated total and HDL cholesterol levels are associated with presence of two X chromosomes. Concentrations of total cholesterol (TC), unesterified cholesterol (UC), high-density lipoprotein (HDL) cholesterol, triglyceride (TG), and free fatty acids (FFA) were measured in gonadectomized XY* chow fed mice with the sex chromosome genotypes indicated (n = 7-8). Low-density lipoprotein (LDL) cholesterol values were calculated by subtracting HDL from TC. Values represent the mean ± standard deviation. Significant comparisons by one-way ANOVA with Duncan's multiple-comparison test are denoted by brackets. *, P≤0.05; **, P≤0.01.
In general, dosage of X chromosome gene expression is normalized between XX and XY cells through inactivation of one X chromosome in XX cells. However, a small subset of genes escape X chromosome inactivation and exhibit higher expression levels in XX compared to XY cells.23 Genes that are well established to escape inactivation in both mice and humans include Ddx3x, Eif2s3x, Kdm5c, and Kdm6a. The expression levels of these genes have the potential to influence numerous downstream cellular processes through their roles as histone methylases (Kdm5c, Kdm6a), a DNA helicase (Ddx3x), and a translation initiation factor (Eif2s3x). To assess whether the X-inactivation escapee genes have enhanced expression levels in the four cohorts of mice studied here in a relevant metabolic tissue, we quantitated gene expression in liver of both intact and gonadectomized FCG mice on chow and high cholesterol diets. These genes were expressed at higher levels in XX mice compared to XY mice in nearly all cohorts (Table 1 and Suppl. Fig. V). In some cases, male–female dimorphism was also observed. The higher hepatic expression levels of X chromosome escapee genes in XX compared to XY liver raise the possibility that altered X chromosome gene dosage may contribute to sexual dimorphism in HDL-C levels, and likely other metabolic traits.
Discussion
We used the FCG mouse model to investigate the relative contributions of gonadal secretions and sex chromosome complement to lipid levels. Using this model, we were able to detect sex chromosome complement as a determinant of sexual dimorphism in plasma lipids and lipoproteins, particularly HDL-C.
In gonadally intact mice fed a chow diet, total and HDL-C, as well as TG and FFA levels, were higher in male mice (XX and XY) compared to female mice (XX and XY). In addition, HDL-C levels were influenced by sex chromosome complement, with higher levels in XX compared to XY mice. Thus, even in the presence of endogenous gonadal hormones, the effect of sex chromosomes on HDL-C levels was apparent. To further explore the influence of sex chromosome complement on lipid levels, we reduced hormone levels by gonadectomy, which eliminated male–female differences observed in the intact mice, and amplified the XX vs. XY effects on HDL-C levels. Gonadectomy also revealed that XX chromosome complement promotes higher total cholesterol and FFA levels. These results suggest that sex chromosome complement may become a particularly important determinant of lipid levels under conditions characterized by reduced gonadal hormones, such as middle age and post-menopause in humans.
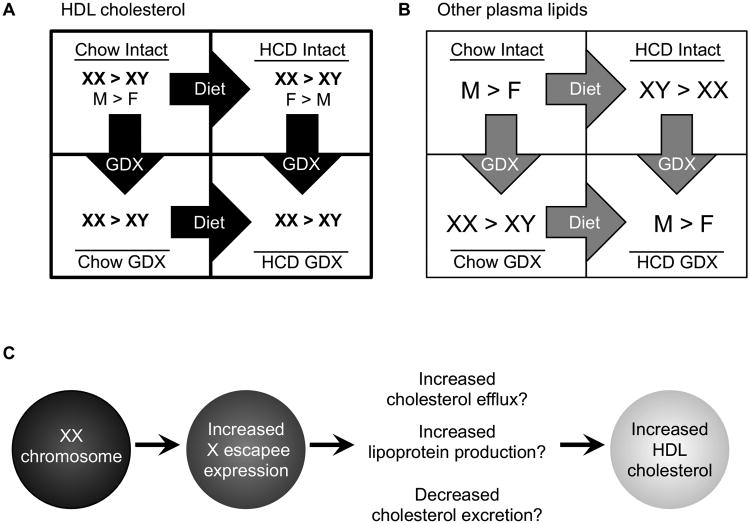
We detected interactions between sex chromosome complement and diet. Gonadally intact XX mice fed a diet enriched in cholesterol had higher HDL-C levels than XY mice, as was observed in chow fed mice. Additionally, the cholesterol-enriched diet brought out a novel sex chromosome effect on TG and FFA levels, with higher levels in XY compared to XX mice. Following gonadectomy, male mice had significantly higher levels than females for total cholesterol, LDL-C, TG and FFA. Since mice were gonadectomized 5 months prior to lipid measurements, the observed male–female dimorphism must be a result of long-lasting (i.e., organizational) effects of gonadal hormones, or non-gonadal effects of Sry that are confounded with gonadal sex in this model. Together, our results indicate that sex differences in lipid levels are determined by a combination of hormonal and sex chromosome effects, and further, these differences are dependent on hormonal (high or low gonadal hormone concentrations) and diet (chow or high cholesterol) context (Fig. 6A).
Figure 6.
Factors influencing circulating HDL cholesterol. A, HDL cholesterol levels are higher in XX mice compared to XY mice, regardless of diet or sex hormone presence status. Male/female gonad status also influences HDL cholesterol levels under some conditions. B, Plasma lipid other than HDL exhibit sex differences that depend on the diet and gonadal status. High cholesterol diet (HCD); gonadectomy (GDX); F, gonadal female; M, gonadal male. C, Mice with two X chromosomes have increased X escapee gene expression in metabolic tissues such as liver. These genes may influence several metabolic pathways to increase circulating HDL cholesterol.
Regardless of diet or sex hormone milieu, HDL-C levels were elevated in mice with two X chromosomes. The potential benefits of elevated HDL-C levels include cholesterol efflux from peripheral tissues, anti-inflammatory and anti-oxidative activities, and protection from infection.24,25 One metric of HDL activity that has been correlated with metabolic syndrome in humans and atherosclerosis in rabbits is the efficiency of apoA-I exchange from HDL particles.21 We found that total HDL-apoA-I exchange activity was greater in XX than XY mice, concordant with the increased HDL-C concentration in XX animals. These results suggest that XX chromosome complement may be a factor in determining both the levels and the protective activity of HDL.
By altering the number of X and Y chromosomes using the Y* mouse model, we determined that the presence of two X chromosomes is associated with increased HDL-C. We previously determined that the presence of two X chromosomes also leads to increased adiposity and fatty liver development on a high fat diet.16 Thus, a future goal of broad importance is to understand how increased X chromosome dosage impacts metabolism. Here we demonstrate that genes that escape X chromosome inactivation are expressed at higher levels in XX compared to XY tissues. The proteins encoded by these genes—which have roles in transcriptional regulation, RNA processing, and protein translation26–33—could conceivably influence lipid homeostasis (Fig. 6B). The specific targets of X chromosome escapee gene activity are not well characterized at present, but ongoing studies are focused on their identification using large-scale transcriptional and epigenomic profiling.
Our data using the Y* mouse model revealed that in addition to effects of two X chromosomes on HDL-C levels, the presence of the Y chromosome may influence LDL-C levels. The Y chromosome has traditionally been viewed to harbor genes restricted to male gonad development and spermatogenesis. However, a recent study using consomic mouse strains with Y chromosomes derived from distinct inbred strains suggests that genetic variation in Y chromosome genes influences plasma lipoprotein levels.34 The Y chromosome carries a set of genes that encode Y-specific proteins that are similar to paralogous genes on the X chromosome. These include the Y chromosome counterparts of the X chromosome escapee genes that exhibit increased dosage in mice with two vs. a single X chromosome. In our study, all X and Y chromosomes were genetically identical, derived from the C57BL/6 strain, so that dosage alone was manipulated. It is interesting to speculate that both dosage and genetic variation in these X and Y chromosome genes may influence lipid levels.
Our genetic analysis of sexual dimorphism of lipoprotein levels has limitations imposed by the necessity of using a mouse model. It is well established that mice and humans differ in several aspects of lipoprotein metabolism.35 When fed a chow diet, mice carry approximately two-thirds of plasma cholesterol in HDL particles, whereas humans carry a similar proportion in LDL particles. In the current study, some mice were fed a cholesterol-enriched diet to increase the levels of LDL cholesterol, but since mice do not express cholesteryl ester transfer protein, which promotes the transfer of cholesteryl esters and triglycerides between lipoprotein particles, species differences in the metabolism of circulating lipoproteins persist. Relevant to the effects of gonadal hormones on lipoprotein metabolism, it has been demonstrated that estradiol administration produces different effects on HDL-C levels in mice and humans.36,37 Despite the known species differences in lipoprotein metabolism, the genetic loci that control HDL-C levels in mouse and human exhibit a substantial degree of overlap,38 raising the possibility that fundamental genetic influences on lipoprotein physiology are shared between mouse and man.
In conclusion, our studies demonstrate that sexual dimorphism in lipid levels is a result of interactions between gonadal hormones, sex chromosome complement, and diet. The results further indicate that XX chromosome complement has a major influence on HDL-C levels, irrespective of diet or gonadal status. Future studies with FCG mice will facilitate the identification of sex-dependent biomarkers of disease associated with altered lipid levels, such as atherosclerosis. Such studies are crucial for improving the assessment and treatment of cardiovascular disease risk in men and women.
Supplementary Material
Significance.
Lipid profiles are an important indicator of the metabolic syndrome. Reports of sexually dimorphic LDL and HDL-C levels suggest regulation by sex hormones. Here, we show that the sex chromosome complement is also a key factor in modulating plasma lipid levels. HDL-C is consistently elevated in mice with two X chromosomes compared to mice with XY sex chromosomes, regardless of diet or circulating sex hormone levels. These findings are important for understanding cardiovascular disease risk in both men and women.
Acknowledgments
Mouse antibody against ApoB was a generous gift from Dr. Stephen G. Young (UCLA).
Sources of Funding: The authors gratefully acknowledge support from the National Institute of Diabetes and Digestive and Kidney Diseases (National Public Health Service R01 DK83561 to A.P.A. X.C., and K.R.), Ruth L. Kirschstein National Research Service Award (GM007185 to J.C.L.), and California Tobacco-related Disease Research Program (22RT-0095 to M.N.O.). Mouse Metabolic Phenotyping Center, Vanderbilt University, Nashville, TN was supported in part by grant U24 DK059637.
Abbreviations
- HDL
high density lipoprotein
- HDL-C
high density lipoprotein cholesterol
- LDL
low density lipoprotein
- LDL-C
low density lipoprotein cholesterol
- TG
triglyceride
- FFA
free fatty aci
- FCG
four core genotypes
Footnotes
Disclosures: Michael N. Oda is founder an holds an ownership stake in Seer BioLogics, Inc. This did not influence his or his laboratory's interpretation or presentation of results.
References
- 1.Kooner JS, Chambers JC, Aguilar-Salinas CA, Hinds DA, Hyde CL, Warnes GR, Gómez Pérez FJ, Frazer KA, Elliott P, Scott J, Milos PM, Cox DR, Thompson JF. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet. 2008;40:149–151. doi: 10.1038/ng.2007.61. [DOI] [PubMed] [Google Scholar]
- 2.Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D'Agostino RB, Wilson PWF, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. 2004;50:1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert CM, Rogers NL, Remsberg KE, Sun SS, Chumlea WC, Demerath EW, Czerwinski SA, Towne B, Siervogel RM. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes (Lond) 2006;30:251–260. doi: 10.1038/sj.ijo.0803129. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 11.Lenfant F, Trémollières F, Gourdy P, Arnal JF. Timing of the vascular actions of estrogens in experimental and human studies: why protective early, and not when delayed? Maturitas. 2011;68:165–173. doi: 10.1016/j.maturitas.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20:342–353. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 13.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A Model System for Study of Sex Chromosome Effects on Sexually Dimorphic Neural and Behavioral Traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao S, Cole TG, Kitchens RT, Pfleger B, Schonfeld G. Genetic heterogeneity of lipoproteins in inbred strains of mice: Analysis by gel-permation chromatography. Metabolism. 1990;39:155–160. doi: 10.1016/0026-0495(90)90069-o. [DOI] [PubMed] [Google Scholar]
- 18.Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigena B. Atherosclerosis and Plasma and Liver Lipids in Nine Inbred Strains of Mice. Lipids. 1993;28:599–605. doi: 10.1007/BF02536053. [DOI] [PubMed] [Google Scholar]
- 19.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavigiolio G, Geier EG, Shao B, Heinecke JW, Oda MN. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: a potential target for oxidative generation of dysfunctional high density lipoproteins. J Biol Chem. 2010;285:18847–18857. doi: 10.1074/jbc.M109.098434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borja MS, Zhao L, Hammerson B, Tang C, Yang R, Carson N, Fernando G, Liu X, Budamagunta MS, Genest J, Shearer GC, Duclos F, Oda MN. HDL-apoA-I exchange: rapid detection and association with atherosclerosis. In: Kocher O, editor. PLoS One. Vol. 8. 2013. p. e71541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eicher E, Hale D, Hunt P, Lee B, Tucker P, King T, Eppig J, Washburn L. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontush A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc Res. 2014;103:341–349. doi: 10.1093/cvr/cvu147. [DOI] [PubMed] [Google Scholar]
- 25.Oda MN. High-density lipoprotein cholesterol: origins and the path ahead. Curr Opin Endocrinol Diabetes Obes. 2015;22:133–141. doi: 10.1097/MED.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 26.Soulat D, Bürckstümmer T, Westermayer S, Goncalves A, Bauch A, Stefanovic A, Hantschel O, Bennett KL, Decker T, Superti-Furga G. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J. 2008;27:2135–2146. doi: 10.1038/emboj.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schröder M, Baran M, Bowie AG. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008;27:2147–2157. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder M. Human DEAD-box protein 3 has multiple functions in gene regulation and cell cycle control and is a prime target for viral manipulation. Biochem Pharmacol. 2010;79:297–306. doi: 10.1016/j.bcp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, Li J, Rao A, Shi Y. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447:601–605. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- 30.Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007;318:447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- 31.Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, Shi Y. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–94. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 32.Hong S, Cho YW, Yu LR, Yu H, Veenstra TD, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci U S A. 2007;104:18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borck G, Shin BS, Stiller B, et al. eIF2γ mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol Cell. 2012;48:641–646. doi: 10.1016/j.molcel.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suto J, Satou K. Effect of the Y chromosome on plasma high-density lipoprotein-cholesterol levels in Y-chromosome-consomic mouse strains. BMC Res Notes. 2014;7:393. doi: 10.1186/1756-0500-7-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaefer EJ, Foster DM, Zech LA, Lindgren FT, Brewer HB, Levy RI. The effects of estrogen administration on plasma lipoprotein metabolism in premenopausal females. J Clin Endocrinol Metab. 1983;57:262–267. doi: 10.1210/jcem-57-2-262. [DOI] [PubMed] [Google Scholar]
- 37.Tang JJ, Srivastava RA, Krul ES, Baumann D, Pfleger BA, Kitchens RT, Schonfeld G. In vivo regulation of apolipoprotein A-I gene expression by estradiol and testosterone occurs by different mechanisms in inbred strains of mice. J Lipid Res. 1991;32:1571–1585. [PubMed] [Google Scholar]
- 38.Wang X, Paigen B. Genetics of variation in HDL cholesterol in humans and mice. Circ Res. 2005;96:27–42. doi: 10.1161/01.RES.0000151332.39871.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.