Abstract
[Purpose] The purpose of this study was to investigate whether early hyperbaric oxygen is useful in rats with permanent cerebral ischemia, and whether its mechanism relates to the inhibition of the tumor necrosis factor-alpha-protein kinase C-alpha pathway. [Subjects] Healthy, male Sprague-Dawley rats (N = 108) were the subjects. [Methods] After middle cerebral artery occlusion models were successfully made, rats were randomly divided into sham-operated, cerebral ischemia, and hyperbaric oxygen groups. At 4 and 12 hours after modeling, the volume of cerebral infarction was determined by triphenyltetrazolium chloride staining, and brain water content was measured using the dry and wet method. The expression of tumor necrosis factor-alpha and protein kinase C-alpha in the ischemic penumbra tissue was measured using Western blot analysis. [Results] The data showed that at 4 and 12 hours after modeling, cerebral infarct volume and brain water content decreased in the hyperbaric oxygen group, and expression of tumor necrosis factor-alpha and phospho-protein kinase C-alpha in the ischemic penumbra tissue also decreased. [Conclusion] Our study demonstrates that early hyperbaric oxygen therapy has protective effects on brain tissue after cerebral ischemia, possibly via inhibition of tumor necrosis factor-alpha and phospho-protein kinase C-alpha.
Key words: Hyperbaric oxygen therapy, Cerebrovascular protection, Cerebral ischemic rats
INTRODUCTION
Cerebral ischemia is the third most prevalent disease that threatens human health. It seriously affects the patient’s quality of life and increases the burden on the family. There are few effective therapies for cerebral ischemia except recombinant tissue plasminogen activator which has strict indications for use as medication. The application of hyperbaric oxygen (HBO) in cerebral ischemia has shown to have certain effects on motor function, aphasia, cognition, and post-stroke depression1). HBO can improve tissue hypoxia, maintain cellular energy metabolism, protect the blood-brain barrier, and relieve cerebral edema. Many recent studies have examined HBO treatment of cerebral ischemia, based on experimental models of ischemia and reperfusion. However, in the clinic, cerebral ischemia in humans is most likely permanent ischemia and has an optimal time window for HBO treatment within 2 hours after the ischemic attack2,3,4,5); 3–6 hours after the ischemic attack, HBO has no effect on the infarct volume and neurological function6). In this experiment, we created permanent cerebral ischemia in rat model to explore whether HBO has a protective effect on brain tissue, and whether its protective mechanism involves the inhibition of phospho-protein kinase C-alpha (p-PKCα) and tumor necrosis factor-alpha (TNF-α) expression.
SUBJECTS AND METHODS
Adult male Sprague-Dawley rats (weight, 250–300 g) were purchased from the Experimental Animal Center of China Medical University. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the China Medical University. Rats were housed in laboratory cages and maintained on a 12-hour light/dark cycle with free access to food and water throughout the study period.
One hundred and eight healthy Sprague-Dawley rats were randomly divided into six groups of 18: sham-operated (sham) 4-hour and 12-hour groups, cerebral ischemia (CI) 4-hour and 12-hour groups, and HBO 4-hour and 12-hour groups. After middle cerebral artery occlusion (MCAO) models were successfully made, rats in the HBO group were immediately given HBO therapy (2 ATA for 1 hour). Rats in the CI group were not given HBO therapy. Rats in the sham-operated group were only sutured with 1 cm thread without blocking the middle cerebral artery or altering the HBO therapy.
As monitored by laser Doppler blood flow assessments, a rat model of focal CI was made by improved Longa suture method7). Briefly, rats were anesthetized by being given an intraperitoneal injection of 10% chloral hydrate (360 mg/kg), and their body temperatures (as monitored with a rectal probe) were maintained at 37 ± 0.5 °C, using a heating lamp during the experiment. After successful anesthesia, the rats were fixed in a supine position. Through a ventral midline incision in the neck, the left common carotid artery (CCA), the internal carotid artery (ICA), and the external carotid artery (ECA) were exposed. A nylon filament (diameter 0.286 mm, length 5 cm) with a head-end grinding round and covered with silica gel was inserted into the CCA and gently advanced into the ICA, approximately 18–20 mm from the carotid bifurcation, until meeting slight resistance. The nylon filament was then fixed in the CCA and the skin was sutured.
To confirm proper occlusion of the left middle cerebral artery, a laser Doppler probe (moorLAB, Moor Instruments, UK) was fixed on the skull (1 mm posterior to the bregma and 5 mm from the midline on the left side) to monitor regional cerebral blood flow (rCBF) in the area supplied by the middle cerebral artery. rCBF flux measurements were taken immediately before and after occlusion. The occlusion was considered successful if there was a 30% decrease in local cortical blood flow compared to the baseline pre-occlusion value8, 9). The animals that did not comply with the above criteria were excluded from the study.
Cerebral infarct volume was measured using triphenyltetrazolium chloride (TTC) staining10). At corresponding time points, rats in each group were sacrificed by giving them an overdose of chloral hydrate. Their brains were rapidly removed, frozen at −20 °C, and sectioned into six coronal slices (2 mm thick) that were immediately immersed in 2% 2,3,5- TTC (Sigma-Aldrich, USA) saline solution at 37 °C for 30 minutes. Normal brain tissue was stained red, whereas infarct areas were not stained and remained white. Stained tissues were then fixed in 4% paraformaldehyde, photographed on both sides, and quantified for infarct area using an image analysis system (NIS-Elements BR 3.0, Nikon Instruments, Melville, NY, USA). The degree of infarct volume was calculated as the percentage of the damaged area of the total area.
Brain water content was determined using a dry and wet method. Rats in each group were sacrificed by decapitation at corresponding time points. After the brains were removed in total, the hemispheres were separated along the interhemispheric plane. Both hemispheres were weighed to assess their wet weights and then dried for 24 hours at 100 °C to determine their dry weights. Water content in both ischemic and nonischemic hemispheres was obtained by the following calculations: Hemispheric water content (%) = (wet weight − dry weight) / wet weight × 100%.
Western blots were used to detect the protein expression levels of TNF-α, p-PKCα, and PKCα. The rats in each group were sacrificed immediately by decapitation at corresponding time points. Brain tissue in ischemic penumbra was obtained immediately. Equal amounts of proteins were separated by size in 6% and 12% SDS-polyacrylamide gels, electrphoretically transferred to nitrocellulose, and then probed overnight at 4 °C with rabbit polyclonal anti-TNF-α (diluted 1:1000) (Abcam,USA), rabbit polyclonal anti-p-PKCα (diluted 1:1000) (Abcam, USA), and rabbit monoclonal anti-PKCα (diluted 1:2000) (Abcam, USA) antibodies. The next day, the membranes were incubated with their respective secondary horseradish peroxidase-conjugated antibodies for 2 hours at room temperature. Protein bands were visualized by chemiluminescence (ECL Kit, Beyotime Inc., China) and scanned with MicroChemi 4.2 (Bio-Imaging Systems Ltd., Jerusalem, Israel). Integrated density values (IDVs) were calculated using a computerized image analysis system (Gel-Pro Analyzer 32) and normalized to that of β-actin (β-actin antibody 1:500, Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
The experiments were repeated at least three times, and all results were presented as mean ± SD. Differences between the two groups were statistically analyzed using t-tests. Differences among multiple groups were statistically analyzed using one-way ANOVA, followed by the Bonferroni test; p<0.05 was considered statistically significant.
RESULTS
Early HBO therapy reduced cerebral infarct volume in rats with permanent CI. At 4 and 12 hours, the percentage of infarct volume in the HBO group was reduced significantly compared with the CI group (p<0.05) (Table 1). Values represent means ± SD (n = 6 for each group).
Table 1. Infarct volume percentage in each group.
| 4 hour | 12 hour | |
|---|---|---|
| Sham-operated group | 0 | 0 |
| CI group | 8.40±1.46% | 21.15±1.89% |
| HBO group | 2.40±0.23%﹡ | 14.54±0.88%﹡ |
*p<0.05. CI: cerebral ischemia; HBO: hyperbaric oxygen
Early HBO reduced brain water content. The brain water content in the ischemic hemisphere of the CI 4-hour and 12-hour groups was significantly higher than in the sham-operated and HBO groups (p<0.05) (Table 2). Values represent means ± SD (n = 6 for each group).
Table 2. Average brain water content percentage in each group.
| 4 hour | 12 hour | ||
|---|---|---|---|
| Sham-operated group | Left | 78.2 ± 0.1% | 78.2 ± 0.2% |
| Right | 78.2 ± 0.1% | 78.3 ± 0.1% | |
| CI group | Left | 79.6 ± 0.4%* | 81.7 ± 0.4%* |
| Right | 78.3 ± 0.2% | 78.3 ± 0.1% | |
| HBO group | Left | 78.6 ± 0.4% | 80.9 ± 0.4% |
| Right | 78.2 ± 0.1% | 78.3 ± 0.2% |
*p<0.05. CI: cerebral ischemia; HBO: hyperbaric oxygen
Figure 1 shows that early HBO decreased the expression of TNF-α and p-PKCα. Western blot analysis showed that, compared with the sham-operated group, the expression of TNF-α and p-PKCα in the CI group increased significantly. In contrast, the expression of TNF-α and p-PKCα decreased significantly in the HBO group compared with the CI group. An analysis of TNF-α to β-actin IDVs and p-PKCα to PKCα IDVs revealed that the expression of TNF-α and p-PKCα in ischemic penumbra tissue in CI group rats increased significantly compared with the sham and HBO groups (p<0.05) (Tables 3 and 4).
Fig. 1.
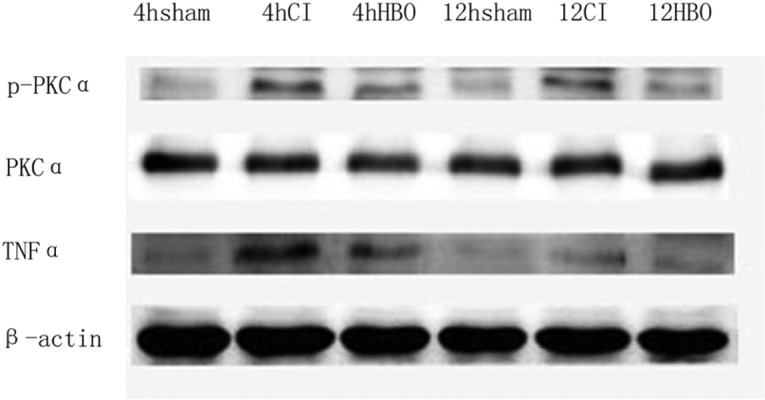
Western blot-detected expression of TNF-α, β-actin, PKCα, and p-PKCα in each group. Representative photographs of Western blot bands in each group. TNF-α: tumor necrosis factor-alpha; β-actin: beta-actin; PKCα: protein kinase C-alpha; p-PKCα: phospho-protein kinase C-alpha; CI: cerebral ischemia; HBO: hyperbaric oxygen
Table 3. The ratios of TNF-α to β-actin integrated density values in each group.
| 4 hour | 12 hour | |
|---|---|---|
| Sham-operated group | 0.12 ± 0.02 | 0.12 ± 0.02 |
| CI group | 0.27 ± 0.03* | 0.19 ± 0.021* |
| HBO group | 0.14 ± 0.125 | 0.13 ± 0.018 |
*Compared with the sham and HBO groups, the ratios of TNF-α integrated density value in the CI group significantly increased (p<0.05). Values represent means±SD (n=6 for each group). TNF-α: tumor necrosis factor-alpha; β-actin: beta-actin; CI: cerebral ischemia; HBO: hyperbaric oxygen
Table 4. The ratios of p-PKCα to PKCα integrated density values in each group.
| 4 hour | 12 hour | |
|---|---|---|
| Sham-operated group | 0.09 ± 0.03 | 0.10 ± 0.02 |
| CI group | 0.45 ± 0.018* | 0.37 ± 0.022* |
| HBO group | 0.18 ± 0.025 | 0.15 ± 0.028 |
*Compared with the sham and HBO groups, the ratios of p-PKCα IDV increased significantly (p<0.05). Values represent means ±SD (n=6 for each group). PKCα: protein kinase C-alpha; p-PKCα: phospho-protein kinase C-alpha; CI: cerebral ischemia; HBO: hyperbaric oxygen
DISCUSSION
HBO refers to breathing pure oxygen in an environment in which the atmospheric pressure is higher than normal; the method of inhaling HBO in disease treatment is called HBO therapy. HBO can significantly increase the uptake and utilization of oxygen in organisms to increase their blood oxygen content, increase oxygen partial pressure, and enhance oxygen-diffusing capacity; thus, HBO is an effective means of treating various hypoxic diseases. Tissue hypoxia after focal CI is one of the main causes of cell injury. HBO can improve tissue oxygen supply, maintain cellular energy metabolism, protect the blood-brain barrier, and relieve cerebral edema; therefore, it is considered to be a promising method for treating CI. It is well known that the ischemic penumbra surrounding the ischemic core can turn into normal area with blood and oxygen supplies, rather that turning into an area of infarct. Consequently, improving the supply of oxygen in the ischemic penumbra as soon as possible is the theoretical reason for using HBO in the treatment of CI11). In this experiment, HBO therapy was given immediately after modeling, thereby maintaining oxygen and blood supplies in ischemic brain tissue during the formation of CI, and consequently reducing the ischemic penumbra. At 4 and 12 hours after modeling, TTC (a marker of cellular respiration) staining was detected. It was found that cerebral infarct volumes in rats in the HBO group were obviously reduced. Similar studies showed that HBO therapy should be performed in the early stages of CI. Sunami et al. found that infarct volume decreased 18% in rats with permanent CI following administration of HBO 10 minutes after ischemia5). Similarly, Schäbitz et al. confirmed on magnetic resonance imaging (DWI, PI, T2) that HBO therapy was effective in permanent CI if given within 2 hours after ischemia and led to no oxidative damage4). Badr et al. also performed HBO experiments at different time points after CI and found that only HBO therapy was able to improve nerve function in transient CI within 6 hours12). Thus, HBO for the treatment of CI has a short therapeutic time window. If patients’ condition permits, HBO should be given as early as possible to reduce the volume of cerebral infarction and symptoms.
TNF-α is a cytokine with a wide range of biological functions, including immune response and inflammatory reaction. After CI, TNF-α increases rapidly and infiltrates the cortex and striatum, playing a protective role in brain tissue in early ischemic stages. However, overexpression of TNF may increase the permeability of the blood-brain barrier and injure brain tissue13). It has been shown that repeated HBO therapy in rats with 3-hour reperfusion after 90 minutes of CI could reduce ischemic brain tissue inflammation, downregulate the expression of inflammatory factors TNF-α and interleukin (IL)-1β, and upregulate the anti-inflammatory factor IL-1014). HBO for traumatic brain injury could also inhibit expression of the inflammatory factor TNF-α, inhibit glial proliferation, and promote the regeneration of blood vessels and nerves15). PKC, an important protein kinase in vivo, is closely connected to the formation and regulation of tight junctions. PKC comprises a variety of isozymes with different biological characteristics. Activated PKCα, a PKC isoform expressed in almost all vessels16), can damage cytoskeletal components, facilitating the formation of brain edema after CI. Prior research showed that increased endothelial permeability due to ischemia and inflammatory factors was PKCα-dependent17, 18). One previous study showed that the activation of TNF-α could cause phosphorylation and translocation of downstream PKCα19). Our Western blot analysis results likewise showed that the expression of TNF-α and p-PKCα both increased during CI. Under the therapeutic protection of HBO, the expression of TNF-α and p-PKCα decreased significantly, verifying that TNF-α and PKCα signaling might be one of the mechanisms through which HBO protects brain tissue.
REFERENCES
- 1.Yan D, Shan J, Ze Y, et al. : The effects of combined hyperbaric oxygen therapy on patients with post-stroke depression. J Phys Ther Sci, 2015, 27: 1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corkill G, Van Housen K, Hein L, et al. : Videodensitometric estimation of the protective effect of hyperbaric oxygen in the ischemic gerbil brain. Surg Neurol, 1985, 24: 206–210. [DOI] [PubMed] [Google Scholar]
- 3.Lou M, Zhang H, Wang J, et al. : Hyperbaric oxygen treatment attenuated the decrease in regional glucose metabolism of rats subjected to focal cerebral ischemia: a high resolution positron emission tomography study. Neuroscience, 2007, 146: 555–561. [DOI] [PubMed] [Google Scholar]
- 4.Reitan JA, Kien ND, Thorup S, et al. : Hyperbaric oxygen increases survival following carotid ligation in gerbils. Stroke, 1990, 21: 119–123. [DOI] [PubMed] [Google Scholar]
- 5.Schäbitz WR, Schade H, Heiland S, et al. : Neuroprotection by hyperbaric oxygenation after experimental focal cerebral ischemia monitored by MRI. Stroke, 2004, 35: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 6.Sunami K, Takeda Y, Hashimoto M, et al. : Hyperbaric oxygen reduces infarct volume in rats by increasing oxygen supply to the ischemic periphery. Crit Care Med, 2000, 28: 2831–2836. [DOI] [PubMed] [Google Scholar]
- 7.Longa EZ, Weinstein PR, Carlson S, et al. : Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 1989, 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura Y, Ito T, Saavedra JM: Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke, 2000, 31: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 9.Takagi K, Ginsberg MD, Globus MY, et al. : Changes in amino acid neurotransmitters and cerebral blood flow in the ischemic penumbral region following middle cerebral artery occlusion in the rat: correlation with histopathology. J Cereb Blood Flow Metab, 1993, 13: 575–585. [DOI] [PubMed] [Google Scholar]
- 10.Bederson JB, Pitts LH, Germano SM, et al. : Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke, 1986, 17: 1304–1308. [DOI] [PubMed] [Google Scholar]
- 11.Michalski D, Härtig W, Schneider D, et al. : Use of normobaric and hyperbaric oxygen in acute focal cerebral ischemia—a preclinical and clinical review. Acta Neurol Scand, 2011, 123: 85–97. [DOI] [PubMed] [Google Scholar]
- 12.Badr AE, Yin W, Mychaskiw G, et al. : Dual effect of HBO on cerebral infarction in MCAO rats. Am J Physiol Regul Integr Comp Physiol, 2001, 280: R766–R770. [DOI] [PubMed] [Google Scholar]
- 13.Gong C, Qin Z, Betz AL, et al. : Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res, 1998, 801: 1–8. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Duan XS, Xu LJ, et al. : Interleukin-10 mediates the neuroprotection of hyperbaric oxygen therapy against traumatic brain injury in mice. Neuroscience, 2014, 266: 235–243. [DOI] [PubMed] [Google Scholar]
- 15.Lin KC, Niu KC, Tsai KJ, et al. : Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg, 2012, 72: 650–659. [DOI] [PubMed] [Google Scholar]
- 16.Salamanca DA, Khalil RA: Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol, 2005, 70: 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta D, Rahman A, Malik AB: Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem, 2001, 276: 22614–22620. [DOI] [PubMed] [Google Scholar]
- 18.Sandoval R, Malik AB, Minshall RD, et al. : Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol, 2001, 533: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, Hannun YA, Obeid LM: Functional dichotomy of protein kinase C (PKC) in tumor necrosis factor-α (TNF-α ) signal transduction in L929 cells. Translocation and inactivation of PKC by TNF-α. J Biol Chem, 2000, 275: 29290–29298. [DOI] [PubMed] [Google Scholar]


