Abstract
OBJECTIVE
To determine the degree to which susceptibility to different types of semantic interference may reflect the earliest manifestations of early Alzheimer disease (AD) beyond the effects of global memory impairment.
METHODS
Normal elderly (NE) subjects (n= 47), subjects with amnestic mild cognitive impairment (aMCI: n=34) and 40 subjects with probable AD were evaluated using a unique cued recall paradigm that allowed for an evaluation of both proactive and retroactive interference effects while controlling for global memory impairment (LASSI-L procedure).
RESULTS
Controlling for overall memory impairment, aMCI subjects had much greater proactive and retroactive interference effects than NE subjects. LASSI-L indices of learning using cued recall evidenced high levels of sensitivity and specificity with an overall correct classification rate of 90%. These provided better discrimination than traditional neuropsychological measures of memory function.
CONCLUSION
The LASSI-L paradigm is unique and unlike other assessments of memory in that items presented for cued recall are explicitly presented, and semantic interference and cuing effects can be assessed while controlling for initial level of memory impairment. This represents a powerful procedure allowing the participant to serve as his or her own control. The high levels of discrimination between subjects with aMCI and normal cognition that exceeded traditional neuropsychological measures makes the LASSI-L worthy of further research in the detection of early AD.
Keywords: Early Detection, Early Alzheimer’s, MCI, Semantic Interference, Semantic Cuing, Proactive interference, Memory
INTRODUCTION
The early diagnosis of neurodegenerative conditions such as Alzheimer’s disease (AD) is extremely important given its increasing prevalence with age and the continued development of pharmacological intervention approaches that will most likely have the greatest efficacy in the earliest stages of the disorder (1). Despite recent advances in the identification of biomarkers such as amyloid imaging and cerebral spinal fluid analysis as diagnostic tools, these techniques are not available or feasible for clinicians in routine clinical settings. Thus clinicians typically rely on traditional neuropsychological screening and diagnostic measures. One of the primary limitations of many of these measures is that they were initially developed for the assessment of dementia and not well suited to assess the earliest stages of cognitive impairment. As such, more sensitive neuropsychological assessment measures are required to identify the earliest neurocognitive deficits associated with AD and other neurodegenerative disorders of the brain (2).
Amnestic Mild Cognitive Impairment (aMCI) is now commonly accepted to reflect a prodrome of Alzheimer’s disease (3). Further, Loewenstein et al., (4) have identified PreMCI states in community dwelling elderly, which confer a greater risk for cognitive decline over time. Declines in memory as measured by delayed recall or rate of forgetting on verbal episodic memory tasks have been found to be a sensitive indicator of mild AD (5,6,7,8) and a predictor of progression to dementia among elders who do not meet criteria for dementia upon initial evaluation (9,10,11). However, it has become increasingly recognized that failures in learning across repeated trials may be as sensitive as or more sensitive than delayed recall measures in the identification of MCI (12, 13). In fact, AD patients have been found to have specific deficiencies on memory measures after the provision of semantic cues at both acquisition and retrieval of to-be-remembered targets. Semantic cues involve providing categories to help facilitate recall such as providing category cues at encoding and recall such as animal, fruits or another category to which the too-be remembered material belongs. Failure to benefit from semantic cuing has proven to be superior to standard memory measures in the detection of early AD, since AD patients do not benefit as much as cognitively normal elderly from these techniques (14, 15, 16). The inability of AD patients to profit from semantic cues may reflect the degradation of semantic memory networks (17, 18).
An extremely promising area of research in aMCI and early AD diagnosis is the role of semantic interference effects on memory performance in incipient and mild AD patients. Proactive interference (PI) refers to old learning interfering with new learning while retroactive interference (RI) refers to new learning interfering with old learning. These interference effects can be best observed when the subject has to learn two competing lists of targets that share semantic categories. Bondi and co-workers (19) showed that measures of proactive interference (PI) and retroactive interference (RI), based on overlap of 8 (of 16) targets on two learning lists of the California Verbal Learning Test (CVLT) (20), could distinguish between MCI and cognitively normal patients and between MCI and AD patients. Other investigations have shown the presence of PI in mild AD patients (21, 22,23, 24, 25). In contrast, other studies evaluating the build-up and release from PI have suggested lack of PI effects (26, 27) or even less vulnerability to PI (28) in AD patients as compared to cognitively normal subjects or Parkinson disease subjects (29, 30). It has been suggested that sufficient global memory impairment in certain AD groups may simply preclude the detection of semantic interference effect in this population. Indeed, this may account for the mixed results found in other studies (24).
Previous paradigms have largely failed to account for differences in initial recall among aMCI and mild AD subjects and have relied on passive rather than more active encoding of to-be-remembered information (i.e., CVLT-II, memory for story passages). Passive encoding involves simply providing to-be-remembered information to the participant while active encoding directly involves the subject in the learning process such as identifying some aspect of the stimuli (e,g., musical instrument versus clothing) that will aid in future recall. In addition, the evaluation of interference effects have been typically based on a limited number of shared semantic categories without explicitly specifying the shared category (i.e., CVLT-II).
To address these concerns, we previously developed a validated paradigm called the Loewenstein-Acevedo Scales of Semantic Interference and Learning (LASSI-L) (31). The LASSI-L addresses the aforementioned difficulties in previous studies by: a) explicitly identifying to the person, the semantic categories around which learning should be organized; b) using a second list in which every to-be-remembered target is semantically related to targets in the original list; c) more active encoding of information to be remembered by increasing depth of initial processing; d) evaluating free recall versus semantic cues at the end of retrieval trials; and e) allowing for the adjustment of initial memory strength in evaluating the ability to benefit from semantic cues and susceptibility to proactive and retroactive interference.
In an initial validation study of the LASSI-L subscales, we found high test-retest reliabilities (r=.60 to r=.89) among elderly persons with aMCI and early dementia attributable to AD as well as high concurrent and discriminative validities (31).
In the current investigation, we evaluated the comparative performance of cognitively intact normal elderly (NE) subjects with aMCI and mild AD across subscales of the LASSI-L to examine differences in various memory processes while accounting for degree of initial memory deficits. We hypothesized that even after adjusting for initial deficits in retrieval capacity, aMCI subjects relative to NE subjects would show less benefit from semantic cuing provided by giving the participants categories (e.g., fruits, articles of clothing, musical instruments) to both organize initial learning and subsequent recall. We also hypothesized decreased learning effects, and increased susceptibility to proactive interference (initial learning and recall of List A interfering with learning and recall of List B) and retroactive interference (new learning and recall of List B interfering with subsequent recall of List A) under cued recall conditions.
METHODS
We recruited 121 subjects from a study investigating longitudinal changes associated with mild cognitive impairment and normal aging as well as from the memory disorders clinic at the Wien Center for Alzheimer’s Disease and Memory Disorders at Mount Sinai Medical Center. Thirty-four subjects (23 males and 11 females) were diagnosed with amnestic mild cognitive impairment (aMCI) by Petersen’s (33) criteria. This includes a memory complaint by the patient and preferably an informant, objective memory deficits on clinical evaluation and cognitive deficits not sufficient to interfere with social and/or occupational function by DSM-IV criteria. All of these subjects obtained a global Clinical Dementia Rating Score (CDR) (34) of .5, equivalent to MCI. The mean age of this group was 77.9 years (SD= 6.8) while mean level of education was 14.32 years (SD=4.3). Forty subjects (22 males and 18 females) met DSM-IV (35) criteria for dementia and NINCDS-ADRDA (36) criteria for probable Alzheimer’s disease (AD). The mean age of this group was 80.7 years (SD= 6.4) while mean level of education was 12.82 years (SD=4.4). Forty-seven individuals (17 males and 30 females) were normal elderly (NE) participants with no evidence of cognitive impairment on clinical examination, a Folstein Mini-Mental State (MMSE) (37) score of 27 or above, and no scores one half standard deviation or lower on the Fuld Object Memory Evaluation (38). The mean age of subjects in the NE group was 78.0 years (SD=4.7) while mean level of education was 14.02 years (SD=3.7). Among aMCI subjects, 67.6% spoke English as their primary language, 50.0% of AD subjects spoke English as their primary language and 70.8% of NE subjects spoke English as their primary language.
There were no significant group differences with regards to age [F (2, 119) = 2.92; p=.06] or educational attainment [F (2, 119) = 2.92; p=.22]. There were statistically significant group differences with regards to MMSE scores [F (2, 119) = 173.88; p<.001. As expected, the cognitively normal group (MMSE scores of 27 or above) had the highest MMSE scores (MMSE= 28.5: SD=.9: range=27–30); followed by the aMCI group (MMSE= 25.6:SD=2.0; range=23–30) and the dementia group (MMSE= 21.0:SD=2.5; range=16–27). A significant effect was observed for gender [X2 (df=2)=8.7; p<.02) indicating a greater percentage of males in the aMCI group than the AD and normal control group. There was no significant effect for primary language (English versus Spanish) [X2 (df=2)=4.5; p>.10).
PROCEDURES
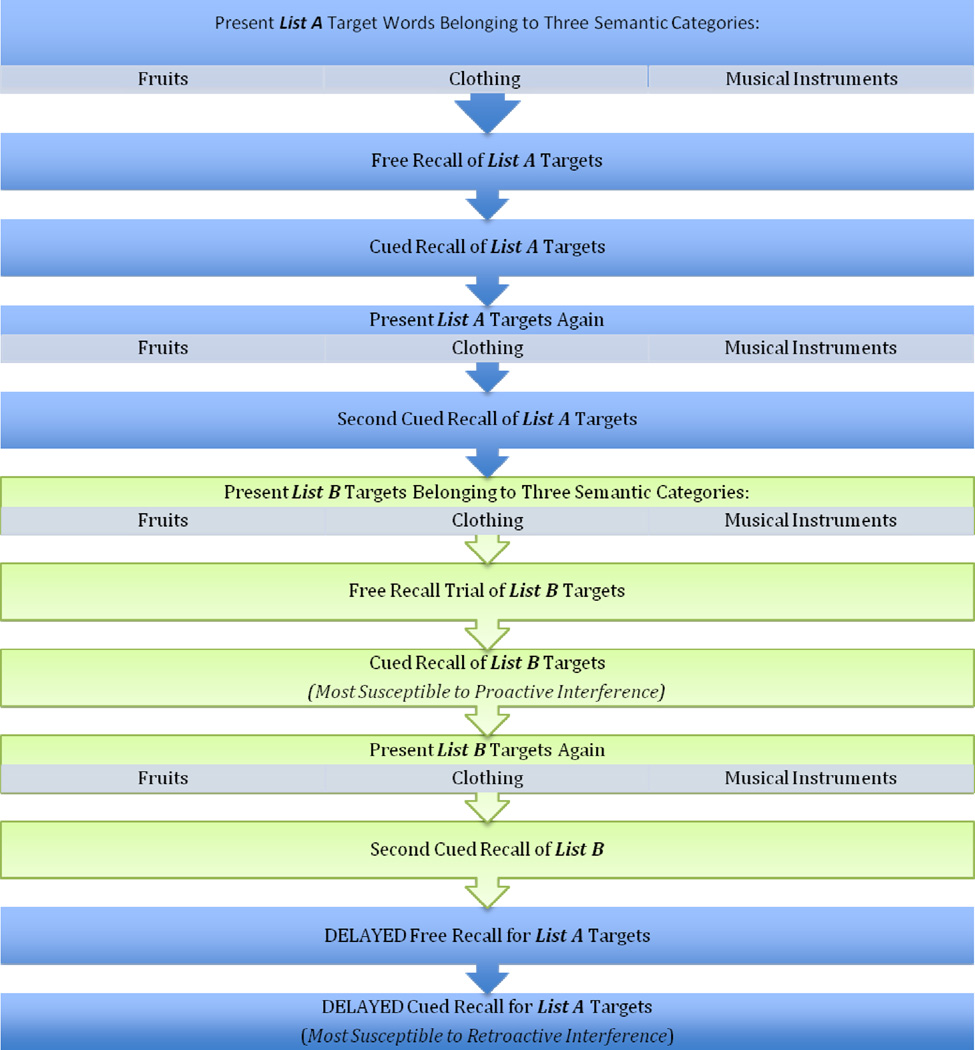
All subjects were administered the Loewenstein-Acevedo Scales of Semantic Interference and Learning (LASSI-L) (31). The LASSI-L instructs a person to remember a list of 15 common words that are fruits, musical instruments or articles of clothing (five words per category). The person is asked to read the words for the target list out loud as each is presented individually at 4-second intervals. The words are printed using a 48-size font. In the unlikely event that the person cannot correctly read the word, the word is read by the examiner and the person is asked to repeat. If a person does not know the meaning of one of the words (also unlikely), the examiner tells the person what category the word belongs to (e.g., “Lime is a fruit”) and the person is asked to repeat the word. After the person has read all 15 words, they are asked to recall the words. After free recall has ended, they are presented with each category cue (e.g., clothing) and asked to recall the words that belonged to that category. Participants are then presented with target stimuli for a second learning trial with subsequent cued recall to strengthen the acquisition and recall of the List A Targets.
The exposure to the semantically related list (i.e., List B) is then conducted in the same manner as exposure to List A. List B consists of 15 words different from List A, 5 of which belong to each of the three categories used in List A (i.e., fruits, musical instruments, and articles of clothing). Following the presentation of the List B words, the person is asked to free recall the List B words, assessing proactive interference effects. Then, each category cue is given and they are asked to recall each of the List B words that belonged to each of the categories. List B words are presented again, followed by a second category-cued recall trial. Finally, to assess retroactive interference they are asked to free recall the original List A words. This is followed by a category-cued recall trial of List A words. The exposure time for each word in all learning trials of Lists A and B is 4 seconds per word, that maximum allotted time for each free recall trials of Lists A and B is 60 seconds, and that maximum allotted time for cued recall of each of the individual categories for Lists A and B is 20 seconds. The sequence of LASSI-L administration is presented in Figure 1.
FIGURE 1.
LASSI-L ADMINISTRATION PROCEDURES
Statistical Methods
Subtests of the LASSI-L for different subject groups were analyzed using analyses of covariance (ANCOVAs) with initial free-recall entered in as a covariate. Post-hoc tests of means were conducted using the Scheffe’ procedure where the criteria for significance was p<.05. Intrusion errors were also analyzed using ANCOVA procedures. Sensitivity and specificity analyses were conducted using step-wise regression procedures. ROC analyses were conducted to examine the comparative areas under the ROC area for LASSI-L versus standard neuropsychological measures.
RESULTS
There were statistically significant group differences between groups with regards to initial free recall of the 15 words on List A [F (2,116) = 69.95; p<.001]. In fact, all measures of the LASSI-L showed high levels of statistical significance in distinguishing between groups (all ps <.001). However, our primary interest was to evaluate different aspects of semantic cuing and semantic interference effects, adjusted for initial free recall (global memory deficit). This was accomplished by entering the participant’s initial free recall trial for List A as a covariate in a series of one-way analyses of variance (ANOVA) models. Post-hoc tests of adjusted means were evaluated using the Sidak Procedure at p≤.05. For purposes of depicting average raw scores on the LASSI-L subscales, unadjusted means are presented in Table 1. Even after adjusting for initial free recall, there were statistically significant differences in List A1 cued recall, List A2 cued recall, List B1 cued recall and List B2 cued recall (See Table1). Post-hoc tests revealed that dementia subjects scored lower than aMCI and NE subjects on cued recall List A1. All diagnostic groups were statistically different with regards to cued List A2 with NE subjects scoring the highest and dementia subjects scoring the lowest. NE scored higher than aMCI and AD groups with regards to cued List B1 and cued List B2. After controlling for initial overall recall of List A1, there were no statistically significant differences between groups with regards to cued delay recall of List A (subject to retroactive interference due to presentation of List B).
Table 1.
LASSI-L Performance Among Different Diagnostic Groups
| Normal Elderly (NE: N=47) |
aMCI (N=34) | Dementia (N=40) | F-Value (2,116) | |
|---|---|---|---|---|
| Cued Recall 1 List A | 11.36a(SD=2.2) | 8.24a (SD=1.3) | 5.98b (SD=2.4) | 11.31*** |
| Cued Recall 2 List A | 13.83a (SD=1.2) | 10.50b (SD=2.0) | 8.10c (SD=2.9) | 16.33*** |
| List B Free Recall | 7.09 (SD=2.5) | 4.97 (SD=2.2) | 3.53 (SD=1.6) | 2.15 |
| List B Cued Recall 1 | 8.09a (SD=2.3) | 4.65b (SD=2.3) | 3.97b (SD=2.2) | 4.73** |
| List B Cued Recall 2 | 11.47a (SD=2.0) | 7.42b (SD=2.0) | 5.80b (SD=2.5) | 15.22*** |
| Short-Delay Recall List A Free Recall | 6.53a (SD= 3.1) | 2.67b (SD=2.3) | 1.64b (SD=1.7) | 5.43** |
| Short Delay Recall List A Cued Recall | 8.57 (SD=2.9) | 5.58 (SD=2.4) | 5.48 (SD= 2.4) | 1.65 |
Note: 1) All other LASSI-L indices are adjusted for scores on initial free recall for List A with Sidak Tests conducted at p≤.05 on adjusted means; 3) For clarity of presentation, all mean scores presented are unadjusted although post-hoc tests of means were conducted on mean values adjusted for the covariate (Initial free Recall-List A)
Intrusion Errors Controlling for Degree of Overall Memory Impairment
We examined intrusion errors for the different diagnostic groups by ANOVA models. As indicated in Table 2, there were significant group differences with regards to intrusion errors for Cued List A1 [F (2,119) 13.5; p< 001], Cued List A2 [F (2,196) =15.97; p<. 001], Short Delay Recall List A [F (2,118) =31.52; p<.001], Cued List B1 Intrusions [F (2, 116) =4.14; p<. 02]. In contrast, there were statistically significant differences for Cued List B1 Intrusions [F (2,119) =21.05; p<. 001] and Cued List B2 Intrusions [F (2,118) =19.94; p<. 001]. Post-hoc tests of means by the Sidak procedure indicated that both aMCI and dementia subjects patients made a greater number of intrusions than normal elderly during all of the cued recall trials. Further, dementia subjects committed a greater number of intrusion errors relative to a-MCI patients on Short Delay Recall List A and Cued List B2 Recall.
Table 2.
LASSI-L Intrusive Errors Among Different Diagnostic Groups
| Normal Elderly (NE: N=47) |
aMCI (N=34) |
Dementia (N=40) |
F-Value (2,116) |
|
|---|---|---|---|---|
| List A Cued Recall 1 Intrusions | .38a (SD=.53) | 2. 09b (SD=2.9) | 2.68b (SD=2.6) | 13.50*** |
| List A Cued Recall 2 Intrusions | .17a (SD=.38) | 1. 15b (SD=1.4) | 1.78b (SD=1.9) | 15.97*** |
| List B Cued Recall 1 Intrusions | 2.17a (SD=1.8) | 5.12b (SD=3.4) | 5.73b (SD= 3.1) | 21.05*** |
| List B Cued Recall 2 Intrusions | .90a (SD=1.0) | 2.36b (SD=2.5) | 3.93c (SD= 3.0) | 19.94*** |
| List A Delay Cued Recall Intrusions | 1.9a (SD=1.8) | 4.24b (SD=2.2) | 6.38c (SD= 3.5) | 31.52*** |
Note: 1) ***p<.001; 2) Post-hoc Sidak Tests conducted at p≤.05 on adjusted means
Do Diagnostic Groups Differ in their Degree of Proactive and Retroactive Interference Effects After Adjusting for Initial Recall Performance?
To further disentangle specific effects of proactive interference (PI) from the effects of general memory impairment, we calculated a free recall PI change score derived by subtracting the free recall score of List B from the obtained initial recall score of List A. ANCOVA models were employed to examine these change scores while adjusting for initial performance on Free List A to control for initial differences in memory performance. There was no group effect for the free recalls PI change score [F (2,116) =2.49; p>.08]. Similarly, a cued recall PI change score derived by subtracting the cued recall score for List B1 from the initial cued recall score of List A1. ANCOVA models were employed to examine these change scores while adjusting for initial performance on Cued List A. This yielded a statistically significant effect for cued recall PI change scores [F (2,116) < 5.04; p<.008]. Post-hoc test of means by the Sidak procedure indicated that the aMCI group evidenced greater proactive interference effects than NE subjects. Mild AD patients did not differ from the other study groups indicating that proactive interference effects appeared to be specific to the aMCI diagnostic group. A free recall retroactive interference (RI) change score derived by subtracting the short delay free recall score of List A from the obtained initial free recall score of List A. ANCOVA models were employed to examine these change scores while adjusting for initial performance on initial Free Recall of List A to control for initial differences in memory performance. This yielded a statistically significant group effect for the free recall RI change score [F (2,115) =5.65; p≤. 005]. Post-hoc test of means by the Sidak procedure indicated that the aMCI and mild AD patients showed greater deterioration in performance on short-delay free recall of List A relative to NE participants. We then calculated a cued recall RI change score by subtracting the delay cued recall of List A from the initial cued recall for List A. ANCOVA models were employed to examine these change scores while adjusting for initial performance on initial cued recall for List A to control for initial differences in memory performance. A statistically significant effect for cued recall RI change scores [F (2,115) =3.68; p<. 03] was observed. Post-hoc test of means by the Sidak procedure indicated that the aMCI group evidenced greater deterioration in performance on short delay cued recall of List A relative to NE participants. Mild AD patients did not differ in performance relative to those in the other study groups.
COMPARATIVE SENSITIVITY AND SPECIFICITY ANALYSES
We attempted to distinguish between aMCI and NE subjects by entering LASSI-L subscales into a step-wise logistic regression analyses. List A2 Cued Recall entered first in the model yielding a sensitivity of 87.9%, a specificity of 89.4% and an overall correct classification rate of 88.8%. When List B cued recall was entered into the model a sensitivity of 87.9%, a specificity of 91.5% and an overall correct classification rate of 90.0% was obtained. No other variables entered into the model.
Nineteen aMCI subjects and forty-six NE subjects had delayed recall scores for both memory for passages and visual memory of the Wechsler Memory Scale (4th Edition) that could be compared to LASSI-L results. For the total combined List A2 Cued Recall Score and List B2 Cued Recall Score, the area under the ROC curve (AUC) was .977 (SE=.02) compared to delayed memory for passages ROC= .887 (SE=.04). This difference in the AUCs was statistically significant (z = 2.2, p < 0.024). The AUC for delayed visual reproduction was .863 (SE=. 06) which approached statistical significance (Z=1.90;p<. 057) when compared to the AUC for the combined List A2 Cued recall and List B2 Cued Recall Score.
DISCUSSION
The present study demonstrated that even when adjusting for initial levels of overall memory impairment, aMCI patients have impaired ability to learn new information, are more susceptible to proactive and retroactive interference effects and evidence a greater number of intrusion errors on all cued recall measures than normal elderly (NE) subjects. In fact, the numbers of intrusions errors were equivalent between aMCI and AD dementia subjects on most cued recall trials.
While all groups exhibited the presence of learning effects upon repeated trials, second cued recall for List A and List B were the strongest predictors in logistic regression models, yielding excellent sensitivity (87.5%) and specificity (91.5%) in distinguishing between aMCI subjects and NE participants (overall accuracy= 90.0%). In addition, a combination of the second cued recall for List A and List B had significantly greater discriminatory power on ROC analyses relative to delayed memory for passages, a measure that is employed in the Alzheimer’s Disease Neuroimaging Initiative Study (ADNI) and major clinical trials for aMCI.
Due to an increasing emphasis on early detection of AD to optimize developing treatments, there is a pressing need for cognitive paradigms and measures that are sensitive to the earliest cognitive manifestations of AD in the MCI or pre-MCI stages of the disease. Previously, there has been controversy in the literature about the importance of semantic proactive interference and retroactive interference as early markers of incipient AD (26,27,24,23,29,30). In our view, discordance in previous findings has been attributed to: the use of relatively passive encoding strategies, assumptions that individuals have implicitly understood overlapping semantic categories and the presence of sufficient retrieval strength to produce proactive and retroactive interference effects.
A strength of the paradigm used in this study was to explicitly identify semantic categories for the participant to guide recall. All targets on List A were designed to have semantic relatedness to targets on List B. Another strength of our approach was to evaluate proactive and retroactive interference effects by adjusting for the strength of initial memory performance in statistical models.
Indeed, the obtained findings in this study indicate that both proactive and retroactive interference effects seem to be a primary feature of amnestic MCI even after adjusting for a participant’s level of global memory impairment. The fact that proactive interference effects (old learning interfering with new learning) were observed on cued recall rather than free-recall trials lends support that the cuing procedure likely puts greater demands on source memory and pulled for semantic intrusions. Further support for this hypothesis can be found by post-hoc analyses indicated that when B1 intrusions were subtracted from the total cued recall score, the mean resultant score for aMCI and AD were −.47 and −1.75 respectively This indicates that on average, these groups actually committed more semantic intrusions errors rather than providing correct responses. On the other hand, cognitively normal individuals averaged scores a score of 5.94 on this measure [F (2,119) =51.58 p<. 001] (indicating that the number of correct category responses was significantly greater than the number of semantic intrusion errors). Only 6.25% of cognitively normal elderly made an equivalent or greater number of intrusion errors that correct responses on Cued Recall List B compared to 52.9% for aMCI participants and 72.5% AD participants. Virtually all of the intrusions exhibited were errors where the subjects intruded items from the other list of semantically related items. There were occasional non-list intrusions for a category (e.g., clothing), which was semantically related but not part of the two to-be remembered lists.
It is likely that the large number of semantic intrusions made by aMCI and mild AD patients on these tasks and the larger number of semantic intrusions compared to correct responses, reflects the specific problems in early AD related to processing specific competing exemplars within retrieval of exemplars within a specific semantic category. In the current study, retroactive interference effects occurred in free and cued recall tasks among aMCI and mild AD participants. Interestingly, the strongest group effects were observed in the free-recall condition. There may have been several reasons for this. First, cued recall for aMCI and mild AD groups for List B items may have been sufficiently weak so that a minimal retroactive interference effect was observed when List A objects had to be subsequently recalled. A second explanation may be related to the intervening time period between the Short Delay Recall and the initial recall trials of List A that operated above and beyond interference effects.
Mild AD patients exhibited the strongest deficits in learning relative to the other groups on both List A and List B cued recall tasks. One surprising finding is that aMCI and NE subjects seemed to equally benefit from cued recall after their initial free recall from List A was taken into account. This runs contrary to our initial hypotheses and previous studies that have found that the inability to benefit from cues is a defining feature of aMCI and early AD (39, 40). It is likely that our findings may have reflected our adjustment for initial differences in free recall abilities between aMCI and NE participants, which has not been addressed in previous studies.
Overall our findings indicate that the LASSI-L holds promise as a diagnostic tool that can be used by clinicians for identifying early cognitive manifestations of AD Distinct advantages of our paradigm relative to routinely employed memory measures is that a) it required active encoding of to-be-remembered information; b) cued recall of all items over learning trials enhanced the depth of encoding; c) proactive and retroactive interference could be assessed; d) initial strength of free recall could be considered in data analyses; and e) stimuli was used that was appropriate to varied educational levels as well as different cultural/language groups.
Future studies are needed with larger numbers of subjects to determine whether vulnerability to semantic interference is specific to AD or whether it is a common feature of other neurodegenerative disorders. As such, further investigation in this area with the LASSI-L can have potentially important implications for further clinical practice and research in this area.
TABLE 3.
Sensitivity and Specificity of LASS-L Measures to Distinguish aMCI from NE Subjects
| Predicted | |||||
|---|---|---|---|---|---|
| REALTRUEDX | Percentage Correct |
||||
| Observed | NE | aMCI | |||
| Step 1 | Actual Dx | NE | 42 | 5 | 89.4 |
| aMCI | 4 | 29 | 87.9 | ||
| Overall Percentage | 88.8 | ||||
| Step 2 | Actual Dx | NE | 43 | 4 | 91.5 |
| aMCI | 4 | 29 | 87.9 | ||
| Overall Percentage | 90.0 | ||||
The cut value is .500
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cummings JL, Doody L, Clark C. Disease modifying therapies for Alzheimer’s disease: Challenges to early intervention. Neurology. 2007;69:1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- 2.Brooks LG, Loewenstein DA. Assessing the progression of mild cognitive impairment to Alzheimer’s disease: current trends and future directions. Alzheimer’s Res Ther. 2010;2:28–38. doi: 10.1186/alzrt52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 4.Loewenstein DA, Greig M, Schinka J, et al. An investigation of PreMCI: subtypes and longitudinal outcomes. Alzheimer’s Dement. 2012;8:172–179. doi: 10.1016/j.jalz.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almkvist O. Neuropsychological features of early Alzheimer's disease: Preclinical and clinical stages. Acta Neurol Scand. 1996;93:63–71. doi: 10.1111/j.1600-0404.1996.tb05874.x. [DOI] [PubMed] [Google Scholar]
- 6.Buschke H, Kulansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology. 1999;52:231–238. doi: 10.1212/wnl.52.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Linn RT, Wolf PA, Bachman DL, et al. The ”preclinical phase” of probable Alzheimer’s disease: A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 8.Locasio J, Growdon J, Corkin S. Cognitive test performance in detecting, staging, and tracking Alzheimer’s disease. Arch Neurol. 1995;52:1087–1099. doi: 10.1001/archneur.1995.00540350081020. [DOI] [PubMed] [Google Scholar]
- 9.Bondi MW, Monsch AU, Galasko D, et al. Episodic memory changes are associated with the APOE-epsilon4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- 10.Devanand DP, Folz M, Gorlyn M, et al. Questionable dementia: clinical course and predictors of outcome. J Am Geriatr Soc. 1997;45:321–328. doi: 10.1111/j.1532-5415.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC. Mild cognitive impairment: Transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 12.Greenaway MC, Lacrit LH, Binegar D, et al. Patterns of verbal memory performance in mild cognitive impairment, Alzheimer’s disease and normal aging. Cogn Behav Neurol. 2006;19:79–84. doi: 10.1097/01.wnn.0000208290.57370.a3. [DOI] [PubMed] [Google Scholar]
- 13.Loewenstein DA, Acevedo A, Schram L, et al. Semantic interference in mild Alzheimer’s disease: Preliminary findings. Am J Geriatr Psychiatry. 2003;11:252–255. [PubMed] [Google Scholar]
- 14.Buschke H, Sliwinski M, Kuslansky G, et al. Aging, encoding specificity and memory change in the Double Memory Test. J Int Neuropsychol Soc. 1995;1:483–493. doi: 10.1017/s1355617700000576. [DOI] [PubMed] [Google Scholar]
- 15.Buschke H, Sliwinski M, Kuslansky G, et al. Diagnosis of early dementia by the Double Memory Test encoding specificity improves diagnostic sensitivity and specificity. Neurology. 1997;48:989–997. doi: 10.1212/wnl.48.4.989. [DOI] [PubMed] [Google Scholar]
- 16.Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annu Rev Psychol. 2009;60:257. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beatty WW, Testa JA, English S, et al. Influences of clustering and switching on the verbal fluency performance of patients with Alzheimer’s disease. Aging Neuropsychol Cogn. 1997;4:273–279. doi: 10.1080/13825589708256652. [DOI] [PubMed] [Google Scholar]
- 18.Salmon DP, Butters N, Chan AS. The deterioration of semantic memory in Alzheimer’s disease. Canadian Journal of Experimental Psychology. 1999;53:108–117. doi: 10.1037/h0087303. [DOI] [PubMed] [Google Scholar]
- 19.Bondi MW, Monsch AU, Galasko D, et al. Preclinical cognitive markers of dementia of the Alzheimer’s type. Neuropsychology. 1994;8:374–384. [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test: Adult Version. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 21.Binetti G, Magni E, Padovani A, et al. Release from proactive interference in early Alzheimer's disease. Neuropsychologia. 1995;33:379–384. doi: 10.1016/0028-3932(94)00118-9. [DOI] [PubMed] [Google Scholar]
- 22.Kopelman MD. Frontal dysfunction and memory deficits in the alcoholic Korsakoff Syndrome and Alzheimer’s type dementia. Brain. 1991;114:117–137. [PubMed] [Google Scholar]
- 23.Multhaup KS, Balota DA, Faust ME. Exploring semantic memory by investigating build up and release of proactive interference in healthy older adults and individuals with dementia of the Alzheimer’s type. J Int Neuropsychol Soc. 2003;9:830–838. doi: 10.1017/S1355617703960024. [DOI] [PubMed] [Google Scholar]
- 24.Loewenstein DA, Acevedo A, Luis CA, Crum T, Barker WW, Duara R. Semantic interference deficits and the detection of mild Alzheimer’s Disease and mild cognitive Impairment without dementia. Journal of the International Neuropsychological Society. 2004;(1):91–100. doi: 10.1017/S1355617704101112. [DOI] [PubMed] [Google Scholar]
- 25.Loewenstein DA, Acevedo A, Agron J, et al. Vulnerability to proactive semantic interference and progression to dementia among older adults with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;24:363–368. doi: 10.1159/000109151. [DOI] [PubMed] [Google Scholar]
- 26.Bellenville S, Peretz I, Argunin M, et al. Assessment of semantic processing in patients with Alzheimer’s type dementia: The release of proactive interference paradigm. Neuropsychology. 1992;6:29–41. [Google Scholar]
- 27.Cushman LA, Como PG, Booth H, et al. Cued recall and release from proactive interference in Alzheimer’s disease. J Clin Exp Neuropsychol. 1988;10:685–692. doi: 10.1080/01688638808402807. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RL, Bacon LD, Fox JH, et al. Primary memory and secondary memory in dementia of the Alzheimer’s type. J Clin Neuropsychol. 1983;5:337–344. doi: 10.1080/01688638308401181. [DOI] [PubMed] [Google Scholar]
- 29.Helkela EL, Laulumaa V, Soininen H, et al. Different pattern of episodic and semantic memory in Alzheimer’s disease and Parkinson’s disease with dementia. Neuropsychologia. 1989;27:1241–1248. doi: 10.1016/0028-3932(89)90036-5. [DOI] [PubMed] [Google Scholar]
- 30.Rouleau I, Imbault H, Laframboise M, et al. Patterns of intrusions in verbal recall: Comparison of Alzheimer’s disease, Parkinson’s disease and frontal lobe dementia. Brain Cogn. 2001;46:244–249. doi: 10.1016/s0278-2626(01)80076-2. [DOI] [PubMed] [Google Scholar]
- 31.Loewenstein DA, Acevedo A. Loewenstein Acevedo Scale for Semantic Interference and Learning: Manual of Procedures and Interpretation. Miami Beach, Florida: 2005. [Google Scholar]
- 32.Loewenstein DA, Acevedo A, Schram L, Ownby R, Whiter G, Mogosky B, Barker WW, Duara R. Semantic interference in mild Alzheimer’s disease: Preliminary findings. American Journal of Geriatric Psychiatry. 2003;11:252–255. [PubMed] [Google Scholar]
- 33.Petersen RC. Mild cognitive impairment. New England Journal of Medicine. 2004;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC. The Clinical Dementia Rating (CDR): Current versions and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington: American Psychiatric Association; 1994. [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA workgroup under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 37.Folstein M, Folstein S, McHugh P. Mini-mental state: A practical method for grading the cognitive state of patients for the physician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Loewenstein DA. The Fuld OME as a more culture fair memory test in the elderly. Clin Gerontol. 1995;15:52–54. [Google Scholar]
- 39.Dierckx E, Engelborghs S, De Raedt R. Vulnerability to proactive semantic interference and progression to dementia among older adults with mild cognitive impairment. Int J Geriatr Psychiatry. 2009;24:1094–1100. doi: 10.1002/gps.2228. [DOI] [PubMed] [Google Scholar]
- 40.Carlesimo GA, Perri R, Caltagirone C. Category cued recall following controlled encoding as a neuropsychological tool in the diagnosis of Alzheimer’s disease: A Review of the evidence. Neuropsychol Rev. 2011;21:54–65. doi: 10.1007/s11065-010-9153-7. [DOI] [PubMed] [Google Scholar]