Abstract
Context
18F-FDG PET/CT has been proved to be a highly sensitive method for pheochromocytomas/paragangliomas (PHEOs/PGLs) associated with succinate dehydrogenase (SDH) mutations. This finding has been attributed to altered tumor cell metabolism resulting from these mutations and does not provide additional prognostic information to genotype. Therefore, identification of new biomarkers for aggressiveness is needed. A high Ki-67 index was proposed to be an additional prognostic factor.
Objectives
This pilot study aimed to evaluate 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) PET/CT, a PET proliferation tracer, as a potential imaging agent in a series of 12 PHEO/PGL patients with different genetic backgrounds, to compare 18F-FLT uptake with 18F-FDG PET/CT, and to evaluate classical factors of aggressiveness.
Patients and Methods
Twelve patients (7 metastatic and 5 non-metastatic) were prospectively evaluated with 18F-FDG and 18F-FLT and followed for at least 2 years after the initial imaging work-up.
Outcome measures
Uptake was assessed at a lesion level, visually and quantitatively by maximum standard uptake values (SUVmax) for both tracers. 18F-FLT uptake was compared to risk factors known to be linked with a poor prognosis in PGLs (SDHB-mutated status, lesion size, dopaminergic phenotype) and with 18F-FDG uptake.
Results
In 12 patients, 77 lesions were assessed. All lesions had low 18F-FLT uptake (median SUVmax, 2.25; range, 0.7–4.5). There was no apparent superiority of 18F-FLT uptake in progressive lesions and most of the lesions showed a mismatch, with high 18F-FDG uptake (median SUVmax, 10.8; range, 1.1–79.0) contrasting with low 18F-FLT uptake.
Conclusions
This study suggests that PHEOs/PGLs—even those that progress—do not exhibit intense 18F-FLT uptake. It provides the first in vivo demonstration that proliferation may not be a major determinant of 18F-FDG uptake in these tumors. These findings provide new insight into the biological behavior of PGL and suggest that antiproliferative agents may be suboptimal for treatment of these tumors.
Key Terms: pheochromocytoma and paraganglioma, 18F-fluorothymidine, 18F-fluorodeoxyglucose, positron emission tomography, proliferation, glycolysis
Introduction
Pheochromocytomas (PHEOs) and extraadrenal paragangliomas (PGLs) are neural crest cell-derived tumors associated with either the sympathetic (thoraco-abdominal PGLs) or parasympathetic (mainly head and neck PGLs) nervous systems.
Approximately 40% of PHEOs and PGLs carry a germline mutation in one of at least 18 genes (1). These mutations are associated with transcriptome changes that are currently subdivided into 2 main clusters. Cluster 1 is enriched with genes (i.e., succinate dehydrogenase complex, subunits A-D (SDHx)) that are associated with the hypoxic response (mainly HIF-2α) and cluster 2 contains tumors mutated for genes that activate kinase signaling and protein translation (i.e., Ret Proto-Oncogene-MEN2) (2).
Overall, the malignancy risk for PHEOs/PGLs has been estimated to be 10%, with an increased risk in sympathetic PGLs belonging to the cluster 1 subgroup. Interestingly, these tumors also exhibit increased 2-deoxy-2-[18F]-fluoro-D-glucose (18F-FDG) uptake on PET/CT compared to cluster 2 tumors (3). 18F-FDG PET/CT has been proven to provide prognostic information in several endocrine cancers such as thyroid carcinomas of follicular origin (4), medullary thyroid carcinoma (5), and gastroenteropancreatic neuroendocrine tumors (GEP-NETs) (6). In these tumors, acquisition of the 18F-FDG metabolic pattern is progressive during the dedifferentiation process. In contrast, the 18F-FDG PET/CT phenotype was found to be mainly dependent on the presence of a pseudohypoxic phenotype (activation of the HIF-signaling pathway despite normal oxygen supply) in PHEOs/PGLs, a finding that is in large part dependent on the genetic background (cluster 1 genes) (2, 3, 7, 8). Therefore, the 18F-FDG PET/CT phenotype does not provide prognostic information in PHEOs/PGLs, and SDHx-tumors may have an indolent course even with highly elevated uptake values of FDG.
Classical indicators of poor prognosis include: SDHB mutation, tumor size > 5 cm, tumor location (extra-adrenal), age < 30 years at first presentation, and metastatic disease (9-11). Recently, some new studies have proposed to predict metastatic potential and/or tumor aggressiveness using characteristics such as a dopaminergic phenotype (i.e. detection of dopamine or its metabolite methoxytyramine) (12, 13), the presence of tumor necrosis, high Ki-67 index and/or mitotic count (14), overexpression of HIF-α and its target genes in tumors (15, 16), or extremely high mRNA copy numbers of a variant of carboxypeptidase E in tumors (17). Identification of in vivo biomarkers of aggressiveness would be of particular interest in the assessment of these tumors.
3′-deoxy-3′-18F-fluorothymidine (18F-FLT) has been proposed as a PET proliferation tracer even though it is not incorporated into DNA due to phosphorylation by cytosolic thymidine kinase-1 (TK1). The assumption is that the concentration of FLT nucleotides in cells is proportional to TK1 activity and, therefore, to cellular proliferation. The role of 18F-FLT in oncology is still debated but several studies have shown promising results for tumor grading and in the evaluation of treatment response (18).
The aims of the present study were to evaluate 18F-FLT PET/CT in a series of 12 PHEO/PGL patients with varying genetic backgrounds, compare 18F-FLT uptake with a metabolic pattern on 18F-FDG PET/CT, and evaluate classical factors of aggressiveness.
Materials and Methods
Patients
Twelve non-consecutive adult patients (10 men and 2 women; median age, 43 years; range, 27–70 years) with PGLs (as defined by the reference standard—see below) were prospectively included between January and July 2012 (and followed up over the course of at least two years). All patients were studied at the National Institutes of Health (NIH). The protocol (NCT00004847) was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. All patients provided written informed consent. The inclusion criteria were at least one PGL (as defined by the reference standard—see below) at the time of the study. Exclusion criteria included: age below 18 years, pregnancy, or recent (< 2 months) systemic treatment.
Reference Standard to define PGL
PGL lesions were confirmed histologically when surgery was performed on patients with nonmetastatic disease (patients # 1, 2, 5, 8 and 9). When surgery was not indicated due to the presence of metastases (patients # 3, 4, 6, 7, 10, 11, 12), lesions were characterized as PGL-related (either primary or metastatic) based on the positivity (characteristic appearance) on conventional imaging—either contrast-enhanced computed tomography (ceCT) or contrast-enhanced magnetic resonance imaging (ceMRI)—that corresponded with positivity on either 18F-FDOPA or 18F-FDA PET/CT (3, 19, 20); these findings were further supported by positive PGL-specific biochemistry and positive genetic testing results.
The following data were collected: metastatic status (defined by presence of tumor cells at non-neural crest-derived sites), genetic testing, adrenal versus extra-adrenal location, tumor size (patient carrying at least one tumor diameter > 5 cm, which is the optimal cutoff from Eisenhofer's analysis (13)), dopaminergic phenotype (i.e., presence of dopamine and/or its metabolite methoxytyramine in urine and/or plasma (13)), and age at diagnosis. Patients were followed (clinically, biochemically, and with imaging) for at least two years after imaging.
Imaging protocol for 18F-FDG and 18F-FLT PET/CT scans
All patients underwent 18F-FDG and 18F-FLT PET/CT scanning. 18F-FLT was provided by Cardinal Health. A fixed 18F-FLT dose of 195 MBq was injected. For 18F-FDG PET scanning, patients fasted for at least 4 hours before intravenous injection of 5 MBq/kg 18F-FDG (median total injected dose, 390 MBq; range, 340–590 MBq) and blood glucose levels were measured just before injection to ensure a value below 200 mg/dL. For both radiopharmaceuticals, PET/CT acquisitions were performed on a Biograph-128 mCT PET/CT scanner (Siemens Medical Solutions). For both studies, PET data acquisition started at 60 minutes (min) after injection. Emission images were obtained in 3-dimensional mode, with 3-min/bed-position (BP) and 5-min/BP rates for 18F-FDG and 18F-FLT, respectively. The acquisitions were done from the thighs to the head. Co-registered CT studies for attenuation correction and anatomic co-registration were performed with the following imaging parameters: 120 kV, 115 mA and a 1.5 mm section thickness. Co-registered images were displayed on a workstation (DeltaViewer - MedImage), with 3D fused navigation along the axial, coronal, and sagittal planes and maximum intensity projection (MIP) rendering.
18F-FLT and 18F-FDG PET/CT scanning were performed within 1 month of each other in all but one case, with 18F-FLT performed first in 2 patients (#2 and 6) and 18F-FDG performed first in the 10 other patients. For patient #10, the 18F-FLT scan was performed 4 months after the 18F-FDG scan.
18F-FDG and 18F-FLT PET image analysis
On 18F-FLT and 18F-FDG PET/CT images, uptake was assessed for each lesion (determined on the basis of CT) visually (intensity of uptake in comparison to the surrounding background and to reference organs) and quantitatively. Uptake values were classified according to their location: soft-tissue for lesions located in head and neck area, mediastinum, lung, liver, extrahepatic abdomen and pelvis, and bone. In the case of multiple lesions, a cut-off of 5 lesions per area was fixed.
For quantitative assessment, body weight maximum standardized uptake values (SUVmax) were calculated using the following formula: SUV equals decay-corrected tracer tissue concentration (in Bq/g) injected dose (in Bq) normalized by the patient's body weight (in g). In both 18F-FLT and 18F-FDG PET, volumes of interest (VOI) were determined manually over the lesions using co-registered CT. Some small lesions that could not be separated easily from surrounding physiologic uptake (such as in the cases of small liver and bone lesions), were considered ‘non-measurable. The largest diameter of each lesion was measured.
Physiological 18F-FLT uptake was also quantified by SUVmax in the following normal organs: muscle (right para-vertebral muscle at the level of L4), for use as an intra-patient non-proliferative negative control, and bone marrow (in the L4 vertebral body) and lymphatic tissue (including non-specific inflammatory lymph nodes, tonsils, and thymic remnants) for use as intra-patient positive controls; indeed hematopoietic cells in bone marrow and (to a lesser extent) lymphatic tissue were highly 18F-FLT positive, as expected for active proliferative tissues (21, 22). Liver uptake was also measured.
Histopathologic analyses and immunohistochemical staining
Histopathologic analyses and immunohistochemical staining were performed in 5 patients with nonmetastatic disease in whom tumor tissue were collected prospectively after 18F-FLT PET scan. Cell and tissue morphology and immunohistochemical staining for (chromogranin and synaptophysin) confirmed the diagnosis of PGL and Ki-67 levels were assessed. No patient with metastatic disease underwent surgery for ethical reasons: PGL diagnosis could non-invasively be confirmed using imaging, biochemistry, and medical history, and PGL biopsy can lead to severe adverse events related to catecholamine release.
Genetic testing
Genetic testing included gene sequencing of SDHB, SDHD, RET, and VHL as well as assessment of large gene rearrangements of SDHB and SDHD. When all these were negative, patients were qualified ‘apparently sporadic’. Patients were not tested for mutations in the recently described genes SDHA, SDHAF2, and TMEM127.
Statistical analysis
Descriptive quantitative data were expressed as either a median or given a range. The correlation between 18F-FLT SUV and 18F-FDG SUV was assessed using the Spearman rank correlation coefficient. A P-value <0.05 was considered statistically significant.
Results
Patients
Twelve patients with PHEO/PGL were studied. The genetic statuses represented were as follows: 5 SDHB, 2 SDHD, 4 apparently sporadic, and 1 MEN2. 7 were carrying metastatic lesions and 5 had only primary lesions (1 adrenal, 4 extra-adrenal). 4 patients were at the initial stages of PGL disease, 2 were evaluated for recurrence, and 6 for disease progression. 3 patients had previously received 131I-MIBG radiation therapy (1 had his last dose 4 months prior), 2 CVD chemotherapy (1 received his last dose 2 months prior), and 2 external beam radiation therapy (EBRT) over the rachis. 2 patients had a dopaminergic phenotype. One patient had an adrenal PGL and 11 patients had extra-adrenal PGLs. 7 patients had a history of a voluminous (larger than 5 cm) primary PHEO/PGL lesion. Patient characteristics are detailed in Table 1.
Table 1. Patient clinical characteristics.
| Patient | Age (yrs), sex | Germline mutation | Age at intial diagnosis | Status at the time of FLT & FDG PET | Size of the largest lesion at Time of FLT/FDG | Dopamine secretion | Reason for evaluation at the time of FLT & FDG PET | Previous systemic treatments (delay between end of last treatment and PET scan) | 2 year follow-up (progression or death from PGL) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 43, M | sporadic | 43 | primary | 3.8 cm | Negative | Suspected primary | NONE | no |
| #2 | 43, M | SDHD | 43 | primary | 4.8 cm | Negative | Suspected primary | NONE | no |
| #3 | 43, M | SDHD | 41 | primary | 4.7 cm | Negative | Restaging | NONE | no |
| #4 | 48, F | MEN2 | 35 | metastatic | 12 cm | Negative | Restaging | 131I-MIBG (>2 years) | no |
| #5 | 47, M | sporadic | 30 | metastatic | unknown | Negative | Suspected recurrence | NONE | no |
| #6 | 70, M | sporadic | 67 | metastatic | 24 cm | Negative | Suspected recurrence | NONE | no |
| #7 | 50, F | sporadic | 43 | metastatic | 8 cm | Negative | Restaging | 131I-MIBG (8 months), sandostatin therapy (on) | no |
| #8 | 30, M | SDHB | 30 | primary | 2.5 cm | Negative | Initial staging | NONE | no |
| #9 | 27, M | SDHB | 27 | primary | 3 cm | Negative | Initial staging | NONE | no |
| #10 | 35, M | SDHB | 19 | metastatic | 8 cm | Positive | Restaging | NONE | yes |
| #11 | 35, M | SDHB | 25 | metastatic | 7 cm | Positive | Restaging | CVD (>2 years), 131I-MIBG (4 months) | Yes |
| #12 | 47, M | SDHB | 41 | metastatic | 3 cm | Negative | Restaging | CVD (2 months) | yes |
CVD: cyclophosphamide, vincristine and dacarbazine
Three patients (patients #10-12) had progressive disease (all associated with the SDHB mutated genotype, 2/3 with dopaminergic phenotype). Other patients had stable or slowly progressive disease.
18F-FLT uptake in PGL lesions
Seventy-seven lesions were assessed. Of those, 14 lesions were in patients with only primary lesions and 63 lesions were in patients carrying metastatic lesions. Metastatic sites included: soft tissue (lymph nodes) (19), bones (16), liver (10), and lungs (18). Of the 77 lesions, 64 were measurable using SUVmax. Thirteen small lesions were non-measurable due to surrounding tissue activity: 2 primary PGLs, 6 bone lesions, and 5 liver lesions.
All lesions had rather low 18F-FLT uptake, with an average SUVmax of 2.25 [0.7– 4.5]. In contrast, 18F-FLT uptake in normal bone marrow was high in all patients, with an average SUVmax of 16.6 [12.9–22.1]. (In patients #7, 11, and 12, this was non-measurable due to disseminated metastatic bone disease). In 4 patients (#2, 3, 5 and 9). FLT uptake was noted in 13 lymph nodes/lymphatic tissue-related sites (including tonsils and thymic remnants) thought unrelated to presence of PHEO/PGL (based on negativity of 18F-FDOPA and 18F-FDA studies). These lesions had an average SUVmax of 6.0 [3.9–10.9].
We observed high 18F-FLT uptake in normal liver (average SUVmax 9.8 [6.8–16.0]) in all patients, reflecting hepatic glucuronidation of FLT.
Quantification of background 18F-FLT uptake in muscle, the nonproliferative control, showed an average SUVmax of 1.7 [1.2–2.3]. Figure 1 displays the 18F-FLT SUVmax in lesions and reference organs.
Figure 1. FLT SUVmax in lesions and reference organs.
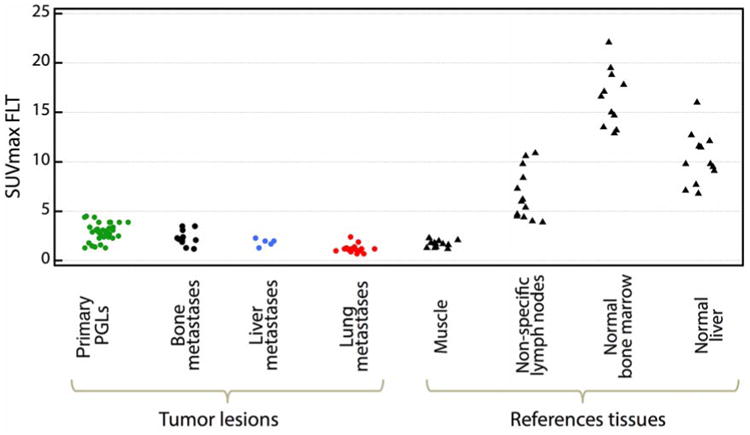
PGLs: includes all PGLs (primary tumos only) from patients with metastatic and non metastatic.disease. The median number of primary PGLs assessed per patient was 3 (range : 1-9).
18F-FLT in PGL compared with clinical criteria of poor prognosis (per-patient analysis)
No significant differences in tumor FLT SUVmax values were observed between patients segregated by presence or absence of poor prognostic factors, including SDHB vs. non-SDHB (2.9 [0.9-3.9] vs 1.9 [0.7-4.5]), age ≤30 vs >30 year at diagnosis (2.35 [0.9-3.9] vs 2.05 [0.7-4.5]), metastatic vs nonmetastatic disease (2.05 [0.7-4.5] vs 2.45 [1.4-3.1]), presence or absence of large (>5 cm) lesion (3.25 [1.3-4.5] vs 2.45 [1.4-3.1], and elevated vs normal dopamine secretion (median 2.9 [range : 0.9-3.9] vs 2.0 [0.7-4.5]). There was also no significant difference in uptake of lesions in patients who had progressive vs. stable disease during follow-up (2.4 [0.9-3.9] versus 2.0 [0.7-4.5]). Figure 2 displays the average 18F-FLT SUVmax in lesions according to these prognostic factors and follow-up.
Figure 2. Lesion FLT SUVmax compared with criteria of poor prognosis.
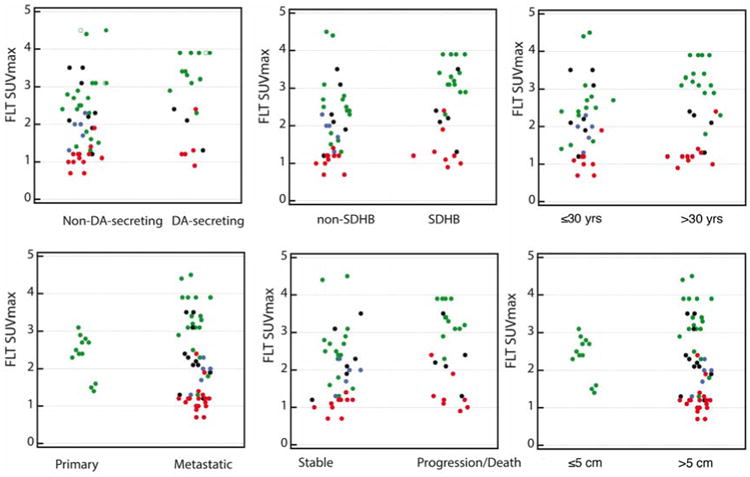
The color-code of the dots is the same as in figure 1.
Comparison of 18F-FLT uptake in PGL with 18F-FDG uptake
On visual analysis, the majority of lesions (66/77) showed a discrepancy between 18F-FLT and 18F-FDG uptake, with high 18F-FDG uptake contrasting with low or absent 18F-FLT uptake. Figure 3 shows an example of an SDHB patient with a primary abdominal PGL, and Figure 4, an example of an SDHB patient with widespread metastatic disease.
Figure 3. Single retroperitoneal primary PGL in an SDHB patient (#3).
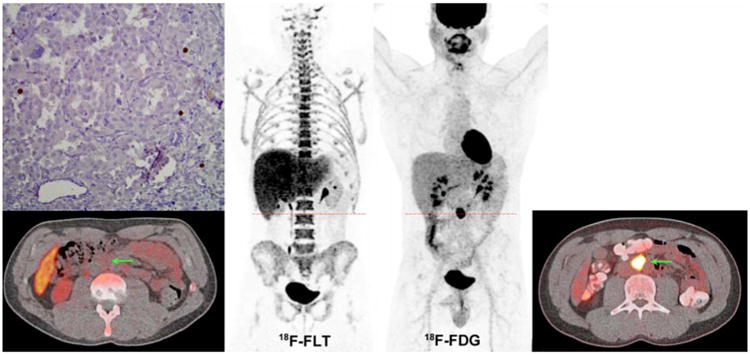
18F-FDG (MIP image and axial CT-fused image at the level of the PGL lesion) shows particularly high uptake (18F-FDG SUVmax of 59.8). This lesion was corresponded to a 2.6-cm abdominal extra-adrenal mass (arrows). 18F-FLT (MIP image and axial CT-fused image at the level of the PGL lesion) shows low uptake (18F-FLT SUVmax of 2.5). Low Ki-67 staining.
Figure 4. Disseminated metastatic disease (soft-tissue, bone, liver, and lung lesions) in an SDHB patient.
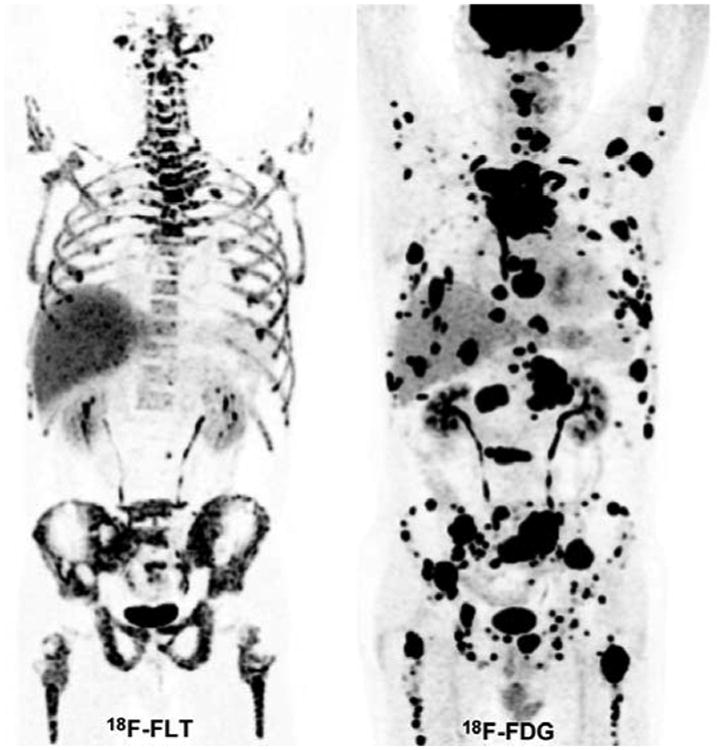
18F-FDG (MIP image) shows high tumor burden with very high 18F-FDG uptake in the vast majority of the lesion. 18F-FLT (MIP image) shows very low 18F-FLT uptake. Bone lesions (notably in both femoral heads and bilateral iliac bones) appear to have relatively low FLT uptake compared to the surrounding background uptake in marrow.
Overall, the average 18F-FDG SUVmax of the 77 lesions was 10.8 [1.1-79.0], compared to an average SUVmax of 2.25 [0.7–4.5] for 18F-FLT. Correlation analysis demonstrated a weak positive correlation between 18F-FLT and 18F-FDG uptake expressed by SUVmax values (Spearman's rho 0.62, P-value <0.05 – Figure 5 shows the scatter plot). Similarly, correlation analysis conducted on subgroups (SDHB-related tumors, non-SDHB-related tumors) demonstrated a weak positive correlation between the 18F-FLT and 18F-FDG SUVmax values (Spearman's rho 0.40, P-value 0.11 for SDHB and Spearman's rho 0.59, P-value <0.05 for non-SDHB).
Figure 5. Scatter-plot displaying tumor 18F-FLT SUVmax values in relation to their 18F-FDG SUVmax.
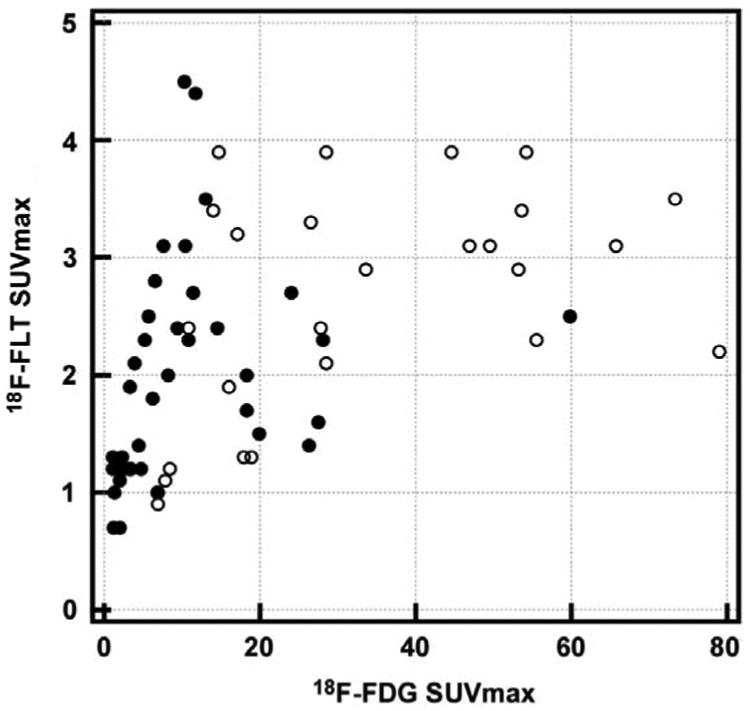
Black rounds correspond to lesions from non-SDHB patients. White rounds correspond to lesions from non-SDHB patients. 18F-FLT SUVmax values in these lesions are low (average 2.25 [0.7-4.5]) whereas their corresponding 18F-FDG SUVmax values are high (average 10.8 [1.1-79.0]).
Ki-67 immunostaining
In vitro proliferative activity as assessed by Ki-67 index was low (less than 5%) in all surgically resected lesions (patients # 1, 2, 5, 8 and 9). Average 18F-FLT and 18F-FDG SUVmax were 2.8 and 6.5 in patient # 1, 2.7 and 14.5 in patient # 2, 3.1 and 10.4 in patient # 5, 2.9 and 53.2 in patient # 8 and 3.1 and 46.9 in patient # 9.
Discussion
This study shows that in our 12 patients, PHEOs/PGLs did not exhibit intense 18F-FLT uptake, even those that progressed rapidly and/or exhibited high 18F-FDG uptake. It also provides the first in vivo demonstration that proliferation is probably not a major determinant of 18F-FDG uptake in these lesions.
These findings differ from highly proliferative cancers (i.e., lymphoma, lung cancer) that exhibit high 8F-FLT tumor uptake values (18). However, the low 18F-FLT uptake found in PGL is concordant with findings observed in well-differentiated GEP tumors. In one study, none of the tumors (primary lesions or metastases) were positive on 18F-FLT PET/CT whereas 18F-FDG PET/CT was positive in 7/10 cases (23). 18F-FLT PET/CT was also found to be less sensitive than 18F-FDG PET/CT in the diagnosis of residual lymph node and distant metastases from differentiated thyroid carcinoma (25). A case of primary papillary thyroid carcinomas detected by 18F-FDG PET/CT were also found to have low 18F-FLT uptake, a finding that was attributed to a low proliferation activity (24).
To our best knowledge, 18F-FLT PET/CT has not been evaluated in other endocrine malignancies. Our results are consistent with the low proportion of PHEOs/PGLs with high Ki-67 indices (26-28) and provide in vivo evidence of low proliferation rates of PHEOs/PGL metastases. This is also consistent with preclinical models showing that PHEOs/PGL tumor cells were mostly at the resting state of the cell cycle (the so-called G0/G1 transition) (29). 18F-FLT SUVmax was also not associated with presence of certain criteria for poor prognosis.
Our study also provides the first in vivo demonstration that proliferation is not a major determinant of 18F-FDG uptake in these tumors, including SDHx-related tumors which often exhibit highly elevated uptake values. Several studies have found that 18F-FDG tumor uptake is higher in patients with SDHx germline mutations compared to other tumor types (RET, NF1), regardless of the tumor location (3, 7). This finding has been attributed to activation of the HIF-α pathway despite normal or even high oxygen supply (also called the pseudohypoxic phenotype). However, it is notable that there are some discrepancies with in vitro studies that failed to identify an overexpression of glycolytic enzymes or HIF-α proteins in SDHx-related tumors in comparison to other subtypes (30). The present study provides preliminary in vivo evidence that increased 18F-FDG uptake in these patients is unrelated to proliferation and the role of glucose uptake by PHEOs/PGLs is currently largely unexplained. Based on our recent metabolomic study, it has been proposed that glucose might be directly or indirectly involved in the metabolism of myoinositol/ascorbate, glutamine/glutamate, methionine/taurine and catecholamines (31).
We acknowledge several limitations to our study: 1) absence of pathological confirmation of all lesions. Nevertheless, this is not a serious issue since PHEOs/PGLs concentrate specific radiotracers; 2) PET/CT acquisition was performed at a single study point (starting at about 60 min). However, pharmacokinetic studies showed that tracer washout is negligible and that a SUV at 60 min is correlated with the net influx constant Ki (32); 3) very small number of patients with widely heterogeneous characteristics. Some patients had also been treated in the past with therapeutic approaches that may have modified 18F-FLT uptake (table 1). However, all of these patients were refractory to these therapies.
Our findings also provide new insight into the biological behavior of PGL and suggests that antiproliferative agents may not be very effective in treatment of these tumors.
Conclusion
In this limited pilot study, 18F-FLT uptake was relatively low in PGL tumors, even progressive ones. This signifies a slow proliferative rate despite high 18F-FDG uptake present in the same lesions. This suggests that antiproliferative drugs may not be effective in the treatment of these patients.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Martucci VL, Pacak K. Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment. Curr Probl Cancer. 2014 Jan-Feb;38(1):7–41. doi: 10.1016/j.currproblcancer.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Berkel A, Rao JU, Kusters B, Demir T, Visser E, Mensenkamp AR, et al. Correlation Between In Vivo 18F-FDG PET and Immunohistochemical Markers of Glucose Uptake and Metabolism in Pheochromocytoma and Paraganglioma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014 Jun 12;55(8):1253–1259. doi: 10.2967/jnumed.114.137034. [DOI] [PubMed] [Google Scholar]
- 3.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012 May 2;104(9):700–708. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006 Feb;91(2):498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 5.Adams S, Baum R, Rink T, Schumm-Drager PM, Usadel KH, Hor G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998 Jan;25(1):79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 6.Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009 Jun;50(6):858–864. doi: 10.2967/jnumed.108.057505. [DOI] [PubMed] [Google Scholar]
- 7.Taieb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009 May;50(5):711–717. doi: 10.2967/jnumed.108.060731. [DOI] [PubMed] [Google Scholar]
- 8.Taieb D, Timmers HJ, Shulkin BL, Pacak K. Renaissance of (18)F-FDG positron emission tomography in the imaging of pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2014 Jul;99(7):2337–2339. doi: 10.1210/jc.2014-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007 Oct;92(10):3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 10.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011 Mar;96(3):717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 11.Schovanek J, Martucci V, Wesley R, Fojo T, Del Rivero J, Huynh T, et al. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer. 2014;14:523. doi: 10.1186/1471-2407-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peitzsch M, Prejbisz A, Kroiss M, Beuschlein F, Arlt W, Januszewicz A, et al. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann Clin Biochem. 2013 Mar;50(Pt 2):147–155. doi: 10.1258/acb.2012.012112. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012 Jul;48(11):1739–1749. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wailly P, Oragano L, Rade F, Beaulieu A, Arnault V, Levillain P, et al. Malignant pheochromocytoma: new malignancy criteria. Langenbecks Arch Surg. 2012 Feb;397(2):239–246. doi: 10.1007/s00423-011-0850-3. [DOI] [PubMed] [Google Scholar]
- 15.Pinato DJ, Ramachandran R, Toussi ST, Vergine M, Ngo N, Sharma R, et al. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. British journal of cancer. 2013 Feb 5;108(2):429–437. doi: 10.1038/bjc.2012.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Span PN, Rao JU, Oude Ophuis B, Lenders JW, Sweep F, Wesseling P, et al. Overexpression of the natural antisense hypoxia-inducible factor-1alpha transcript is associated with malignant phaeochromocytoma/paraganglioma. Endocr Relat Cancer. 2011 Mar 21; doi: 10.1530/ERC-10-0184. [DOI] [PubMed] [Google Scholar]
- 17.Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cell Mol Neurobiol. 2010 Nov;30(8):1377–1381. doi: 10.1007/s10571-010-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Tehrani OS, Shields AF. PET imaging of proliferation with pyrimidines. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013 Jun;54(6):903–912. doi: 10.2967/jnumed.112.112201. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel S, Blanchet EM, Sebag F, Chen CC, Fakhry N, Deveze A, et al. Functional characterization of nonmetastatic paraganglioma and pheochromocytoma by (18) F-FDOPA PET: focus on missed lesions. Clin Endocrinol (Oxf) 2013 Aug;79(2):170–177. doi: 10.1111/cen.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-Fluoro-L-DOPA, 18F-Fluoro-Deoxyglucose, and 18F-Fluorodopamine PET and 123I-MIBG Scintigraphy in the Localization of Pheochromocytoma and Paraganglioma. Journal of Clinical Endocrinology and Metabolism. 2009 Oct 28;94(12):4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agool A, Schot BW, Jager PL, Vellenga E. 18F-FLT PET in hematologic disorders: a novel technique to analyze the bone marrow compartment. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006 Oct;47(10):1592–1598. [PubMed] [Google Scholar]
- 22.Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ, et al. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007 May;48(5):726–735. doi: 10.2967/jnumed.106.037473. [DOI] [PubMed] [Google Scholar]
- 23.Giammarile F, Billotey C, Lombard-Bohas C, Le Bars D, Bournaud C, Masson S, et al. 18F-FLT and 18F-FDG positron emission tomography for the imaging of advanced well-differentiated gastro-entero-pancreatic endocrine tumours. Nucl Med Commun. 2011 Feb;32(2):91–97. doi: 10.1097/MNM.0b013e3283412143. [DOI] [PubMed] [Google Scholar]
- 24.Nakajo M, Kajiya Y, Jinguji M, Mori S, Aridome K, Suenaga T, et al. High FDG and low FLT uptake in a thyroid papillary carcinoma incidentally discovered by FDG PET/CT. Clin Nucl Med. 2012 Jun;37(6):607–608. doi: 10.1097/RLU.0b013e318252d80f. [DOI] [PubMed] [Google Scholar]
- 25.Nakajo M, Jinguji M, Tani A, Kajiya Y, Tanabe H, Fukukura Y, et al. Diagnosis of metastases from postoperative differentiated thyroid cancer: comparison between FDG and FLT PET/CT studies. Radiology. 2013 Jun;267(3):891–901. doi: 10.1148/radiol.13121546. [DOI] [PubMed] [Google Scholar]
- 26.Strong VE, Kennedy T, Al-Ahmadie H, Tang L, Coleman J, Fong Y, et al. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 2008 Jun;143(6):759–768. doi: 10.1016/j.surg.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 27.van der Harst E, Bruining HA, Jaap Bonjer H, van der Ham F, Dinjens WN, Lamberts SW, et al. Proliferative index in phaeochromocytomas: does it predict the occurrence of metastases? J Pathol. 2000 Jun;191(2):175–180. doi: 10.1002/(SICI)1096-9896(200006)191:2<175::AID-PATH615>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 28.Brown HM, Komorowski RA, Wilson SD, Demeure MJ, Zhu YR. Predicting metastasis of pheochromocytomas using DNA flow cytometry and immunohistochemical markers of cell proliferation: A positive correlation between MIB-1 staining and malignant tumor behavior. Cancer. 1999 Oct 15;86(8):1583–1589. [PubMed] [Google Scholar]
- 29.Martiniova L, Lu J, Chiang J, Bernardo M, Lonser R, Zhuang Z, et al. Pharmacologic modulation of serine/threonine phosphorylation highly sensitizes PHEO in a MPC cell and mouse model to conventional chemotherapy. PLoS One. 2011;6(2):e14678. doi: 10.1371/journal.pone.0014678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imperiale A, Moussallieh FM, Roche P, Battini S, Cicek AE, Sebag F, et al. Metabolome Profiling by HRMAS NMR Spectroscopy of Pheochromocytomas and Paragangliomas Detects SDH Deficiency: Clinical and Pathophysiological Implications. Neoplasia. 2015 Jan;17(1):55–65. doi: 10.1016/j.neo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visvikis D, Francis D, Mulligan R, Costa DC, Croasdale I, Luthra SK, et al. Comparison of methodologies for the in vivo assessment of 18FLT utilisation in colorectal cancer. Eur J Nucl Med Mol Imaging. 2004 Feb;31(2):169–178. doi: 10.1007/s00259-003-1339-2. [DOI] [PubMed] [Google Scholar]


