Abstract
Disease relapse in cancer patients many years after clinical remission, often referred to as cancer dormancy, is well documented but remains an incompletely understood phenomenon on the biological level. Recent reviews have summarized potential models that can explain this phenomenon, including angiogenic, immunologic, and cellular dormancy. We focus on mechanisms of cellular dormancy as newer biological insights have enabled better understanding of this process. We provide a historical context, synthesize current advances in the field, and propose a mechanistic framework that treats cancer cell dormancy as a dynamic cell state conferring a fitness advantage to an evolving malignancy under stress. Cellular dormancy appears to be an active process that can be toggled through a variety of signaling mechanisms that ultimately down-regulate the Ras/MAPK and PI(3)K/AKT pathways, an ability that is preserved even in cancers that constitutively depend on these pathways for their growth and survival. Just as unbridled proliferation is a key hallmark of cancer, the ability of cancer cells to become quiescent may be critical to evolving malignancies, with implications for understanding cancer initiation, progression, and treatment resistance.
I. Introduction
Despite significant advancements in cancer therapeutics over the past several decades, relapse following long periods of remission after treatment remains a persistent problem in many patients. Fatal recurrences for a variety of cancers can arise years and even decades later, often in the form of metastatic disease, the major cause of cancer-related deaths (1-3). The extensive period of time in which patients remain asymptomatic prior to relapse represents the clinical observation known as cancer dormancy, a loosely defined phenomenon that has garnered increasing interest but remains poorly understood. Efforts at linking the clinical phenomenon of cancer dormancy to underlying cellular mechanisms remains challenging since conceptual models of dormancy, generated through experimental studies, are difficult to validate in patients. While several recent reviews have approached the subject (4-6), interest in dormancy has been growing quickly and new insights are rapidly being made.
In this review, we use the phrase “cancer dormancy” to describe the clinical phenomenon of slowly growing primary or metastatic tumors that are often seen as the culprit for relapsed disease. While cancer dormancy can be explained through different biological mechanisms, we will focus primarily on the concept of “cellular dormancy” or “solitary cell dormancy”, one such model that has gained more momentum due to its explanatory power and novel advances in the field. Thus, this review has four primary aims: 1) examine the historical context, clinical evidence, and relevance of cancer dormancy broadly defined, 2) discuss prevailing models that have been developed to explain clinical behavior, 3) synthesize the body of literature on cellular dormancy and propose a logical framework, and 4) outline current barriers to studying this phenomenon and discuss potential therapeutic implications.
Evidence for cancer dormancy through clinical observation
Evidence of cancer dormancy has historically been viewed through a clinical lens as an attempt to explain relapse in post-treatment cancer patients who have been asymptomatic for a period of time. One of the earliest observations of cancer relapse following tumor removal can be traced to ancient Rome when the physician Celsus (25 B.C. to 50 A.D.) noted the recurrence of certain types of cancers, which he referred to as carcinomas, stating that “after excision, even when a scar has formed, none the less the disease has returned, and caused death” (7). The concept of cancer dormancy was not formalized and widely noted, however, until the early 20th century, after the pioneering of anesthesia and the use of more sophisticated surgical techniques facilitated the extirpation of tumors and the study of post-operative cancer recurrence. In 1934, Rupert Willis, an Australian pathologist who examined cancer metastases in autopsies, noted that delayed metastases in patients who had no local recurrence following removal of the primary tumor suggested that “neoplastic cells must have lain dormant in the tissues in which they were arrested” (8). It was further proposed by the English pathologist Geoffrey Hadfield in 1954 that the appearance of secondary tumors over 5 years following surgery is likely the outcome of cancer cells lying in a state of “temporary mitotic arrest” (9). Over the past several decades, extreme cases of time to relapse have been documented in a handful of patient series. Examples of recurrent melanoma over 10 years after initial diagnosis are rare, but have been reported several times in literature (10-13). In one series, recurrence of up to 20 years after diagnosis was observed in patients who initially only had one primary cutaneous melanoma (13). In studies of breast cancer patients, deaths attributable to relapse have been observed up to 25 years following surgery, after which the mortality rate was no longer significantly different from that of the general population (1,14). Examination of circulating tumor cells (CTCs) in breast cancer patients without evidence of disease following mastectomy has also shown that CTCs could be detected up to 22 years later (15).
Cancer dormancy is a heterogeneous phenomenon that should be viewed as a specific property of an evolving malignancy that reflects differences in underlying biology even within the same tumor type. For example, it is well known that women with “basal-like” breast cancers, an aggressive histological subtype with poor clinical prognosis, have shorter relapse-free survival times than women with other types of breast cancers (16). In a cohort of over 1,600 patients, those with triple-negative breast cancers, which have a high degree of overlap with the “basal-like” histopathology, were shown to have a significantly higher rate of recurrence within the first several years of diagnosis compared to other types of breast cancers (17). However, this risk rapidly declined thereafter, with no recurrences after 8 years of follow-up, while other breast cancer subtypes continue to carry a low but steady risk even up to nearly 20 years after diagnosis. These predictable trends raise the idea that cancer dormancy is governed by distinguishing biological characteristics of evolving tumors that have significant clinical implications for prognosis and treatment.
Interest in cancer dormancy has primarily involved examining metastastic behavior and relapsed disease, which is ultimately responsible for the vast majority of cancer deaths (18). While there remains some controversy as to when metastases develop with respect to the primary growth of the tumor, there is genomic evidence derived from comparison of primary to secondary tumors that metastatic spread can occur very early on in the evolving disease (19,20). Mathematical modeling of late recurrence suggests that the most likely explanation is the presence of a prolonged period of dormancy as opposed to slow but uninterrupted growth (21), which can potentially be explained by 1) individual disseminated cells entering a state of prolonged dormancy at a distant site, or 2) the presence of micrometastatic lesions in a growth equilibrium defined by a balance between cellular proliferation and death. As a variety of models can account equally well for different trends in long-term follow-up relapse data (22), however, further insight into this phenomenon can only be gained through understanding the driving biological pathways.
Dormant cancer cells also plays an important role in the evolution of primary tumors both in determining the overall growth rate and response to therapy. It is well known that there is proliferative heterogeneity in a tumor and that variable fractions of cells exist in an out-of-cycle (23,24). While slow proliferators may retard overall tumor growth, they also may offer an overall fitness advantage under conditions of iatrogenic stress. Work in this field emphasizes the need to better understand cancer development and therapeutic resistance from the standpoint of cellular heterogeneity with respect to epigenetic and metabolic changes (25-27), as models of treatment resistance through acquired genetic mutation remain incomplete (28). Insight into dormancy, however, may also be critical for understanding how functional cellular heterogeneity contributes to tumor dynamics, progression, and treatment resistance.
II. Current Models of Cancer Dormancy
Current experimental models of cancer dormancy can be subdivided into two general categories reflecting distinct growth kinetics and have been recently reviewed (4-6). The first category, known as tumor mass dormancy, involves stagnation of overall tumor growth due to the equilibrium of proliferation and cell death. The prevailing models that comprise this category include angiogenic dormancy (29-31) and immunologic dormancy (32-34). The second category, referred to as cellular dormancy or solitary cell dormancy, involves the ability for individual cancer cells to enter a state of temporary cell cycle arrest (4,35,36). As recent reviews have discussed these models in detail, we provide a brief historical and conceptual overview (Figure 1).
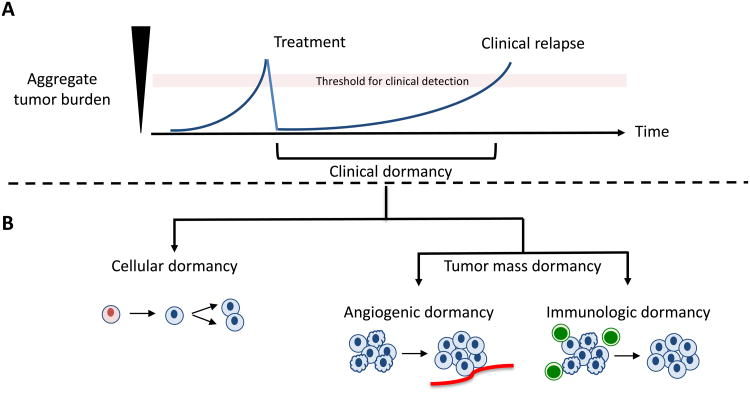
Figure 1. Biological Mechanisms of Cancer Dormancy.
A) Clinical dormancy is depicted in the graph as the period of time after treatment when residual malignant cells become detectable either as recurrent local or metastatic disease. B) Cancer dormancy can be distinguished physiologically as cellular dormancy or tumor mass dormancy (rate of proliferation is balanced by rate of apoptosis). Progression of cancer occurs in cellular dormancy when individual dormant malignant cells re-enter the cell cycle. Likewise, progression in tumor mass dormancy occurs when an angiogenic switch occurs or when tumor cells evade immune surveillance, after which the equilibrium between proliferation and apoptosis shifts in favor of the former.
Angiogenic Dormancy
The hypothesis that tumors are dependent on the formation of new vasculature to sustain their growth was developed in the 1970's, generating increasing amount of interest in understanding the mechanism behind angiogenesis (37). When a growing tumor mass expands beyond 1-2 mm, it relies on the formation of new vascular beds in order to attain sufficient oxygen and nutrient for growth by inducing expression of the hypoxia-inducible factor-1 (HIF-1) (29,31,38). This limitation has been cited as an explanatory model for how microscopic pockets of malignant cells can remain clinically undetectable for years prior to an “angiogenic switch” that is regulated by a balance between pro- anti-angiogenic factors produced by the tumor and its microenvironment (31,39). An early pioneer of this model, Judah Folkman suggested that human bodies are constantly keeping small tumors in check by preventing their ability to recruit new blood supply (39). In particular, Folkman invoked angiogenic dormancy as a way to explain the discrepancy between the high prevalence of undetected breast, prostate, and thyroid tumors found at autopsy and the low rate of which these cancers become clinically significant over the course of a lifetime. In concordance with this idea, microscopic and macroscopic tumors that were stained with an endothelial cell marker demonstrated that large tumors had well-organized vascular structures while small tumors had few small vessels (31).
There is a large body of evidence demonstrating the mechanism of angiogenic dormancy in mouse models (4,31,40,41) through the evolution of growing tumor masses. However, the role of angiogenic dormancy in metastatic dormancy is less convincing. Live-cell imaging experiments of metastatic models using GFP-tagged cancer cells in the patient have suggested that circulating tumor cells most likely initially seed and grow in an oxygen-rich environment close to endothelial vessels, suggesting that dormancy at this single-cell level must therefore be explained by some other mechanism (42).
Immunologic Dormancy
The role of immunity in shaping cancer growth, first hypothesized by Ehrlich in the early 1900's and further developed several decades ago by Burnet and Thomas (43), has been extensively reviewed in recent years (44-46). A current model explaining the role of the immune system in cancer progression is a three-stage process: elimination (where cancer cells are recognized and eliminated by the immune system), equilibrium (a less-well defined process where the immune system controls but does not completely eliminated malignant growth), and escape (when tumors that reside in a less-immunogenic state are no longer susceptible to immunosurveillance) (44,46). Proponents of immunologic dormancy have worked on elucidating the role of the equilibrium phase in cancer progression, and multiple lines of evidence in mouse studies aimed at understanding this phase have demonstrated that the innate and adaptive immunity can indeed hold a growing tumor in check (33,45,47,48).
The transmission of cancer from organ transplant donors to recipients has been cited as evidence of immunologic dormancy in the clinical setting (44). In a recent case study, metastatic melanoma developed 1-2 years post-transplant in two allograft recipients receiving kidneys from the same donor who had been treated for primary melanoma 16 years prior to death and who was considered disease free at time of organ donation (49). Several other studies of malignant melanoma in allograft recipients corroborate this observation (50-52). In another study involving pediatric acute myeloid leukemia, the propensity for relapse in patients treated with chemotherapy with or without autologous bone marrow transplant was strongly correlated with anti-tumor CD8+ T-lymphocyte levels (53). Patients in remission had detectable anti-tumor CTLs following induction, and none of these patients relapsed, with follow-up times up to 74 months. On the other hand, seven of eight patients without detectable anti-tumor CTLs suffered from relapse ranging from 2-17 months following treatment.
Given the extensive clinical data on post-transplantation patients with induced immunosuppression, one would predict such patients to have a higher rate of occurrence of a broad spectrum of tumor types as the innate and adaptive immunity are compromised (32). However, in two major studies of over 40,000 combined patients, there was only a significant elevation of viral-associated cancers and no elevation in the rate of common carcinomas such as lung, breast, prostate, and colon (54,55). It remains unclear what cellular processes governs these clinical observations, as the immune system has been shown to play both pro and anti-proliferative functions in tumor evolution (56).
Cellular Dormancy
While several lines of evidence have demonstrated the presence of dormant cancer cells, the underlying mechanism behind cellular dormancy is perhaps the least well-defined, as characterizing individual cancer cells in vivo remains a significant technical hurdle (4). Identification of dormant cancer cells in vivo has primarily relied on static immunohistochemistry such as Ki67 or TUNEL stain, which provides limited insight into a dynamic process (36). More recent techniques such as live-cell imaging, however, has been able to offer additional information about the growth kinetics on a single-cell level in experimental models, and in one study using in vivo videomicroscopy, the survival of dormant cancer cells up to 11 weeks following injection was shown in mouse models of metastasis (57).
Clinical evidence of cellular dormancy has been documented in both primary tumor and metastases and has also suggested that dormant cells can be refractory treatment. In a recent study analyzing human tumor tissue samples of breast cancer patients undergoing neoadjuvant chemotherapy, there was a significant enrichment for dormant cells in tissue samples of patients after exposure to chemotherapy compared to those in the same individual before treatment (58). Although it remains unclear whether these cells were induced or selected by chemotherapy, the study suggests a direct relevance to disease treatment. Isolation and characterization of circulating tumor cells (CTCs) from patients have also demonstrated that many of these cells are dormant or have limited proliferative capacity (59-61). In one study, the presence of Ki-67 negative circulating tumor cells isolated from patients with metastatic breast cancer was correlated with disease progression and elevation of tumor marker levels (59). Furthermore, in a cohort of patients who had undergone adjuvant chemotherapy and were initially negative for circulating tumor cells, repeat examination during chemotherapy revealed the presence of Ki-67 negative circulating tumor cells, suggesting that these non-proliferative cells may be resistant to therapy (59). These circulating cells were likely not just a result of shedding of non-viable cells from treatment, as subset of patients who were initially positive for circulating tumor cells became negative following chemotherapy.
Given the inherent difficulties in detecting and characterizing individual cells or micrometastatic lesions in the patient, the relative contribution of various mechanisms of dormancy in the clinical setting is difficult to assess. Recent advancements in the underlying mechanism governing cellular dormancy, however, has refined our understanding of how this complex phenomenon may play an increasingly important role in the development and progression of human cancers.
III. Cellular Dormancy at a Molecular and Cellular Level
Although a detailed review of the canonical cell cycle is beyond the scope of this review, a basic understanding of signaling pathways in relation to the canonical cell cycle is necessary for synthesizing upstream pathways involved in cancer cell dormancy (35). While mechanisms controlling entry and progression through the G1 phase of the cell cycle vary depending on cell type and context, different pathways must converge on activation of specific cyclin-dependent kinases (CDKs), which subsequently drive entry into S, G2, and M phase through association with various cyclins. Evidence accumulated over the past several decades has suggested that the key decision point for a cell entering the cell cycle occurs in late G1, where dissociation of the transcription factor E2F from Rb enables cell cycle progression. The timing of this event is governed by the interactions between D-type cyclins, CDK4/6, and CDK inhibitors p21Cip1/WAF1, p27Kip1, p57Kip2, p16 and INK4 proteins (35,62).
Many recent lines of investigations suggest that down-regulation of two of the most well-studied pathways activated during oncogenesis, the Ras-MEK-ERK/MAPK and PI(3)K-AKT signaling cascades, play a critical role in governing cancer cell dormancy (4,58,63-65). This is perhaps not surprising given that both of these pathways directly activate the canonical cell cycle pathway. For example, the Ras-MEK-ERK/MAPK kinase cascade plays a pivotal role in cellular proliferation through stabilization of c-Myc, which induces the expression of cyclin D1 and suppresses CDK inhibitors, thereby promoting CDK activation (35). Similarly, the PI(3)K-AKT signaling axis facilitates CDK activity via inhibiting glycogen synthase kinase 3-beta, which normally inhibits proliferation by destabilizing cyclin D, and preventing nuclear localization of FOXO transcription factors, which activates CDK inhibitors p21 and p27 (35).
A logical question follows: given that mutation, overexpression, or aberrant regulation of key players in the Ras and PI(3)K pathways are present in the majority of human tumors, what role does cellular dormancy play under these circumstances? Interestingly, it has been shown that cancer cells with mutations that constitutively activate the PI(3)K-AKT axis can still downregulate this pathway upon entering a quiescent state, for example, through ubiquitin-mediated degradation (58,66,67). The fact that mechanisms exist to abrogate supposed driver pathway of mutagenesis argues for the hypothesis that cellular dormancy offers an evolutionary advantage towards tumor progression.
Moving upstream: lessons learned from integrin signaling and microenvironmental cues
The influence of microenvironmental factors on cancer cell dormancy has been best characterized through the study of integrin signaling. Integrins are a family of heterodimeric transmembrane receptors that transduce signals from the extracellular matrix (ECM), by activating signaling intermediaries including cytosolic tyrosine kinases, to promote proliferation, survival, and motility of both cancer and normal cells. Several lines of evidence have demonstrated that modulation of integrin signaling, and in particular, beta-1 integrin, plays an important role in inducing cancer cell dormancy (64,65,67-70).
Down-regulation of beta-1 integrin signaling appears to promote cell cycle transition from a proliferative to a quiescent state. In an early study using a three-dimensional basement membrane assay, culturing HMT-3522 mammary cancer cells with inhibitory beta-1 integrin antibody induced growth arrest and phenotypic differentiation such as reformation of acini morphology and basement membrane reassembly (64). Removal of inhibitory antibody was able to reverse this phenotype. Beta-1 integrin signaling relies on the phosphorylation of focal adhesion kinase (PTK2/FAK), a cytosolic tyrosine kinase. In an MMTV transgenic mouse model of human breast cancer that utilized a Cre/LoxP1 system to disrupt integrin function, complete abrogation of beta-integrin led to a decrease in the phosphorylation of FAK tyrosine residues, inhibition of cell proliferation, and cessation of tumor growth in vivo (65). Similarly, up-regulation of beta-1 signaling enables dormant cancer cells to re-enter into the cell cycle. In vitro experiments using the highly metastatic D2A1 mammary carcinoma cell line revealed that their growth ability in three-dimensional cell-culture was dependent on presence of fibronectin, beta-1 integrin signaling, and downstream phosphorylation of the myosin light chain complex (69).
The connection between beta1-integrin/FAK and the MAPK pathway in cancer cell growth was shown in vivo by demonstrating the dependency of metastatic spread of mouse mammary cancer cell lines D2.0R and D2A1 on the density of collagen I / beta1-integrin signaling (71), which led to the activation of MAPK and subsequent phosphorylation of myosin light chain and actin stress fiber formation. Similarly, beta-1 integrin/FAK signaling also modulates the AKT pathway to drive cancer cell proliferation. In a recent study using both MCF7 human mammary epithelial cancer cells and HCT-116 colon cancer cell lines, in vitro proliferative heterogeneity was regulated by beta-1 integrin/FAK signaling, which preserved cytosolic AKT1 levels by preventing ubiquitin-mediated proteasomal degradation driven by the mTORC2 complex (67). It remains to be seen if the effect of integrin signaling on cancer cell dormancy is unique to beta1-integrin or if other subtypes may also play a similar role. Available data suggests that there is likely some degree of specificity, as knockdown of beta-1 containing integrins but not alpha-V containing integrins significantly hinder the proliferation of metastatic D2 cells under three-dimensional culturing conditions (69,70).
The complex interplay of microenvironmental cues that influence cellular dormancy has also been recently examined with respect to cancer cell-niche interactions (72-74). In a recent study using engineered microvascular niches derived from primary human umbilical endothelial cells, the authors examined the behavior of disseminated tumor cells and demonstrated that microvasculature niches that tend to promote cellular quiescence express increased thrombospondin-1, a glycoprotein known to interact with ECM proteins including fibronectin, collagen, and beta-1 integrin (72). Niches that promoted cellular proliferation were found to excrete TGF-beta1 and periostin, a ligand for several integrin subtypes. Another study utilizing intravital microscopy to perform imaging analysis of calvarial bone marrow demonstrated that local secretion of osteopontin, an integrin ligand, was able to promote quiescent behavior (73). While further work is needed to better characterize the various extracellular signaling events that can contribute to cancer cell dormancy, these results represent an important step forward in our understanding of cancer dormancy as it provides both a framework to explore the effect of microenvironmental factors on tumor progression as well as offer potential therapeutic targets (75).
Internal cues triggering cancer cell dormancy – towards a quantitative model
Internal cues such as stress signaling may also contribute to the induction of cellular dormancy. Aguirre-Ghiso and colleagues have demonstrated using the human D-HEp3 head and neck cancer model that dormancy can result from a near complete inhibition of the Raf-MEK-MAPK(ERK) pathways in conjunction with activation of the stress pathway involving p38 (68). They demonstrated in vivo using a GFP-reporter system for ERK and p38 activity that proliferation requires a high ERK:p38 ratio while a low ERK:p38 ratio favors dormancy. Through a proteomic search of p38-regulated genes in the D-HEp3 cell line, the authors found that the molecule upregulates endoplasmic reticulum-resident chaperones normally induced during adaption to a type of stress signaling known as the unfolded protein response (76). Autophagy, another form of stress response, has also been implicated in the induction of dormancy in human ovarian cancer cell lines and was shown to be correlated with the downregulation of the PI(3)K-AKT pathway (63). This body of work suggests that loss of mitogenic signaling alone may not be sufficient to reprogram cells into a dormant phenotype, and that other internal cues such as stress signaling may be necessary to ultimately alter levels of CDK inhibitors and other cell cycle players to favor cell cycle arrest.
Quantitative aspects of cell signaling in the induction of cancer cell dormancy
The proliferative capacity of cancer cells is a fine-tuned process as the degree and duration of signaling from proliferative pathways can determine the balance between proliferation, dormancy, and cell death. Toggling of the PI(3)K-AKT signaling pathway in recent studies nicely illustrates this concept. In one study examining the autophagic process in human ovarian cancer cell lines with a inducible ARHI (aplasia Ras homolog member I) system, the authors demonstrate that expression of ARHI in tissue culture leads to autophagic cell death through inhibition of PI(3)K/AKT/mTOR and MAPK signaling pathways (63). Interestingly, when this cell line was introduced in mouse xenografts, induction of ARHI resulted in dormancy that was reversible upon removal of ARHI stimuli as opposed to causing cell death. Further tissue culture analysis indicated that autophagic cell death was reduced when these ARHI-induced cells were treated with growth factors (IGF-1, M-CSF), angiogenic factors (VEGF, IL-8), and matrix proteins found in xenografts. From these studies, the outcome of autophagy appeared to be correlated with the degree of PI(3)K signaling: chronic and severe PI3K downregulation caused autophagic death while the presence of lower levels of PI(3)K signaling contributed by microenvironmental factors promoted autophagic dormancy (77).
Along similar lines, Dey-Guha et al. demonstrated in actively proliferating MCF-7 breast and HCT-116 colon cancer cells, which have up-regulated the AKT/PKB pathway, that there are rare sub-populations of temporarily dormant cells characterized by decreased AKT/PKB signaling (58). Treatment with allosteric AKT inhibitors in the bulk population of these cells induced apoptosis when given at a higher dose while treatment with a lower dose induced a state of reversible dormancy. These studies suggest that precise levels of proliferation and survival signals may be mediate a fine balance between cell dormancy and death.
The variable growth kinetics of cellular dormancy
The sensitivity of cell growth in response to fluctuating intra and extracellular cues suggests that the temporal aspect of cellular dormancy is highly variable. While technical barriers limit our ability to track individual cancer cells in the patient for long period of time, current studies have so far been consistent with this hypothesis. As mentioned earlier, survival of dormant cancer cells up to 11 weeks in mouse models of metastasis have been demonstrated using in vivo videomicroscopy (57). A back-of-the envelope calculation suggests that with a doubling time of two to three months, it would take roughly four to five years for a single cell to form a clinically detectable 1mm mass (below the angiogenic limit of dormancy) comprised of approximately one million cells. While this calculation obviously makes many assumptions about the growth kinetics, it nevertheless demonstrates that cellular dormancy can in fact operate on a time scale on par with clinical evidence.
Several recent lines of work have also suggested that dormancy can also operate on a shorter time frame. For example, work on the uPAR/p38/ERK(MAPK) pathway as previously noted described the ability to interrupt dormancy using the same cell lines in a matter of days. Perturbation of the uPAR/p38/ERK network by inhibition of p38 kinase activity for 48 hours or transient transfection with uPAR cDNA resulted in reprogramming the cells into a proliferative state (78,79). Dey-Guha and colleagues have also shown that dormant cells can resume a proliferative stance within two to three days in tissue culture media replete with growth factors upon upregulation of the AKT pathway (58). The length of cellular dormancy in a developing cancer can thus vary dramatically depending on the environmental cues. Thus, this phenomenon cannot be thought of as an on-or-off phenotype but rather as a behavior that exists along a continuous spectrum that is constantly influenced by internal and external variables in flux.
Relationship between cellular dormancy and the cancer stem cell model
The intersection between cellular dormancy and the cancer stem cell model may offer insight into underlying biology in both realms, but is poorly characterized. Conceptually, the phenomena are linked as they represent two important models in which cancer cells can evade therapy and relapse despite clinical remission. Perhaps the simplest description of the relationship between the two phenomena is that cancer stem cells represent a subset of dormant cancer cells in that while cancer stem cells have the ability to remain dormant, not all dormant cancer cells have been shown to carry the stem-like property of self-renewal and differentiation. This relationship is more complex, however, in light of evidence challenging the strict hierarchical behavior of stem cells, as it suggests that dormancy, the ability to self-renew, and the ability to differentiate may represent aspects of tumor heterogeneity that may or may not overlap depending on the individual circumstance.
The possibility of transient stem-like behavior in a growing tumor was recently shown in a study where small subpopulations of slowly-cycling melanoma cells with division time greater than 4 weeks were isolated using an H3K4 demethylase biomarker (26). These population of cells could give rise to highly proliferative progeny and were shown to be essential for continuous tumor growth, as knockdown of the demethylase initially led to increased tumor growth but was ultimately followed by exhaustion. However, the demethylation status was reversible and subpopulation of cells initially lacking the demethylase could gain the marker over time. While these slowly-cycling cells harbor the ability to self-renew, they do not follow a hierarchical cancer stem cell model with respect to proliferation. In another recent study using human breast cancer cells, isolation of cancer cells that were “stem-like” using specific surface markers produced subpopulations that ultimately reproduced initial population characteristics over serial passaging (80). The authors were able to show that this behavior can be mathematically modeled as a stochastic process, suggesting that these stem-like properties were simply part of the dynamic behavior of a heterogeneous tumor population. Other features of stem-cell behavior such as the ability to differentiate have also shown to be reversible in cancers. Shachaf et al. used a transgenic mouse model conditionally expressing the MYC proto-oncogene in liver cells to show that MYC inactivation resulted in differentiation of tumor cells into hepatocytes and biliary cells that can form bile duct structures (81). Reactivation of MYC abolishes the cellular architecture and restored the neoplastic features.
While the subject of cancer stem cells is not meant to be a focus of this review, we make the assertion that whether or not dormant cancer cells are “stem-like” is not as relevant as the observation that dormancy is a dynamic phenotype that can achieved through a variety of situations, including the scenario where cancer cells assuming a “stem-like” state. We thus propose a quantitative model of cellular dormancy where the proliferative capacity of cancer cells are determined by the combination of external and internal cues (Figure 2). There is strong evidence that the interplay between microenvironmental factors (e.g. through integrin signaling) and internal cues in response to cell stress plays an important role in dictating the ability for individual cancer cells to enter or exit dormancy. In theory, any process that can alter the core balance of the cell-cycle components can influence the proliferative behavior of the cell, and it is likely that other important cues will be uncovered moving forward. One major challenge that remains is determining which of these factors are relevant or play a predominant role under specific clinical situations.
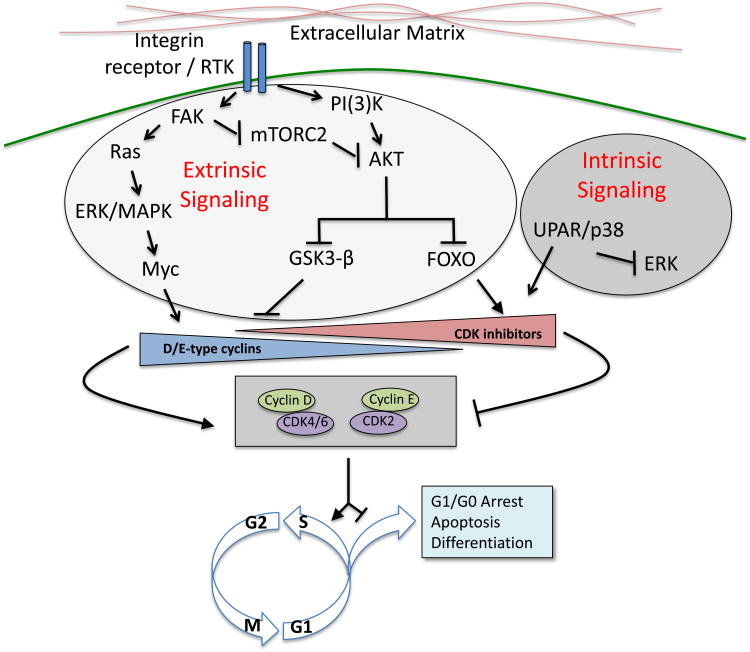
Figure 2. Pathways of cellular dormancy.
Cellular dormancy is governed by a combination of external and internal cues. Current literature supports the role of integrin receptor signaling as a pivotal event that leads to the activation of Ras-ERK/MAPK and PI(3)K-AKT pathways, thereby maintaining cell cycle progression. Down-regulation of integrin signaling is a critical driver of cellular dormancy and can be influenced by the composition of extracellular matrix. Intrinsic signaling through the stress pathway uPAR/p38 can also shift the balance towards cellular dormancy through downregulation of the MAPK pathway.
IV: Moving Forward
Functional characterization of dormant tumor cells in a physiologic setting is important for bridging the gap between our limited biological insight and the clinically observed phenomenon of cancer dormancy. While there are obvious limitations in studying this process in tissue culture, some progress has been made to improve the relevance of in vitro work, for example, by utilizing a 3-dimentional culture system constituted from basement membrane that more accurately reflect in vivo ECM components (69), and more recently, by directly engineering microenvironmental niches (72,74). Newer approaches also need to focus on characterizing the dynamic process of cellular dormancy. Much of our current understanding of cellular dormancy is based on static histology or retention of fluorescent dye that fade after each successive cell division (26,57,58). Utilizing advanced in vivo imaging modalities such as quantum dot imaging (82,83) MRI labeling techniques (84), and intravital microscopy (73), for example, could enable a better understanding of the time scale in which this phenomenon operates.
Given that long-term cellular imaging in the human poses a significant technical hurdle, the application of mouse models of cancer is another promising avenue that can expand our understanding of cancer cell dormancy by examining different aspects of cancer progression. For example, the continued reliance of late-stage tumors on initial proliferative signaling events was demonstrated using a transgenic mice carrying tetracycline-inducible c-Neu receptor tyrosine kinase (85). These mice were shown to develop multiple invasive carcinomas following transgene induction, which essentially regressed to a clinically undetectable state after de-induction. Similar experiments aimed at studying the kinetics of cancer cells entering and exiting a dormant state may be observed by quantitatively manipulating candidate pathways (such as integrin signaling) in mouse models.
Therapeutic implications of cancer cell dormancy
It is critical that our understanding of cancer dormancy shifts from a primarily clinical phenomenon to one characterized by a clear grasp of underlying cellular pathways, as the knowledge gained will have important implications on disease management for several reasons (3). First, novel therapeutics may be designed to manipulate the transition in and out of this state. For example, while dormant cancer cell populations have been shown in vivo to resist conventional chemotherapy (86), there are a variety of methods that can promote the reentrance into the cell cycle and increase drug sensitivity. Some of these methods have been used for decades, though the precise biologic implications may not be fully appreciated until now: in a study examining treatment of leukemia, agents such as interferon-alpha, G-CSF, and arsenic trioxide, all of which are efficient at promoting cycling of dormant hematopoietic and leukemic stem cells, were used as a way to sensitize these cells for killing by various chemotherapeutic agents (87). Given our increasing understanding of the various pathways involved in cellular dormancy, targeted therapy may offer a more effective approach. For example, manipulating integrin signaling has been demonstrated to eliminate slow proliferators in vitro (67). As epigenetic events play a critical role in maintaining cellular dormancy (26,58), another approach might involve using histone-modifying drugs to prevent such dynamic cell-state transitions (27).
Second, understanding the survival mechanisms of dormant tumor cells may reveal ways to specifically target these cells given their refractory nature. The challenge with this approach, however, lies in the selectivity of eliminating dormant cancer cells while leaving quiescent non-cancerous cells (such as normal stem cells). This may be possible as we focus on identify dependent pathways that help maintain dormancy. For example, as up-regulation of specific stress signaling responses has shown to be a critical factor in the maintenance of dormancy as discussed earlier, targeting these pathways may cause dormant cells to lose their ability to reenter the cell cycle and either senesce or undergo apoptosis. Further characterization of dormant cells will be required to gain insights on unique ways to target this population. High throughput technologies such as small molecule screens may be used to find candidate targets, but this approach would rely on the challenging task of having to isolate or assay a sufficient number of dormant cells.
Many unanswered questions remain. First, what is the role of the various types of cancer dormancy in the clinical setting? For example, perhaps cellular dormancy plays a more important role in the initial establishment of solitary metastatic cells whereas angiogenic dormancy and immunologic surveillance occur later in the continuum as the tumor grows. On the other hand, cellular dormancy may also play a major role in explaining the ability for bulk tumors to survive cytotoxic stress. What genetic or epigenetic factors influence the ability for cancer cells to transition in and out of cellular dormancy? Certain tumor types or mutational profile may intrinsically alter a specific cancer cell's ability to enter and maintain a dormant state. Given that several different pathways have already been shown to be involved in influencing cellular dormancy, manipulations in one or another may bias the subsequent events necessary to achieve such a state. Understanding the biological mechanism governing each situation would impact potential therapies targeting this phenomenon. An increased understanding of cancer dormancy will likely change our current paradigms of cancer treatment and disease progression.
Acknowledgments
This work was supported by awards from the National Cancer Institute, Susan G. Komen for the Cure, and Prostate Cancer Foundation (to S. Ramaswamy). S. Ramaswamy was supported with a Stand Up to Cancer Innovative Research Grant, a program of the Entertainment Industry Foundation (SU2C-AACR-IRG0411). S. Ramaswamy and A.C. Yeh were supported by awards from the Howard Hughes Medical Institute (Physician-Scientist Early Career Award, Medical Student Research Fellowship).
References
- 1.Friedl W, Herfarth C. The long-term prognosis of breast cancer Retrospective study of 973 patients. Langenbecks Archiv fur Chirurgie. 1992;377(3):168–73. doi: 10.1007/BF00184375. [DOI] [PubMed] [Google Scholar]
- 2.Leman JA, Mac Kie RM. Late (> 10 years) recurrence of melanoma: the Scottish experience. The British journal of dermatology. 2003;148(2):372–3. doi: 10.1046/j.1365-2133.2003.05097_8.x. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nature reviews Cancer. 2010;10(12):871–7. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 4.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nature reviews Cancer. 2007;7(11):834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155(4):750–64. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature reviews Cancer. 2014;14(9):611–22. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celsus AC, Spencer WG. De medicina. London: W. Heinemann ltd.; 1935. [Google Scholar]
- 8.Willis RA. The spread of tumours in the human body. London: J. & A. Churchill; 1934. [Google Scholar]
- 9.Hadfield G. The dormant cancer cell. British medical journal. 1954;2(4888):607–10. doi: 10.1136/bmj.2.4888.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley NJ, Seigler HF. Late recurrence of malignant melanoma. Analysis of 168 patients. Annals of surgery. 1990;212(2):173–7. doi: 10.1097/00000658-199008000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansel G, Schonlebe J, Haroske G, Wollina U. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). A single-centre analysis of 1881 patients with a follow-up of 10 years or more. Journal of the European Academy of Dermatology and Venereology: JEADV. 2010;24(7):833–6. doi: 10.1111/j.1468-3083.2009.03536.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmid-Wendtner MH, Baumert J, Schmidt M, Konz B, Holzel D, Plewig G, et al. Late metastases of cutaneous melanoma: an analysis of 31 patients. Journal of the American Academy of Dermatology. 2000;43(4):605–9. doi: 10.1067/mjd.2000.107234. [DOI] [PubMed] [Google Scholar]
- 13.Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79(12):2361–70. [PubMed] [Google Scholar]
- 14.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. Journal of the National Cancer Institute. 1999;91(1):80–5. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 15.Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, et al. Circulating tumor cells in patients with breast cancer dormancy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(24):8152–62. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 16.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 17.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 18.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nature reviews Cancer. 2004;4(6):448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 19.Klein CA, Holzel D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell cycle. 2006;5(16):1788–98. doi: 10.4161/cc.5.16.3097. [DOI] [PubMed] [Google Scholar]
- 20.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nature genetics. 2003;33(1):49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 21.Demicheli R, Terenziani M, Bonadonna G. Estimate of tumor growth time for breast cancer local recurrences: rapid growth after wake-up? Breast cancer research and treatment. 1998;51(2):133–7. doi: 10.1023/a:1005887422022. [DOI] [PubMed] [Google Scholar]
- 22.Willis L, Graham TA, Alarcon T, Alison MR, Tomlinson IP, Page KM. What can be learnt about disease progression in breast cancer dormancy from relapse data? PloS one. 2013;8(5):e62320. doi: 10.1371/journal.pone.0062320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loddo M, Kingsbury SR, Rashid M, Proctor I, Holt C, Young J, et al. Cell-cycle-phase progression analysis identifies unique phenotypes of major prognostic and predictive significance in breast cancer. British journal of cancer. 2009;100(6):959–70. doi: 10.1038/sj.bjc.6604924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. The lancet oncology. 2010;11(2):174–83. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 25.Lambert G, Estevez-Salmeron L, Oh S, Liao D, Emerson BM, Tlsty TD, et al. An analogy between the evolution of drug resistance in bacterial communities and malignant tissues. Nature reviews Cancer. 2011;11(5):375–82. doi: 10.1038/nrc3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141(4):583–94. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borst P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open biology. 2012;2(5):120066. doi: 10.1098/rsob.120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29(6 Suppl 16):15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 30.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature medicine. 1995;1(2):149–53. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 31.Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. Journal of the National Cancer Institute. 2006;98(5):316–25. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 32.Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(30):12396–400. doi: 10.1073/pnas.1106613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinhold KJ, Goldstein LT, Wheelock EF. The tumor dormant state. Quantitation of L5178Y cells and host immune responses during the establishment and course of dormancy in syngeneic DBA/2 mice. The Journal of experimental medicine. 1979;149(3):732–44. doi: 10.1084/jem.149.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature reviews Cancer. 2005;5(4):263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 35.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432(7015):298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 36.Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell cycle. 2006;5(16):1744–50. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- 37.Folkman J. Tumor angiogenesis. Advances in cancer research. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews Cancer. 2003;3(10):721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 39.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427(6977):787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 40.Giuriato S, Ryeom S, Fan AC, Bachireddy P, Lynch RC, Rioth MJ, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16266–71. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Indraccolo S, Stievano L, Minuzzo S, Tosello V, Esposito G, Piovan E, et al. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4216–21. doi: 10.1073/pnas.0506200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature medicine. 2000;6(1):100–2. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 43.Burnet FM. The concept of immunological surveillance. Progress in experimental tumor research. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 44.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 46.Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. Journal of leukocyte biology. 2008;84(4):988–93. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 47.Farrar JD, Katz KH, Windsor J, Thrush G, Scheuermann RH, Uhr JW, et al. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-gamma in establishing and maintaining the tumor-dormant state. Journal of immunology. 1999;162(5):2842–9. [PubMed] [Google Scholar]
- 48.Matsuzawa A, Takeda Y, Narita M, Ozawa H. Survival of leukemic cells in a dormant state following cyclophosphamide-induced cure of strongly immunogenic mouse leukemia (DL811) International journal of cancer Journal international du cancer. 1991;49(2):303–9. doi: 10.1002/ijc.2910490227. [DOI] [PubMed] [Google Scholar]
- 49.MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. The New England journal of medicine. 2003;348(6):567–8. doi: 10.1056/NEJM200302063480620. [DOI] [PubMed] [Google Scholar]
- 50.Elder GJ, Hersey P, Branley P. Remission of transplanted melanoma--clinical course and tumour cell characterisation. Clinical transplantation. 1997;11(6):565–8. [PubMed] [Google Scholar]
- 51.Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996;61(2):274–8. doi: 10.1097/00007890-199601270-00019. [DOI] [PubMed] [Google Scholar]
- 52.Suranyi MG, Hogan PG, Falk MC, Axelsen RA, Rigby R, Hawley C, et al. Advanced donor-origin melanoma in a renal transplant recipient: immunotherapy, cure, and retransplantation. Transplantation. 1998;66(5):655–61. doi: 10.1097/00007890-199809150-00020. [DOI] [PubMed] [Google Scholar]
- 53.Montagna D, Maccario R, Locatelli F, Montini E, Pagani S, Bonetti F, et al. Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood. 2006;108(12):3843–50. doi: 10.1182/blood-2006-05-021535. [DOI] [PubMed] [Google Scholar]
- 54.Penn I. Post-transplant malignancy: the role of immunosuppression. Drug safety: an international journal of medical toxicology and drug experience. 2000;23(2):101–13. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 55.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA: the journal of the American Medical Association. 2006;296(23):2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 56.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes & development. 2011;25(24):2559–72. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer research. 2002;62(7):2162–8. [PubMed] [Google Scholar]
- 58.Dey-Guha I, Wolfer A, Yeh AC, J GA, Darp R, Leon E, et al. Asymmetric cancer cell division regulated by AKT. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12845–50. doi: 10.1073/pnas.1109632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(10):3678–85. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- 60.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature reviews Cancer. 2008;8(5):329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 61.Solakoglu O, Maierhofer C, Lahr G, Breit E, Scheunemann P, Heumos I, et al. Heterogeneous proliferative potential of occult metastatic cells in bone marrow of patients with solid epithelial tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2246–51. doi: 10.1073/pnas.042372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes & development. 1999;13(12):1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 63.Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. The Journal of clinical investigation. 2008;118(12):3917–29. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. The Journal of cell biology. 1997;137(1):231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer cell. 2004;6(2):159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 66.Endo H, Okuyama H, Ohue M, Inoue M. Dormancy of cancer cells with suppression of AKT activity contributes to survival in chronic hypoxia. PloS one. 2014;9(6):e98858. doi: 10.1371/journal.pone.0098858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dey-Guha I, Alves CP, Yeh AC, Salony, Sole X, Darp R, et al. A mechanism for asymmetric cell division resulting in proliferative asynchronicity. Molecular cancer research: MCR. 2015;13(2):223–30. doi: 10.1158/1541-7786.MCR-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer research. 2004;64(20):7336–45. doi: 10.1158/0008-5472.CAN-04-0113. [DOI] [PubMed] [Google Scholar]
- 69.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer research. 2008;68(15):6241–50. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10290–5. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer research. 2010;70(14):5706–16. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15(7):807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM, Sipkins DA. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood. 2013;121(24):4821–31. doi: 10.1182/blood-2012-12-475483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer research. 2013;73(23):6886–99. doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 75.Barkan D, Chambers AF. beta1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(23):7219–23. doi: 10.1158/1078-0432.CCR-11-0642. [DOI] [PubMed] [Google Scholar]
- 76.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer research. 2006;66(3):1702–11. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amaravadi RK. Autophagy-induced tumor dormancy in ovarian cancer. The Journal of clinical investigation. 2008;118(12):3837–40. doi: 10.1172/JCI37667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer research. 2003;63(7):1684–95. [PubMed] [Google Scholar]
- 79.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Molecular biology of the cell. 2001;12(4):863–79. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–44. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 81.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431(7012):1112–7. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 82.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature biotechnology. 2004;22(8):969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 83.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature medicine. 2004;10(9):993–8. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 84.Murrell DH, Foster PJ, Chambers AF. Brain metastases from breast cancer: lessons from experimental magnetic resonance imaging studies and clinical implications. Journal of molecular medicine. 2014;92(1):5–12. doi: 10.1007/s00109-013-1108-z. [DOI] [PubMed] [Google Scholar]
- 85.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer cell. 2002;2(6):451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 86.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast cancer research and treatment. 2003;82(3):199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 87.Essers MA, Trumpp A. Targeting leukemic stem cells by breaking their dormancy. Molecular oncology. 2010;4(5):443–50. doi: 10.1016/j.molonc.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]