Abstract
Elevated oxidative stress is an aberration seen in many solid tumors, and exploiting this biochemical difference has the potential to enhance the efficacy of anti-cancer agents. Homeostasis of reactive oxygen species (ROS) is important for normal cell function, but excessive production of ROS can result in cellular toxicity and therefore ROS levels must be balanced finely. Here, we highlight the relationship between the extracellular matrix and ROS production by reporting a novel function of the matricellular protein Fibulin-5 (Fbln5). We employed genetically engineered mouse models of pancreatic ductal adenocarcinoma (PDA) and found that mutation of the integrin-binding domain of Fbln5 led to decreased tumor growth, increased survival, and enhanced chemoresponse to standard PDA therapies. Through mechanistic investigations, we found that improved survival was due to increased levels of oxidative stress in Fbln5 mutant tumors. Furthermore, loss of the Fbln5-integrin interaction augmented fibronectin signaling, driving integrin-induced ROS production in a 5-lipooxygenase-dependent manner. These data indicate that Fbln5 promotes PDA progression by functioning as a molecular rheostat that modulates cell-ECM interactions to reduce ROS production and thus tip the balance in favor of tumor cell survival and treatment-refractory disease.
Introduction
Tumors develop and progress in the context of the extracellular matrix (ECM). In fact, structural ECM proteins can promote tumor cell survival and stimulate invasive tumor cell programs (1-6). This is particularly evident in desmoplastic tumors, such as pancreatic ductal adenocarcinoma (PDA) (7), where ECM proteins including fibronectin (FN) and collagen activate signaling pathways that drive cell survival, proliferation and migration (1, 2). ECM-mediated signaling is governed by expression of the ECM proteins, the presence of cell surface receptors and the expression and activity of matricellular proteins that function as extracellular adaptors to reduce ECM-cell interaction (1, 8, 9). Fibulin-5 (Fbln5), a member of the fibulin family of proteins, is particularly important in this regard, as it binds to α4β1 and α5β1 integrins via an RGD sequence, but does not support integrin activation (8). Thus, Fbln5 competes with structural ECM ligands, principally FN, that would otherwise activate these integrins.
ECM proteins stimulate the generation of reactive oxygen species (ROS) in an integrin-dependent manner (2, 10, 11). ROS generation in this context is generally transient and serves primarily as a signaling intermediate that enhances cellular activity (12). We reasoned that a chronic increase in integrin-induced ROS would negatively affect tumor growth. FN-driven ROS generation is an attractive pathway to exploit for this strategy because FN ligation of β1 integrins is governed in part by Fbln5 (8, 13). We reported previously that Fbln5 reduced FN-mediated integrin-induced ROS production by competing with FN for binding to α5β1 integrin (13). Mutation of the three amino acid RGD sequence in Fbln5 to RGE abolishes integrin binding yet preserves other functions of Fbln5 (14, 15). An essential function of Fbln5 is elastic fiber formation (16). As a result, Fbln5-/- mice exhibit disorganized elastic fibers throughout the body leading to tortuous great vessels, emphysematous lungs and loose skin, resembling cutis laxa syndrome in humans (17, 18). In contrast, Fbln5RGE/RGE (RGE) mice have intact elastic fibers and are essentially indistinguishable from wild-type (WT) littermates (15). However, RGE mice show increased levels of ROS compared to WT animals in tissues where FN is abundant (15). Given the high expression of FN in the stroma of PDA and increasing evidence supporting enhanced ROS production as an anti-cancer strategy (19), we evaluated the consequence of ablating the integrin binding ability of Fbln5 in robust spontaneous models of PDA. Our results show that Fbln5-integrin interaction promotes aggressive tumor growth and progression in mice and that 5-lipooxygenase (5-LOX) activity was required for ROS induction in the absence of functional Fbln5. Additionally, we found that Fbln5 was expressed abundantly in the stroma of human PDA tumors. These data provide insight into the function of Fbln5 in PDA and reveal how ECM signaling might be exploited to drive pro-oxidant therapy.
Materials and Methods
Mouse models
Fbln5RGE/RGE (RGE), Fbln5-/- (KO), LSL-KrasG12D/+; Cdkn2aLox/Lox (KI) and Cdkn2aLox/Lox; p48Cre (IC) mice were generated as previously described (1, 15, 45, 46). RGE mice were used to breed with KI and IC mice to generate genetically matched LSL-KrasG12D/+; Cdkn2aLox/Lox; p48Cre (KIC) mice and RGE-KIC mice. LSL-Trp53R172H/+ mice were obtained from National Cancer Institute (NCI) Mouse Repository (22). RGE mice were also used to breed with LSL-KrasG12D/+; LSL- Trp53R172H/+ (KP) and p48Cre mice to generate genetically matched LSL-KrasG12D/+; LSL-Trp53R172H/+; p48Cre (KPC) mice and RGE-KPC mice. All mice were housed in a pathogen-free facility and all experiments were performed under written protocols approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern (UTSW) Medical Center at Dallas.
Animal studies
For endpoint studies, KIC and RGE-KIC mice were sacrificed and entire tissues including pancreas/tumor, liver and spleen were harvested and weighed at 1, 1.5 and 2 months old. KPC and RGE-KPC mice were sacrificed at 3 and 5 months, n≥8 mice per time point per group. For all survival studies, mice were carefully monitored and sacrificed when they appeared moribund. For antioxidant treatment, N-acetyl cysteine (NAC) (Sigma Aldrich) was given to mice at 7 mg/ml in the drinking water from 4 weeks old until moribund. For endpoint therapy experiments, KIC and RGE-KIC mice were treated for 3 weeks starting at 7 weeks (1.5 months) of age with intraperitoneal (i.p.) injection of low dose Gemcitabine (GemL; 12.5 mg/kg 3×/week) or Abraxane (Abx; 5 mg/kg 2×/week). Mice were sacrificed and tissues were isolated for analysis, n ≥6 mice per group. For survival studies with therapy, cohorts of KIC and RGE-KIC mice were treated similarly with GemL, high dose Gemcitabine (GemH; 50 mg/kg 1×/week i.p.) or Abx until moribund.
Histology, immunohistochemistry (IHC) and immunofluorescence (IF) staining
Tissues were snap frozen and embedded in OCT (Tissue-Tek) for frozen sections or fixed with 4% paraformaldehyde (PFA) overnight and embedded in paraffin for sectioning. Frozen sections were fixed in ice-cold acetone for 5 minutes (min), air dried for 10 min followed by 10 min incubation with PBS to dissolve the OCT. Paraffin sections were deparaffinized and rehydrated with xylene and serial dilutions of ethanol followed by antigen retrieval with 0.01 M citric acid buffer (pH 6.0). Sections were blocked with 20% aquablock and incubated with primary antibodies in blocking solution (5% BSA in TBST) at 4°C overnight. Primary antibodies used for frozen sections were: rabbit polyclonal anti-mouse Fbln5 (1:100) (purified polyclonal IgG by our lab, 1.6 mg/ml) (17), rat anti-Meca32 (1:100) (purified IgG from hybridoma by our lab) (47), goat anti-Amylase (1:2000) (sc-12821, Santa Cruz Biotechnology), rabbit anti-fibronectin (1:100) (DP3060, Acris) and rabbit anti-γH2AX (1:50) (NB100-2280, NOVUS). Primary antibodies used for paraffin sections were: rabbit anti-human Fbln5 (1:75) (HPA000868, Sigma Aldrich), rabbit anti-Phospho-Histone H3 (PH3) (1:100) (06-570, Millipore), rabbit anti-Amylase (1:2000) (3796S, Cell Signaling) and rat anti-endomucin (1:100) (sc-65495, Santa Cruz Biotechnology). Fluorescein Isothiocyanate (FITC)-conjugated donkey anti-rabbit, rat IgGs, Cyanine 3 (Cy3)-conjugated donkey anti-rat, mouse, rabbit IgGs and Horseradish Peroxidase (HRP)-conjugated donkey anti-rabbit IgGs from Jackson ImmunoResearch were used as secondary antibodies.
Slides with sections of FFPE de-identified human pancreatic cancer tissue were obtained from the UT Southwestern Tissue Resource and the Department of Pathology, UT MD Anderson Cancer Center. Human PDA sections were stained for Fbln5 expression using rabbit anti-Fbln5 (HPA000868, Sigma Aldrich) as indicated above.
Western blot analysis
Western blots were performed as previously described (49). In brief, protein lysates from cell culture or tumor tissues were extracted in ice-cold RIPA buffer (50 mM Tris-Cl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing cocktails of protease (Thermo Scientific) and phosphatase inhibitors (Sigma-Aldrich), by centrifugation (13000g, 15 min) at 4°C following 3 freeze-thaw cycles. Proteins were separated by SDS-PAGE and transferred to methanol activated polyvinylidene difluoride (PVDF) membrane (VWR). The primary antibodies used include the following: rabbit anti-mouse Fbln5 (1:1000), rabbit anti-human Fbln5 (1:500) (HPA000868, Sigma Aldrich), anti-Nqo1 (1:1000) (ab34173, Abcam), anti-α-Tubulin (1:1000) (ab4047, Abcam) and anti-β-actin (1:5000) (A2066, Sigma-Aldrich). HRP-conjugated donkey anti-rabbit IgG (1:10000) (Jackson Immunoresearch) as secondary antibodies were used.
Cell culture
Cell lines used include mouse endothelial cell line bEnd.3 (13), mouse pancreatic cancer cell lines Pan02 (13) and mPLR B9 (1), and human pancreatic cell lines MiaPaCa-2, AsPC-1 and Panc1 (all purchased from ATCC). NG2+ cells were isolated using anti-NG2 antibody conjugated magnetic beads from a KIC tumor (50). Fbln5+/+ (WT), Fbln5-/- (KO) and Fbln5RGE/RGE (RGE) mouse embryonic fibroblasts (MEFs) were isolated from embryonic day E12.5-E14.5 embryos and genotypes were confirmed by PCR. bEnd.3 cells were treated with 100 μM H2O2 or 10 μg/ml α5β1 integrin activating antibody and lysates were collected for Western blot analysis. MEFs were cultured in reduced serum medium Opti-MEM (Life Technologies) overnight before being plated on plastic, FN (Sigma Aldrich) or collagen I (Fisher Scientific), each at 10 μg/ml unless otherwise noted. After plating, MEFs were grown in serum free medium (SFM) supplemented with FN, collagen, β1 integrin blocking antibody (each at 10 μg/ml) or various chemicals. Inhibitors used for various ROS sources include Rotenone (R8875-1G, Sigma Aldrich), Diphenyleneiodonium chloride (DPI) (D2926, Sigma-Aldrich) and nordihydroguaiaretic acid (NDGA) (479975, Millipore). All cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Inc.) with 10% fetal bovine serum (FBS) and were grown in 37°C humidified incubator with 5% CO2. MEFs were used between passages 2-5 for all experiments.
ROS detection
The detailed protocol on ROS detection and quantification has been described previously (13). In brief, for tissues, 5 μM dihydroethidium (DHE) (D11347, Life technologies) was applied to freshly sectioned tissues and incubated at 37°C for 30 minutes. 6-10 images were taken randomly from each tissue and at least 3 tissues were included in each group. Fluorescence intensity was quantified by the software NIS-Elements. To visualize ROS in cells, 10 μM of 2′-7′-dicholordihydrofluorescein diacetate (DCF-DA) (D-399, Life Technologies) was added to cells grown on fibronectin (FN)-coated chamber slides. 8-10 pictures were taken randomly from each condition and area fraction was quantified and normalized to cell number by DAPI using the software NIS-Elements. Three independent experiments for each condition were evaluated.
qPCR array and Real-time PCR
WT and RGE MEFs were cultured in reduced serum medium Opti-MEM overnight before plating on FN. Upon plating, MEFs were grown in serum free medium (SFM) supplemented with FN for 4, 16 and 24 hrs. RNA lysates were isolated using RNeasy plus mini kit (Qiagen). RT2 first strand kit (Qiagen) was used for cDNA synthesis. Then cDNA samples were subjected to RT2 Profiler™ PCR array to analyze gene expression changes related to mouse oxidative stress and antioxidant defense pathways (Qiagen, PAMM-065A). Experiments were performed and data were analyzed following manufacturer's instructions. All the candidate genes were further checked and confirmed by Real-time PCR. Ribosome protein S6 (RPS6) was used as the internal control. Following primers were used for real-time PCR: Nqo1 (forward): 5′-AGACCTGGTGATATTTCAGTTCCCATTG-3′; Nqo1 (reverse): 5′-CAAGGTCTTCTTATTCTGGAAAGGACCGT-3′; RPS6 (forward): 5′-AAGCTCCGCACCTTCTAT-3′; RPS6R (reverse) :5′-TGACTGGACTCAGACTTAGAAGTAGAAGC-3′.
Nqo1 activity assay
Nqo1 enzyme activity was measured in a reaction mixture containing 200 μM NADH (Sigma Aldrich) as an electron donor and 10 μM menadione (Sigma Aldrich) as an Nqo1 substrate and intermediate electron acceptor as described (51, 52). Cytochrome c serves as the terminal electron acceptor. Therefore, the measured rate of cytochrome c reduction correlates with Nqo1 enzymatic activity. To prepare lysates, cells were scraped in PBS and samples were sonicated. Lysate was added to the reaction mixture and the reduction of cytochrome c (Sigma Aldrich) over two minutes was monitored by absorbance at 550 nm. Dicoumarol, a selective inhibitor of Nqo1, was added as a negative control. Enzyme activity units were calculated as nmol of cytochrome c reduced/min/μg lysate.
Statistical analysis
For statistical analysis, unpaired t-test was used for comparison between genotypes and various groups. Log-rank (Mantel-Cox) test was used for all the mouse survival studies. Overall, P value less than 0.05 was considered as statistically significant. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001.
Results
Fbln5 expression in pancreatic cancer
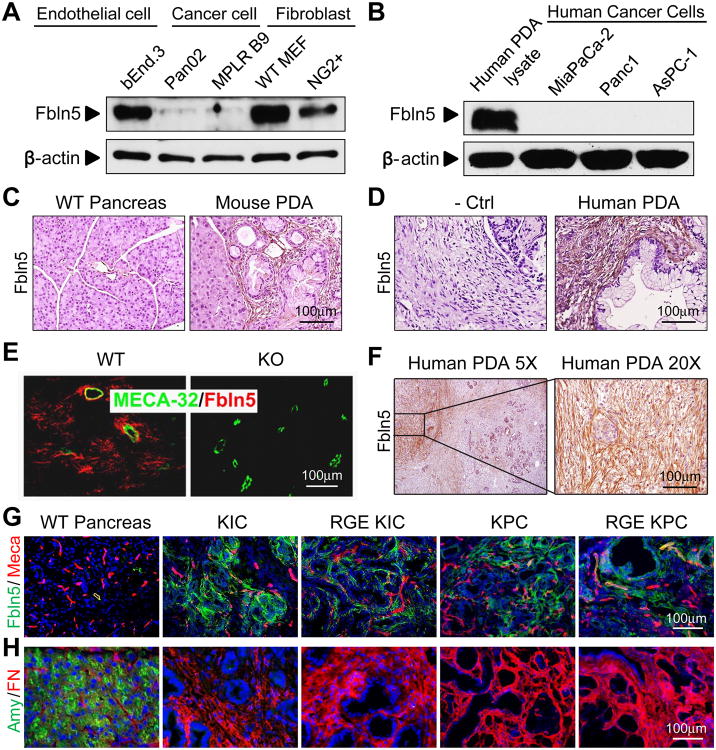
The expression of Fbln5 is prominent in developing arteries and is diminished in most adult organs, but can be reactivated in injured vessels (14). We examined Fbln5 expression in multiple mouse and human cell lines and tumor lysates. The mouse endothelial cell line bEnd.3 and fibroblasts, including mouse embryo fibroblasts (MEFs) and fibroblasts isolated from mouse PDA (NG2+ cells), expressed Fbln5 (Fig. 1A and S1B) as did human and mouse PDA lysates (Fig. 1B and S1C). In contrast, pancreatic cancer cell lines including three human (MiaPaCa-2, Panc1 and AsPC-1) (Fig. 1B) and four mouse lines (Pan02 and three isogenic lines isolated from mouse PDA) did not express detectable levels of Fbln5 protein (Fig. 1A, S1B and data not shown). However, all cell lines and PDA tumor lysates examined express α5β1 integrin (Fig. S1A and S1C), which serves as the cell surface receptor for Fbln5 and FN (8).
Figure 1. Expression pattern of Fbln5 in mouse and human.
(A-B) Protein lysates from mouse endothelial cells, cancer cells and fibroblasts (A) and human PDA tissue and cancer cell lines (B) were probed for indicated targets by Western blot. (C-D) Immunohistochemical (IHC) staining for Fbln5 on mouse (C) and human (D) PDA sections. (E) Immunofluorescence (IF) staining of Fbln5 (Red) and MECA-32 (Green) on subcutaneously grown pancreatic tumor (Pan02) in Fbln5 WT and KO mice. (F) Representative images of FBLN5 expression in human PDA, showing heterogeneous expression in the stroma. (G-H) Immunofluorescence (IF) staining of WT pancreas, KIC and RGE KIC tumors, KPC and RGE KPC tumors for Fbln5 (green) and endothelial cell marker Meca32 (Meca) (red) in panel (G), acinar cell marker amylase (Amy) (green) and FN (red) in panel (H). Nucleuses were counterstained with DAPI (blue). Scale bars are presented as indicated.
Fbln5 immunohistochemistry (IHC) in syngenic pancreatic Pan02 tumors grown subcutaneously in Fbln5+/+ (WT) or Fbln5-/- (KO) mice revealed that Fbln5 is produced by stromal cells (Fig. 1E). Co-staining of Fbln5 with the endothelial cell marker Meca32 shows that Fbln5 can be produced by endothelial cells within the tumor (Fig. 1E). IHC analysis of Fbln5 expression in multiple mouse models of PDA revealed Fbln5 reactivity mainly in the stroma (Fig. 1C-D and data not shown). We also examined FBLN5 in human PDA by IHC and found the protein was expressed in all human PDAs examined (n=25). The staining pattern was confined largely to the stromal compartment; however, not all regions of stroma were positive for FBLN5 protein (Fig. 1F). The nature of the heterogeneous stromal staining pattern is unclear but suggests that FBLN5 expression is controlled tightly.
Characterization of KIC and KPC mice
To evaluate the contribution of Fbln5 to PDA development and progression we utilized KIC and KPC mice, two well established conditional genetically engineered mouse models (GEMMs) of PDA based on the p48Cre (also known as Ptf1a) driver, which is expressed in pancreatic bud progenitor cells (20-22). KIC animals express an active form of Kras and have biallelic inactivation of the Cdkn2a locus (LSL-KrasG12D/+; Cdkn2aLox/Lox; p48Cre) (21). KPC animals express the same activating G12D mutation in Kras and also harbor a R172H point mutation in p53, the Li-Fraumeni human ortholog (LSL-KrasG12D/+; LSL-Trp53R172H/+; p48Cre) (22). Histological examination of KIC and KPC pancreatic tissue by a pathologist revealed that each model developed early pancreatic intraepithelial neoplasias (PanINs) and highly infiltrative adenocarcinomas ranging from well-differentiated areas with clear malignant gland formation to areas that were more poorly differentiated (Fig. S2A). Similar to human PDA, Masson's trichrome staining showed extensive collagen deposition in the area of PanINs and PDA in KIC and KPC tumors (Fig. S2B).
Ablation of Fbln5-integrin interaction reduces tumor growth and prolongs survival
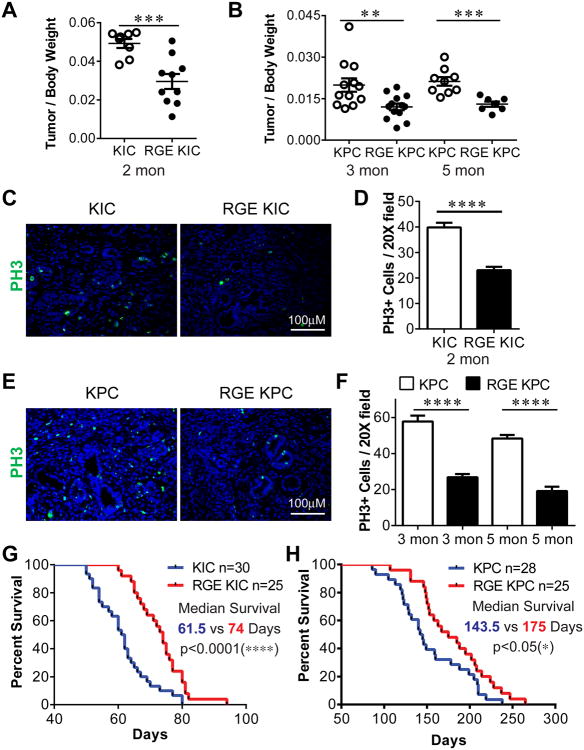
Mutation of the Fbln5 integrin binding sequence from RGD to RGE renders the protein incapable of binding to integrins (14). Fbln5RGE/RGE (RGE) mice are viable, fertile and phenotypically normal compared to WT animals (15). To study the contribution of Fbln5 to PDA development, we crossed RGE mice with KIC or KPC animals to generate genetically matched KIC and RGE-KIC, KPC and RGE-KPC mice. There was no difference in Fbln5 expression levels between KIC and RGE-KIC or KPC and RGE-KPC tumors (Fig. 1G and S1D). However, tumors had significantly increased Fbln5 expression compared to normal pancreas (Fig. 1C, 1G and S1D). Pancreatic and mouse body weights were determined in KIC and RGE-KIC mice at 1, 1.5 and 2 months (Fig. S2C and 2A). There is no significant difference for tumor/body weight at 1 and 1.5 months (Fig. S2C). RGE-KIC mice exhibited significantly lower tumor/body weight at 2 months than KIC mice, with tumor weights ranging from 0.24 to 0.86 gram for RGE-KIC mice and 0.72 to 1.11 gram for KIC mice (Fig. 2A). This is consistent with significantly reduced proliferating cells in RGE-KIC tumors (Fig. 2C-D). Similar results were observed in the KPC model, which were analyzed at 3 and 5 months of age (Fig. 2B and 2E-F).
Figure 2. RGE KIC and RGE KPC mice show reduced tumor growth and prolonged survival compared to KIC and KPC mice.
(A-B) Whole tumors were isolated and weighed and normalized against body weight at 2 months for KIC and RGE KIC mice (A) or 3 and 5 months for KPC and RGE KPC mice (B). n≥7 tumors per group. (C, E) IF staining on tumor sections for phospho-Histone H3 (PH3) (green). n≥4 tumors per group. (D, F) Quantification of PH3 positive (+) cells per 20× field from 4-5 tumors per group with 8-10 pictures per tumor. Results are shown as mean±s.e.m. (G-H) Kaplan-Meier survival curve of KIC and RGE KIC mice (G), KPC and RGE KPC mice (H). Scale bars are presented as indicated. For statistical analysis, unpaired t test was used for panel (A), (B), (D) and (F). Log-rank test was used for panel (G) and (H). *, p<0.05, **, p<0.01, ***, p<0.001, ****, p<0.0001.
Survival analysis revealed that RGE-KIC mice lived significantly longer than KIC mice (Fig. 2G) with a median survival of 74 days for RGE-KIC mice and 61.5 days for KIC mice. KIC and RGE-KIC mice appeared normal with no obvious phenotype up to 1.5 months of age but later became moribund, usually accompanied by weight loss. Moreover, some mice developed jaundice or ascites. Autopsies revealed the presence of large solid tumors with limited gross metastases. Liver micrometastasis was seen in the majority of KIC and RGE-KIC mice in the survival study necropsies (Fig. S2D). Similarly, RGE-KPC show significantly prolonged survival compared to KPC mice (175 days vs 143.5 days) (Fig. 2H). KPC and RGE-KPC mice appeared healthy up to 3 month old and were sacrificed between 3 to 9 months, commonly presenting with body weight loss, jaundice or ascites. Gross liver metastasis was seen in 30-40% of animals (Fig. S2E-F).
Increased oxidative stress in RGE-KIC and RGE-KPC tumors
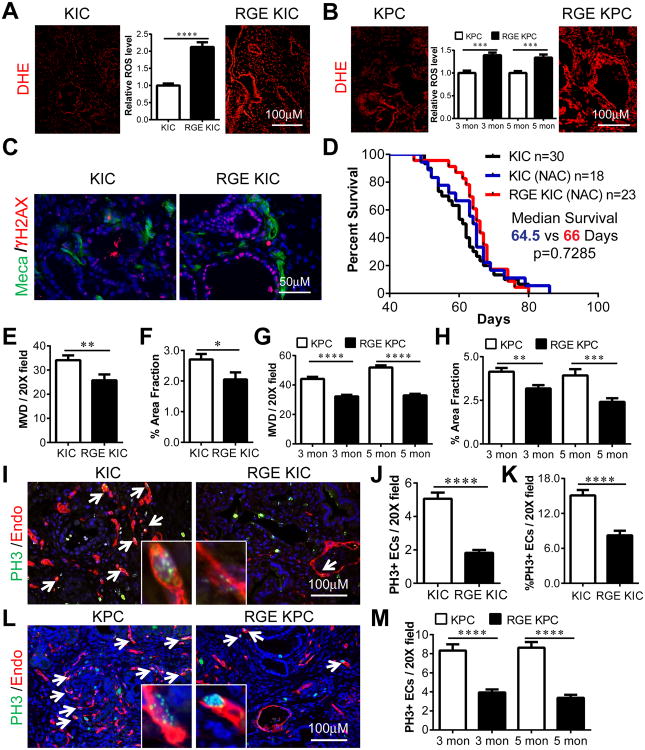
We reported previously that the growth of subcutaneous Pan02 tumors in Fbln5-/- mice was significantly reduced compared to WT animals due to increased ROS (13). Dihydroethidium (DHE) staining of tumors from the GEMMs showed that the Fbln5 RGE mutation significantly induced ROS levels in KIC and KPC tumors (Fig. 3A-B). Accordingly, the level of γH2AX, a commonly used marker for oxidative stress-induced DNA damage (23), was higher in RGE-KIC tumors than KIC tumors (Fig. 3C). However, in the context of normal pancreatic tissue, the Fbln5 RGE mutation did not alter ROS levels (Fig. S3A-B). This is consistent with the level of FN expression, which was elevated in PDA compared to normal pancreatic tissue (Fig. 1H). To determine whether ROS induction contributed to the prolonged survival in RGE animals, KIC and RGE-KIC mice were treated with the antioxidant N-acetylcysteine (NAC) and survival was examined (Fig. 3D). Prolonged NAC treatment decreased survival of RGE-KIC mice but did not affect the survival of KIC mice (Fig. 3D). ROS production was also examined in NAC-treated KIC and RGE-KIC tumors, which revealed no difference between the two groups (Fig. S3C-D). Collectively, ROS induction driven by the Fbln5 RGE mutation resulted in reduced tumor growth and prolonged survival.
Figure 3. Increased oxidative stress and reduced microvessel density (MVD) and endothelial cell (EC) proliferation in RGE KIC and RGE KPC tumors compared to KIC and KPC tumors.
(A-B) Dihydroethidium (DHE) (red) staining on freshly cut frozen sections of KIC and RGE KIC (A), KPC and RGE KPC (B) tumors for in situ detection of ROS. Relative ROS level was quantified by fluorescence intensity using the software NIS-Elements and shown inside images. Quantification was from 3 tumors per group with 6-10 images per tumor. (C) IF staining on KIC and RGE KIC tumor sections for Meca32 (Meca) (green) and γ-H2AX (red). n=3 tumors per group. (D) Kaplan-Meier survival curve of KIC and RGE KIC mice treated with the antioxidant N-acetyl-Cysteine (NAC) by drinking water starting at 4 weeks old. (E, G) MVD was counted per 20× field from 4-5 KIC and RGE KIC (E), KPC and RGE KPC (G) tumors with 8-10 pictures per tumor. (F, H) Quantification of MVD for KIC and RGE KIC (F), KPC and RGE KPC (H) tumors using the software NIS-Elements. Endomucin (Endo) stained areas are counted as % area fraction. (I, L) IF staining on 2 month old KIC and RGE KIC (I) and 3 month old KPC and RGE KPC (L) tumor sections for PH3 (green) and Endo (red). Arrows indicate double labeled ECs, one of which was enlarged and shown in an insert box for each image. (J, M) Quantification of PH3 and Endo co-stained cells (PH3+ ECs) per 20× field in KIC and RGE KIC (J) and KPC and RGE KPC (M) tumors at indicated ages. (K) Quantification of % PH3+ ECs over total number of ECs in 20× field in KIC and RGE KIC tumors. Scale bars are presented as indicated. All the results shown are mean±s.e.m. For statistical analysis, unpaired t test was used for panel (E-H), (J-K) and (M). *, p<0.05, **, p<0.01, ***, p<0.001 ****, p<0.0001.
Angiogenesis is reduced in RGE-KIC and RGE-KPC tumors
Prior studies indicate that Fbln5 can modulate angiogenesis (24) and we reported that loss of Fbln5 resulted in decreased angiogenesis in pancreatic tumors (13). Therefore, we examined microvessel density (MVD) in KIC and RGE-KIC, KPC and RGE-KPC tumors by co-immunostaining with the endothelial cell marker endomucin and the acinar cell marker amylase. MVD was significantly reduced in RGE-KIC compared to KIC in tumor regions as marked by loss of amylase reactivity (Fig. 3E-F and S4A). Immunostaining and quantification of MVD from 3 and 5 month old KPC and RGE-KPC tumors also revealed significantly reduced MVD in RGE-KPC tumors (Fig. 3G-H and S4B-C). We also examined the MVD of KIC and RGE-KIC tissues at 1 month old. At this time point, more than 90% of the tissue retained amylase expression. There was no difference in MVD in amylase positive areas between KIC and RGE-KIC tissues (Fig. S5A-C). Additionally, pancreas tissue from non-tumor bearing WT and RGE mice were analyzed for MVD (Fig. S5D). Again, no significant difference between the two groups was observed (Fig. S5E-F). We found that the number of proliferating endothelial cells co-stained with phospho-histone H3 and endomucin was decreased in tumors in RGE animals compared to tumors in WT mice, supporting the reduction of MVD in RGE-KIC and RGE-KPC tumors (Fig. 3I-M and S4D-E). Overall, the reduction of MVD correlated with tumor specific induction of ROS in RGE animals (Fig. 3A and S4A, 3B and S4B-C). These data suggest that the absence of functional Fbln5 impairs endothelial cell survival specifically in the tumor microenvironment.
Induction of the oxidative stress responsive gene Nqo1 by FN-induced ROS in vitro and in vivo
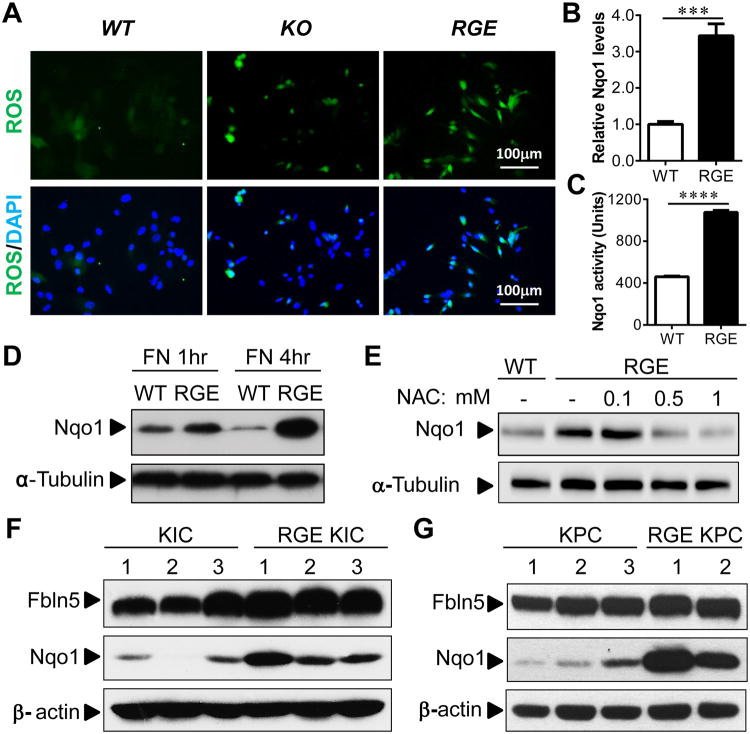
Our data suggested that Fbln5 controls ROS production through FN–β1 integrin interaction. To elucidate the underlying molecular mechanism of ROS generation, we isolated WT, KO and RGE primary MEFs. Fbln5 expression levels were equivalent between WT and RGE MEFs (Fig. S6). We found that ROS was elevated in KO and RGE MEFs but not in WT MEFs when cells were plated on FN (Fig. 4A). Next, we performed qPCR arrays to screen for oxidative stress and antioxidant response pathway related genes using RNA harvested from WT and RGE MEFs. From these arrays, NADP(H):quinone oxidoreductase 1 (Nqo1) was a reproducible and reliable target that was increased in RGE MEFs after plating on FN. Nqo1 is an antioxidant enzyme that is responsible for the reduction of quinones to hydroquinones utilizing NAD(P)H as an electron donor (25). Reducing quinone levels lowers the occurrence of ROS generation as a result of redox cycling (25). Induction of Nqo1 can be mediated by the Keap1/Nrf2/ARE pathway (26). The induction of Nqo1 in RGE MEFs when plated on FN was confirmed by quantitative real-time PCR (Fig. 4B), enzymatic activity (Fig. 4C) and Western blotting (Fig. 4D). Concordantly, the induction of Nqo1 expression was reversed by antioxidant treatment (with NAC) in a dose-dependent manner (Fig. 4E), showing that the elevation of Nqo1 is a consequence of increased ROS status in RGE MEFs. In addition, Nqo1 induction was elevated in tumors from RGE animals (Fig. 4F-G).
Figure 4. Fbln5 RGE mutation induces ROS production and oxidative stress responsive protein Nqo1 in vitro and in vivo.
(A) MEFs harvested from Fbln5 WT, KO and RGE mice were grown on FN for 16hr and stained with DCF-DA (green) to detect ROS. Nucleus was counterstained in blue with DAPI. (B) Real time PCR result with RNA isolated from WT or RGE MEFs plated on FN for 24hr. (C) Enzymatic activity of Nqo1 was measured and normalized against protein concentration with samples isolated from WT or RGE MEFs plated on FN for 4hr. (D) Western blot using lysates harvested from WT or RGE MEFs plated on FN for 1 or 4hr. (E) Western blot using lysates harvested from WT or RGE MEFs plated on FN for 4hrs and treated with increasing concentration of antioxidant N-acetyl cysteine (NAC). (F, G) Western blot using lysates harvested from several randomly selected KIC and RGE KIC tumors (F) or KPC and RGE KPC tumors (G). α-tubulin or β-actin was used as loading control. Scale bars are presented as indicated. All the results in (B) and (C) are mean±s.e.m. For statistical analysis, unpaired t test was used for panel (B) and (C). ***, p<0.001, ****, p<0.0001.
Nqo1 induction is dependent on FN-β1 integrin interaction and 5-lipooxygenase (5-Lox) activity
It has been reported that integrin activation by FN can induce ROS production (12). Accordingly, when the endothelial cell line bEnd.3 was treated with a α5β1 integrin-activating antibody, Nqo1 levels were induced (Fig. 5A). The induction of Nqo1 was specific to activation by FN and was not present when cells were plated on plastic or collagen (Fig. 5B). Induction was partially blocked by β1 integrin blockade (Fig. 5B). Given this data, we conclude that the induction of Nqo1 is responsive to ROS production induced by FN mediated β1 integrin ligation.
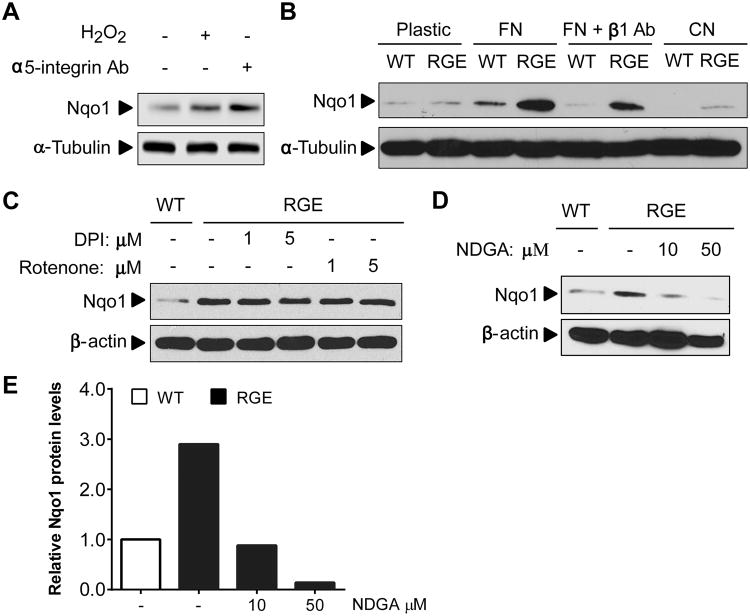
Figure 5. 5-LOX activation through FN-integrin interaction is responsible for ROS induction in RGE MEFs.
(A) bEnd.3 cells were plated on FN for 4 hr and treated with 100 μM H2O2 or 10 μg/ml α5 integrin activating antibody at time of plating and probed for Nqo1 by Western blot. (B) WT or RGE MEFs were plated on plastic, FN, FN + β1 integrin blocking antibody (10 μg/ml) or Collagen (CN) for 4 hr. Lysates were then harvested and subjected to Western blot. (C) RGE MEFs were plated on FN and treated with the NADPH Oxidase (NOX) inhibitor DPI or the mitochondrial electron transport chain inhibitor Rotenone at the time of plating. Lysates were harvested after 4 hrs for Western blot. (D) RGE MEFs were plated on FN and treated with a 5-lipoxygenase (5-LOX) inhibitor (NDGA) at the time of plating and harvested 4 hrs later for Western blot. (E) Quantification results of relative Nqo1 protein levels from panel (D) using the software Image Studio Digits. α-tubulin or β-actin was used as loading control for all the Western blots.
To determine the cellular source of ROS production in the absence of Fbln5-integrin interaction, we used inhibitors for various ROS sources including the mitochondrial respiratory chain inhibitor Rotenone, NADPH oxidase (NOX) inhibitor Diphenyleneiodonium chloride (DPI) and 5-lipoxygenase (5-Lox) inhibitor, nordihydroguaiaretic acid (NDGA). Treatment with Rotenone or DPI did not suppress the induction of Nqo1, suggesting mitochondria and NOX are not the intracellular source of ROS production (Fig. 5C). In addition, there was no induction of NOX enzymatic activity in RGE MEFs compared to WT MEFs by FN (Fig. S7). In contrast, inhibition of 5-Lox by NDGA reduced Nqo1 levels, indicating 5-Lox as the potential source of ROS (Fig. 5D). The quantification results were shown in Fig. 5E. This is consistent with a previous discovery that 5-Lox contributes to a strong burst of ROS production by FN-integrin engagement (11).
ROS induction has an additive therapeutic effect when combined with standard chemotherapy agents and development of Fbln5 targeted agents
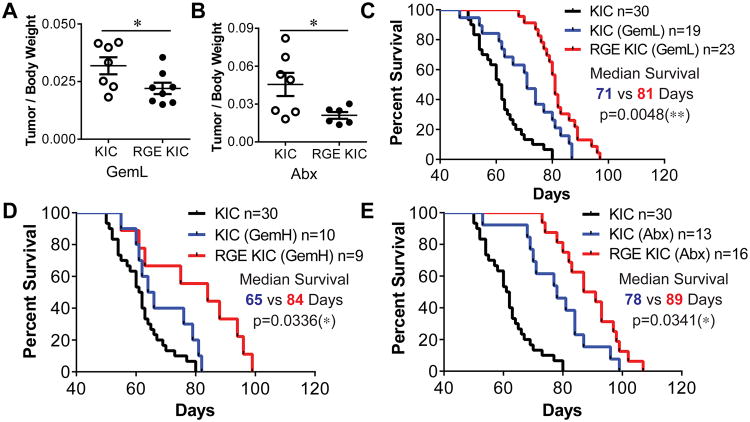
To determine if increased integrin-induced ROS improved response to chemotherapy we compared the efficacy of standard chemotherapy agents Gemcitabine (Gem) and Abraxane (Abx) in KIC and RGE-KIC mice. Due to poor diagnosis for pancreatic cancer, most patients received therapy in later stages. To mimic patient condition, all therapy started at 1.5 month old when both KIC and RGE-KIC mice have established solid tumors (Fig.S2C). We found that low-dose Gemcitabine (GemL) and Abx were more effective in the context of mutant Fbln5 (Fig. 6A-B). Survival studies were also performed with cohorts of KIC and RGE-KIC mice treated with GemL, high-dose Gemcitabine (GemH) and Abx. RGE-KIC mice consistently survived significantly longer in all three treatment groups than similarly treated KIC mice (Fig. 6C-E). These data suggest that increasing integrin-induced ROS augments the activity of standard chemotherapy.
Figure 6. RGE KIC mice have increased survival compared to KIC mice when given chemotherapy.
(A, B) KIC and RGE KIC mice were treated with 12.5 mg/kg Gemcitabine (Gem) 3×/wk (GemL) (A) or 5 mg/kg Abraxane (Abx) 2×/wk (B) for 3 wks starting at 7 week old. Mice were then sacrificed and tissues were isolated for analysis. Tumor size is presented as the mean ratio of tumor/body weight ±s.e.m. n≥6 tumors per group. (C-E) Kaplan-Meier survival curve of KIC control, KIC and RGE KIC mice treated with GemL (C), GemH (D) or Abx (E). All therapies were given to mice from 7 week old until moribund. For GemL, 12.5 mg/kg Gem was given to mice 3×/wk by intraperitoneal (i.p.) injection. For GemH, 50 mg/kg Gem was given to mice 1×/wk by i.p. injection. For Abx, 5 mg/kg was given to mice 2×/wk by i.p. injection. For statistical analysis, unpaired t test was used for panel (A-B). Log-rank test was used for panel (C-E). *, p<0.05, **, p<0.01.
Discussion
We demonstrated that Fbln5 expression is induced in a significant percentage of pancreatic cancers and that it promotes tumor progression by competing with FN for integrin ligation. Global loss of Fbln5-integrin interaction resulted in decreased tumor growth and prolonged survival of tumor-bearing mice with no apparent adverse effects in normal tissues. The decrease in tumor burden was dependent on increased FN-mediated integrin activation, which increased ROS production through 5-Lox activity and resulted in reduced angiogenesis in the tumor microenvironment. These findings are summarized in Figure 7.
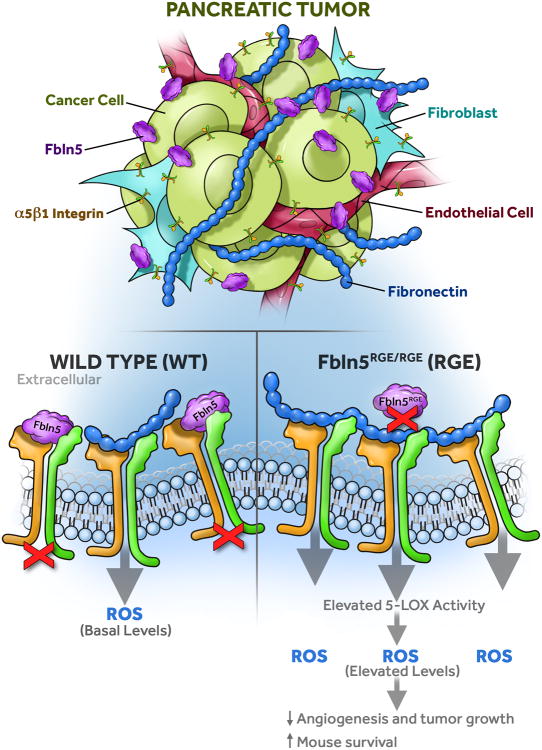
Figure 7. Fbln5 controls ROS production in the tumor microenvironment.
Fbln5 is mainly secreted into the tumor microenvironment by tumor-associated fibroblasts (TAFs) and ECs. Fbln5 competes with FN for integrin binding. In the absence of Fbln5-integrin interaction (RGE), more FN will bind to integrin receptors and increase ROS production, resulting in increased 5-LOX activity and reduced angiogenesis and tumor growth.
The ECM provides a structural framework in which tumors develop and progress. ECM signaling contributes to cell survival, proliferation and migration, thus regulation of cellular events initiated by the ECM is critical for tumor progression. To date, pharmacologic modification of the ECM in PDA has not resulted in improvement of chemoresponse or overall survival in patients (27-29). However, preclinical studies focused on inhibiting pathways that stimulate ECM deposition (e.g., TGFβ) have shown promise in promoting tumor control (30). Here, we have highlighted that increased integrin activation can result in decreased tumor growth by elevating integrin-induced ROS production. The extent of cell-ECM interaction is regulated in part by matricellular proteins, including Fbln5. Yet, the contribution of Fbln5 to cancer has been limited largely to expression studies (31-34), which have not defined a clear function for Fbln5 in tumorigenesis. We found that FBLN5 protein was expressed in all of the human PDA samples (n=25) we evaluated. Expression was largely restricted to the stromal compartment, yet the pattern of expression was not uniform as there were some areas of stroma that were negative or only weakly reactive. This heterogeneity suggests that evaluation of FBLN5 and potentially other matricellular proteins in human tissue microarrays could be challenging. Additional studies on the expression of Fbln5 protein in clinically annotated tumor samples are needed to elucidate if Fbln5 expression has predictive value.
To extend our studies, we sought to understand how Fbln5-integrin interaction functions in the context of the microenvironment of PDA. We employed two distinct but related GEMMs of PDA that recapitulate common mutations observed in the human disease (35-37). The expression of Fbln5 in each model is similar to the expression level and pattern of Fbln5 expression in human PDA. Furthermore, FN and α5β1 integrin are expressed abundantly in animal models of PDA as well as human PDA (38, 39). To study Fbln5-integrin interaction we took advantage of the fact that 1) Fbln5 binds but does not activate α5β1 (8, 14), suggesting that it can function to reduce binding of other ligands of the integrin; and 2) knockin mice carrying a point mutation in the integrin binding domain of Fbln5 (RGE mice) are viable and fertile (15). The described essential function of Fbln5 is in elastic fiber assembly as shown by Fbln5-deficient animals (17, 18) and biochemical analysis (16, 40). However, RGE mice have intact elastic fibers (15), indicating that Fbln5-integrin binding is not required for elastic fiber assembly. These data strongly suggest that the phenotype in the RGE animals is not due to changes in elastic fiber assembly but a result of an increase in integrin activation by ligands other than Fbln5. Given the dramatic increase in FN expression as well as other stromal components in PDA we postulated that the tumor microenvironment would provide a biologically meaningful stress to ascertain the functional consequences of increased integrin ligation in RGE animals.
ROS production as a result of integrin ligation is a well-established (11, 12), although underappreciated signalling pathway. Previously, we discovered that the loss of Fbln5-integrin interaction results in increased integrin-induced ROS production (13). Cellular redox homeostasis is tightly regulated by the balance between ROS scavenging and eliminating systems (19). Cancer cells often generate higher levels of ROS due to metabolic abnormality, activation of oncogenes or loss of functional p53 (19). For example, increased levels of ROS, particularly H2O2 are highly mutagenic and contribute to elevated mutation levels and heterogeneity. Thus an imbalance in ROS scavenging and eliminating systems is likely to result in acute consequences in the tumor microenvironment. For example, increasing ROS levels might result in inhibition of cell proliferation and ultimately cell death (41). However, cancer cells have developed adaptive mechanisms to manage increased ROS levels (19). One adaptive mechanism is the induction of the antioxidant response transcription factor Nrf2 to increase the expression of the ROS detoxification enzyme Nqo1 (42, 43). We found that the loss of Fbln5-integrin interaction induces Nqo1 and that this response is ROS-dependent. Nqo1 levels as a result further validated the elevation of oxidative stress in tumors grown in RGE mice and also provided a tractable biochemical endpoint to evaluate the signaling cascade induced by FN in the absence of integrin binding Fbln5. Furthermore, using Nqo1 levels as an endpoint, we discovered that ablation of Fbln5-integrin interaction increased 5-Lox activity in a FN-dependent manner. Pharmacologic inhibition of 5-Lox rescued the FN-driven phenotype in vitro implicating that 5-Lox is downstream of integrin activation. This is consistent with previous reports showing that integrin activation by FN can stimulate ROS production primarily through 5-Lox (11, 12). FN has also been reported to stimulate intracellular ROS in pancreatic cancer cells through NOX and the mitochondria (10), although we did not find evidence of this in our system.
We found that stromal and tumor cells express the integrin profile required for FN-induced ROS production. However, changes in endothelial cells were the most apparent phenotype in tumors from RGE mice. Endothelial cells are sensitive to elevated ROS (44) and this was evident by the consistent reduction in microvessel density and reduction in proliferating endothelial cells in tumors grown in RGE animals. Fibroblasts from RGE mice produce elevated levels of ROS in culture in a FN and integrin-dependent manner. Yet, surprisingly, we found no significant changes in the presence or activation of fibroblasts in tumors from RGE mice (data not shown). Global analysis of ROS using DHE indicates that many cell types, including tumor cells, display elevated ROS levels in tumors from RGE mice. However, in vitro studies suggest that Fbln5 does not affect ECM-mediated ROS induction in tumor cells (data not shown). It is plausible that long lived ROS molecules (e.g., H2O2) travel from stromal cells and increase oxidative stress in tumor cells resulting in decreased proliferation and reduced tumor growth. It is also feasible that endothelial cells in the tumor microenvironment succumb to elevated ROS induced by mutant Fbln5 and the decreased tumor growth is akin to an anti-angiogenic effect. In contrast, Fbln5 null mice display an exaggerated vascular response after subcutaneous implantation of polyvinyl alcohol sponges (24). However, the mechanism of how Fbln5 directly affects endothelial cell function and the contribution of integrins in this phenotype is poorly understood. In our model, non-tumor bearing pancreata of WT and RGE mice show similar microvessel density (Fig. S5). However, in the context of the tumor microenvironment the basal level of ROS is increased compared to normal pancreas, therefore inducing further ROS by mutation of Fbln5 may explain the negative effect on endothelial cell function. If so, this suggests that the mutation in Fbln5 functions as an endogenous inhibitor of angiogenesis selectively in the tumor microenvironment. These hypotheses are currently being evaluated.
Our studies show that Fbln5 functions as a rheostat to dampen integrin-mediated ROS production. This function of reducing cell-ECM interaction is similar to what has been observed for other matricellular proteins. For example, SPARC reduces the binding of fibrillar collagens to discoidin domain receptors thereby reducing collagen induced cell signaling and attachment (1). Current studies are focused on understanding factors that drive Fbln5 expression in the tumor microenvironment and identification of the integrin-mediated signaling pathway that activates 5-Lox in the context of mutant Fbln5. Overall, our study illustrates how the matricellular protein Fbln5 functions to reduce FN-integrin interaction and suggests that Fbln5 is a novel therapeutic target for pancreatic cancer.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the American Cancer Society (ACS, RSG-10-244-01-CSM to R.A.B.), The Joe and Jessie Crump Medical Research Foundation (to R.A.B.), NIH (R01 CA118240 to R.A.B., R01 CA137181 to D.H.C. and T32 GM008203 to M. T.), the Effie Marie Cain Scholarship in Angiogenesis Research (to R.A.B.) and Remeditex Ventures. The UT Southwestern Tissue Resource is supported by the NCI (5P30 CA142543). The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript. We acknowledge helpful discussions with Drs. Michael Dellinger and Adi Gazdar and members of the Brekken laboratory.
Footnotes
Disclosure of Potential Conflicts of Interest: RA Brekken, co-founder of Tuevol Therapeutics, a company that is developing therapeutics that target the tumor microenvironment
References
- 1.Aguilera KY, Rivera LB, Hur H, Carbon JG, Toombs JE, Goldstein CD, et al. Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1032–44. doi: 10.1158/0008-5472.CAN-13-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoudjit F, Vuori K. Integrin signaling in cancer cell survival and chemoresistance. Chemother Res Pract. 2012;2012:283181. doi: 10.1155/2012/283181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71:3453–8. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebens Muerkoster S, Kotteritzsch J, Geismann C, Gast D, Kruse ML, Altevogt P, et al. alpha5-integrin is crucial for L1CAM-mediated chemoresistance in pancreatic adenocarcinoma. International journal of oncology. 2009;34:243–53. [PubMed] [Google Scholar]
- 5.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature medicine. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 6.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Molecular biology of the cell. 1993;4:953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem J. 2007;405:417–28. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong GS, Rustgi AK. Matricellular proteins: priming the tumour microenvironment for cancer development and metastasis. Br J Cancer. 2013;108:755–61. doi: 10.1038/bjc.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–47. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 11.Taddei ML, Parri M, Mello T, Catalano A, Levine AD, Raugei G, et al. Integrin-mediated cell adhesion and spreading engage different sources of reactive oxygen species. Antioxid Redox Signal. 2007;9:469–81. doi: 10.1089/ars.2006.1392. [DOI] [PubMed] [Google Scholar]
- 12.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, et al. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–44. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, et al. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Dis Model Mech. 2010;3:333–42. doi: 10.1242/dmm.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura T, Ruiz-Lozano P, Lindner V, Yabe D, Taniwaki M, Furukawa Y, et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem. 1999;274:22476–83. doi: 10.1074/jbc.274.32.22476. [DOI] [PubMed] [Google Scholar]
- 15.Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, et al. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J Clin Invest. 2011;121:2048–59. doi: 10.1172/JCI45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Q, Davis EC, Richardson JA, Starcher BC, Li T, Gerard RD, et al. Molecular analysis of fibulin-5 function during de novo synthesis of elastic fibers. Mol Cell Biol. 2007;27:1083–95. doi: 10.1128/MCB.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–71. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–5. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 19.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–86. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan KM, Bissonnette R, Yanagisawa H, Hussain SN, Davis EC. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab Invest. 2007;87:818–27. doi: 10.1038/labinvest.3700594. [DOI] [PubMed] [Google Scholar]
- 25.Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, et al. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–47. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–38. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proceedings of the National Academy of Sciences. 2014;111:E3091–E100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhim Andrew D, Oberstein Paul E, Thomas Dafydd H, Mirek Emily T, Palermo Carmine F, Sastra Stephen A, et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2014;25:735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano Paolo P, Cuevas C, Chang Amy E, Goel Vikas K, Von Hoff Daniel D, Hingorani Sunil R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostapoff KT, Kutluk Cenik B, Wang M, Ye R, Xu X, Nugent D, et al. Neutralizing murine TGFβR2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Research. 2014 doi: 10.1158/0008-5472.CAN-13-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue W, Sun Q, Landreneau R, Wu C, Siegfried JM, Yu J, et al. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res. 2009;69:6339–46. doi: 10.1158/0008-5472.CAN-09-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29:2243–51. doi: 10.1093/carcin/bgn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Z, Ai Q, Xu H, Ma X, Li HZ, Shi TP, et al. Fibulin-5 is down-regulated in urothelial carcinoma of bladder and inhibits growth and invasion of human bladder cancer cell line 5637. Urol Oncol. 2011;29:430–5. doi: 10.1016/j.urolonc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Li XG, Zhang Y, Cao LQ, Deng ZH, Chen Y. Expression of EVEC in ovarian carcinoma and its biological significance. Zhonghua Zhong Liu Za Zhi. 2010;32:676–80. [PubMed] [Google Scholar]
- 35.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh M, Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotechnol. 2012;30:648–57. doi: 10.1038/nbt.2286. [DOI] [PubMed] [Google Scholar]
- 37.Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–87. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- 38.Ansari D, Aronsson L, Sasor A, Welinder C, Rezeli M, Marko-Varga G, et al. The role of quantitative mass spectrometry in the discovery of pancreatic cancer biomarkers for translational science. Journal of translational medicine. 2014;12:87. doi: 10.1186/1479-5876-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagadeeshan S, Krishnamoorthy YR, Singhal M, Subramanian A, Mavuluri J, Lakshmi A, et al. Transcriptional regulation of fibronectin by p21-activated kinase-1 modulates pancreatic tumorigenesis. Oncogene. 2014 doi: 10.1038/onc.2013.576. [DOI] [PubMed] [Google Scholar]
- 40.Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, et al. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176:1061–71. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kardeh S, Ashkani-Esfahani S, Alizadeh AM. Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur J Pharmacol. 2014;735C:150–68. doi: 10.1016/j.ejphar.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Awadallah NS, Dehn D, Shah RJ, Russell Nash S, Chen YK, Ross D, et al. NQO1 expression in pancreatic cancer and its potential use as a biomarker. Appl Immunohistochem Mol Morphol. 2008;16:24–31. doi: 10.1097/PAI.0b013e31802e91d0. [DOI] [PubMed] [Google Scholar]
- 43.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. American journal of physiology Endocrinology and metabolism. 2012;302:E481–95. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- 45.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70:2852–61. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostapoff KT, Awasthi N, Cenik BK, Hinz S, Dredge K, Schwarz RE, et al. PG545, an angiogenesis and heparanase inhibitor, reduces primary tumor growth and metastasis in experimental pancreatic cancer. Mol Cancer Ther. 2013;12:1190–201. doi: 10.1158/1535-7163.MCT-12-1123. [DOI] [PubMed] [Google Scholar]
- 47.Hallmann R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–32. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- 48.Weng S, Wang H, Chen W, Katz MH, Chatterjee D, Lee JE, et al. Overexpression of protein phosphatase 4 correlates with poor prognosis in patients with stage II pancreatic ductal adenocarcinoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:1336–43. doi: 10.1158/1055-9965.EPI-12-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, et al. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera LB, Brekken RA. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-beta1 activity. J Cell Biol. 2011;193:1305–19. doi: 10.1083/jcb.201011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275:5416–24. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 52.Fitzsimmons SA, Workman P, Grever M, Paull K, Camalier R, Lewis AD. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996;88:259–69. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.