Abstract
Purpose of review
Neutrophils have traditionally been viewed in the context of acute infection and inflammation forming the first line of defence against invading pathogens. Neutrophil trafficking to the site of inflammation requires adhesion and transmigration through blood vessels, which is orchestrated by adhesion molecules, such as β2- and β1-integrins, chemokines and cytokines. This review focuses on recent advances in understanding the regulators of neutrophil recruitment during inflammation in both acute and chronic settings.
Recent findings
Recent findings suggest that besides the established pathways of selectin or chemokine-mediated integrin activation, signaling by distinct TLRs (especially TLR2, TLR4 and TLR5) can activate integrin-dependent neutrophil adhesion. Moreover, the integrin α3β1 has been vitally implicated as a new player in neutrophil recruitment and TLR-mediated responses in septic inflammation. Furthermore, several endogenous inhibitory mechanisms of leukocyte recruitment have been identified, including the secreted molecules Del-1, PTX3, and GDF-15, which block distinct steps of the leukocyte adhesion cascade, as well as novel regulatory signaling pathways, involving the protein kinase AKT1 and IFN-λ2/IL-28A.
Summary
The leukocyte adhesion cascade is a tightly regulated process, subjected to both positive and negative regulators. Dysregulation of this process and hence neutrophil recruitment can lead to the development of inflammatory and autoimmune diseases.
Keywords: Neutrophil recruitment, leukocyte adhesion, integrins, inflammation, α3β1, Del-1, JAM-C
Introduction
Neutrophils are the first line of the immune response against infectious agents or injury. These cells rapidly extravasate from the circulation into sites of infection or sterile tissue injury [1–3]. Neutrophil recruitment is orchestrated by the leukocyte adhesion cascade, which represents a series of tightly regulated, interdependent adhesive interactions that take place between neutrophils and the vessel wall endothelium. This adhesion cascade ultimately leads to transmigration (also called diapedesis) of the inflammatory cells through the endothelial cells [2, 4]. The initial rolling of neutrophils on the endothelium is mediated by endothelial selectins binding to their counter-receptors on neutrophils; the rolling step serves to decrease neutrophil velocity and allows neutrophils to interact with chemokines exposed on the endothelial cell surface [2, 4]. The subsequent steps of firm adhesion and crawling of neutrophils on endothelial cells are largely dependent on leukocyte integrins, especially of the β2-integrin family, for instance, LFA-1 (Lymphocyte Function-associated Antigen-1; αLβ2; CD11a/CD18) and Mac-1 (Macrophage-1 antigen; αMβ2; CD11b/CD18), which interact with their endothelial counter-receptors, such as ICAM-1 and ICAM-2 [2, 4–6]. Finally, neutrophils migrate through the endothelium, predominantly through endothelial junctions; the process of transmigration also depends on β2-integrins as well as on molecules located in endothelial junctions, such as PECAM-1 or junctional adhesion molecules (JAM) [2, 4, 7–9]. Besides the prototypical paradigm of the leukocyte adhesion cascade, alternative paradigms for neutrophil recruitment seem to exist, which may be specific for distinct vascular beds. For instance, Devi et al have recently described a new mechanism for leukocyte recruitment in the glomerulus [10]. In particular, neutrophils constitutively adhere or migrate intravascularly in the glomerular capillaries; acute inflammation predominantly enhances the duration of neutrophil retention in the capillaries thereby triggering further inflammation [10]. The different steps of the cascade are interconnected with each other, as intracellular signals induced by an adhesive interaction may promote the next adhesive step; for instance, signaling pathways activated by chemokines or selectin ligation of P-selectin glycoprotein ligand-1 (PSGL-1) on neutrophils can stimulate inside-out activation of integrins and consequently integrin-dependent adhesion [4, 11]. In this review, we summarize and discuss recent advances in the understanding of neutrophil recruitment, as well as the fate of recruited neutrophils and their potential role within the infiltrated tissue.
The interplay of selectins, chemokines and integrins in the regulation of neutrophil recruitment
The initial step of neutrophil extravasation, rolling of neutrophils on the endothelial lumen, is mediated by endothelial P-selectin and E-selectin, the abundance of which on the luminal endothelial surface is increased via exocytosis or transcriptional upregulation, respectively [4, 12]. These selectins interact with PSGL-1 as well as CD44 and E-selectin ligand on neutrophils [4, 12]. In addition, neutrophils express L-selectin, and the proteolytic cleavage thereof modulates neutrophil migration [13]. Recently, Stadtmann et al identified an association between L-selectin and PSGL-1 on the neutrophil surface, which is crucial for LFA-1-integrin activation via signaling that requires Src family kinases and ITAM domain-containing adaptor proteins [14]. Moreover, neutrophils engage L-selectin and PSGL-1 in concert with Mac-1 and LFA-1 integrins, and CXCR4 to traffic to lymph nodes via high endothelial venules [15]. Except from its involvement in the initial rolling, PSGL-1 was recently shown to be critical for the interaction between neutrophils and activated platelets in the inflamed venules. This interaction is required for the proper homogenous distribution of Mac-1, which promotes neutrophil crawling onto endothelial cells, thereby contributing to thromboinflammatory injury [16].
The contact of rolling neutrophils with the endothelial cell surface facilitates chemokine-dependent activation of neutrophil integrins and subsequently neutrophil firm arrest [2, 4]. Chemokines are bound on the endothelial cell surface via glycosaminoglycans [17]. Chemokines often derive from interstitial inflammatory cells and may reach the apical endothelial surface via specific transcytosis systems, such as the Duffy antigen receptor for chemokines [18]. For instance, CXCL1 and CXCL2 (CXC chemokine ligand 1/2) chemokines are produced by tissue macrophages and mast cells in response to lipopolysaccharide stimulation and induce neutrophil recruitment [19]. Interestingly, CXCL1 and CXCL2 are pre-formed in granules of mast cells, and their rapid TLR-4-dependent release upon inflammatory stimulation allows for rapid neutrophil recruitment [19]. Furthermore, CXCL1 is central for neutrophil migration and contributes to bactericidal functions of neutrophils during polymicrobial sepsis [20].
Integrins of the β2 family, such as LFA-1 and Mac-1, mediate neutrophil adhesion, crawling and transendothelial migration [2, 4]. To accomplish their function in the leukocyte adhesion cascade, integrins have to be activated, that is, to adopt a high affinity conformation. Integrin activation is triggered by internal signals generated by other receptors (e.g., PSGL-1 or chemokine receptors) and is therefore designated as inside-out signaling [4, 21]. In this context, the guanine-nucleotide-exchange factor (GEF), P-Rex-1, was demonstrated to mediate selectin-triggered activation of the intermediate affinity state of LFA-1, thereby promoting slow rolling of neutrophils, as well as Mac-1-dependent intravascular crawling [22]. Consistently, in a model of acute kidney injury, P-Rex-1 deficiency resulted in reduced neutrophil recruitment and decreased kidney damage [22].
Novel regulatory mechanisms of neutrophil adhesion
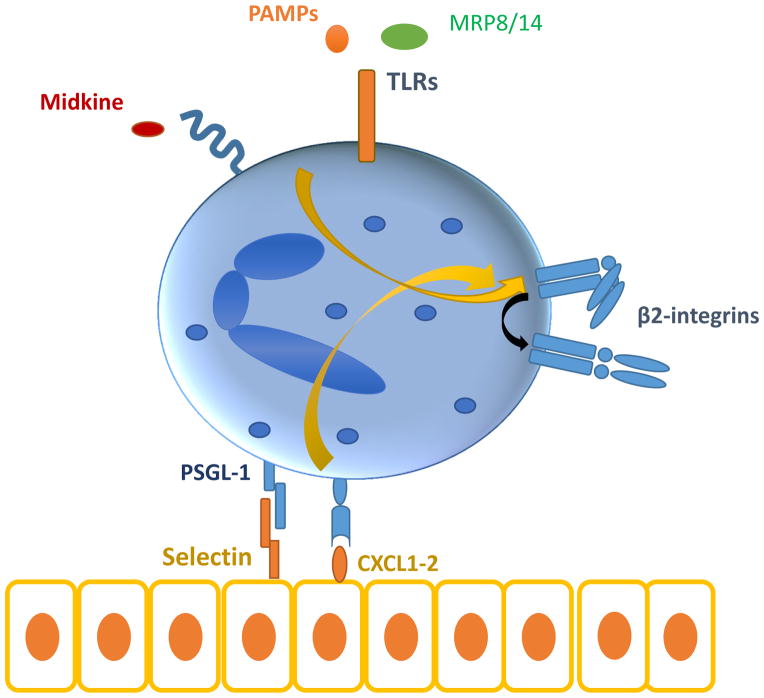
In addition to the well-established pathways of selectin- or chemokine-mediated integrin activation, recent evidence suggest that signaling by Toll-like receptors (TLRs) may also activate integrins and integrin-dependent leukocyte adhesion (figure 1) [23–25]. In particular, TLR2 and TLR5 ligation rapidly induces activation of leukocyte integrins and neutrophil adhesion to ICAM1 [24]. This pathway was dependent on activation of Rap1 GTPase, which is a well-established player in inside-out signal activation of integrins [4, 26, 27]; increased Rap1 activity was mediated by Rac1 activation, and NADPH oxidase 2-dependent reactive oxygen species production [24]. Such a mechanism for TLR-dependent neutrophil infiltration may be operative in graft-versus-host disease, whereby intestinal neutrophil recruitment is triggered by local microbial flora in a manner dependent on neutrophil TLRs [28]. Moreover, the E-selectin–PSGL-1 interaction during neutrophil rolling triggers neutrophil secretion of myeloid-related protein (MRP)8/14, which in turn acts in an autocrine fashion via its receptor TLR4 to mediate activation of neutrophil β2-integrins in a GTPase Rap1-dependent manner [23]. Consistently, MRP8/14 (also designated S100A8/A9) cooperates with TLR2 and CXCL2 to promote neutrophil recruitment in CCl4-induced liver injury [29].
Figure 1. Signals that result in leukocyte integrin activation.
Chemokine- and selectin-dependent signalling, activation of TLRs by pathogen-associated molecular patterns or endogenous signals, like MRP8/14 as well as further endogenous stimuli, such as midkine, activate β2 integrins in an inside-out signalling fashion by inducing changes in integrin affinity.
Conformational changes in β2-integrins are also induced by the cytokine midkine (also known as neurite growth-promoting factor 2), which thereby induces neutrophil adhesion and recruitment [30]. Midkine-deficient mice show diminished leukocyte accumulation in the cremaster model of acute inflammation and in a model of hind limb ischemia [30]. Neutrophil recruitment to necrotic tissues is mediated by high-mobility group box 1 (HMGB1) and its receptor, receptor for advanced glycation end products (RAGE) [31]; here, a potential link to integrin-dependent neutrophil recruitment may exist, as RAGE interacts with the β2-integrin Mac-1 [32, 33]. An important player in leukocyte integrin activation is kindlin-3, which interacts with the cytoplasmic tail of β2-integrins [34], and the absence of which causes leukocyte adhesion deficiency (LAD) Type III [35]. In genetically modified mice that express a mutant form of kindlin-3 that is incapable of interacting with integrins, neutrophil adhesion and recruitment in the course of systemic inflammation is decreased [34]. Interestingly, the ability of neutrophils bearing this mutant kindlin-3 to form neutrophil extracellular traps (NETs) was also inhibited, which implies that the kindlin-3 /β2-integrin interaction may contribute to neutrophil functions over and above recruitment [34].
A tight balance between the different steps of the leukocyte adhesion cascade is required for an appropriate response to infection. For instance, the receptor-like protein tyrosine phosphatase CD45 was recently shown to modulate integrin activation in inflammation [36]. Specifically, in mice which carry a point mutation that constitutively activates CD45, neutrophil recruitment to the lung following Escherichia coli lung infection was reduced due to enhanced Mac-1 adhesiveness which impaired crawling and transmigration [36]. Moreover, during Staphylococcus aureus skin infection, increased neutrophil crawling in capillaries was observed which was mediated by β2- and α4-integrins [37]. In the context of streptococcal infection, the transcription factor nuclear factor of activated T cells (NFAT) mediated the Streptococcus pyogens-derived M1 protein-induced upregulation of neutrophil Mac-1 and of CXC chemokines and thereby neutrophil infiltration [38].
Another transcription factor, activation transcription factor 3 (ATF3), regulates neutrophil recruitment during lung inflammation [39]. ATF3-deficient neutrophils displayed defective chemotaxis, which was associated with reduced expression of TIAM2, which is known to regulate Rac1-dependent focal adhesions and cell motility [39]. Neutrophil lung infiltration is also promoted by the GTPase Rab27b, which contributes to neutrophil migration in response to chemoattractants, such as CXCL2 (also designated macrophage inflammatory protein-2) and leukotriene B4 (LTB4) [40]. Interestingly, CXCR2-mediated chemotaxis of neutrophils is dependent on the presence of transient receptor potential channel family 6 (TRPC6), which regulates signaling downstream of the CXCR2 receptor [41].
A recent study has identified that, besides β2-integrins, the integrin α3β1 (VLA-3, CD49c/CD29) contributes critically to neutrophil recruitment during experimental sepsis in mice [42]. Consistently, neutrophils from patients with severe sepsis exhibit enhanced surface expression of α3β1, as compared to neutrophils from healthy subjects. The presence of α3β1-integrin on neutrophils allowed for the distinction of two neutrophil populations in human and murine sepsis with different functional properties. The α3β1high neutrophils exhibit a hyper-inflammatory phenotype associated with enhanced MPO activity, elevated secretion of IL-6 and decreased IL-10 secretion, as compared to the α3β1low neutrophils. Accordingly, α3β1-deficient neutrophils displayed an attenuated TLR-induced inflammatory response. Pharmacologic blockade of α3β1-integrin diminished neutrophil infiltration and protected mice from sepsis lethality. Therefore, α3β1-integrin emerges as an important adhesion receptor in neutrophil recruitment and activation during septic inflammation [42, 43].
Neutrophil transmigration
The final step in leukocyte recruitment is transmigration [4, 7, 8]. Leukocytes primarily transmigrate via the paracellular route, whereas transcellular migration is less common [4, 7, 8]. Leukocyte transmigration requires bidirectional interactions between leukocytes and adhesion molecules expressed on endothelial junctions [7, 8]. After neutrophils have passed the endothelial cell monolayer, they crawl along pericytes via interactions mediated by the β2-integrins LFA-1 and Mac-1 and their counter-receptor ICAM-1 on pericytes. Crawling continues until neutrophils find gaps between pericytes, which they use as exit points through the vessel wall thereby completing transendothelial migration [44, 45]. NG2 proteoglycan-expressing pericytes that surround capillaries and arterioles upregulate ICAM-1 and the chemoattractant MIF (macrophage migration inhibitory factor) in response to inflammatory stimuli. By this mechanism, pericytes serve to guide extravasated neutrophils, hence facilitating tissue inflammation [46]. Abtin et al have shown that perivascular macrophages represent a major source of neutrophil chemoattractants, thereby instructing neutrophil infiltration into Staphylococcus aureus-infected skin [47]. Interestingly, staphylococcal hemolysin-mediated killing of perivascular macrophages adds a yet another strategy to the long list of mechanisms, by which this pathogen evades host immunity [47–49].
Furthermore, Junctional adhesion molecules (JAMs) play an essential role in leukocyte transmigration, in part via regulating endothelial barrier integrity as well as via interactions with integrins, e.g., JAM-A and JAM-C bind respectively to LFA-1 and Mac-1 [4, 50]. In addition to neutrophils exiting the circulation through endothelial junctions, the process of reverse transendothelial migration (rTEM) of neutrophils back to the vascular lumen has been described [51]. Neutrophil rTEM is observed predominantly during ischemia reperfusion (IR) injury and is thought to promote dissemination of inflammation to secondary sites, although in zebrafish, reverse chemotaxis of neutrophils has also been linked to resolution of inflammation [52]. JAM-C was identified as an important player of the polarized transmigration of neutrophils from the vascular lumen to the subendothelial space. While the presence of JAM-C at endothelial junctions was reduced upon IR injury, pharmacologic inhibition of JAM-C or endothelial-specific JAM-C deficiency enhanced the frequency of rTEM of neutrophils in response to IR injury, establishing JAM-C as a major regulator of rTEM [51]. In this regard, JAM-C in endothelial junctions undergoes proteolytic cleavage following IR injury [53]. This proteolytic event involves LTB4-dependent neutrophil elastase cleavage of endothelial JAM-C and is facilitated by the β2-integrin Mac-1, which presents neutrophil elastase to JAM-C, thereby promoting rTEM [53].
Modulators and inhibitors of neutrophil recruitment
In recent years, several local endogenous modulators and inhibitors of the leukocyte adhesion cascade have been identified [54], the first of which was the developmental endothelial locus-1 (Del-1), an inhibitor of β2-integrin-dependent leukocyte adhesion [55–58]. Interestingly, the downregulation of endothelial Del-1 expression appears to be an important mechanism by which IL-17 promotes neutrophil recruitment and inflammatory disease [56, 59, 60]. Other significant endogenous regulators of the leukocyte adhesion cascade include pentraxin-3, which interferes with P-selectin/PSGL-1-mediated neutrophil rolling [61], and growth differentiation factor-15 (GDF-15), which blocks chemokine-induced integrin activation [62]. Moreover, the paired immunoglobulin-like type 2 receptor alpha (PILRα), which is an inhibitory receptor with immunoreceptor tyrosine-based inhibitory motifs, inhibits inside-out activation of β2-integrins and neutrophil adhesion to ICAM-1 [63]. Consistently, PILRα-deficient mice display enhanced neutrophil infiltration and increased susceptibility to endotoxic shock [63].
Several novel regulatory pathways involved in neutrophil recruitment have been identified recently. The protein kinase AKT1, which is downregulated in activated neutrophils, diminishes neutrophil migration during inflammation by interfering with the CXCL1/2-CXCR2 axis in a STAT1-dependent manner [64]. In the context of group B Streptococcus-induced neonatal sepsis, TLR2-induced IL-10 production acts as a negative regulator of neutrophil recruitment [65]. The plasminogen-derived cleavage product angiostatin, which exerts anti-angiogenic properties, was shown to also act as an inhibitor of neutrophil recruitment in vitro and in acute inflammation by interfering with β1- and β2-integrin-dependent adhesion to extracellular matrix proteins and endothelium [66]. More recently, the anti-inflammatory actions of angiostatin were expanded, as it was shown to block neutrophil recruitment by inhibiting MAPK signaling and reactive oxygen species production [67]. Furthermore, in the context of autoimmune arthritis, Blazek et al showed that IFN-λ2/IL-28A can block neutrophil recruitment by acting via its cognate receptor IL-28RA on neutrophils [68].
Recruited neutrophils in tissue homeostasis
Upon arrival in the inflamed tissue, neutrophils are activated by a multitude of inflammatory mediators, which triggers the release of several factors with antimicrobial and pro-inflammatory actions, including reactive oxygen species, granule proteases, cytokines, chemokines as well as NETs [69]. Despite their anti-microbial potential, these neutrophil functions may contribute to collateral tissue damage, especially when inflammation is not timely resolved. Being relatively short-lived, extravasated neutrophils commonly undergo apoptosis in tissues [70]. Clearance of apoptotic neutrophils by macrophages is considered a major mechanism triggering resolution of inflammation and as a regulatory feedback mechanism for the control of granulopoiesis [1, 70–72]. Stark et al have shown that phagocytosis of apoptotic neutrophils by macrophages acts a negative regulator of granulopoiesis by inhibiting expression of IL-23, which in turn leads to decreased T cell production of IL-17; IL-17 contributes to granulopoiesis through regulation of G-CSF [71]. The importance of this negative feedback loop in the regulation of granulopoiesis is evident in patients with leukocyte adhesion deficiency type I (LAD-I) [73]. LAD-I patients have inactivating mutations in the common β2-integrin subunit CD18, thereby diminishing neutrophil adhesion and transendothelial migration to peripheral tissues, resulting in neutrophilia and recurrent infections. Intriguingly, the disrupted negative feedback loop in LAD-I patients due to abrogated neutrophil tissue infiltration results in dysregulated upregulation of the IL-23-IL-17 axis, which is responsible for IL-17-dependent inflammatory bone loss and development of an aggressive form of periodontitis in early age [73, 74]. A similar mechanism involving IL-17-dependent inflammatory bone loss and periodontitis is observed in LFA-1-deficient mice [74].
The interplay between IL-17-dependent inflammation and neutrophil recruitment is also operative in various pathologies, such as neuroinflammation, arthritis and psoriasis [75]. For example, in murine experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis, adoptive transfer of IL-17-producing Th17 cells resulted in increased myelopoisis and infiltration of neutrophils into the central nervous system (CNS), affecting disease severity [76]. Interestingly, neutrophil-associated markers, such as CXCL1 or neutrophil elastase correlated with multiple sclerosis lesion burden [76]. Similarly, increased IL-17 expression in the CNS due to Del-1 deficiency was associated with increased neutrophil recruitment and increased disease severity [60]. Furthermore, a recent study by Zenaro et al. revealed the implication of neutrophil recruitment in the pathogenesis of Alzheimer’s disease. Neutrophils infiltrate areas of amyloid-β deposition and exert a pathogenic role through the release of IL-17 and neutrophil extracellular traps. Inhibition of neutrophil recruitment by means of LFA-1 deficiency protected mice from cognitive decline, suggesting the importance of neutrophil recruitment to the pathogenesis of this neurodegenerative syndrome [77].
Together, these examples illustrate that neutrophils, over and above their role in acute infection and inflammation, may also contribute to the pathogenesis of chronic inflammatory and autoimmune disorders.
Conclusion
Neutrophils are the innate immune cells that initially infiltrate in vast numbers into the sites of infectious or sterile inflammation [1, 2]. A deregulation of neutrophil recruitment is linked to the development of inflammatory and autoimmune diseases [69, 70]. Infiltrating neutrophils have a significant contribution to not only the regulation of inflammation and immune responses in peripheral tissue, but also to the regulation of myelopoiesis [71]. For this reason, neutrophil adhesion to inflamed endothelium is a highly coordinated process comprising a series of intertwined adhesive events [2, 3]. Recent experimental findings summarized in the present review have identified and added new players in the cascade of neutrophil recruitment, which have increased the complexity of this process. The identification of TLR signalling as a regulator of neutrophil integrin activation has enhanced the repertoire of factors triggering neutrophil recruitment [23, 24]. The recently emerged role of α3β1 integrin for neutrophil recruitment in sepsis [42] supports the existence of different subsets of neutrophils and the idea that these cells may respond differently to inflammatory insults. Except from the positive regulatory pathways, the recently identified negative regulators and inhibitors of neutrophil adhesion cascade further enable the fine tuning of neutrophil infiltration and the maintenance of tissue homeostasis [54–63].
Neutrophil recruitment is the initial response to infectious and inflammatory insults and depends on adhesive interactions between neutrophils and the inflamed endothelium.
Besides integrin activation by signals deriving from chemokines and selectin ligands, recent evidence suggests that TLR ligation by pathogen-derived or endogenous factors (e.g. MRP8/14) can activate leukocyte integrins, thereby promoting neutrophil recruitment.
Recently, α3β1 integrin was identified as an important player for neutrophil infiltration in sepsis.
A novel role of neutrophils in chronic inflammatory diseases, for instance in neuroinflammatory disorders, is being increasingly recognized.
Del-1, PTX-3 and GDF-15 act as endogenous inhibitors of neutrophil recruitment.
Acknowledgments
Financial support and sponsorship: TC is supported by the European Community's Seventh Framework Programme under grant agreement n°602699 (DIREKT) and by the Deutsche Forschungsgemeinschaft (SFB-TRR 127 Project A3) and GH is supported by NIH grants DE015254, DE024716 and DE021685.
Footnotes
Conflicts of interest None
References
- 1.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 2013;34(1):1–6. doi: 10.1016/j.it.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitroulis I, et al. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–35. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorina R, et al. beta2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J Immunol. 2014;192(1):324–37. doi: 10.4049/jimmunol.1300858. [DOI] [PubMed] [Google Scholar]
- 6.Phillipson M, et al. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203(12):2569–75. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013;5(4):336–47. doi: 10.1159/000346659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi EY, Santoso S, Chavakis T. Mechanisms of neutrophil transendothelial migration. Front Biosci (Landmark Ed) 2009;14:1596–605. doi: 10.2741/3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50(1):7–22. doi: 10.1177/0300985812469883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devi S, et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19(1):107–12. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 11.Mocsai A, Walzog B, Lowell CA. Intracellular signalling during neutrophil recruitment. Cardiovasc Res. 2015;107(3):373–85. doi: 10.1093/cvr/cvv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarbock A, et al. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–51. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venturi GM, et al. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19(5):713–24. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 14.Stadtmann A, et al. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J Exp Med. 2013;210(11):2171–80. doi: 10.1084/jem.20130664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorlino CV, et al. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J Immunol. 2014;193(4):1966–74. doi: 10.4049/jimmunol.1301791. [DOI] [PubMed] [Google Scholar]
- 16.Sreeramkumar V, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346(6214):1234–8. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marki A, et al. Role of the endothelial surface layer in neutrophil recruitment. J Leukoc Biol. 2015 doi: 10.1189/jlb.3MR0115-011R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruenster M, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10(1):101–8. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Filippo K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–7. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 20.Jin L, et al. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J Immunol. 2014;193(7):3549–58. doi: 10.4049/jimmunol.1401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alon R. Chemokine arrest signals to leukocyte integrins trigger bi-directional-occupancy of individual heterodimers by extracellular and cytoplasmic ligands. Cell Adh Migr. 2010;4(2):211–4. doi: 10.4161/cam.4.2.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herter JM, et al. Integrin activation by P-Rex1 is required for selectin-mediated slow leukocyte rolling and intravascular crawling. Blood. 2013;121(12):2301–10. doi: 10.1182/blood-2012-09-457085. [DOI] [PubMed] [Google Scholar]
- 23.Pruenster M, et al. Extracellular MRP8/14 is a regulator of beta2 integrin-dependent neutrophil slow rolling and adhesion. Nat Commun. 2015;6:6915. doi: 10.1038/ncomms7915. This study implicates signalling stimulated by MRP8/14 via TLR4 in the activation of β2 integrins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung KJ, et al. A novel pathway of rapid TLR-triggered activation of integrin-dependent leukocyte adhesion that requires Rap1 GTPase. Mol Biol Cell. 2014;25(19):2948–55. doi: 10.1091/mbc.E14-04-0867. This study revealed the direct activation of β2 integrins and leukocyte adhesion through TLR ligation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harokopakis E, et al. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176(12):7645–56. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 26.Mor A, Dustin ML, Philips MR. Small GTPases and LFA-1 reciprocally modulate adhesion and signaling. Immunol Rev. 2007;218:114–25. doi: 10.1111/j.1600-065X.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 27.Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb Haemost. 2009;102(2):191–7. doi: 10.1160/TH08-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab L, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014;20(6):648–54. doi: 10.1038/nm.3517. This study demonstrates that neutrophil infiltration in the intestine can have a detrimental effect in the immune response after transplantation. [DOI] [PubMed] [Google Scholar]
- 29.Moles A, et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J Hepatol. 2014;60(4):782–91. doi: 10.1016/j.jhep.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weckbach LT, et al. The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via beta2 integrins (CD11/CD18) Blood. 2014;123(12):1887–96. doi: 10.1182/blood-2013-06-510875. This study proposes a novel role for the cytokine midkine in the activation of β2 integrins. [DOI] [PubMed] [Google Scholar]
- 31.Huebener P, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125(2):539–50. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavakis T, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198(10):1507–15. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlova VV, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26(4):1129–39. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z, et al. Interaction of kindlin-3 and beta2-integrins differentially regulates neutrophil recruitment and NET release in mice. Blood. 2015;126(3):373–7. doi: 10.1182/blood-2015-03-636720. In this study the critical role of kindlin-3 for integrin-dependent neutrophil recruitment and neutrophil extracellular traps release is demonstrated. [DOI] [PubMed] [Google Scholar]
- 35.Harris ES, Weyrich AS, Zimmerman GA. Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr Opin Hematol. 2013;20(1):16–25. doi: 10.1097/MOH.0b013e32835a0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germena G, et al. Mutation in the CD45 inhibitory wedge modulates integrin activation and leukocyte recruitment during inflammation. J Immunol. 2015;194(2):728–38. doi: 10.4049/jimmunol.1401646. [DOI] [PubMed] [Google Scholar]
- 37.Harding MG, et al. Neutrophil crawling in capillaries; a novel immune response to Staphylococcus aureus. PLoS Pathog. 2014;10(10):e1004379. doi: 10.1371/journal.ppat.1004379. This study adds important novel insights into the ability of neutrophils to crawl in capillaries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, et al. Streptococcal M1 protein triggers chemokine formation, neutrophil infiltration, and lung injury in an NFAT-dependent manner. J Leukoc Biol. 2015;97(6):1003–10. doi: 10.1189/jlb.3HI0214-123RR. [DOI] [PubMed] [Google Scholar]
- 39.Boespflug ND, et al. ATF3 is a novel regulator of mouse neutrophil migration. Blood. 2014;123(13):2084–93. doi: 10.1182/blood-2013-06-510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh RK, et al. A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biol. 2014;15:39. doi: 10.1186/s12860-014-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindemann O, et al. TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J Immunol. 2013;190(11):5496–505. doi: 10.4049/jimmunol.1201502. [DOI] [PubMed] [Google Scholar]
- 42.Lerman YV, et al. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin alpha3beta1-dependent. Blood. 2014;124(24):3515–23. doi: 10.1182/blood-2014-01-552943. In this study, the novel role of α3β1 integrin in neutrophil recruitment and activation in sepsis is shown. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitroulis I, Chavakis T. alpha3beta1 is INTEGRAL to septic neutrophils. Blood. 2014;124(24):3507–8. doi: 10.1182/blood-2014-10-606632. [DOI] [PubMed] [Google Scholar]
- 44.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Proebstl D, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209(6):1219–34. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stark K, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol. 2013;14(1):41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 47.Abtin A, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15(1):45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chavakis T, Preissner KT, Herrmann M. The anti-inflammatory activities of Staphylococcus aureus. Trends Immunol. 2007;28(9):408–18. doi: 10.1016/j.it.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Athanasopoulos AN, et al. The extracellular adherence protein (Eap) of Staphylococcus aureus inhibits wound healing by interfering with host defense and repair mechanisms. Blood. 2006;107(7):2720–7. doi: 10.1182/blood-2005-08-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orlova VV, Chavakis T. Regulation of vascular endothelial permeability by junctional adhesion molecules (JAM) Thromb Haemost. 2007;98(2):327–32. [PubMed] [Google Scholar]
- 51.Woodfin A, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12(8):761–9. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathias JR, et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80(6):1281–8. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 53.Colom B, et al. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity. 2015;42(6):1075–86. doi: 10.1016/j.immuni.2015.05.010. This study identified the critical role of the leukotriene B4-elastase axis in regulating neutrophil reverse transmigration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chavakis T. Leucocyte recruitment in inflammation and novel endogenous negative regulators thereof. European Journal of Clinical Investigation. 2012;42(6):686–691. doi: 10.1111/j.1365-2362.2012.02677.x. [DOI] [PubMed] [Google Scholar]
- 55.Mitroulis I, et al. Developmental endothelial locus-1 attenuates complement-dependent phagocytosis through inhibition of Mac-1-integrin. Thromb Haemost. 2014;111(5):1004–6. doi: 10.1160/TH13-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maekawa THK, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, Hajishengallis G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3β–C/EBPβ pathway. Nature Communications. 2015 doi: 10.1038/ncomms9272. in press. In this paper, the antagonistic effect of pro-inflammatory and pro-resolving mediators in the regulation of Del-1, an endogenous inhibitor of neutrophil recruitment, is demonstrated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin JMT, Abe T, Hajishengallis E, Hosur K, Pyaram K, Mitroulis I, Chavakis T, Hajishengallis G. The homeostatic factor Del-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Science Translational Medicine. 2015 doi: 10.1126/scitranslmed.aac5380. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi EY, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322(5904):1101–4. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eskan MA, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13(5):465–73. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi EY, et al. Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol Psychiatry. 2015;20(7):880–8. doi: 10.1038/mp.2014.146. This study demonstrates that Del-1 blocks neutrophil recruitment into the CNS and thereby ameliorates neuroinflammation in an animal model of multiple sclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deban L, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11(4):328–34. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 62.Kempf T, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17(5):581–8. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, et al. Neutrophil infiltration during inflammation is regulated by PILRalpha via modulation of integrin activation. Nat Immunol. 2013;14(1):34–40. doi: 10.1038/ni.2456. [DOI] [PubMed] [Google Scholar]
- 64.Liu G, et al. Kinase AKT1 negatively controls neutrophil recruitment and function in mice. J Immunol. 2013;191(5):2680–90. doi: 10.4049/jimmunol.1300736. [DOI] [PubMed] [Google Scholar]
- 65.Andrade EB, et al. TLR2-induced IL. –10 production impairs neutrophil recruitment to infected tissues during neonatal bacterial sepsis. J Immunol. 2013;191(9):4759–68. doi: 10.4049/jimmunol.1301752. [DOI] [PubMed] [Google Scholar]
- 66.Chavakis T, et al. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood. 2005;105(3):1036–43. doi: 10.1182/blood-2004-01-0166. [DOI] [PubMed] [Google Scholar]
- 67.Aulakh GK, et al. Angiostatin inhibits activation and migration of neutrophils. Cell Tissue Res. 2014;355(2):375–96. doi: 10.1007/s00441-013-1753-0. [DOI] [PubMed] [Google Scholar]
- 68.Blazek K, et al. IFN-lambda resolves inflammation via suppression of neutrophil infiltration and IL-1beta production. J Exp Med. 2015;212(6):845–53. doi: 10.1084/jem.20140995. This study shows the anti-inflammatory role of IFNs through the suppression of neutrophil recruitment in arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantovani A, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 70.Witko-Sarsat V, et al. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011;32(3):117–24. doi: 10.1016/j.it.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 72.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5(5):661–74. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hajishengallis G, et al. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol. 2014 doi: 10.1189/jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moutsopoulos NM, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 2014;6(229):229ra40. doi: 10.1126/scitranslmed.3007696. This study identified local IL-17 production in the periodontal tissue, resulting from defective neutrophil recruitment, as the pathomechanism of severe periodontitis in patients with LAD-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isailovic N, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Rumble JM, et al. Neutrophil-related factors as biomarkers in EAE and MS. J Exp Med. 2015;212(1):23–35. doi: 10.1084/jem.20141015. This study provides evidence for the previously underestimated role of neutrophils in T cell mediated neuroinflammation and multiple sclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zenaro E, et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21(8):880–6. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]