Abstract
In recent years evidence has accumulated to suggest that neuroinflammation might be an early pathology of schizophrenia that later leads to neurodegeneration, yet the exact role in the etiology, as well as the source of neuroinflammation, are still not known. The hypothesis of neuroinflammation involvement in schizophrenia is quickly gaining popularity, and thus it is imperative that we have reliable and reproducible tools and measures that are both sensitive, and, most importantly, specific to neuroinflammation. The development and use of appropriate human in vivo imaging methods can help in our understanding of the location and extent of neuroinflammation in different stages of the disorder, its natural time-course, and its relation to neurodegeneration. Thus far, there is little in vivo evidence derived from neuroimaging methods. This is likely the case because the methods that are specific and sensitive to neuroinflammation are relatively new or only just being developed. This paper provides a methodological review of both existing and emerging positron emission tomography and magnetic resonance imaging techniques that identify and characterize neuroinflammation. We describe how these methods have been used in schizophrenia research. We also outline the shortcomings of existing methods, and we highlight promising future techniques that will likely improve state-of-the-art neuroimaging as a more refined approach for investigating neuroinflammation in schizophrenia.
INTRODUCTION
Schizophrenia is a severe psychiatric disease, noted for its chronic and often debilitating processes, characterized by delusions and hallucinations, cognitive impairment, and blunted affect (Bleuler, 1950; Kraepelin, 1971). Onset is during adolescence or young adulthood, and it is often lifelong and chronic. Although progress has been made in delineating brain abnormalities in schizophrenia (Fitzsimmons et al., 2013), the etiology, pathogenesis, and biological course still remain elusive, with evidence suggesting a variety of deficiencies and abnormalities, including neurodevelopmental and neurodegenerative abnormalities, as well as a number of dopaminergic, myelin, oligodendrocyte, and volumetric alterations in a number of brain regions that are not proximal but may reflect an underlying anatomical and or functional connection (e.g., see reviews in Harrison, 1999; Jaaro-Peled et al., 2010; Kubicki et al., 2005; Shenton et al., 2001).
The involvement of neuroinflammation in schizophrenia has long been hypothesized (DeLisi et al., 1984; Ganguli et al., 1987; Pandey et al., 1981; Torrey and Peterson, 1973; Vartanian et al., 1978). It is only recently, however, that evidence from neuropathological and neuroimaging studies has emerged to suggest the possible role of neuroinflammation in the etiology of schizophrenia (see recent reviews: Chew et al., 2013; Kahn and Sommer, 2014; Monji et al., 2009; Monji et al., 2013; Najjar and Pearlman, 2015). Neuroinflammation is a normal, albeit, nonspecific response of the brain’s immune system to harmful stimuli such as tissue damage or pathogen invasion (Streit et al., 2004).
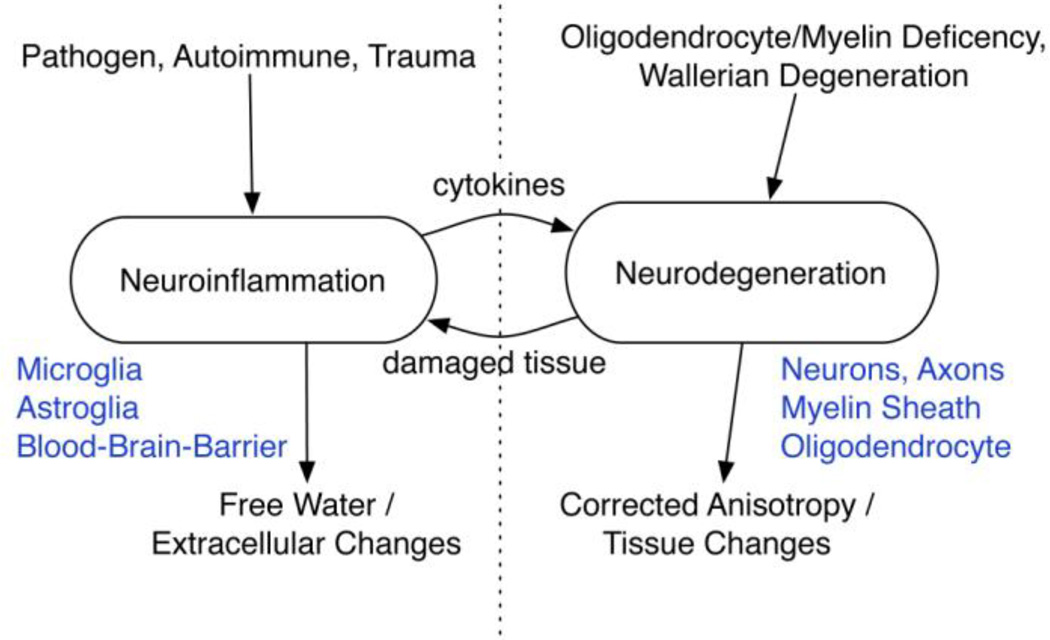
While neuroinflammation is important for a healthy functioning brain, it has been suggested that in neurodegenerative disorders, chronic neuroinflammation likely induces adverse effects, and may be responsible for some of the symptoms that persist for many years during the course of schizophrenia (Streit, 2006; Streit et al., 2004). Similarly, recent studies in schizophrenia have investigated the hypothesis that neuroinflammation is an early indicator of pathology in the etiology of schizophrenia, which may later lead to neurodegeneration (Feigenson et al., 2014; Muller et al., 2004; Najjar and Pearlman, 2015).
Neuroinflammation is observed in many brain disorders, especially in those with a neurodegenerative course such as Multiple Sclerosis, Alzheimer’s disease and Parkinson’s disease (Schwartz, 2003; Weiner and Selkoe, 2002). The brain parenchyma is separated from the periphery by the blood brain barrier (BBB), which, under normal conditions, prevents immune cells that are in the blood from entering brain tissue (Schultzberg et al., 2007; Zlokovic, 2008). Instead, the brain has its own innate immune system that operates mainly through the function of astrocytes and microglia, where neuroinflammation is defined as the activation of this system (Schwartz, 2003). Excellent reviews of the structure and function of glia cells are available and the reader is referred to these for further details (e.g., Allaman et al., 2011; Fontana et al., 1987; Rock et al., 2004; Schwartz et al., 2006; Streit et al., 1988; Tilleux and Hermans, 2007). Briefly, microglia are the resident macrophages of the brain and are usually the initial responders to tissue insult or damage. Receptors on the microglia respond and activate the cells. When active, the cells change their shape and function and initiate phagocytosis. In addition, activated microglia, in concert with astrocytes, emit cytokines that lead to a cascade of events that modulate the neuroinflammatory response. As part of this process, the glia cells also emit oxidative and nitrosative products, as well as excitotoxic metabolites that can damage surrounding tissue. An acute, or short-term neuroinflammatory response is likely important for a healthy functioning brain and contributes to the repair of damaged or infected tissue. However, when the inflammatory process continues for a long period of time (weeks, months or even years), damage to the surrounding brain tissue may become substantial. For example, a prolonged neuroinflammatory response in the white matter may damage oligodendrocytes, and the myelin sheath surrounding axons, thereby affecting network connectivity in the brain (Chew et al., 2013; Deng, 2010). Exposure to oxidative, nitrosative, and excitotoxic metabolites may also result in the loss of neuronal cell bodies and reduced extracellular matrix, evident as brain atrophy (Bigler, 2013; Frodl and Amico, 2014; Jacobs et al., 2012; Versijpt et al., 2005). Tissue degeneration, in turn, reactivates neuroinflammation, forming a “vicious circle” of neuroinflammation and neurodegeneration (Jacobs et al., 2012).
The cause of neuroinflammation and its association with neurodegenerative disorders is not yet known. Moreover, in most neurodegenerative disorders (e.g., Multiple Sclerosis, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease), the reason for the initial inflammatory response is also not well known. This is also the case in schizophrenia, where maternal and prenatal infections (Anderson and Maes, 2013), brain injury (Chew et al., 2013), autoimmune disorder (Fineberg and Ellman, 2013), and stress response (Vogel et al., 2011), have all been hypothesized, among other possible causes (Kirkpatrick and Miller, 2013; Meyer, 2013), as the source of neuroinflammation. The duration of neuroinflammation, whether it is chronic or acute, its location and extent, as well as its relation to symptoms, are also not known in schizophrenia. Nevertheless, if neuroinflammation is indeed an earlier pathology that may lead to neurodegeneration, and if it can be reliably detected, then it can potentially be treated, making the study of neuroinflammation in schizophrenia an important and active field of research.
A key to understanding the involvement of neuroinflammation in schizophrenia, as well as in other brain disorders, is our ability to monitor neuroinflammation with respect to when it begins and as it progresses. However, monitoring neuroinflammation in vivo in the brain is challenging. Currently inflammatory signs in schizophrenia are mainly identified in blood and cerebrospinal fluid (CSF) markers. Yet these markers may be limited in their sensitivity and specificity (Feigenson et al., 2014), with CSF markers requiring highly invasive lumbar punctures. Additionally, these markers cannot identify the location and extent of neuroinflammation in the brain. It is therefore important to develop in vivo imaging methods that are sensitive and specific to neuroinflammation.
This paper is focused on existing and emerging neuroimaging methods that can identify and characterize neuroinflammation in vivo, by targeting different chemical, physical, and geometrical changes that occur in the neuroinflammatory cascade. Methods reviewed include positron emission tomography (PET), magnetic resonance spectroscopy (MRS), anatomical and quantitative magnetic resonance imaging (MRI), and diffusion MRI.
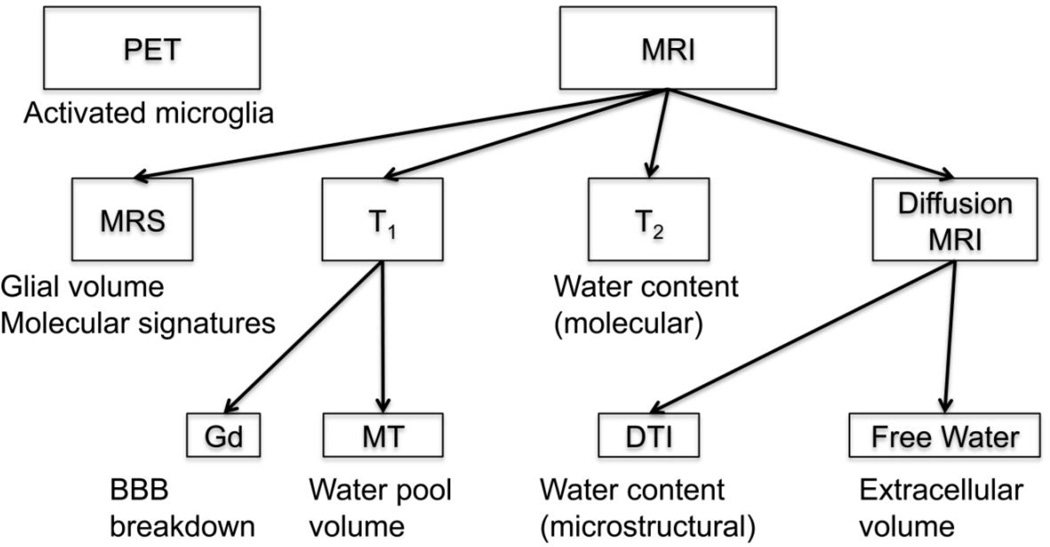
These methods are depicted in Figure 1. To date, most of the evidence for neuroinflammation in schizophrenia comes from ex vivo neuropathological studies, with only a small number of studies that have tried to utilize neuroimaging for the identification of neuroinflammation in schizophrenia (Feigenson et al., 2014; Najjar and Pearlman, 2015). The advancement of such neuroimaging methods is critical for our ability to identify and to monitor neuroinflammation in vivo in schizophrenia, as well as in other brain disorders.
Figure 1. Overview of imaging methods useful for neuroinflammation identification.
Positron emission tomography (PET) uses ligands that bind to activated microglia, which initiate the inflammatory cascade. Magnetic Resonance Imaging (MRI) provides several methods: MR spectroscopy (MRS) can identify metabolites that are sensitive to glial volume, and to other molecular signatures of neuroinflammation; and Gadolinium (Gd) enhanced T1 can identify blood-brain-barrier (BBB) breakdown, which occurs in severe cases of neuroinflammation. Other MRI based methods can identify changes in water content, which is expected to increase due to neuroinflammation, these include: Magnetization transfer (MT) that can identify changes in the volume of the water pool versus the macromolecular pool; T2 weighted imaging that can identify water content changes through molecular interactions; and diffusion tensor imaging (DTI) that can identify the water content changes through microstructural changes that affect the diffusivity of water molecules. More advanced diffusion MRI based methods, such as free-water imaging, can identify the extracellular volume, which is likely more specific to neuroinflammation than water content changes.
IDENTIFYING NEUROINFLAMMATION WITH PET
PET is currently the main neuroimaging modality used for the identification of neuroinflammation in vivo (Jacobs et al., 2012). The PET scanner produces 3D images representing levels of detected gamma rays emitted by tracers, which are radionuclides chemically incorporated into a biologically active molecule and introduced to the body (Muehllehner and Karp, 2006)
PK11195 targeting activated microglia
PET can detect neuroinflammation by using tracers that are ligands specific for the translocator protein-18-kd (TSPO), which is a protein of the outer mitochondrial membrane (Venneti et al., 2013). Currently, the main tracer used for in vivo studies is the Isoquinoline ligand 1-[2-chlorophenyl]-N-methyl-N-[1-methyl-propyl]-3-isoquinoline carboxamide (PK11195). Most human studies use [11C](R)-PK11195, which is the (R) enantiomer labeled with carbon-11. The normal healthy brain expresses TSPO in areas such as the choroid plexus, the ependymal layer, and in perivascular cells (Kannan et al., 2009). However, in the injured nervous system TSPO is highly expressed in activated microglia. TSPO is also expressed albeit to a lesser extent in reactive astrocytesand, and in neuroblastoma and glioblastoma cell lines (Rupprecht et al., 2010).
In an insulted brain there is a robust and widespread increase in [11C](R)-PK11195 binding, indicating the presence of activated microglia, and thus neuroinflammation. Increases in [11C](R)-PK11195 estimates of binding potentials have been reported in patients with stroke (Gerhard et al., 2005; Thiel et al., 2010), traumatic brain injury (Ramlackhansingh et al., 2011), and in patients with chronic neurodegenerative conditions including Huntington's disease (Politis et al., 2011) and Parkinson's disease (Gerhard et al., 2006). Binding potential is estimated by fitting time-activity curves with a kinetic model, which estimates efflux of [11C](R)-PK11195 between the modeled compartments (Kropholler et al., 2005). The identification of increased estimated binding potential of [11C](R)-PK11195 in neurodegenerative diseases, as well as in animal models, has made PET the most applied imaging modality for the identification of neuroinflammation.
PK11195 studies in schizophrenia research
To date there are only two published studies that have quantified [11C](R)-PK11195 in schizophrenia subjects to identify neuroinflammation. Van Berckel et al. (van Berckel et al., 2008) compared the estimated binding potential of [11C](R)-PK11195 between ten patients diagnosed with recent-onset schizophrenia and ten healthy controls, and found significantly increased estimated binding potential in the entire gray matter of patients diagnosed with schizophrenia. Doorduin et al. (Doorduin et al., 2009) compared seven patients diagnosed with an acute episode of psychosis with eight age-matched controls. They found a significant regional estimated binding potential increase in the hippocampus of patients, and a trend level increased estimated binding potential in the basal ganglia, midbrain, cerebellum, and pons. Estimated binding potential in the entire gray matter was increased, albeit not significantly. These findings suggest that in the early stages of schizophrenia there may be a subtle neuroinflammatory effect, which is hard to detect with statistical significance using [11C](R)-PK11195.
Emerging ligands for neuroinflammation detection
While PK11195 is currently the most utilized tracer to identify neuroinflammation, there are debates about its affinity to TSPO in in vivo human studies, as well as about its specificity to activated microglia. Studies, in fact, show that in vivo [11C](R)-PK11195 imaging suffers from poor signal to noise characteristics (Venneti et al., 2013). Various analysis methods have been used to increase the specificity and sensitivity of the output parameters to binding potential. However, specificity is limited by, for example, the level of the TSPO receptor density in the normal brain, which is ubiquitous (Venneti et al., 2013), whereas sensitivity is limited by poor penetration of the BBB (Bartels et al., 2010). Furthermore, since TSPO is sensitive both to activated microglia and activated astrocytes, the two may not be distinguished (Lavisse et al., 2012), which is a limitation since the different cell types have different roles in neuroinflammation and disease progression. Reliability and proper selection of kinetic models, especially for use in vivo, are also under debate (Turkheimer et al., 2007).
In the past 15 years, a multitude of candidate second-generation TSPO tracers have been developed in an attempt to obtain higher affinity and higher specificity to TSPO than PK11195 (Chauveau et al., 2008). However, a limitation of second-generation tracers is that they appear to have variable binding affinity in humans (Owen et al., 2011), likely related to genetic variation (Guo et al., 2012). As a result, the analysis of second-generation TSPO tracers is complicated by the need to account for three different groups: high affinity binders, mixed affinity binders, and low affinity binders (Guo et al., 2012). This is opposed to PK11195, which appears to bind with similar affinity across subjects (Owen et al., 2011).
Utilizing the second-generation tracer (N-5-fluoro-2-phenoxyphenyl)-N-(2,5-dimethoxyl-benzyl) acetamide (DAA1106), which has been shown to have higher affinity to TSPO than PK11195 (Maeda et al., 2004), Takano et al. (Takano et al., 2010) compared the estimated binding potential of DAA1106 in 14 patients with chronic schizophrenia versus 14 healthy controls. In this study there were no significant differences between groups in gray matter. Nonetheless, the estimated binding potential in patients was correlated with positive symptoms. Another study utilized the second-generation TSPO tracer N-acetyl-N-(2-[18F]fluoroethoxybenzyl)-2-phe-noxy-5-pyridinamine ([18F]-FEPPA) to identify neuroinflammation in gray and white matter in patients with chronic schizophrenia (Kenk et al., 2015). In this study no group differences were reported between patients and controls. This is despite the fact that a larger number of patients was scanned, and despite the expected higher SNR and affinity of [18F]-FEPPA compared with [11C](R)-PK11195. Taken together, these four studies that applied PET for the identification of neuroinflammation in schizophrenia provide inconclusive evidence for the involvement of neuroinflammation in schizophrenia. This is in contrast to PET studies in neurodegenerative diseases demonstrating a robust identification of an inflammatory component. The lack of findings in schizophrenia may be explained, however, either by a lack of microglial activation, or by an activation that is subtle, and is not detected even with the more sensitive second-generation tracers. The fact that the two latter studies (Kenk et al., 2015; Takano et al., 2010) tested chronic subjects might also explain the lack of findings, perhaps suggesting that neuroinflammation might be more extensive in the earlier stages of the disorder, or that antipsychotic medication reduces the inflammatory effect (Doorduin et al., 2009; Kenk et al., 2015). Future studies using more sensitive PET ligands need to include patients diagnosed with schizophrenia early in the course of illness.
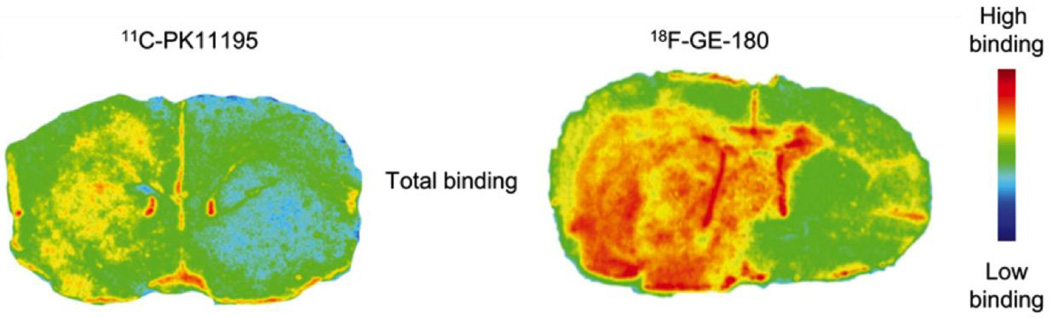
Thus while PET ligands show promise in the identification of neuroinflammation, there are major limitations in the applicability of PET studies in general, which are likely the reason for the small number of published studies. PET scanners and cyclotrons, in which the radioligands are being synthesized, are also expensive (Berger et al., 2003) and not widely available in many clinical settings. In addition, and importantly, the injection of the radioligand exposes subjects to radiation, which makes longitudinal studies more problematic, and where longitudinal studies could shed more light on the trajectory of neuroinflammation, and its association with neurodegeneration. Nevertheless, technical improvements in the PET scanner, and, more importantly, new ligands that may have higher sensitivity and specificity to neuroinflammation are being developed (Jacobs et al., 2012), such as the promising 18F-GE-180 (Dickens et al., 2014; see Fig. 2). Other advances focus on identifying targets other than TSPO that can identify neuroinflammation, and appropriate ligands for the detection of these targets (Pulli and Chen, 2014). These promising developments, when applied to patients diagnosed with schizophrenia, at different stages of the disease, will likely lead to a further understanding of the role of neuroinflammation in schizophrenia.
Figure 2. PET ligands binding in neuroinflammation.
Representative in vitro autoradiography images from coronal striatal sections of a rat injected with lipopolysaccharide (LPS) demonstrate that the second generation TSPO tracers 18F-GE-180 provides better binding to glial activation than PK11195. This figure was originally published in JNM (Dickens et al., 2014) ©by the Society of Nuclear Medicine and Molecular Imaging, Inc.
IDENTIFYING NEUROINFLAMMATION WITH MRS
MRS is one of the basic MRI experiments, providing probes of brain structure and function. In MRI, the subject is inserted into a large and homogenous magnetic field. Radio frequency (RF) coils are then used to introduce magnetic manipulations that provide electrical signal reconstructed into images with contrasts that are sensitive to different molecular properties (Haacke et al., 1999; Mansfield and Morris, 1982). Unlike PET, MRI does not impose ionizing radiation, and most MRI acquisitions in the research arena do not require an injection of a contrast agent. In addition, with MRI images it is possible to have higher resolution than PET.
In MRS, the MR signal is used to measure metabolite concentrations. Typically a fairly large region of interest (ROI), in the order of a few cm3 is defined, and the acquisition results in a spectrum that quantifies the abundance of different molecules within that ROI (Oz et al., 2014). Both phosphorus (31P) and proton (1H) MRS have been used to study changes in brain metabolite levels associated with neuroinflammation and neurodegenerative diseases. Of these two options, 1H MRS is used in the clinical setting in the overwhelming majority of clinical studies (Chang et al., 2013), since the 1H nucleus forms the most sensitive, and most abundant naturally occurring nucleus. The higher sensitivity of 1H-MRS makes it better suited for examining specific small brain regions, as opposed to 31P-MRS which necessitates larger voxel and longer scan times for adequate signal to noise ratio (SNR) in regions of similar sizes (Stanley et al., 2000).
Metabolites targeting glia changes
The 1H MRS spectra is dominated by a water peak, which is about 100,000 times higher than the signals of brain metabolites. Once the water resonance is suppressed, the spectra has several peaks that can be robustly identified and related to specific metabolites, some of which have a role in the neuroinflammatory process (Chang et al., 2013). These metabolites include N-acetyl-aspartate (NAA), which is widely used as a marker of neuronal density, since NAA is found within mature neurons (Rigotti et al., 2007). The resonance peak for myo-inositol (MI) is a putative glial marker, since it is primarily present in glial cells, functioning as an osmolyte that maintains glial cell volumes (Kantarci et al., 2008). Hence activated glia, with enlarged cell volumes, tend to evince elevated MI. The peak for choline compounds (Cho) is a marker for cell membrane metabolism and cellular turnover (Chang et al., 2013; Oz et al., 2014). The total creatine (tCr) resonance peak includes proton resonances from creatine and phosphocreatine, which reflects the levels of cellular energy metabolites (Chang et al., 2013). Both Cho and tCr have two to three fold higher concentrations in glial cells than in neurons (Brand et al., 1993), and may be elevated in conditions that involve neuroinflammation.
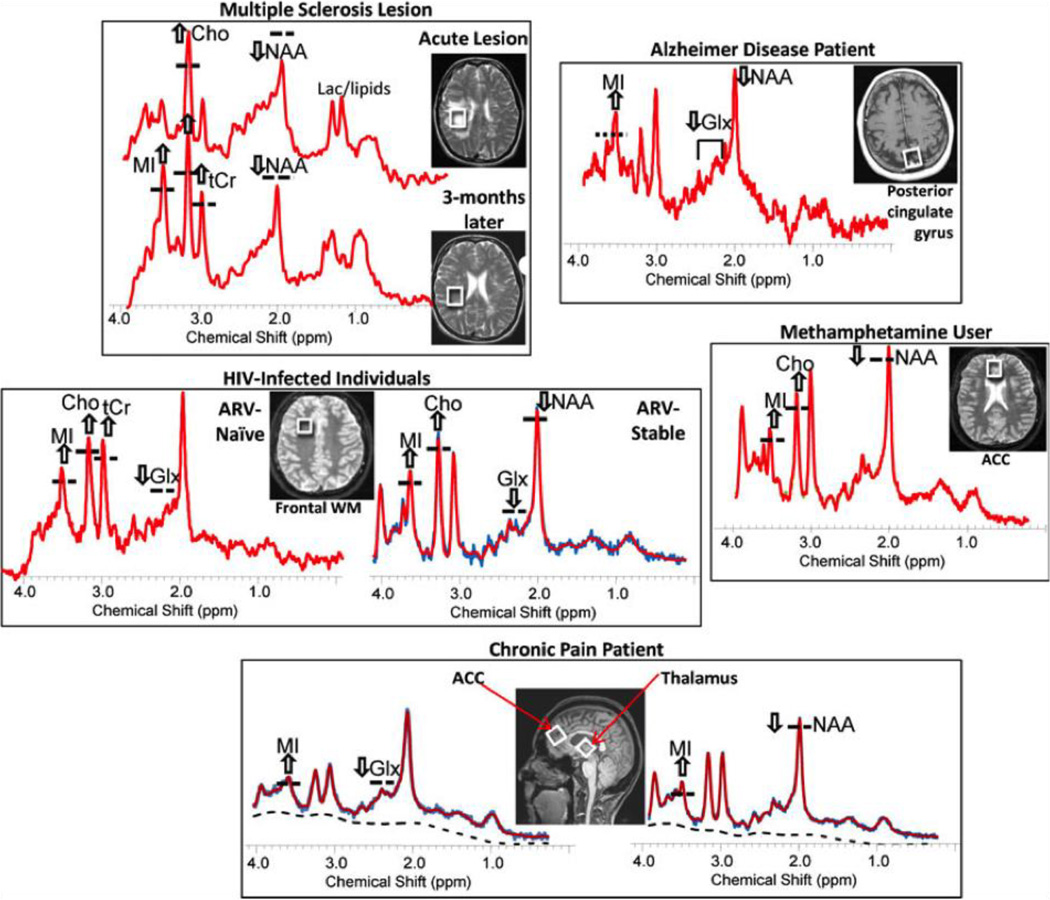
The main utility of MRS for neuroinflammation detection is in identifying larger glia cell content (Fig. 3), following the activation or migration of glia cells to the inflamed area, in which case MI, tCr, and Cho are expected to increase (Bitsch et al., 1999; Chang et al., 2013). Using MRS, glia content can also be distinguished from neuronal integrity, which is expected to show decreased NAA concentrations. Although recent studies suggest that NAA may also appear, and be synthesized, in glia cells as well (Amaral et al., 2013).
Figure 3. Identifying neuroinflammation with MRS.
Representative MR spectra and MRIs showing the voxel locations of the spectra from the brains of patients with various neuroinflammatory disorders. The spectra demonstrate elevated glial markers, especially myo-inositol (MI) and less often choline-containing compounds (Cho) and total creatine (tCr). In addition, the neuronal markers, N-acetylaspartate (NAA) and glutamate (Glu), may be decreased, which may be related directly to the disorder, but also may be exacerbated by neuroinflammation. Reprinted with permission from Chang et al. (2013).
Increased levels of MI, tCr, and Cho, as well as correlations with other neuroinflammatory markers have been reported in a variety of neurodegenerative disorders, including human immunodeficiency virus, Multiple Sclerosis and Alzheimer’s disease (see, e.g., reviews in Chang et al., 2013; Oz et al., 2014). However, other research shows evidence that metabolite changes are not specific to neuroinflammation, and, in addition, may not always appear in neuroinflammatory scenarios (Zahr et al., 2014), with the conclusion being that elevated levels of metabolites must be interpreted within the context of the disease examined.
MRS studies in schizophrenia
Studies of MRS are very common in schizophrenia patients (e.g., see reviews in Port and Agarwal, 2011; Schwerk et al., 2014), yet to the best of our knowledge there are no MRS studies that are specifically aimed at identifying neuroinflammation in schizophrenia.
Nevertheless, some of the findings reported in the literature may be attributed to neuroinflammation. For example, in a recent review it was concluded that NAA reductions, especially in the dorsolateral prefrontal cortex, are the most robust metabolite changes expected in MRS, and that NAA reductions implicate illness progression (Schwerk et al., 2014). In this comprehensive review, Schwerk et al. noted that changes in MI, tCr, and Cho, which might be associated with neuroinflammation, were inconsistent. Inconsistencies may be due to using different sample sizes, often not sufficiently large to pick up subtle metabolite changes. Other explanations for such inconsistencies include different cohorts of schizophrenia patients that might be in different stages of the disorder (e.g., first-episode versus chronic patients), and differences in types of medications that likely affect the metabolite concentrations, as well as the neuroinflammatory process (Sommer et al., 2012; Sommer et al., 2014). Further, different types of analyses might explain the lack of reproducible results. It is now also becoming apparent that MRS studies need to correct for partial volume and morphology in the analysis, they need to refrain from normalizing metabolites by the tCr ratio (since tCr is expected to change), and they need to account for several covariates such as age and gender in the analyses (Schwerk et al., 2014).
Emerging MRS techniques
There are several advances in the MRS acquisition and analysis field that allow for better coverage, potentially smaller regions of interest and better spectral resolution, which could improve sensitivity and specificity of MRS (Ng et al., 2014; Posse et al., 2013). These newer more sophisticated acquisition methods utilize additional excitation pulses and scan time can now provide information regarding spatial variations of metabolite within a large region of interest (chemical shift imaging). Spectral editing methods can also increase chemical specificity. More specifically, two-dimensional correlated spectroscopy (2D COSY) can provide richer spectral information, making possible the identification of metabolites that are hidden in conventional analyses (Ramadan et al., 2011). Finally, novel analysis methods improve water suppression, decrease partial volume effects, and resolve other artifacts such as those induced by motion.
These advances provide a more robust estimation of metabolites that are difficult to estimate using conventional analysis methods. In addition, some of these metabolites could potentially have higher specificity to neuroinflammatory processes. For example, glutathione (GSH) is the main antioxidant of the brain, and it is easier to identify using 2D COSY. Since oxidative stress can promote neuroinflammation via activation of nuclear factor kappa B (NFkB) transcription factor (Najjar et al., 2013), GSH might be an additional indirect neuroinflammatory marker (Anderson and Maes, 2013).
To conclude, MRS has the potential to identify molecular signatures of neuroinflammation, and, unlike PET, MRS is a non-invasive imaging method that has high clinical availability. Since neuroinflammation likely changes the levels of metabolites, there is always a necessity to compare values across a group of patients with a group of healthy subjects. In schizophrenia, these comparisons have not yielded consistent results that directly indicate a neuroinflammatory process. However, recent developments in the acquisition and analysis of MRS show promise in increasing sensitivity and specificity of MRS to neuroinflammation.
IDENTIFYING NEUROINFLAMMATION WITH ANATOMICAL MRI
The main restriction of MRS is its low spatial resolution. Therefore, the identification of small or localized neuroinflammatory processes are limited using MRS. Anatomical MRI, on the other hand, provides high resolution in the order of 1 mm3, which is much superior to MRS as well as to PET in resolution. Many excellent books and reviews of MR physics are available (see e.g., Haacke et al., 1999; Mansfield and Morris, 1982). Briefly, there are two main relaxation times in MRI that can be evaluated, T1 (also spin-lattice) relaxation time and T2 (also spin-spin) relaxation time. Images weighted by T1 have contrast sensitive to macromolecular volume and the physicochemical environment of macromolecules. Thus, T1 weighted images provide nice contrast between different types of tissue (e.g., gray matter, white matter, and CSF), and are useful for the segmentation and volume measure of various brain areas. Images weighted by T2 are sensitive to molecular motion and interactions between neighboring molecules. The contrast is similar for gray and white matter, but is higher for CSF and pathologies in which water is accumulating within tissue, such as vasogenic edema, which is an extreme form of neuroinflammation (Stamatovic et al., 2008).
T1 based contrasts
Two variants of T1-weighted images, namely gadolinium-enhanced T1, and magnetization transfer (MT) images, have been thought to be useful for the identification of neuroinflammation (Jacobs et al., 2012). In a gadolinium-enhanced acquisition, a dose of gadolinium (Gd) is injected. The interaction between Gd and protons lowers the T1, and as a result, areas in which Gd is accumulated have higher contrast comparing to regular tissue. The Gd molecules cannot pass the BBB, and therefore hyper signal outside of blood vessels indicates BBB breakdown, which is a surrogate marker for severe neuroinflammation (Bruck et al., 1997). Gd enhanced T1 weighted images have been used to detect severe neuroinflammatory pathologies such as active lesions in MS (Gonzalez-Scarano et al., 1987; Grossman et al., 1986), and in brain tumors (Claussen et al., 1985), but these images may not detect subtle neuroinflammatory processes in which the BBB does not leak (Rausch et al., 2003). In one study of schizophrenia that investigated Gd enhanced T1, there were no group differences when comparing patients with controls (Szymanski et al., 1991), suggesting that a severe neuroinflammatory response may not be extant in schizophrenia.
In MT, the second variant of T1-weighted images, the signal is sensitive to the exchange of magnetization between mobile protons (e.g., free-water) and motional restricted protons, such as those attached to macromolecules, through chemical processes, diffusion and cross relaxation (Henkelman et al., 2001). MT is sensitive to macromolecules, which are practically invisible to other MRI modalities. This is achieved by applying an off-resonance RF pulse that excites only the motion-restricted pool, i.e., macromolecules.
Some of the magnetization is then transferred from the macromolecules to the mobile pool (e.g., water in tissue) through magnetization interactions (dipolar exchange and chemical exchange) and can be detected. A scalar parameter is often used to quantify the magnetization transfer ratio (MTR), which is the normalized difference between the signal obtained with and without the off-resonance RF pulse (Henkelman et al., 2001). The MTR value is sensitive to the volume ratio between the two pools. Therefore, changes in the water content of tissue due to inflammation would also change the MTR (Laule et al., 2007; Vavasour et al., 2011). This hypothesis has been tested mainly in severe cases of neuroinflammation (e.g., edema in MS (Vavasour et al., 2011)). See below for MTR studies in schizophrenia.
Brain segmentation and volumetric measures are currently the main utility of T1-weighted images. In this context it is interesting to note that while volumetric changes are a robust finding in schizophrenia (e.g., Levitt et al., 2010; Makris et al., 2010), longitudinal studies have reported reversible changes in white matter volume (e.g., Christensen et al., 2004). While most studies explain loss of volume as resulting from atrophy, the reversible nature reported suggests that white matter volumetric measures may also be sensitive to extracellular volume (Garver et al., 2008), and thus excessive water content resulting from neuroinflammation may explain some of the volume changes, including reversible volume changes, reported in schizophrenia.
MTR studies in schizophrenia
Studies using MTR to investigate schizophrenia have been conducted mainly in order to quantify the macromolecule distribution, which may be a surrogate marker of demyelination (Kubicki et al., 2005). However, recent studies suggest that changes in MTR observed in schizophrenia might be explained by both changes in the macromolecule pool as well as in the mobile pool, and thus neuroinflammation might explain some of the differences found between schizophrenia patients and controls (Mandl et al., 2015; Mandl et al., 2010). This means that both neuroinflammation and demyelination may affect the MTR signal, and as such additional input is required in order to distinguish between the two. In general, findings from MTR studies in schizophrenia vary, with some studies finding increased MTR and some finding decreased MTR (Kubicki et al., 2005; Mandl et al., 2015), complicating further the ability to relate MTR changes to a specific pathology that might be present in schizophrenia. Nevertheless, since MT is likely sensitive to neuroinflammation, emerging techniques (see below) could further increase its utility for the identification of neuroinflammation.
Emerging T1 techniques
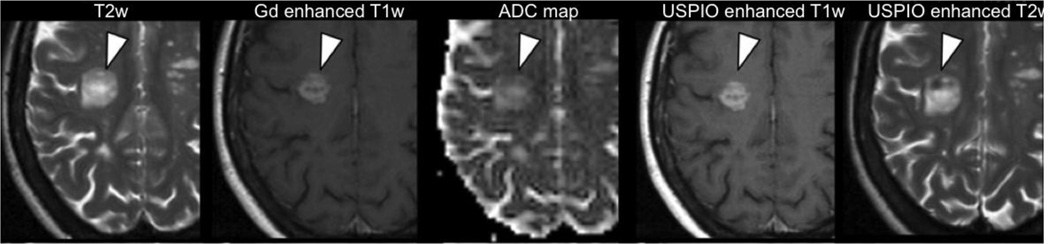
In recent years ultra-small superparamagnetic particles of iron oxide (USPIO) have been developed for clinical MRI as contrast agents that complement Gd (Corot et al., 2006). The iron oxide core of the USPIO induces a signal increase in T1-weighted images and increased T2-weighted contrast. The enhanced contrast visualizes infiltration of macrophages as an aspect of neuroinflammation that may be independent of BBB breakdown (Deddens et al., 2012). Studies in tumors (e.g., Seyfer et al., 2014; Taschner et al., 2005), and in Multiple Sclerosis (Tourdias and Dousset, 2013; Vellinga et al., 2008) show that USPIO provides more enhancement than Gd in lesions, and therefore USPIO may identify more subtle vascular integrity changes than Gd (Fig. 4). With the maturation in this technology, as well as other molecular imaging technologies aimed at identifying neuroinflammation (Pulli and Chen, 2014), these advances will likely become increasingly relevant for understanding the type and role of neuroinflammation in schizophrenia.
Figure 4. Identifying neuroinflammation with MRI.
T2-weighted (T2w), Gd enhanced T1-weighted (T1w), apparent diffusion coefficient (ADC) maps, ultra-small superparamagnetic particles of iron oxide (USPIO) enhanced T1w, and USPIO enhanced T2w maps from a relapsing-remitting multiple sclerosis patient demonstrate a large inflammatory lesion. The Gd enhancement reflects BBB breakdown, associated with USPIO enhancement (i.e., hyperintensity in T1w, and hypointensity in T2w) reflecting concomitant macrophage invasion. At the same time, increased ADC and T2 suggest co-occurrence of vasogenic edema. Figure modified with permission from Tourdias and Dousset (2013).
More elaborate acquisitions of MT with variable off-resonance frequencies are emerging, and these acquisitions enable quantitative models for MT, also called cross-relaxation imaging (Cercignani et al., 2005; Yarnykh, 2002; Yarnykh and Yuan, 2004). Such models then provide separate estimations of the cross-relaxation rate between the two pools, as well as the volume fraction of each pool. The volume fraction can also be estimated from a set of t1-weighted images with varying flip-angle (Mezer et al., 2013). Three pool models may also increase the robustness of parameter estimation (Mossahebi et al., 2014). A recent study evaluated the utility of quantitative MT to identify neuroinflammation (Harrison et al., 2014). In that study exposing subjects to typhoid vaccination experimentally induced low-grade neuroinflammation. As a result, a significant increase in the MT exchange rate was found, consistent with an increase in hydrophilic macromolecules. This finding highlights quantitative MT as a promising modality for the identification of neuroinflammation, which could be applied to schizophrenia patients as well. It should be noted, however, that the findings in Harrison et al. (Harrison et al., 2014) do not support increased water content, likely because the experimentally induced neuroinflammation was acute. This again underscores the need for longitudinal studies that might identify the expected trajectory of neuroinflammation for the acute and chronic stages.
T2 based contrasts
Unlike T1, T2-weighted images are used routinely in the clinic to identify edema, which is a severe case of neuroinflammation. Edema appears hyperintense comparing to healthy brain tissue (Barnes et al., 1987). This hyperintensity is explained by the increased water content (Claudio et al., 1990), and by the fact that water has higher T2 than brain tissue. Fluid attenuation inversion recovery (FLAIR) can further highlight such hyperintensities, as well as separate them from the similarly high T2-weighted signal of CSF (Husstedt et al., 2000). As a result, T2-weighted, or FLAIR images, are widely used to identify edema in tumors, MS (Fig. 4), stroke, and in brain injuries (Castillo and Mukherji, 2000).
In mild cases of neuroinflammation, the increased contrast may not be visible to the naked eye, but it can be identified using statistical methods. However, comparing images between groups requires a quantitative measure, where the T2-weighted and FLAIR images are unit-less and have an arbitrary scale that is likely different across acquisitions. However, quantitative T2 images can be extracted by acquiring a series of T2-weighted images that differ by their echo time (TE). The series of images can then be fitted to an exponential function, with the T2 value as its free-parameter (Haacke et al., 1999). Since the T2 of water is higher than that of tissue, it is expected that in neuroinflammation, as in edema, higher T2 values will be found. Quantitative T2 is therefore another viable option for identifying neuroinflammation (Jacobs et al., 2012).
T2 studies in schizophrenia
Increased T2 has been found in several schizophrenia studies (Andreasen et al., 1991; Du et al., 2012; Pfefferbaum et al., 1999; Supprian et al., 1997; Williamson et al., 1992), and these increases have been hypothesized to be related to neuroinflammation (Pfefferbaum et al., 1999). However, there are many other causes that could increase T2, such as changes in iron, molecular changes in the tissue composition, etc. It is possible to extract more information from the T2 decay by fitting the curve to multiple exponential compartments (Dula et al., 2010), or to other models of T2 distributions (Ropele et al., 2011). These models might potentially explain better the source of T2 changes, yet they require lengthy acquisition time, and depend upon elaborated mathematical fittings that are not always stable. Nevertheless, quantitative T2 may become an important modality for identifying neuroinflammation.
IDENTIFYING NEUROINFLAMMATION WITH DIFFUSION MRI
T2-weighted contrast is also affected by self-diffusion, i.e., Brownian motion, of water molecules. While the interference of diffusion in the T2 acquisition may be negligible, in an adaptation of a T2 acquisition sequence it is possible to measure the signal attenuation attributed to diffusion (Price, 1997). In general, this can be achieved by adding diffusion-sensitizing gradients, which enhance the attenuation caused by the displacement of water molecules along the direction of the gradient (Stejskal and Tanner, 1965). The diffusion MRI signal is a function of the average displacement of all water molecules within a voxel, which, in turn, depends on the diffusion coefficient as well as the geometry of the space in which the molecules traverse during the experiment time -- typically shorter than a few tens milliseconds. This makes the signal sensitive to micron scale displacement of water, detecting geometrical structures in the scale of cellular tissue (Szafer et al., 1995).
Through mathematical modeling, the diffusion signal can be related to the apparent diffusion coefficient (ADC), which depends on the actual diffusion coefficient as well as the geometry of the scanned object. Additional parameters can be extracted with the diffusion tensor imaging (DTI) model (Basser et al., 1994), which requires at least six differently oriented measurements, fitted to a diffusion tensor. Parameters calculated from the diffusion tensor include mean diffusivity (MD), which is the 3D equivalent of ADC, and fractional anisotropy (FA), which measures the orientation dependent variance of the diffusion coefficient, i.e., high in elongated shapes, and low in round shapes (Pierpaoli et al., 1996). Diffusion imaging and especially DTI have been found to be useful in identifying multiple brain disorders, mainly focused on white matter, where FA provides unique information about directionality and consistency of white matter fibers (Assaf and Pasternak, 2008).
Diffusion MRI for the identification of increased water content
The utility of diffusion MRI to identify neuroinflammation is similar to that of T2, where increased water volume indirectly detects neuroinflammation (Alexander et al., 2007). However, unlike T2, which identifies changes in molecular interactions, the diffusion MRI signal detects the geometrical changes that are caused by excessive water entering the tissue from blood vessels, which, in turn, enlarge the extracellular space (Sykova and Nicholson, 2008). More specifically, vasogenic edema can be detected in DTI as having higher MD than tissue, (Fig. 4) and, usually, due to partial volume with restricted tissue, lower MD than that of CSF. Similarly, it is expected that milder cases of neuroinflammation will show increased MD. The MD of edema, much like CSF, is higher than that of tissue, since the water molecules are relatively free to diffuse, whereas water molecules in tissue are hindered or restricted by membranes and other tissue structures (Assaf and Pasternak, 2008; Pasternak et al., 2009). Diffusion MRI can thus indirectly detect neuroinflammation by geometry changes caused by excessive water.
Diffusion MRI in schizophrenia
Increased diffusivities are robust and ubiquitous findings in neurodegenerative diseases (Agosta et al., 2011; Assaf and Pasternak, 2008; Inglese and Bester, 2010; Seppi and Poewe, 2010; Shenton et al., 2012), which are likely associated with neuroinflammation. Diffusion MRI is increasingly a very popular modality for schizophrenia studies, mainly because white matter abnormalities are often reported (e.g., see reviews in Fitzsimmons et al., 2013; Kubicki et al., 2007). The most studied parameter is FA, which is usually lower in schizophrenia, and thought to convey less organization or integrity in white matter bundles. In addition, increased ADC or MD is also often reported in schizophrenia studies. Some of these studies have hypothesized that increased MD may be due to processes related to neuroinflammation, such as increased extracellular space (DeLisi et al., 2006), and also associated with elevations in the proinflammatory cytokine IL-6 (Garver et al., 2008; Garver et al., 2003). Interestingly, Garver et al. reported findings of increased MD during psychosis (Garver et al., 2008) that was partially reversed following drug treatment, further supporting an inflammatory process that was partially resolved. In this context it is worth noting that even though MD is considered sensitive to neuroinflammation, FA decreases may be explained by increased MD, since the two variables are not mathematically independent (Pasternak et al., 2009). Therefore part of the ubiquitous finding of decreased FA in the schizophrenia literature may also be related to increased extracellular space and neuroinflammation.
Diffusion MRI, and its DTI model, remain a powerful and important modality for identifying subtle abnormalities, yet their utility for the identification of neuroinflammation is equivocal. This is mainly due to the lack of specificity of FA and MD measures, which may be sensitive to neuroinflammation but also to many other geometrical changes that occur in tissue, such as demyelination, changes in the organization of fibers, partial volume effects and membrane permeability (Alexander et al., 2007; Assaf and Pasternak, 2008; Jones et al., 2013). Care also must be taken in the acquisition and analysis of diffusion MRI data in order to avoid biases and artifacts that may appear as signs of neuroinflammation (Jones and Cercignani, 2010; O'Donnell and Pasternak, 2015).
Emerging diffusion MRI techniques to identify increased water content
A recent technique, the free-water model, enhances DTI by explicitly modeling the contribution of extracellular free-water, or water molecules that are away from tissue membranes and are free to diffuse, i.e., have isotropic diffusion with a diffusion coefficient of water in body temperature (Pasternak et al., 2009). Initially purported to detect edema (Pasternak et al., 2009), this free-water model monitors changes in the fractional volume of the extracellular free-water, which is likely to be a more direct method for identifying neuroinflammation than are diffusivities (Fig. 5). This is illustrated in a recent free-water study (Fig. 6) of a cohort of first-episode schizophrenia that showed extensive increases in the free-water fractional volume covering large areas of the brain, in both gray and white matter (Pasternak et al., 2012b). When using this same free-water method in a chronic schizophrenia population, the method showed a limited extent of increased free-water, and instead showed more extensive decreases in FA corrected for free-water, which likely indicate neurodegeneration (Pasternak et al., 2015). These two studies are consistent with the PET studies (Doorduin et al., 2009; van Berckel et al., 2008) and a DTI study by Garver et al. (Garver et al., 2008), reviewed above, which show more signs of neuroinflammation in the early stages of the disorder.
Figure 5. Distinguishing neuroinflammation from neurodegeneration.
Both neuroinflammation and neurodegeneration can cause similar changes in water content that cannot be distinguished using measures such as FA and MTR. By explicitly modeling free-water in the extracellular space it is possible to estimate the extracellular volume, providing separate identification of neuroinflammatory changes in the extracellular space and neurodegenerative changes affecting tissue.
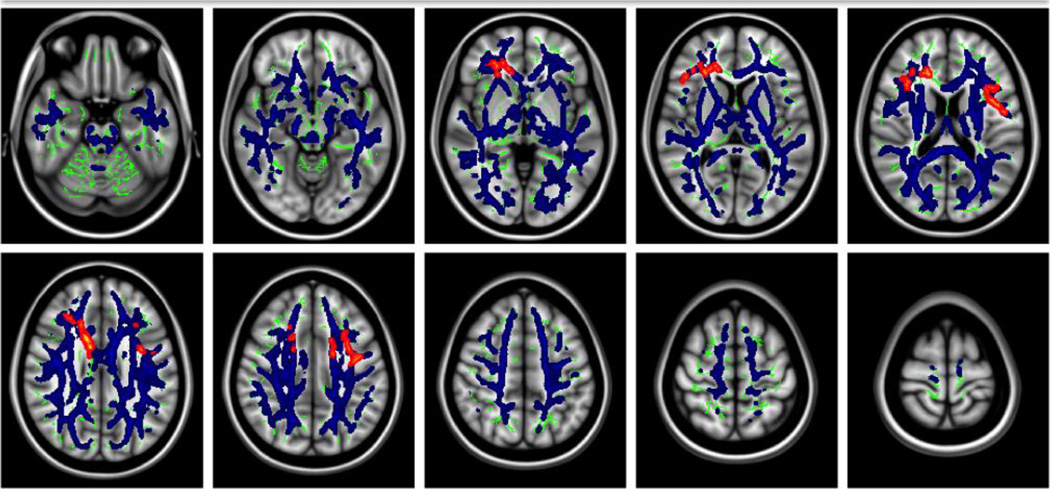
Figure 6. Identifying neuroinflammation with diffusion MRI.
Statistical comparison of free-water maps, derived from diffusion MRI, between first-episode schizophrenia patients and matching controls revealed global significant increase (blue) in extracellular free-water volume. Significant changes in the tissue compartment (red), were found in limited areas in the frontal lobe, which could suggest axonal degeneration. Extracellular free-water volume is an emerging marker that is likely related to neuroinflammation, and enhances current diffusion MRI measures. Figure modified with permission from Pasternak et al. (2012b).
The free-water method requires the same data that a DTI acquisition requires (Pasternak et al., 2009), yet other emerging techniques such as multi-shell based free-water (Hoy et al., 2014; Pasternak et al., 2012a), diffusion basis spectrum imaging (DBSI) (Wang et al., 2011), and neurite orientation dispersion and density imaging (NODDI) (Zhang et al., 2012) may provide an even better evaluation of the extracellular space, providing that more elaborate acquisitions are available. These methods could potentially increase both sensitivity and specificity to extracellular changes. Nevertheless, care must be taken in interpreting extracellular changes as surrogate markers of neuroinflammation. For example, reduction in cell density, which is one of the main signs of atrophy, is also expected to increase extracellular space. In schizophrenia, atrophy is expected to be present, especially in the chronic stages, making distinctions between atrophy and neuroinflammation challenging. For example, in the study of Pasternak et al., reviewed above (Pasternak et al., 2015), the limited increases in extracellular space observed in chronic subjects may be attributed to reduced cell density or to neuroinflammation. More specific measures of neuroinflammation may become available through other emerging diffusion MRI methods that include non-traditional gradient designs, such as double pulsed-field-gradients (double PFG) (Shemesh et al., 2010), multiple PFG (Avram et al., 2013), and q-space trajectory imaging (QTI) (Westin et al., 2014). These methods can further disentangle the contribution of various changes that may appear as increased extracellular space, such as atrophy and permeability effects (Nilsson et al., 2013), by explicitly modeling an extracellular component (Morozov et al., 2013), or by identifying the underlying components that are responsible for variability in the diffusion measures (Westin et al., 2014). These latter measures are not yet available in clinical settings, yet preliminary pilot clinical studies indicate that they may become available in the coming years.
DISCUSSION
Neuroinflammatory responses trigger a complicated cascade of processes yielding a variety of subtle biological, chemical, and physical changes that can all influence imaging signal. It is thus specificity to neuroinflammation that remains the main issue, and also currently limits the interpretation of findings. As a consequence there are very few imaging studies that attempt to directly identify neuroinflammation in schizophrenia. The limited available studies do not provide definite evidence for neuroinflammation (Filiou et al., 2014). However, based on the literature to-date, it can be concluded that if neuroinflammation occurs in schizophrenia it is subtle, especially comparing to other neurodegenerative disorders, and it is more likely to occur in the early stages of the disorder (Pasternak et al., 2015).
The imaging methods covered in this review included PET, MRS, anatomical MRI and diffusion MRI. These methods are also the leading clinically available in vivo and minimally or non-invasive imaging modalities used today. While none of these methods offers an optimal tool for identifying neuroinflammation, emerging technological advances hold promising possibilities. PET currently provides the most direct measures, since the ligands used are specifically designed to identify activated microglia (Pulli and Chen, 2014). Yet, the sensitivity in human studies is under debate, and PET exposes subjects to ionizing radiation. MRS measures do not expose subjects to ionizing radiation, and have relatively high specificity in terms of being able to target metabolites that are in high concentration within glia cells. Yet, poor spatial resolution, and limited coverage of the brain, reduce sensitivity to subtle neuroinflammatory responses. On the other hand, anatomical MRI, and diffusion MRI, are likely more sensitive to changes associated with neuroinflammation, yet their specificity is not as good as that of PET and MRS, since these methods are very sensitive to many types of tissue changes.
In light of the limitations of current technology, the emerging techniques reviewed here are important to facilitate imaging of neuroinflammation, and to improving the specificity and sensitivity of current state-of-the-art. The identification of neuroinflammation may also be improved with multi-modal acquisitions, which combine the benefits of different imaging modalities targeting different aspects of the neuroinflammatory response, such as microglial activation, astroglial and microglial density, myelin destruction, and water content. For example, combinations that hold the most promise include MT with diffusion MRI (Mandl et al., 2015; Mandl et al., 2010), T2 with MRS (Ongur et al., 2010), and MRS with diffusion MRI (Du et al., 2013). Combining information across different imaging modalities is also important in order to understand further the relation between neuroinflammation and other well-documented abnormalities in brains of schizophrenia subjects, such as axonal pathology and oligodendroglia pathology (Najjar and Pearlman, 2015). Here, emerging technologies for the imaging of these additional pathologies (e.g., Oguz et al., 2009) show the most promise.
It is also important to interpret the different imaging modality results within the context of schizophrenia, as measured with clinical or cognitive measures. It is likely that patients in different stages of schizophrenia will show a different extent of changes, and thus care should be taken with study designs to include homogeneous populations. Other measures of importance are blood markers and CSF measures that may support the presence of neuroinflammation in the brain (Miller et al., 2011). For example, emerging blood essay techniques show promise in identifying the occurrence of neuroinflammation with increased specificity (Yeste and Quintana, 2013), and combined with imaging, the extent of neuroinflammation could be validated as well. Further, the interaction of changes observed by the imaging modalities and the effects of medications is a concern, as medications (both anti-psychotic and non-psychotic) likely induce subtle changes that are detectable with imaging, and may overlap with surrogate imaging markers of neuroinflammation (Kirkpatrick and Miller, 2013). Complicating things further is the fact that many of the medicines prescribed to patients diagnosed with schizophrenia may have anti-inflammatory effects (Sommer et al., 2012; Sommer et al., 2014). Thus, care must be taken when interpreting results in medicated patients.
Another issue involved in the development of imaging techniques is the critical need to have appropriate validation methods. This is an issue, since currently validation of neuroinflammation is done via ex vivo histopathology. However, if neuroinflammation in schizophrenia is expected mainly in the early stages of the disorder, ex vivo studies become more improbable (Steiner et al., 2008). Further, with the lack of animal models for schizophrenia, validation of imaging methods can still be conducted with animal models of neuroinflammation. A common model is the injection of lipopolysaccharide (LPS), such as purified endotoxins from Escherichia coli, or Salmonella abortus equi, or typhoid vaccine containing endotoxin from S. typhi, which induces robust microglia activation (Andersson et al., 1992; Espinosa-Oliva et al., 2013; Montero-Menei et al., 1994). The LPS model was used to demonstrate the sensitivity of different PET ligands to neuroinflammation, further validated the use of histological markers that coincide with the PET signal (e.g., Dickens et al., 2014; Dobos et al., 2012; Liraz-Zaltsman et al., 2011). The LPS model has also been applied to other imaging modalities (e.g., Lodygensky et al., 2014; Moshkin et al., 2012; van de Looij et al., 2012), including a small number of human studies (e.g., Harrison et al., 2014; also see review in Schedlowski et al., 2014). Such models could also be used to validate other imaging modalities, although some critics argue that LPS induces much higher levels of microglial activations than are expected in neurodegenerative disorders (Streit et al., 2014). Technologies to validate imaging results using histology are being developed (Budde et al., 2011; Dauguet et al., 2007; Meyer et al., 2006) and, in combination with an appropriate neuroinflammatory model, may provide an essential validation tool.
Additional challenges in the identification of neuroinflammation stem from the analysis methods used to identify abnormalities. Specifically, since current imaging modalities often provide noisy estimations, group comparisons are necessary to demonstrate statistical significant findings. In schizophrenia, it appears that any changes induced by neuroinflammation are subtle, and in addition, we do not know whether the changes should be expected in the same brain areas across different subjects, or whether only a subset of patients present neuroinflammatory markers. If spatial overlap between subjects is not sufficient, findings in any modality may not have sufficient statistical power. New analysis techniques are now emerging in which each individual subject is statistically compared to an atlas composed of a cohort of controls (e.g., Bouix et al., 2013; Pasternak et al., 2014; White et al., 2013; White et al., 2009). With such approaches, it is possible to construct group analyses that tolerate low or even no spatial overlap between subjects. Such approaches could improve dramatically the sensitivity of existing and emerging methods, as well as lay a path towards imaging modalities as a personal diagnostic tool, as well as a tool to monitor treatment efficacy in individual patients over time.
As the neuroinflammation hypothesis in schizophrenia gains popularity, and as technologies improve, the number of imaging studies is bound to increase dramatically in the coming years. The emerging imaging techniques thus offer a new playing field and new armamentarium for understanding further the role of neuroinflammation in schizophrenia. This new focus can lead to possible early intervention at the onset or even in the prodromal stage, in order to curb the progression of brain changes that lead to neurodegenerative pathology and chronic schizophrenia. Neuroinflammation may be the first sign that, if addressed sufficiently early in the course of illness, or even in the prodromal stage, may halt the progression to chronicity and perhaps lead to possible recovery, with a new focus on anti-inflammatory agents.
ACKNOWLEDGMENTS
This work was partially funded by grants from the NIH (nos. R01MH102377-01, R01MH074794, P41RR013218, and P41EB015902) and by a VA Merit Award (MES). OP was partially supported by a NARSAD (National Alliance for Research on Schizophrenia and Depression) Young Investigator grant from the Brain & Behavior Research Foundation.
Role of funding source
The identified funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that there are no competing, financial, or potential conflicts of interests.
Contributors
Ofer Pasternak performed literature search and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
REFERENCES
- Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, Filippi M. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258(3):853–863. doi: 10.1148/radiol.10101284. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34(2):76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Amaral AI, Meisingset TW, Kotter MR, Sonnewald U. Metabolic aspects of neuron-oligodendrocyte-astrocyte interactions. Frontiers in endocrinology. 2013;4:54. doi: 10.3389/fendo.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Maes M. Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:5–19. doi: 10.1016/j.pnpbp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues. Neuroscience. 1992;48(1):169–186. doi: 10.1016/0306-4522(92)90347-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Ehrhardt JC, Swayze VW, Tyrrell G, 2nd, Cohen G, Ku JS, Arndt S. T1 and T2 relaxation times in schizophrenia as measured with magnetic resonance imaging. Schizophrenia research. 1991;5(3):223–232. doi: 10.1016/0920-9964(91)90080-b. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of molecular neuroscience : MN. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Avram AV, Ozarslan E, Sarlls JE, Basser PJ. In vivo detection of microscopic anisotropy using quadruple pulsed-field gradient (qPFG) diffusion MRI on a clinical scanner. NeuroImage. 2013;64:229–239. doi: 10.1016/j.neuroimage.2012.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, McDonald WI, Johnson G, Tofts PS, Landon DN. Quantitative nuclear magnetic resonance imaging: characterisation of experimental cerebral oedema. Journal of neurology, neurosurgery, and psychiatry. 1987;50(2):125–133. doi: 10.1136/jnnp.50.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, Leenders KL. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson's disease? Parkinsonism & related disorders. 2010;16(1):57–59. doi: 10.1016/j.parkreldis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gould MK, Barnett PG. The cost of positron emission tomography in six United States Veterans Affairs hospitals and two academic medical centers. AJR. American journal of roentgenology. 2003;181(2):359–365. doi: 10.2214/ajr.181.2.1810359. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuroinflammation and the dynamic lesion in traumatic brain injury. Brain : a journal of neurology. 2013;136(Pt 1):9–11. doi: 10.1093/brain/aws342. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Bruck W. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR. American journal of neuroradiology. 1999;20(9):1619–1627. [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox; or, The group of schizophrenias. International Universities Press; 1950. [Google Scholar]
- Bouix S, Pasternak O, Rathi Y, Pelavin PE, Zafonte R, Shenton ME. Increased gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. PloS one. 2013;8(6):e66205. doi: 10.1371/journal.pone.0066205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Annals of neurology. 1997;42(5):783–793. doi: 10.1002/ana.410420515. [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain : a journal of neurology. 2011;134(Pt 8):2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M, Mukherji SK. Clinical applications of FLAIR, HASTE, and magnetization transfer in neuroimaging. Seminars in ultrasound, CT, and MR. 2000;21(6):417–427. doi: 10.1016/s0887-2171(00)90034-9. [DOI] [PubMed] [Google Scholar]
- Cercignani M, Symms MR, Schmierer K, Boulby PA, Tozer DJ, Ron M, Tofts PS, Barker GJ. Three-dimensional quantitative magnetisation transfer imaging of the human brain. NeuroImage. 2005;27(2):436–441. doi: 10.1016/j.neuroimage.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(3):576–593. doi: 10.1007/s11481-013-9460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35(12):2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- Chew LJ, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci. 2013;35(2–3):102–129. doi: 10.1159/000346157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry research. 2004;130(1):71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Claudio L, Kress Y, Factor J, Brosnan CF. Mechanisms of edema formation in experimental autoimmune encephalomyelitis. The contribution of inflammatory cells. The American journal of pathology. 1990;137(5):1033–1045. [PMC free article] [PubMed] [Google Scholar]
- Claussen C, Laniado M, Schorner W, Niendorf HP, Weinmann HJ, Fiegler W, Felix R. Gadolinium-DTPA in MR imaging of glioblastomas and intracranial metastases. AJNR. American journal of neuroradiology. 1985;6(5):669–674. [PMC free article] [PubMed] [Google Scholar]
- Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Dauguet J, Peled S, Berezovskii V, Delzescaux T, Warfield SK, Born R, Westin CF. Comparison of fiber tracts derived from in-vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. NeuroImage. 2007;37(2):530–538. doi: 10.1016/j.neuroimage.2007.04.067. [DOI] [PubMed] [Google Scholar]
- Deddens LH, Van Tilborg GA, Mulder WJ, De Vries HE, Dijkhuizen RM. Imaging neuroinflammation after stroke: current status of cellular and molecular MRI strategies. Cerebrovasc Dis. 2012;33(4):392–402. doi: 10.1159/000336116. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, King AC, Targum S. Serum immunoglobulin concentrations in patients admitted to an acute psychiatric in-patient service. The British journal of psychiatry : the journal of mental science. 1984;145:661–665. doi: 10.1192/bjp.145.6.661. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch H, Majcher M, Brown K, Bappal A, Branch CA, Ardekani BA. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry research. 2006;148(1):61–66. doi: 10.1016/j.pscychresns.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010;6(6):328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- Dickens AM, Vainio S, Marjamaki P, Johansson J, Lehtiniemi P, Rokka J, Rinne J, Solin O, Haaparanta-Solin M, Jones PA, Trigg W, Anthony DC, Airas L. Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F–GE-180. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(3):466–472. doi: 10.2967/jnumed.113.125625. [DOI] [PubMed] [Google Scholar]
- Dobos N, de Vries EF, Kema IP, Patas K, Prins M, Nijholt IM, Dierckx RA, Korf J, den Boer JA, Luiten PG, Eisel UL. The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J Alzheimers Dis. 2012;28(4):905–915. doi: 10.3233/JAD-2011-111097. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Du F, Cooper A, Cohen BM, Renshaw PF, Ongur D. Water and metabolite transverse T2 relaxation time abnormalities in the white matter in schizophrenia. Schizophrenia research. 2012;137(1–3):241–245. doi: 10.1016/j.schres.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Ongur D. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biological psychiatry. 2013;74(6):451–457. doi: 10.1016/j.biopsych.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dula AN, Gochberg DF, Valentine HL, Valentine WM, Does MD. Multiexponential T2, magnetization transfer, and quantitative histology in white matter tracts of rat spinal cord. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;63(4):902–909. doi: 10.1002/mrm.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, de Pablos RM, Herrera AJ. Intracranial injection of LPS in rat as animal model of neuroinflammation. Methods Mol Biol. 2013;1041:295–305. doi: 10.1007/978-1-62703-520-0_26. [DOI] [PubMed] [Google Scholar]
- Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neuroscience and biobehavioral reviews. 2014;38:72–93. doi: 10.1016/j.neubiorev.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiou MD, Arefin AS, Moscato P, Graeber MB. 'Neuroinflammation' differs categorically from inflammation: transcriptomes of Alzheimer's disease, Parkinson's disease, schizophrenia and inflammatory diseases compared. Neurogenetics. 2014;15(3):201–212. doi: 10.1007/s10048-014-0409-x. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biological psychiatry. 2013;73(10):951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Current opinion in psychiatry. 2013;26(2):172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Fontana A, Frei K, Bodmer S, Hofer E. Immune-mediated encephalitis: on the role of antigen-presenting cells in brain tissue. Immunol Rev. 1987;100:185–201. doi: 10.1111/j.1600-065X.1987.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Rabin BS, Kelly RH, Lyte M, Ragu U. Clinical and laboratory evidence of autoimmunity in acute schizophrenia. Annals of the New York Academy of Sciences. 1987;496:676–685. doi: 10.1111/j.1749-6632.1987.tb35829.x. [DOI] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2008;11(1):49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- Garver DL, Tamas RL, Holcomb JA. Elevated interleukin-6 in thecerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28(8):1515–1520. doi: 10.1038/sj.npp.1300217. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiology of disease. 2006;21(2):404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. NeuroImage. 2005;24(2):591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Grossman RI, Galetta S, Atlas SW, Silberberg DH. Multiple sclerosis disease activity correlates with gadolinium-enhanced magnetic resonance imaging. Annals of neurology. 1987;21(3):300–306. doi: 10.1002/ana.410210312. [DOI] [PubMed] [Google Scholar]
- Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721–725. doi: 10.1148/radiology.161.3.3786722. [DOI] [PubMed] [Google Scholar]
- Guo Q, Owen DR, Rabiner EA, Turkheimer FE, Gunn RN. Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. NeuroImage. 2012;60(2):902–910. doi: 10.1016/j.neuroimage.2011.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. Wiley; 1999. [Google Scholar]
- Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, Cercignani M. Biological psychiatry. 2014. Quantitative Magnetization Transfer Imaging as a Biomarker for Effects of Systemic Inflammation on the Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain : a journal of neurology. 1999;1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR in biomedicine. 2001;14(2):57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. NeuroImage. 2014;103C:323–333. doi: 10.1016/j.neuroimage.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husstedt HW, Sickert M, Kostler H, Haubitz B, Becker H. Diagnostic value of the fast-FLAIR sequence in MR imaging of intracranial tumors. European radiology. 2000;10(5):745–752. doi: 10.1007/s003300050997. [DOI] [PubMed] [Google Scholar]
- Inglese M, Bester M. Diffusion imaging in multiple sclerosis: research and clinical implications. NMR in biomedicine. 2010;23(7):865–872. doi: 10.1002/nbm.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophrenia bulletin. 2010;36(2):301–313. doi: 10.1093/schbul/sbp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AH, Tavitian B, consortium IN. Noninvasive molecular imaging of neuroinflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(7):1393–1415. doi: 10.1038/jcbfm.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR in biomedicine. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Balakrishnan B, Muzik O, Romero R, Chugani D. Positron emission tomography imaging of neuroinflammation. Journal of child neurology. 2009;24(9):1190–1199. doi: 10.1177/0883073809338063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Knopman DS, Dickson DW, Parisi JE, Whitwell JL, Weigand SD, Josephs KA, Boeve BF, Petersen RC, Jack CR., Jr Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248(1):210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S, Mizrahi R. Imaging Neuroinflammation in Gray and White Matter in Schizophrenia: An In-Vivo PET Study With [18F]-FEPPA. Schizophrenia bulletin. 2015;41(1):85–93. doi: 10.1093/schbul/sbu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophrenia bulletin. 2013;39(6):1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. R. E. Krieger Pub. Co; 1971. [Google Scholar]
- Kropholler MA, Boellaard R, Schuitemaker A, van Berckel BN, Luurtsema G, Windhorst AD, Lammertsma AA. Development of a tracer kinetic plasma input model for (R)-[11C]PK11195 brain studies. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(7):842–851. doi: 10.1038/sj.jcbfm.9600092. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Journal of psychiatric research. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. NeuroImage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C, Vavasour IM, Kolind SH, Li DK, Traboulsee TL, Moore GR, MacKay AL. Magnetic resonance imaging of myelin. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):460–484. doi: 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, Ben Haim L, Lebon V, Remy P, Dolle F, Delzescaux T, Bonvento G, Hantraye P, Escartin C. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. 2012;32(32):10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Bobrow L, Lucia D, Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Current topics in behavioral neurosciences. 2010;4:243–281. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- Liraz-Zaltsman S, Alexandrovich AG, Trembovler V, Fishbein I, Yaka R, Shohami E, Biegon A. Regional sensitivity to neuroinflammation: in vivo and in vitro studies. Synapse. 2011;65(7):634–642. doi: 10.1002/syn.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygensky GA, Kunz N, Perroud E, Somm E, Mlynarik V, Huppi PS, Gruetter R, Sizonenko SV. Definition and quantification of acute inflammatory white matter injury in the immature brain by MRI/MRS at high magnetic field. Pediatr Res. 2014;75(3):415–423. doi: 10.1038/pr.2013.242. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Zhang MR, Okauchi T, Yasuno F, Ikoma Y, Inaji M, Nagai Y, Takano A, Obayashi S, Suzuki K. Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: an imaging tool for glial cells in the brain. Synapse. 2004;52(4):283–291. doi: 10.1002/syn.20027. [DOI] [PubMed] [Google Scholar]
- Makris N, Seidman LJ, Ahern T, Kennedy DN, Caviness VS, Tsuang MT, Goldstein JM. White matter volume abnormalities and associations with symptomatology in schizophrenia. Psychiatry research. 2010;183(1):21–29. doi: 10.1016/j.pscychresns.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RC, Pasternak O, Cahn W, Kubicki M, Kahn RS, Shenton ME, Hulshoff Pol HE. Comparing free water imaging and magnetization transfer measurements in schizophrenia. Schizophrenia research. 2015;161(1):126–132. doi: 10.1016/j.schres.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RC, Schnack HG, Luigjes J, van den Heuvel MP, Cahn W, Kahn RS, Hulshoff Pol HE. Tract-based analysis of magnetization transfer ratio and diffusion tensor imaging of the frontal and frontotemporal connections in schizophrenia. Schizophrenia bulletin. 2010;36(4):778–787. doi: 10.1093/schbul/sbn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield P, Morris PG. NMR imaging in biomedicine. New York: Academic Press; 1982. [Google Scholar]
- Meyer CR, Moffat BA, Kuszpit KK, Bland PL, McKeever PE, Johnson TD, Chenevert TL, Rehemtulla A, Ross BD. A methodology for registration of a histological slide and in vivo MRI volume based on optimizing mutual information. Mol Imaging. 2006;5(1):16–23. [PMC free article] [PubMed] [Google Scholar]
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Mezer A, Yeatman JD, Stikov N, Kay KN, Cho NJ, Dougherty RF, Perry ML, Parvizi J, Hua le H, Butts-Pauly K, Wandell BA. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nature medicine. 2013;19(12):1667–1672. doi: 10.1038/nm.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63(3):257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, Kanba S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115–121. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Montero-Menei CN, Sindji L, Pouplard-Barthelaix A, Jehan F, Denechaud L, Darcy F. Lipopolysaccharide intracerebral administration induces minimal inflammatory reaction in rat brain. Brain Res. 1994;653(1–2):101–111. doi: 10.1016/0006-8993(94)90377-8. [DOI] [PubMed] [Google Scholar]
- Morozov D, Bar L, Sochen N, Cohen Y. Modeling of the diffusion MR signal in calibrated model systems and nerves. NMR in biomedicine. 2013;26(12):1787–1795. doi: 10.1002/nbm.3018. [DOI] [PubMed] [Google Scholar]
- Moshkin MP, Akulov AE, Petrovskii DV, Saik OV, Petrovskii ED, Savelov AA, Koptug IV. [Magnetic resonance spectroscopy of metabolic changes in mice brain after 2-deoxy-D-glucose injection] Ross Fiziol Zh Im I M Sechenova. 2012;98(10):1264–1272. [PubMed] [Google Scholar]
- Mossahebi P, Alexander AL, Field AS, Samsonov AA. Removal of cerebrospinal fluid partial volume effects in quantitative magnetization transfer imaging using a three-pool model with nonexchanging water component. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehllehner G, Karp JS. Positron emission tomography. Physics in medicine and biology. 2006;51(13):R117–R137. doi: 10.1088/0031-9155/51/13/R08. [DOI] [PubMed] [Google Scholar]
- Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, Gruber R, Riedel M. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. European archives of psychiatry and clinical neuroscience. 2004;254(1):14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophrenia research. 2015;161(1):102–112. doi: 10.1016/j.schres.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. Journal of neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TS, Lin AP, Koerte IK, Pasternak O, Liao H, Merugumala S, Bouix S, Shenton ME. Neuroimaging in repetitive brain trauma. Alzheimer's research & therapy. 2014;6(1):10. doi: 10.1186/alzrt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Latt J, van Westen D, Brockstedt S, Lasic S, Stahlberg F, Topgaard D. Noninvasive mapping of water diffusional exchange in the human brain using filter-exchange imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;69(6):1573–1581. doi: 10.1002/mrm.24395. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Pasternak O. Does diffusion MRI tell us anything about the white matter? An overview of methods and pitfalls. Schizophrenia research. 2015;161(1):133–141. doi: 10.1016/j.schres.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz KK, Kurne A, Aksu AO, Karabulut E, Serdaroglu A, Teber S, Haspolat S, Senbil N, Kurul S, Anlar B. Assessment of citrullinated myelin by 1H–MR spectroscopy in early-onset multiple sclerosis. AJNR. American journal of neuroradiology. 2009;30(4):716–721. doi: 10.3174/ajnr.A1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, Jensen JE, Rouse ED, Cohen BM, Renshaw PF, Olson DP. T2 relaxation time abnormalities in bipolar disorder and schizophrenia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;63(1):1–8. doi: 10.1002/mrm.22148. [DOI] [PubMed] [Google Scholar]
- Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52(1):24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dincer A, Dydak U, Emir UE, Frahm J, Gonzalez RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Huppi PS, Hurd RE, Kantarci K, Klomp DW, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjanska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJ, Ratai EM, Ross BD, Scheenen TW, Schuster C, Smith IC, Soher BJ, Tkac I, Vigneron DB, Kauppinen RA, Group MRSC. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–679. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey RS, Gupta AK, Chaturvedi UC. Autoimmune model of schizophrenia with special reference to antibrain antibodies. Biological psychiatry. 1981;16(12):1123–1136. [PubMed] [Google Scholar]
- Pasternak O, Koerte IK, Bouix S, Fredman E, Sasaki T, Mayinger M, Helmer KG, Johnson AM, Holmes JD, Forwell LA, Skopelja EN, Shenton ME, Echlin PS. Hockey Concussion Education Project, Part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. Journal of neurosurgery. 2014;120(4):873–881. doi: 10.3171/2013.12.JNS132090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Shenton ME, Westin CF. Estimation of extracellular volume from regularized multi-shell diffusion MRI. Medical image computing and computer-assisted intervention : MICCAI … International Conference on Medical Image Computing and Computer-Assisted Intervention. 2012a;15(Pt 2):305–312. doi: 10.1007/978-3-642-33418-4_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;62(3):717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012b;32(48):17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Dahlben B, Bouix S, Kubicki M. The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophrenia research. 2015;161(1):113–118. doi: 10.1016/j.schres.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Moseley M, Lim KO. Brain gray and white matter transverse relaxation time in schizophrenia. Psychiatry research. 1999;91(2):93–100. doi: 10.1016/s0925-4927(99)00023-2. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ, Tabrizi SJ, Barker RA, Piccini P. Microglial activation in regions related to cognitive function predicts disease onset in Huntington's disease: a multimodal imaging study. Human brain mapping. 2011;32(2):258–270. doi: 10.1002/hbm.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port JD, Agarwal N. MR spectroscopy in schizophrenia. Journal of magnetic resonance imaging : JMRI. 2011;34(6):1251–1261. doi: 10.1002/jmri.22787. [DOI] [PubMed] [Google Scholar]
- Posse S, Otazo R, Dager SR, Alger J. MR spectroscopic imaging: principles and recent advances. Journal of magnetic resonance imaging : JMRI. 2013;37(6):1301–1325. doi: 10.1002/jmri.23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price WS. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part 1. Basic theory. Concepts in Magnetic Resonance. 1997;9(5):299–336. [Google Scholar]
- Pulli B, Chen JW. Imaging Neuroinflammation - from Bench to Bedside. J Clin Cell Immunol 5. 2014 doi: 10.4172/2155-9899.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan S, Andronesi OC, Stanwell P, Lin AP, Sorensen AG, Mountford CE. Use of in vivo two-dimensional MR spectroscopy to compare the biochemistry of the human brain to that of glioblastoma. Radiology. 2011;259(2):540–549. doi: 10.1148/radiol.11101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Annals of neurology. 2011;70(3):374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rausch M, Hiestand P, Baumann D, Cannet C, Rudin M. MRI-based monitoring of inflammation and tissue damage in acute and chronic relapsing EAE. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003;50(2):309–314. doi: 10.1002/mrm.10541. [DOI] [PubMed] [Google Scholar]
- Rigotti DJ, Inglese M, Gonen O. Whole-brain N-acetylaspartate as a surrogate marker of neuronal damage in diffuse neurologic disorders. AJNR. American journal of neuroradiology. 2007;28(10):1843–1849. doi: 10.3174/ajnr.A0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17(4):942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropele S, Langkammer C, Enzinger C, Fuchs S, Fazekas F. Relaxation time mapping in multiple sclerosis. Expert review of neurotherapeutics. 2011;11(3):441–450. doi: 10.1586/ern.10.129. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Engler H, Grigoleit JS. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav Immun. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Lindberg C, Aronsson AF, Hjorth E, Spulber SD, Oprica M. Inflammation in the nervous system--physiological and pathophysiological aspects. Physiology & behavior. 2007;92(1–2):121–128. doi: 10.1016/j.physbeh.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23(4):385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29(2):68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Schwerk A, Alves FD, Pouwels PJ, van Amelsvoort T. Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. Journal of neurochemistry. 2014;128(1):1–87. doi: 10.1111/jnc.12398. [DOI] [PubMed] [Google Scholar]
- Seppi K, Poewe W. Brain magnetic resonance imaging techniques in the diagnosis of parkinsonian syndromes. Neuroimaging clinics of North America. 2010;20(1):29–55. doi: 10.1016/j.nic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Seyfer P, Pagenstecher A, Mandic R, Klose KJ, Heverhagen JT. Cancer and inflammation: differentiation by USPIO-enhanced MR imaging. Journal of magnetic resonance imaging : JMRI. 2014;39(3):665–672. doi: 10.1002/jmri.24200. [DOI] [PubMed] [Google Scholar]
- Shemesh N, Ozarslan E, Komlosh ME, Basser PJ, Cohen Y. From single-pulsed field gradient to double-pulsed field gradient MR: gleaning new microstructural information and developing new forms of contrast in MRI. NMR in biomedicine. 2010;23(7):757–780. doi: 10.1002/nbm.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia research. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern RA, Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain imaging and behavior. 2012;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. The Journal of clinical psychiatry. 2012;73(4):414–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophrenia bulletin. 2014;40(1):181–191. doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Current neuropharmacology. 2008;6(3):179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JA, Pettegrew JW, Keshavan MS. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings--part I. Biological psychiatry. 2000;48(5):357–368. doi: 10.1016/s0006-3223(00)00949-5. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of psychiatric research. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. The Journal of Chemical Physics. 1965;42(1):288–292. [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. Journal of neuroinflammation. 2004;1(1):14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Xue QS, Tischer J, Bechmann I. Microglial pathology. Acta Neuropathol Commun. 2014;2:142. doi: 10.1186/s40478-014-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supprian T, Hofmann E, Warmuth-Metz M, Franzek E, Becker T. MRI T2 relaxation times of brain regions in schizophrenic patients and control subjects. Psychiatry research. 1997;75(3):173–182. doi: 10.1016/s0925-4927(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiological reviews. 2008;88(4):1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafer A, Zhong J, Anderson AW, Gore JC. Diffusion-weighted imaging in tissues: theoretical models. NMR in biomedicine. 1995;8(7–8):289–296. doi: 10.1002/nbm.1940080704. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Ashtari M, Zito J, Degreef G, Bogerts B, Lieberman J. Gadolinium-DTPA enhanced gradient echo magnetic resonance scans in first episode of psychosis and chronic schizophrenic patients. Psychiatry research. 1991;40(3):203–207. doi: 10.1016/0925-4927(91)90011-e. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y, Suhara T. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13(7):943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- Taschner CA, Wetzel SG, Tolnay M, Froehlich J, Merlo A, Radue EW. Characteristics of ultrasmall superparamagnetic iron oxides in patients with brain tumors. AJR. American journal of roentgenology. 2005;185(6):1477–1486. doi: 10.2214/AJR.04.1286. [DOI] [PubMed] [Google Scholar]
- Thiel A, Radlinska BA, Paquette C, Sidel M, Soucy JP, Schirrmacher R, Minuk J. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(9):1404–1412. doi: 10.2967/jnumed.110.076612. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85(10):2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Peterson MR. Slow and latent viruses in schizophrenia. Lancet. 1973;2(7819):22–24. doi: 10.1016/s0140-6736(73)91952-1. [DOI] [PubMed] [Google Scholar]
- Tourdias T, Dousset V. Neuroinflammatory imaging biomarkers: relevance to multiple sclerosis and its therapy. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10(1):111–123. doi: 10.1007/s13311-012-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer FE, Edison P, Pavese N, Roncaroli F, Anderson AN, Hammers A, Gerhard A, Hinz R, Tai YF, Brooks DJ. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48(1):158–167. [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van de Looij Y, Lodygensky GA, Dean J, Lazeyras F, Hagberg H, Kjellmer I, Mallard C, Huppi PS, Sizonenko SV. High-field diffusion tensor imaging characterization of cerebral white matter injury in lipopolysaccharide-exposed fetal sheep. Pediatr Res. 2012;72(3):285–292. doi: 10.1038/pr.2012.72. [DOI] [PubMed] [Google Scholar]
- Vartanian ME, Kolyaskina GI, Lozovsky DV, Burbaeva GS, Ignatov SA. Aspects of humoral and cellular immunity in schizophrenia. Birth defects original article series. 1978;14(5):339–364. [PubMed] [Google Scholar]
- Vavasour IM, Laule C, Li DK, Traboulsee AL, MacKay AL. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? Journal of magnetic resonance imaging : JMRI. 2011;33(3):713–718. doi: 10.1002/jmri.22441. [DOI] [PubMed] [Google Scholar]
- Vellinga MM, Oude Engberink RD, Seewann A, Pouwels PJ, Wattjes MP, van der Pol SM, Pering C, Polman CH, de Vries HE, Geurts JJ, Barkhof F. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain : a journal of neurology. 2008;131(Pt 3):800–807. doi: 10.1093/brain/awn009. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia. 2013;61(1):10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versijpt J, Debruyne JC, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler. 2005;11(2):127–134. doi: 10.1191/1352458505ms1140oa. [DOI] [PubMed] [Google Scholar]
- Vogel M, Meier J, Gronke S, Waage M, Schneider W, Freyberger HJ, Klauer T. Differential effects of childhood abuse and neglect: mediation by posttraumatic distress in neurotic disorder and negative symptoms in schizophrenia? Psychiatry research. 2011;189(1):121–127. doi: 10.1016/j.psychres.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, Tu TW, Trinkaus K, Klein RS, Cross AH, Song SK. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology. 2011;134(Pt 12):3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Selkoe DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420(6917):879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- Westin CF, Szczepankiewicz F, Pasternak O, Ozarslan E, Topgaard D, Knutsson H, Nilsson M. Measurement tensors in diffusion MRI: generalizing the concept of diffusion encoding. Medical image computing and computer-assisted intervention : MICCAI … International Conference on Medical Image Computing and Computer-Assisted Intervention. 2014;17(Pt 3):209–216. doi: 10.1007/978-3-319-10443-0_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Ehrlich S, Ho BC, Manoach DS, Caprihan A, Schulz SC, Andreasen NC, Gollub RL, Calhoun VD, Magnotta VA. Spatial characteristics of white matter abnormalities in schizophrenia. Schizophrenia bulletin. 2013;39(5):1077–1086. doi: 10.1093/schbul/sbs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Schmidt M, Karatekin C. White matter 'potholes' in early-onset schizophrenia: a new approach to evaluate white matter microstructure using diffusion tensor imaging. Psychiatry research. 2009;174(2):110–115. doi: 10.1016/j.pscychresns.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P, Pelz D, Merskey H, Morrison S, Karlik S, Drost D, Carr T, Conlon P. Frontal, temporal, and striatal proton relaxation times in schizophrenic patients and normal comparison subjects. The American journal of psychiatry. 1992;149(4):549–551. doi: 10.1176/ajp.149.4.549. [DOI] [PubMed] [Google Scholar]
- Yarnykh VL. Pulsed Z-spectroscopic imaging of cross-relaxation parameters in tissues for human MRI: theory and clinical applications. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2002;47(5):929–939. doi: 10.1002/mrm.10120. [DOI] [PubMed] [Google Scholar]
- Yarnykh VL, Yuan C. Cross-relaxation imaging reveals detailed anatomy of white matter fiber tracts in the human brain. NeuroImage. 2004;23(1):409–424. doi: 10.1016/j.neuroimage.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Yeste A, Quintana FJ. Antigen microarrays for the study of autoimmune diseases. Clinical chemistry. 2013;59(7):1036–1044. doi: 10.1373/clinchem.2012.194423. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Sullivan EV, Pfefferbaum A. Imaging neuroinflammation? A perspective from MR spectroscopy. Brain pathology. 2014;24(6):654–664. doi: 10.1111/bpa.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]