Abstract
Purpose
To evaluate the 12-month outcome of anti-vascular endothelial growth factor (VEGF) treatment for extrafoveal polypoidal choroidal vasculopathy (PCV).
Methods
This retrospective observational study included 32 eyes of 32 patients newly diagnosed with extrafoveal PCV (polyps located more than 500 µm from the center of the fovea). Patients were treated with intravitreal ranibizumab, bevacizumab, or both. The best-corrected visual acuity (BCVA) and central foveal thickness (CFT) at diagnosis and at 12 months were compared. Eyes were divided into two groups according to the presence of submacular hemorrhage. The BCVA in each group was compared at baseline and at 12 months.
Results
During the 12-month study period, patients received an average of 4.0 ± 1.1 anti-VEGF injections. The BCVA at baseline, three-month post-diagnosis, and 12-month post-diagnosis was 0.59 ± 0.40, 0.34 ± 0.38, and 0.38 ± 0.38, respectively. The BCVA at 12 months was significantly better than the baseline value (p = 0.002). The CFT at baseline, three-month, and 12-month post-diagnosis was 477.1 ± 194.2 µm, 214.5 ± 108.8 µm, and 229.8 ± 106.1 µm, respectively. The CFT at 12 months was significantly lower than the baseline value (p < 0.001). A significant improvement in BCVA was noted in eyes with and without submacular hemorrhage (n = 13, p = 0.032 and n = 19, p = 0.007, respectively).
Conclusions
Anti-VEGF therapy was beneficial in extrafoveal PCV, regardless of the presence of submacular hemorrhage.
Keywords: Age-related macular degeneration, Anti-vascular endothelial growth factor, Extrafoveal, Polypoidal choroidal vasculopathy, Treatment outcome
Intravitreal injection of anti-vascular endothelial growth factor (VEGF) is an effective treatment for exudative age-related macular degeneration (AMD) [1,2,3,4]. Recent reports have demonstrated that anti-VEGF therapy is also effective in treating extrafoveal exudative AMD [5,6], and one study demonstrated that the treatment outcome of anti-VEGF therapy was superior to that of laser photocoagulation in extrafoveal lesions [6].
Polypoidal choroidal vasculopathy (PCV) is a distinct entity characterized by branching vascular networks and polypoidal lesions on indocyanine green angiography (ICGA). Anti-VEGF therapy is one of the treatment options for PCV [7,8,9,10]. However, the efficacy of anti-VEGF therapy in extrafoveal PCV has not been fully elucidated, even though extrafoveal PCV is a frequent finding [10,11,12].
The purpose of the present study was to evaluate treatment outcomes in patients with newly diagnosed PCV with extrafoveal polyps treated with anti-VEGF monotherapy for 12 months. The outcomes of cases with and without submacular hemorrhage were analyzed separately.
Materials and Methods
This retrospective observational case series contained cases from a single center. The study adhered to the tenets of the Declaration of Helsinki, and the study protocol was approved by the institutional review board of Kim's Eye Hospital, Korea.
Extrafoveal PCV cases included in the study had no history of treatment prior to the 12-month anti-VEGF monotherapy. We reviewed fundus photographs and ICGA results from patients at our institution who were initially diagnosed with PCV between January 2011 and December 2012 and were followed for at least 12 months. The diagnosis of PCV was based on ICGA findings, according to the following, previously suggested criteria [13]: single/multiple focal nodular areas of hyperfluorescence from the choroidal circulation within the first six minutes after injection of ICGA with one or more features including, nodular appearance on stereoscopic view of ICGA, hypofluorescent halo surrounding the focal hyperfluorescence, association with a branching vascular network in ICGA, or association with submacular hemorrhage. In the present study, extrafoveal PCV was diagnosed when the polypoidal lesions on ICGA were located more than 500 µm from the center of the fovea. If extrafoveal PCV was diagnosed in both eyes, the eye that was affected first was included in the study.
All subjects in the study were required to undergo a comprehensive ophthalmologic examination, including measurement of best-corrected visual acuity (BCVA), 90-diopter lens slit-lamp biomicroscopy, fundus photography, fluorescein angiography, and ICGA with a confocal laser-scanning system (HRA-2; Heidelberg Engineering, Dossenheim, Germany). Horizontal and vertical cross-hair scans positioned at the center of the fovea were obtained using spectral domain optical coherence tomography (OCT; Spectral OCT/SLO, OTI Ophthalmic Technologies, Miami, FL, USA). Exclusion criteria included a follow-up period shorter than 12 months, a large lesion with a greatest linear dimension >4,500 µm, severe media opacity, previous vitreoretinal surgery, macroaneurysms, proliferative diabetic retinopathy, central retinal vascular occlusion, or any other retinal disorder that might influence macular microstructure and/or function. Eyes that underwent photodynamic therapy or vitrectomy during the 12-month follow-up period were also excluded. Eyes with submacular hemorrhage involving the fovea at the time of diagnosis were classified in the hemorrhage group, and the remaining eyes were in the non-hemorrhage group. If thick submacular hemorrhage impeded visualization of the polypoidal lesions, the case was excluded from the study. Only cases in which accurate localization of polypoidal lesions was possible were included in the hemorrhage group.
Visual acuities in each group were compared at diagnosis and at 12 months. The degree of change in visual acuity was also compared between the two groups.
All eyes in the study were initially treated with three consecutive, monthly intravitreal ranibizumab injections. Patients were subsequently examined every one to three months, as determined by the attending physician. Retreatment with an intravitreal anti-VEGF agent (either ranibizumab or bevacizumab) was performed when intraretinal, retinal, or subretinal hemorrhage developed with an accompanying increase in macular thickness.
Because volume scans were not routinely performed for every patient, central foveal thickness (CFT) was used for analysis. The CFT was defined as the distance between the internal limiting membrane and Bruch's membrane at the fovea. The CFT was manually measured using built-in calipers included in the OCT software program (OTI Ophthalmic Technologies). The greatest linear dimension of the entire PCV lesion on ICGA was measured using built-in calipers of the ICGA software program (Heidelberg Engineering).
The BCVA and CFT at diagnosis were compared with the values at three and 12 months. The BCVA at 12 months was compared between eyes with and without subfoveal retinal pigment epithelial detachment (RPED) at diagnosis. We evaluated the associations between BCVA data and the following factors: age, greatest linear dimension of the lesion, distance between the fovea and the polypoidal lesion located closest to the fovea, and number of anti-VEGF injections. In the multivariate analysis, presence of submacular hemorrhage and that of subfoveal RPED were also included as variables.
Statistics
Data are presented as mean ± standard deviation, where applicable. Statistical analyses were performed with a commercially available software package (SPSS ver. 12; SPSS Inc., Chicago, IL, USA). Differences in values between various time points were tested for statistical significance using a repeated-measures analysis of variance with Bonferroni's correction. Associations between values were analyzed using Pearson's correlation analysis and multiple linear regression analysis. Differences in values within subgroups were analyzed using the Wilcoxon signed ranks test. Differences in values between groups were analyzed using the Mann-Whitney U-test. A p-value less than 0.05 was considered statistically significant.
Results
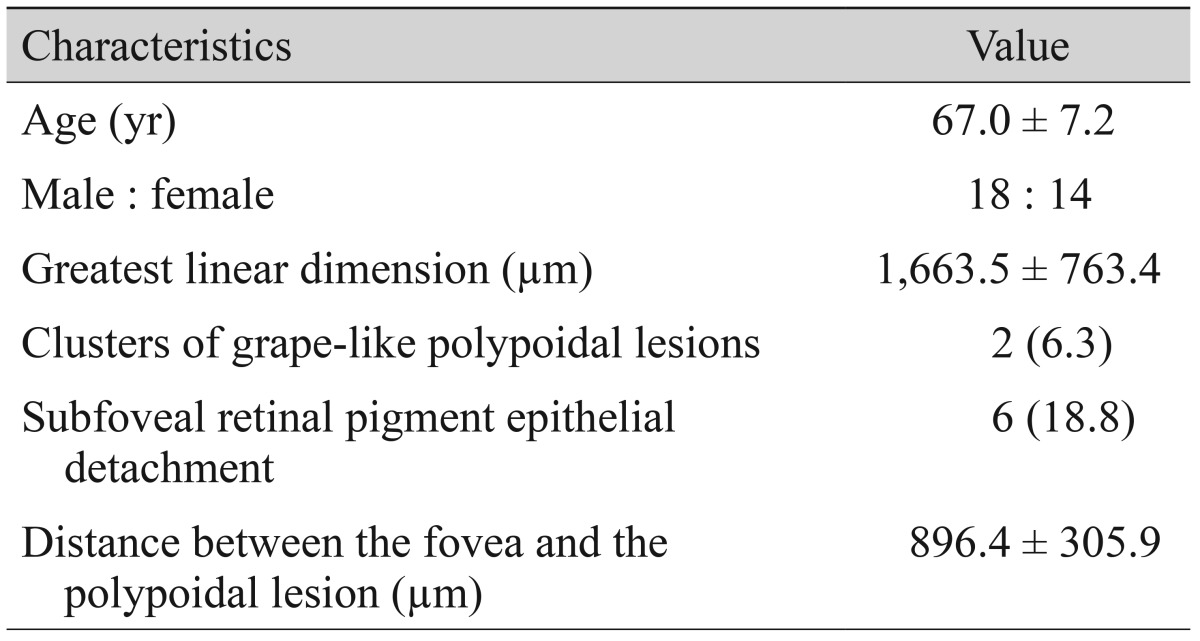
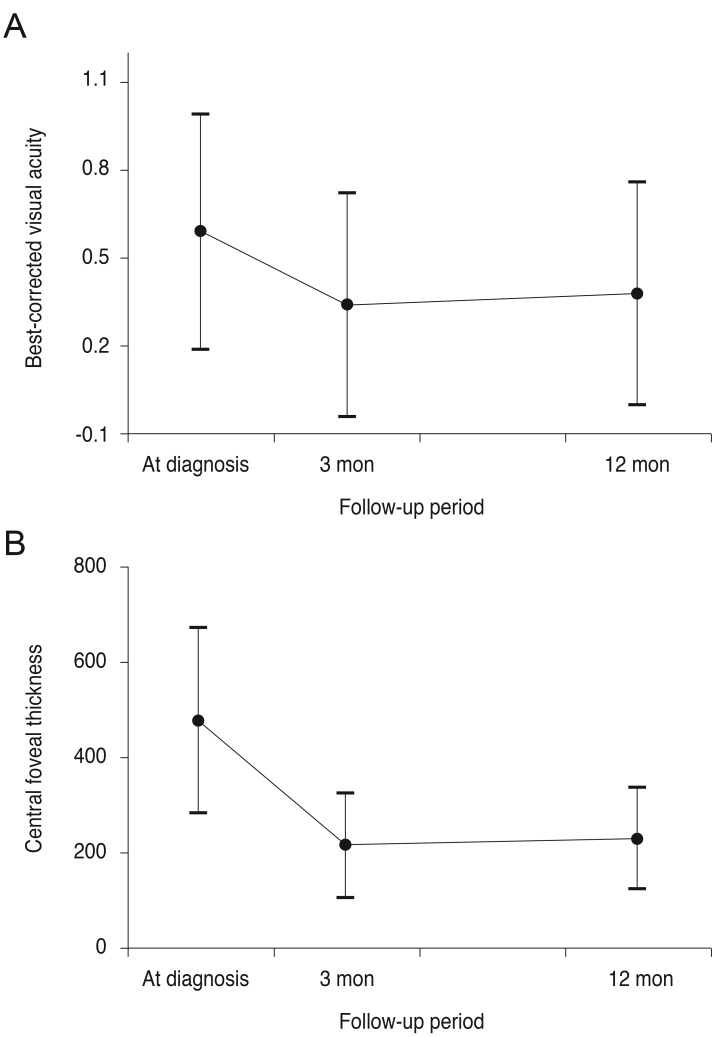
A total of 37 eyes satisfied all eligibility criteria. Of these, OCT results at 12 months were not available for five eyes. As a result, analyses of 32 eyes of 32 patients were ultimately included in the results (Table 1). There were 18 male patients (56.3%) and 14 female patients (43.8%), with a mean age of 67.0 ± 7.2 years. The greatest linear dimension was 1,663.5 ± 763.4 µm. Clusters of grape-like polypoidal lesions were noted in two eyes (6.3%) and subfoveal RPED in six eyes (18.8%). The mean distance between the center of the fovea and the polypoidal lesion located closest to the fovea was 896.4 ± 305.9 µm. In 20 eyes (62.5%), the closest polypoidal lesion was 500 to 1,000 µm from the fovea, whereas the polyps in the remaining 12 eyes (37.5%) were located more than 1,000 µm from the fovea. During the 12-month follow-up period, patients received an average of 4.0 ± 1.1 anti-VEGF injections (3.5 ± 1.6 ranibizumab injections and 0.5 ± 1.1 bevacizumab injections). In 15 eyes (46.9%), recurrence of fovea-involving fluid was not noted after the three initial anti-VEGF injections. The BCVA at baseline, three, and 12 months post-diagnosis was 0.59 ± 0.40 (Snellen equivalent, 20 / 77), 0.34 ± 0.38 (20 / 43), and 0.38 ± 0.38 (20 / 47), respectively (Fig. 1A). The BCVA at three and 12 months was significantly better than the baseline values (p < 0.001 and p = 0.002, respectively). The CFT at baseline, three, and 12 months post-diagnosis was 477.1 ± 194.2, 214.5 ± 108.8, and 229.8 ± 106.1 µm, respectively (Fig. 1B). The three- and 12-month CFTs were significantly lower than the baseline value (p < 0.001 and p < 0.001, respectively). At 12 months, intraretinal or subretinal fluid at the foveal location was noted in seven eyes (21.9%).
Table 1. Baseline characteristics of patients diagnosed with extrafoveal polypoidal choroidal vasculopathy.
Values are presented as mean ± standard deviation, number, or number (%).
Fig. 1. Changes in logarithm of minimal angle of resolution best-corrected visual acuity (A) and central foveal thickness (B) in eyes with extrafoveal polypoidal choroidal vasculopathy treated with anti-vascular endothelial growth factor therapy.
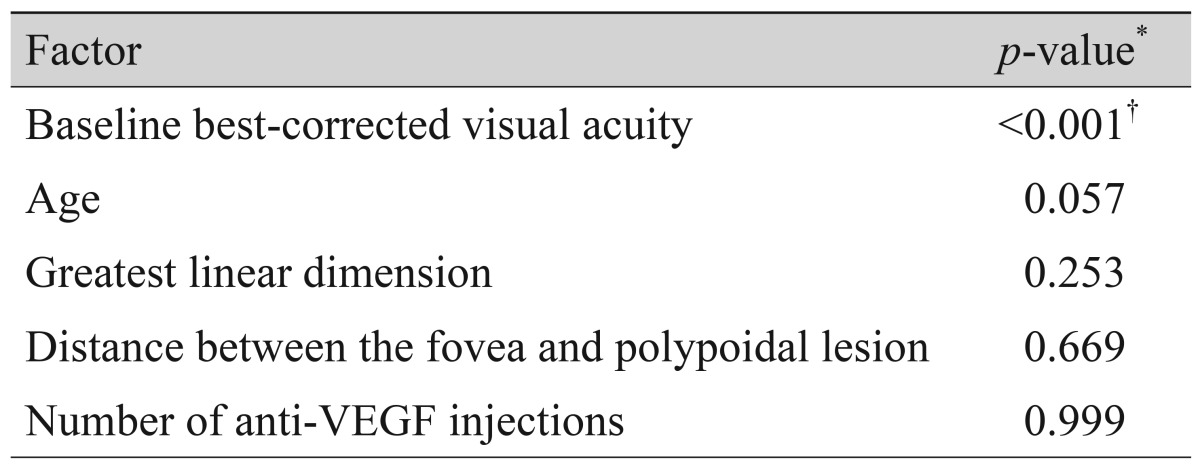
Results of analysis regarding factors associated with BCVA at 12 months are summarized in Table 2. The BCVA at 12 months was significantly associated with BCVA at diagnosis (p < 0.001, r = 0.656), whereas the associations with age (p = 0.057), greatest linear dimension (p = 0.253), distance between the fovea and the polypoidal lesion (p = 0.669), and number of anti-VEGF injections during the 12-month follow-up period (p = 0.999) were not significant. In the multivariate analysis, BCVA at diagnosis was significantly associated with BCVA at 12 months (p < 0.001). The difference in BCVA between eyes with and without subfoveal RPED at diagnosis was not significant at 12 months (p = 0.524).
Table 2. Analysis of factors associated with best-corrected visual acuity at 12 months.
VEGF = vascular endothelial growth factor.
*Pearson's correlation analysis; †Significant association was verified when analyzed with multiple linear regression analysis. Variables included in the analysis were age, greatest linear dimension of the lesion, distance between the fovea and the polypoidal lesion located closest to the fovea, number of anti-vascular endothelial growth factor injections, presence of submacular hemorrhage, and presence of subfoveal retinal pigment epithelial detachment.
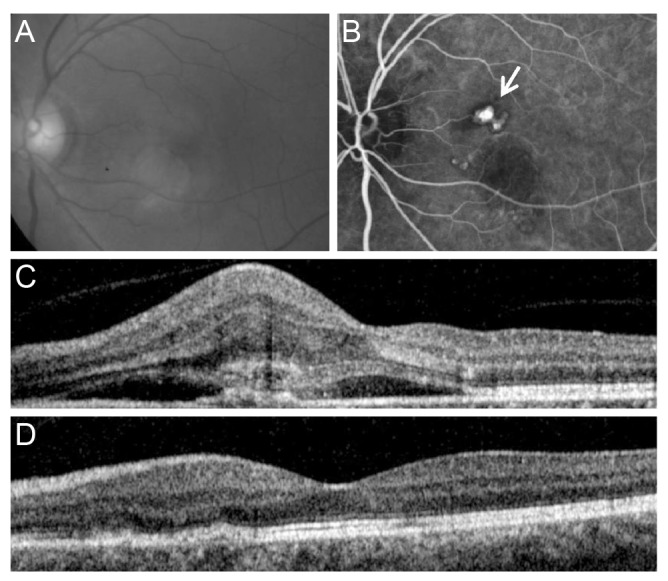
Submacular hemorrhage involving the fovea at the time of diagnosis was noted in 13 eyes (40.6%). These eyes were included in the hemorrhage group, and the remaining 19 eyes (59.4%) were included in the non-hemorrhage group. Fig. 2 illustrates representative cases of eyes from the hemorrhage and non-hemorrhage groups. In the hemorrhage group, the mean distance between the center of the fovea and the polypoidal lesion located closest to the fovea was 754.7 ± 175.3 µm. Patients received an average of 3.8 ± 1.1 anti-VEGF injections during the follow-up period. The BCVA at baseline, at three-month, and 12-month post-diagnosis was 0.76 ± 0.50 (Snellen equivalent, 20 / 115), 0.46 ± 0.54 (20/57), and 0.47 ± 0.54 (20 / 59), respectively. Compared to baseline, the BCVA was significantly better at 12 months (p = 0.032). There were six eyes (46.2%) that gained two or more lines of vision (≥0.2 logarithm of the minimum angle of resolution [logMAR] BCVA) and one eye (7.7%) that lost two or more lines of vision. The remaining six eyes (46.2%) had a stable BCVA.
Fig. 2. Fundus photography (A), indocyanine green angiography (B), and optical coherence tomography (C,D) images from an eye diagnosed with extrafoveal polypoidal choroidal vasculopathy without submacular hemorrhage. The eye was treated with three intravitreal anti-vascular endothelial growth factor injections. The best-corrected visual acuity improved from 20 / 30 at diagnosis to 20 / 25 at 12 months. Images (A), (B), and (C) at diagnosis; image (D) at 12 months. The arrow (B) indicates polypoidal lesions.
In the non-hemorrhage group, the mean distance between the center of the fovea and the polypoidal lesion located closest to the fovea was 993.3 ± 341.0 µm. Patients received an average of 4.1 ± 1.1 anti-VEGF injections during the follow-up period. The BCVA at baseline, three-month, and 12-month post-diagnosis was 0.47 ± 0.26 (Snellen equivalent, 20 / 59), 0.26 ± 0.19 (20 / 36), and 0.33 ± 0.22 (20 / 42), respectively. Compared to the baseline, the BCVA significantly improved at 12 months (p = 0.007). There were seven eyes (36.8%) that gained two or more lines of vision (≥0.2 logMAR BCVA) and one eye (5.3%) that lost two or more lines of vision. The remaining 11 eyes (57.9%) had a stable BCVA. A mean of 2.9 lines of improvement in BCVA was noted in the hemorrhage group, whereas the degree of improvement was 1.4 lines in the no-hemorrhage group. However, the difference was not statistically significant (p = 0.495).
Discussion
Although somewhat controversial [14], the efficacy of anti-VEGF therapy in extrafoveal exudative AMD has been generally encouraging [5,6]. In a study by Parodi et al. [5], the mean logMAR of visual acuity improved from 0.3 at diagnosis to 0.2 at 12 months. In a study by Ladas et al. [6], the mean logMAR of visual acuity improved from 0.46 at baseline to 0.16 at a mean of 19.1 months after diagnosis. The visual acuity at the final follow-up of a previous study was markedly better in the anti-VEGF monotherapy group than the laser photocoagulation group. Moreover, the time of recurrence after initial treatment in that study was relatively longer in the anti-VEGF group (mean, 18 months) than in the laser group (mean, 11.5 months). In those studies, however, ICGA was not routinely performed, and outcomes in cases of extrafoveal PCV could not be analyzed separately.
Argon laser photocoagulation has been used as one of the treatment modalities in cases of extrafoveal PCV. However, the treatment outcome is variable [15,16,17,18]. In addition, laser photocoagulation inevitably damages adjacent retinal tissue. Photodynamic therapy with or without anti-VEGF therapy is another useful treatment option. However, this therapy is not free from possible ocular side effects, including hemorrhage [19], choroidal ischemia [20], or tearing of the retinal pigment epithelium [21]. Non-ocular, systemic side effects, such as dyspnea, flushing, and pruritus have also been reported [22]. In addition, photodynamic therapy is not available in some regions [23]. Although anti-VEGF therapy has shown excellent efficacy in treating exudative AMD with a very low incidence of adverse events, there have been debates regarding the long-term efficacy of anti-VEGF monotherapy in PCV. Encouraging outcomes have been reported by various investigative groups [8,24,25]. However, the efficacy in other studies was limited [26,27]. The sub-retinal pigment epithelial location of PCV and possible differences in the etiology between PCV and typical exudative AMD [27] were postulated to explain this limited efficacy. In the present study, a marked decrease in CFT accompanied by improvement in visual acuity was noted after anti-VEGF therapy in extrafoveal PCV, regardless of whether submacular hemorrhages were present. In approximately half of the cases, recurrence of fovea-involving exudate was not noted after the initial three anti-VEGF injections. One recent study reported the treatment outcome of extrafoveal PCV using argon laser with and without anti-VEGF therapy [11]. In that study, the visual acuity improved from 0.57 at diagnosis to 0.39 at 12 months. The treatment outcome of the present study was comparable to that of the previous study [11] despite the relatively shorter distance between the fovea and polyps in the present study. In the previous study, the polypoidal lesions were located 500 to 1,000 µm from the center of the fovea in 21.2% of eyes and more than 1,000 µm from the center of the fovea in 78.8% of eyes [11], whereas the proportion was 62.5% and 37.5%, respectively, in the present study. This result may suggest the efficacy of anti-VEGF monotherapy in cases of extrafoveal PCV.
These results suggest that anti-VEGF therapy is a useful treatment option for extrafoveal PCV. We postulate that this favorable result might be due to a relatively well-preserved retinal function in the foveal region. In subfoveal PCV cases, a functional deficit of the retina might remain in the foveal region, despite the resolution of exudation or hemorrhage, because growth of the lesion itself can influence the adjacent microstructure. However, the underlying retina at the foveal region might have been less damaged in our patients compared to subfoveal PCV cases because the polypoidal lesions were located relatively further from the fovea. In the present study, subfoveal RPED at diagnosis (known to be associated with a poor prognosis [28]) was noted in only 18.8% of cases, whereas the incidence was 42% in a previous study [28].
Several factors, including baseline visual acuity [29], history of photodynamic therapy [29], clusters of grape-like polypoidal lesions [29], greatest lesion diameter [28], or RPED at diagnosis [28], were found to be associated with long-term visual prognosis in PCV treated with anti-VEGF therapy. In the present study, baseline visual acuity was found to be the only factor predictive of visual outcome in extrafoveal PCV cases at 12 months. Clusters of grape-like polypoidal lesions were found in only two eyes, so this factor was not analyzed.
In the present study, significant improvement in visual acuity was noted regardless of the presence of submacular hemorrhage. However, it is notable that eyes with submacular hemorrhage at initial presentation showed a relatively greater degree of improvement in visual acuity than eyes without hemorrhage. Approximately three lines of marked improvement in visual acuity were noted in the hemorrhage group. This favorable result might be mainly derived from the resolution of hemorrhage. In the present study, however, eyes with extensive hemorrhage that impeded accurate diagnosis of PCV and eyes experiencing vitrectomy requiring severe vitreous hemorrhage were excluded. Thus, our result might not be valid in cases involving extensive hemorrhage.
In the present study, the incidence of subfoveal RPED was 18.8%. RPED in the macular area was observed in a majority of eyes with PCV [4,30]. The primary reason for the low incidence of RPED among our patients might be that we evaluated the incidence of subfoveal RPED only in cases of extrafoveal PCV. In addition, only cross-hair scans centered at the center of the fovea were analyzed to identify RPED. Thus, the incidence of RPED in the macular area might be greater than the incidence of subfoveal RPED in this study.
This study had several limitations in addition to the retrospective design. Initially, there was no single treatment guideline. Treatment strategy, including follow-up interval and examinations, was determined at the discretion of the attending physician. In addition, because ICGA was not a routinely performed examination during the follow-up period, angiographic findings could not be determined in the majority of patients during the study period. Only cases with polyps 500 µm or more distant from the center of the fovea were included. Thus, the efficacy of anti-VEGF therapy in PCVs with polyps 200 to 500 µm from the center of the fovea needs further evaluation. Lastly, because this study was an observational study without a control group, it cannot provide any evidence that the effect of anti-VEGF monotherapy is more or less superior than other treatment modalities.
In conclusion, anti-VEGF therapy was found to be beneficial in extrafoveal PCV in which polyps were located more than 500 µm from the center of the fovea, improving visual acuity and decreasing macular thickness. This benefit was noted regardless of the presence of submacular hemorrhage at diagnosis. Eyes with better visual acuity at diagnosis had a more favorable visual outcome at 12 months. Further prospective, well-controlled studies are required to confirm our findings.
Acknowledgements
This study was supported by Kim's Eye Hospital Research Center.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang YS, Han JI, Yoo SJ, et al. Intravitreal anti-vascular endothelial growth factor for typical exudative age-related macular degeneration in eyes with good baseline visual acuity. Korean J Ophthalmol. 2014;28:466–472. doi: 10.3341/kjo.2014.28.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon YH, Lee DK, Kim HE, Kwon OW. Predictive findings of visual outcome in spectral domain optical coherence tomography after ranibizumab treatment in age-related macular degeneration. Korean J Ophthalmol. 2014;28:386–392. doi: 10.3341/kjo.2014.28.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parodi MB, Iacono P, La Spina C, et al. Intravitreal ranibizumab for naive extrafoveal choroidal neovascularization secondary to age-related macular degeneration. Retina. 2014;34:2167–2170. doi: 10.1097/IAE.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 6.Ladas ID, Chatziralli IP, Kotsolis AI, et al. Intravitreal ranibizumab versus thermal laser photocoagulation in the treatment of extrafoveal classic choroidal neovascularization secondary to age-related macular degeneration. Ophthalmologica. 2012;228:93–101. doi: 10.1159/000337347. [DOI] [PubMed] [Google Scholar]
- 7.Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94:297–301. doi: 10.1136/bjo.2008.150029. [DOI] [PubMed] [Google Scholar]
- 8.Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013;156:644–651. doi: 10.1016/j.ajo.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2008;22:92–99. doi: 10.3341/kjo.2008.22.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho HJ, Baek JS, Lee DW, et al. Short-term effectiveness of intravitreal bevacizumab vs. ranibizumab injections for patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2012;26:157–162. doi: 10.3341/kjo.2012.26.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemmy Cheung CM, Yeo I, Li X, et al. Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;155:295–304.e1. doi: 10.1016/j.ajo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Cackett P, Wong D, Yeo I. A classification system for polypoidal choroidal vasculopathy. Retina. 2009;29:187–191. doi: 10.1097/IAE.0b013e318188c839. [DOI] [PubMed] [Google Scholar]
- 13.Koh AH, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013;33:686–716. doi: 10.1097/IAE.0b013e3182852446. [DOI] [PubMed] [Google Scholar]
- 14.Giacomelli G, Giansanti F, Finocchio L, et al. Results of intravitreal ranibizumab with a prn regimen in the treatment of extrafoveal and juxtafoveal neovascular membranes in age-related macular degeneration. Retina. 2014;34:860–867. doi: 10.1097/IAE.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 15.Lafaut BA, Leys AM, Snyers B, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752–759. doi: 10.1007/s004170000180. [DOI] [PubMed] [Google Scholar]
- 16.Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003;47:379–384. doi: 10.1016/s0021-5155(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Ulla F, Gonzalez F, Torreiro MG. Diode laser photocoagulation in idiopathic polypoidal choroidal vasculopathy. Retina. 1998;18:481–483. doi: 10.1097/00006982-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond) 2009;23:145–148. doi: 10.1038/sj.eye.6702955. [DOI] [PubMed] [Google Scholar]
- 19.Hirami Y, Tsujikawa A, Otani A, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27:335–341. doi: 10.1097/01.iae.0000233647.78726.46. [DOI] [PubMed] [Google Scholar]
- 20.Isola V, Pece A, Parodi MB. Choroidal ischemia after photodynamic therapy with verteporfin for choroidal neovascularization. Am J Ophthalmol. 2006;142:680–683. doi: 10.1016/j.ajo.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein M, Heilweil G, Barak A, Loewenstein A. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye (Lond) 2005;19:1315–1324. doi: 10.1038/sj.eye.6701765. [DOI] [PubMed] [Google Scholar]
- 22.Schnurrbusch UE, Jochmann C, Einbock W, Wolf S. Complications after photodynamic therapy. Arch Ophthalmol. 2005;123:1347–1350. doi: 10.1001/archopht.123.10.1347. [DOI] [PubMed] [Google Scholar]
- 23.Oluleye T, Babalola Y. Pattern of presentation of idiopathic polypoidal choroidal vasculopathy in Ibadan, Sub-Saharan Africa. Clin Ophthalmol. 2013;7:1373–1376. doi: 10.2147/OPTH.S47511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng CK, Peng CH, Chang CK, et al. One-year outcomes of intravitreal bevacizumab (avastin) therapy for polypoidal choroidal vasculopathy. Retina. 2011;31:846–856. doi: 10.1097/IAE.0b013e3181f84fdf. [DOI] [PubMed] [Google Scholar]
- 25.Hikichi T, Higuchi M, Matsushita T, et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol. 2012;154:117–124.e1. doi: 10.1016/j.ajo.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina. 2011;31:1581–1588. doi: 10.1097/IAE.0b013e31820d3f3f. [DOI] [PubMed] [Google Scholar]
- 27.Tsujikawa A, Ooto S, Yamashiro K, et al. Treatment of polypoidal choroidal vasculopathy by intravitreal injection of bevacizumab. Jpn J Ophthalmol. 2010;54:310–319. doi: 10.1007/s10384-010-0813-1. [DOI] [PubMed] [Google Scholar]
- 28.Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;156:652–660. doi: 10.1016/j.ajo.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 29.Hikichi T, Higuchi M, Matsushita T, et al. Factors predictive of outcomes 1 year after 3 monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Retina. 2013;33:1949–1958. doi: 10.1097/IAE.0b013e31828bcafa. [DOI] [PubMed] [Google Scholar]
- 30.De Salvo G, Vaz-Pereira S, Keane PA, et al. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014;158:1228–1238.e1. doi: 10.1016/j.ajo.2014.08.025. [DOI] [PubMed] [Google Scholar]