Abstract
Purpose
To evaluate the effects of bevacizumab on expression of B-cell leukemia/lymphoma (Bcl)-2 and apoptosis in retinal pigment epithelial (RPE) cells under oxidative stress conditions.
Methods
RPE cells were treated with H2O2 (0, 100, 200, 300, and 400 µM) and bevacizumab at or above the doses normally used in clinical practice (0, 0.33, 0.67, 1.33, and 2.67 mg/mL). Cell apoptosis was measured using flow cytometry with annexin V-fluorescein isothiocyanate. The expression of Bcl-2 mRNA was determined using reverse transcription polymerase chain reaction.
Results
Under low oxidative stress conditions (H2O2 100 µM), cell apoptosis was not significantly different at any concentration of bevacizumab, but Bcl-2 mRNA expression decreased with increasing concentration of bevacizumab (0.33, 0.67, 1.33, and 2.67 mg/mL). Under moderate oxidative stress conditions (H2O2 200 µM), Bcl-2 mRNA expression decreased with increasing concentration of bevacizumab (0.33, 0.67, 1.33, and 2.67 mg/mL), but cell apoptosis increased only at 2.67 mg/mL of bevacizumab. Under high oxidative stress (300 µM) conditions, cell apoptosis increased at high concentrations of bevacizumab (1.33 and 2.67 mg/mL), but it did not correlate with Bcl-2 expression.
Conclusions
Withdrawal of vascular endothelial growth factor can lead to RPE cell apoptosis and influences the expression of anti-apoptotic genes such as Bcl-2 under oxidative stress conditions. Since oxidative stress levels of each patient are unknown, repeated injections of intravitreal bevacizumab, as in eyes with age-related macular degeneration, might influence RPE cell survival.
Keywords: Apoptosis, Bcl-2, Bevacizumab, Retinal pigment epithelial cell
Vascular endothelial growth factor (VEGF) has been implicated as a pro-angiogenic factor that stimulates neovascularization in age-related macular degeneration (AMD), and retinal pigment epithelial (RPE) is an important source of VEGF [1]. VEGF expression by RPE is a feature of tissues excised from human eyes showing AMD-related choroidal neovascularization [2]. Oxidants have been reported to increase the deposition of oxidized proteins or other oxidized compounds in Bruch's membrane in a process that might involve complement activation and inflammation, provoking VEGF-A release in patients with exudative AMD [1]. Thus, VEGF-A is induced by chronic exposure to oxidative stress, suggesting a role of this stress in both neovascular and advanced dry AMD [3].]
AMD eyes exhibited increased RPE apoptosis compared with normal eyes [3,4]. In particular, RPE apoptosis is an important feature of advanced forms of AMD; indeed, patchy loss of RPE is one of the classic features of AMD, and this process involves apoptosis of RPE cells [4]. In eyes with exudative AMD, apoptotic cells are present in choroidal neovascular membranes [5]. B-cell leukemia/lymphoma (Bcl)-2 is a key anti-apoptotic member of the Bcl-2 family that regulates the intrinsic apoptosis pathway and is comprised of important survival factors in RPE cells [5,6,7]. Oxidative stress activates the intrinsic apoptosis pathway, a process mediated by enhanced mitochondrial membrane permeability and decreased Bcl-2 [8].
Anti-VEGF therapy plays a significant role in the management of retinal and retinal vascular disorders and has demonstrated the best clinical outcomes of all approaches tested to date. However, VEGF-A is a known survival factor of vascular endothelial cells and the developing and mature retina [9,10,11]. RPE tears and choroidal atrophy in specimens from patients with anti-VEGF treated AMD raise questions about the long-term safety of anti-VEGF treatment [12].
The effects of bevacizumab, which might involve cell apoptosis and regulation of anti-apoptotic protein Bcl-2 expression, have not been fully investigated using RPE cells under oxidative stress. Our research therefore focused on evaluating the effects of bevacizumab on expression of Bcl-2 and apoptosis in RPE cells under various oxidative stress conditions and the possible complications of intravitreal bevacizumab injections in patients.
Materials and Methods
Cell culture and exposure to oxidative stress
A human retinal pigment epithelial (ARPE-19) cell line was obtained from ATCC (Manassas, VA, USA). Cells were maintained in Dulbecco's modified Eagle's medium/Ham's F-12 nutrient medium (Invitrogen-Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin B. The ARPE-19 cells were used for four to six passages and plated in six-well plates at 1.5 × 105 cells per well. They were incubated for 24 hours in a humidified 5% CO2 atmosphere at 37℃ after reaching approximately 70% confluence. We next washed the cells with pH 7.4 phosphate buffered saline (PBS). The cells were serum-starved for four hours before H2O2 exposure and treated with H2O2 (100 to 400 µM) for 16 hours to induce oxidative stress before they were harvested for cell death analysis.
Enzyme-linked immunosorbent assay after exposure to oxidative stress
The cells were treated with various concentrations of H2O2. The supernatants were collected at baseline (0 hours) and at 16 hours, centrifuged to remove cell debris, and stored at -70℃ until enzyme-linked immunosorbent assay was performed according to the manufacturer's instructions. In this analysis, a monoclonal antibody specific for VEGF-A was pre-coated onto a microplate. Standards and samples were pipetted into the wells, and any VEGF-A present was bound by the immobilized antibody. After washing any unbound substances, an enzyme-linked polyclonal antibody specific for VEGF-A was added to the wells. Following a wash to remove any unbound antibody- enzyme reagent, a substrate solution was added to the wells, and color developed in proportion to the amount of VEGF-A bound in the initial step. The color development was then stopped, and the intensity of the color was measured. To determine the optical density of each well, we used a microplate reader at 450 and 540 nm. The concentrations of VEGF-A standard numbers 1 to 8 were 0, 15.6, 31.2, 62.5, 125, 250, 500, and 1,000 pg/mL of recombinant VEGF-A in a buffered protein base with preservatives, respectively. The level of VEGF-A protein was measured in cell-free supernatant using a human VEGF-A Quantikin ELISA kit (catalog no. DVE00; R&D Systems, Minneapolis, MN, USA).
Bevacizumab treatment and flow cytometric analysis of apoptosis after exposure to oxidative stress
The cells were washed with PBS and incubated in serum- free Dulbecco's modified Eagle's medium in the presence of H2O2 (100, 200, 300, and 400 µM) for 16 hours. Bevacizumab (0.33, 0.67, 1.33, and 2.67 mg/mL, respectively) was added 2 hours before H2O2 treatment. An annexin V-fluorescein isothiocyanate (FITC) apoptosis kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to detect phosphatidylserine externalization as an index of apoptosis. The cells were washed and incubated for 15 minutes at room temperature in the presence of annexin V labeled with FITC and propidium iodide (PI). In total, 10,000 cells were excited at 488 nm, and emission was measured at 530 and 584 nm to assess FITC and PI fluorescence, respectively. The cells were a nalyzed with a flow c ytometer (BD Biosciences). The number of gated cells was plotted on a dot plot with reference to both annexin V and PI staining.
Reverse transcription polymerase chain reaction
The cells were diluted to 1 × 105 cell/mL and were incubated for 24 hours in six-well plates (Falcon, BD Biosciences). After washing the culture media twice with PBS, serum-free media was applied. H2O2 and bevacizumab were challenged at different concentrations (0, 100, 200, 300, 400 µM and 0.33, 0.67, 1.33, 2.67 mg/mL) and incubated for 16 hours. Total RNA was isolated using the total RNA purification kit (Invitrogen). Isolated RNA was quantified, and 1 µg of total RNA and 100 pmol of oligo dT were added to the reverse transcription (RT) premix (Bioneer, Seoul, Korea) to prepare 20 µL of cDNA. Preformed cDNA, Bcl-2 primer, and glycerol-3-phosphate dehydrogenase (GAPDH) primer were mixed with polymerase chain reaction (PCR) premix (Bioneer), and PCR was performed. The sequences of the primers are described in Table 1. Next, 10 µL of each amplificate were assessed using 1.5% agarose gel electrophoresis. Quantification of Bcl-2 mRNA content was performed using computer-assisted video densitometry (Eagle Eye II-system; Stratagene, La Jolla, CA, USA).
Table 1. Primer used for reverse transcription polymerase chain reaction.
Bcl = B-cell leukemia/lymphoma; GAPDH = glycerol-3-phosphate dehydrogenase.
Statistical analysis
Data are expressed as percentage of control or mean ± standard deviation of results in three or more independent experiments. Statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The Kruskal-Wallis test was used to compare the difference between control and experimental groups. A p-value ≤0.05 was considered statistically significant.
Results
Influence of bevacizumab on apoptosis of retinal pigment epithelial cells without H2O2
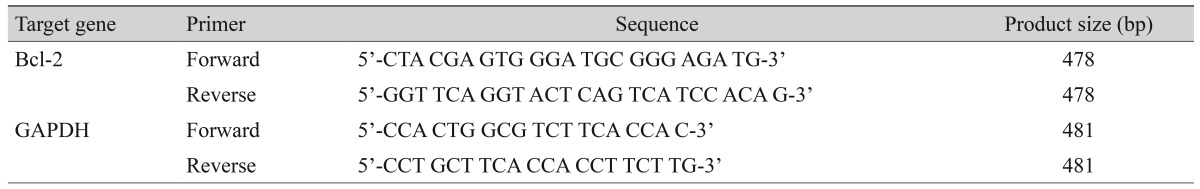
The cells were treated with bevacizumab for 16 hours (0, 0.33, 0.67, 1.33, and 2.67 mg/mL). Cell apoptosis was not significantly different at control, clinical (0.33 and 0.67 mg/mL), or high (1.33 and 2.67 mg/mL) doses of bevacizumab without H2O2 (Fig. 1).
Fig. 1. Influence of bevacizumab on apoptosis of retinal pigment epithelial cells in the absence of H2O2. The retinal pigment epithelial cells were treated with bevacizumab for 16 hours (0, 0.33, 0.67, 1.33, and 2.67 mg/mL). Cellular apoptosis was not significantly different at control, clinical (0.33 and 0.68 mg/mL) and high (1.33 and 2.67 mg/mL) doses of bevacizumab in the absence of H2O2 (p = 0.121, 0.439, 0.221, and 0.063, respectively). Each bar shows the mean ± standard deviation of results of three or more independent experiments.
Effects of H2O2 on apoptosis of retinal pigment epithelial cells
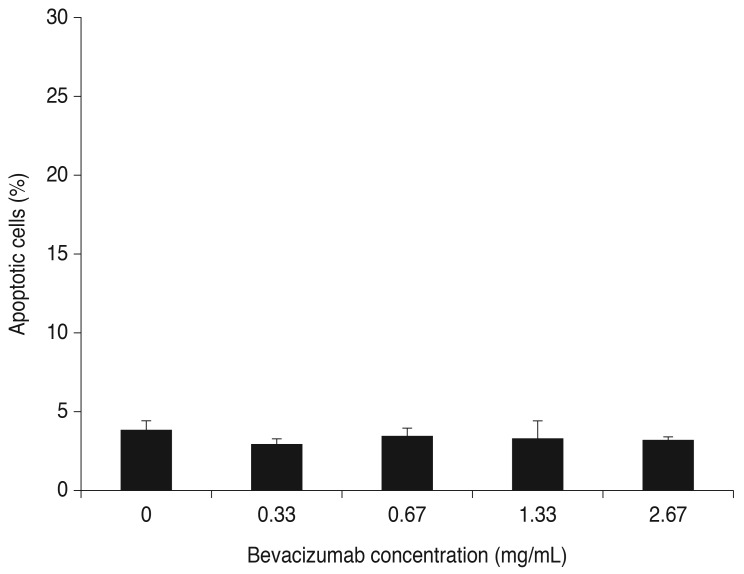
The cells were cultured with H2O2 for 16 hours (0, 100, 200, 300, and 400 µM). Cell apoptosis was 3.26% for RPE at 100 µM H2O2 compared to 3.82% for the controls, but the difference was not significant. Cell apoptosis decreased at 200 µM H2O2 (2.65%) but increased at 300 and 400 µM H2O2 (10.42%, 17.99%). The means at these concentrations were significantly different than the control (p < 0.05) (Fig. 2A).
Fig. 2. Apoptosis and expression of vascular endothelial growth factor (VEGF)-A and B-cell leukemia/lymphoma (Bcl)-2 after treatment of retinal pigment epithelial (RPE) cells with H2O2. (A) Effects of H2O2 on apoptosis of RPE cells. The RPE cells were treated with various concentrations of H2O2 for 16 hours. Cell apoptosis was 3.26% for RPE at 100 µM H2O2 compared to 3.82% for the control, but the difference was not significant. Cell apoptosis decreased at 200 µM H2O2 (2.65%) but increased at 300 and 400 µM of H2O2 (10.42%, 17.99%). Means were significantly different than the control (p < 0.05). Each bar shows the mean ± standard deviation of results of three or more independent experiments. The asterisk indicates a statistically significant difference within the group (*increased, **decreased, p < 0.05). (B) Expression of VEGF-A after exposure to H2O2. VEGF-A excretion into the medium was measured using enzyme-linked immunosorbent assay. VEGF-A expression increased after addition of 50, 100, or 200 µM H2O2 to RPE cells. However, after being treated with 300 and 400 µM H2O2, VEGF-A expression decreased. Data are expressed as the mean ± standard deviation of the results of three or more independent experiments. Means were statistically significant compared to the control at all concentrations of H2O2 (p < 0.05). (C) Expression of Bcl-2 mRNA after exposure to H2O2. Cells were cultured with various concentrations of H2O2 for 16 hours (0, 100, 200, and 300 µM). Expression of Bcl-2 mRNA decreased as oxidative stress increased. Data are expressed as the mean ± standard deviation of the results of three or more independent experiments. Means were statistically significant compared with the control at all concentrations of H2O2 (p < 0.05).
Vascular endothelial growth factor-A level under oxidative stress conditions
VEGF-A expression was increased after addition of 50, 100, and 200 µM H2O2 to RPE cells. However, after being treated with 300 and 400 µM H2O2, VEGF-A expression decreased. Means at these concentrations were significantly different from the control (p < 0.05) (Fig. 2B).
Expression of Bcl-2 mRNA after exposure to oxidative stress
The cells were cultured with H2O2 for 16 hours (0, 100, 200, and 300 µM). Expression of Bcl-2 mRNA decreased as oxidative stress increased (p < 0.05) (Fig. 2C).
Influence of bevacizumab on apoptosis of RPE cells and Bcl-2 mRNA expression under low oxidative stress
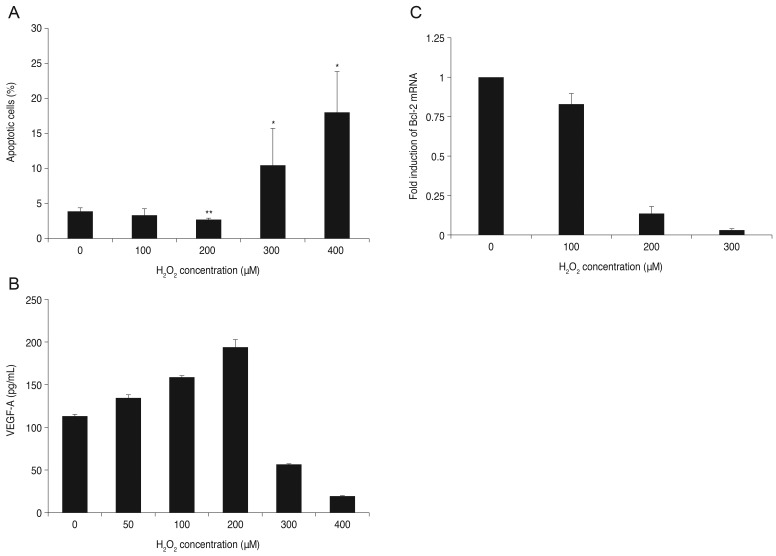
ARPE-19 cells were treated with bevacizumab (0, 0.33, 0.67, 1.33, and 2.67 mg/mL) under low oxidative stress conditions (100 µM of H2O2). Cell apoptosis was not significantly different at any concentration of bevacizumab at 100 µM H2O2. Expression of Bcl-2 mRNA decreased under low oxidative stress conditions, and the decrease was proportional to the increase in bevacizumab. There was a statistically significant difference at all concentrations compared to the result of the control. The mRNA expression of Bcl-2 was normalized to GAPDH (a housekeeping gene). Each bar shows the mean ± standard deviation of results in three or more independent experiments (p < 0.05) (Fig. 3).
Fig. 3. Influence of bevacizumab on apoptosis of retinal pigment epithelial (RPE) cells and B-cell leukemia/lymphoma (Bcl)-2 mRNA expression under low oxidative stress (100 µM H2O2). (A) Influence of bevacizumab on apoptosis of RPE cells under low oxidative stress (100 µM H2O2). The RPE cells were treated with various concentrations of bevacizumab (0, 0.33, 0.67, 1.33, and 2.67 mg/mL) under low oxidative stress (100 µM H2O2). Cell apoptosis was not significantly different at all concentrations of bevacizumab less than 100 µM H2O2. Each bar shows the mean ± standard deviation of results of three or more independent experiments. p < 0.05 vs. control. (B,C) Expression of Bcl-2 mRNA with bevacizumab under low oxidative stress (100 µM H2O2). Expression of Bcl-2 mRNA decreased under low oxidative stress (100 µM H2O2), and this decrease was proportional to the increase in bevacizumab dose. There was a statistically significant difference at all concentrations. The mRNA expression of Bcl-2 was normalized to glycerol-3-phosphate dehydrogenase (GAPDH, housekeeping gene). Each bar shows the mean ± standard deviation of results of three or more independent experiments. p < 0.05 vs. control.
Influence of bevacizumab on apoptosis of RPE cells and Bcl-2 mRNA expression under moderate oxidative stress
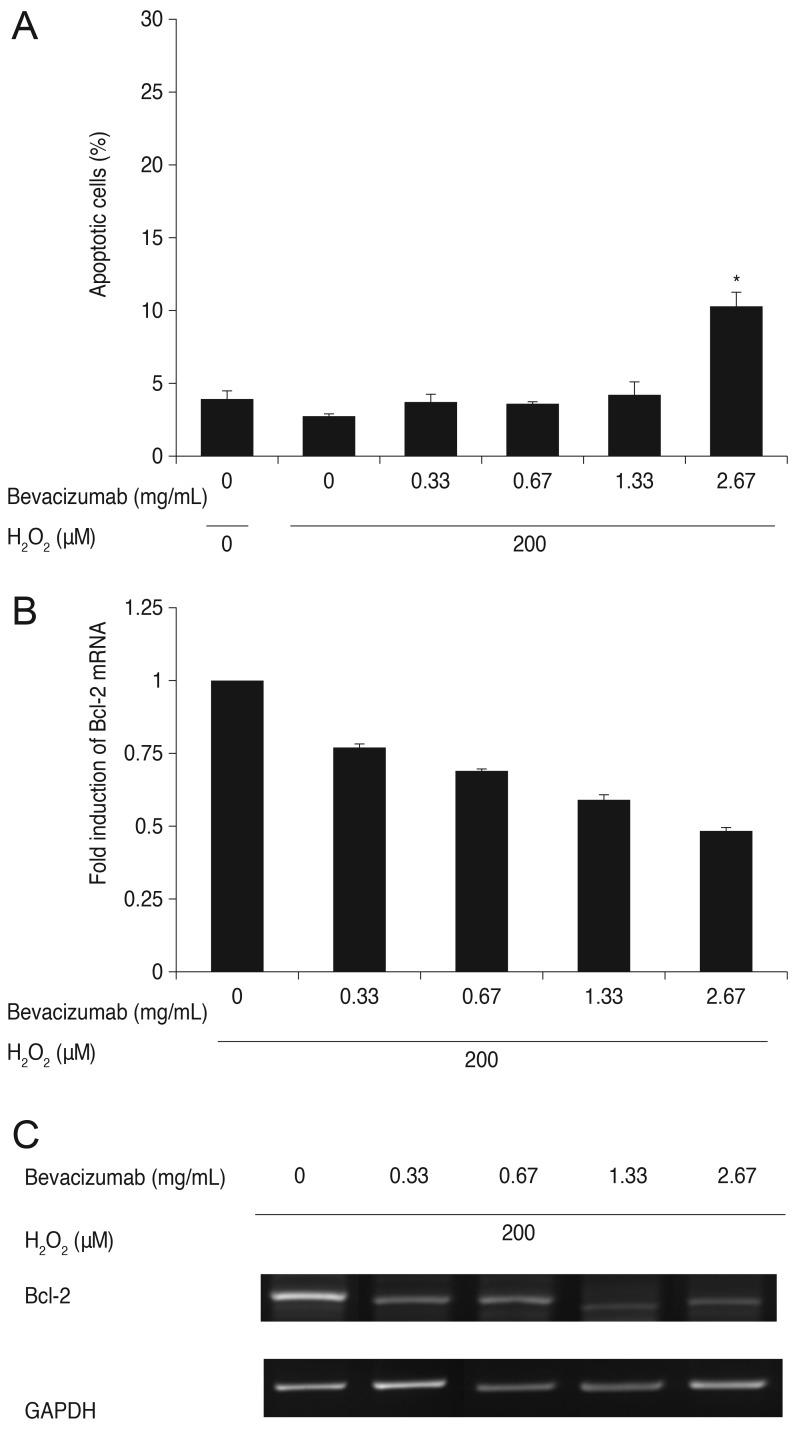
ARPE-19 cells were treated with bevacizumab (0, 0.33, 0.67, 1.33, and 2.67 mg/mL) under moderate oxidative stress conditions (200 µM of H2O2). As mentioned previously, cell apoptosis decreased at 200 µM H2O2 (2.65%) compared with the control (3.82%). Cell apoptosis increased to 3.59%, 3.50%, and 4.13% at bevacizumab concentrations of 0.33, 0.67, and 1.33 mg/mL, respectively, but the differences were not statistically significant. However, cell apoptosis increased significantly to 10.16% at a high dose of bevacizumab (2.67 mg/mL) under the same oxidative stress conditions. Expression of Bcl-2 mRNA decreased proportionally to the increase in bevacizumab dose. There was a statistically significant difference at all concentrations (p < 0.05) (Fig. 4).
Fig. 4. Influence of bevacizumab on apoptosis of retinal pigment epithelial (RPE) cells and B-cell leukemia/lymphoma (Bcl)-2 mRNA expression under moderate oxidative stress (200 µM of H2O2). (A) Influence of bevacizumab on apoptosis of RPE cells under moderate oxidative stress (200 µM H2O2). The RPE cells were treated with various concentrations of bevacizumab (0, 0.33, 0.67, 1.33, and 2.67 mg/mL) under moderate oxidative stress (200 µM H2O2). Cell apoptosis decreased at 200 µM H2O2 (2.65%) compared with the control (3.82%). Cell apoptosis did not show significant change until a bevacizumab concentration of at least 1.33 mg/mL. Apoptosis increased to 10.16% at high doses of bevacizumab (2.67 mg/mL) under the same oxidative stress conditions. Each bar shows the mean ± standard deviation of results of three or more independent experiments. The asterisk indicates a statistically significant difference within the group (*p <0.05). (B,C) Expression of Bcl-2 mRNA with bevacizumab under moderate oxidative stress (200 µM H2O2). Expression of Bcl-2 mRNA decreased under moderate oxidative stress (200 µM H2O2), and that decrease was proportional to the increase in bevacizumab dose. There was a statistically significant difference at all concentrations. The mRNA expression of Bcl-2 was normalized to glycerol-3-phosphate dehydrogenase (GAPDH, housekeeping gene). Each bar shows the mean ± standard deviation of results of three or more independent experiments. p < 0.05 vs. control.
Influence of bevacizumab on apoptosis of RPE cells and Bcl-2 mRNA expression under high oxidative stress
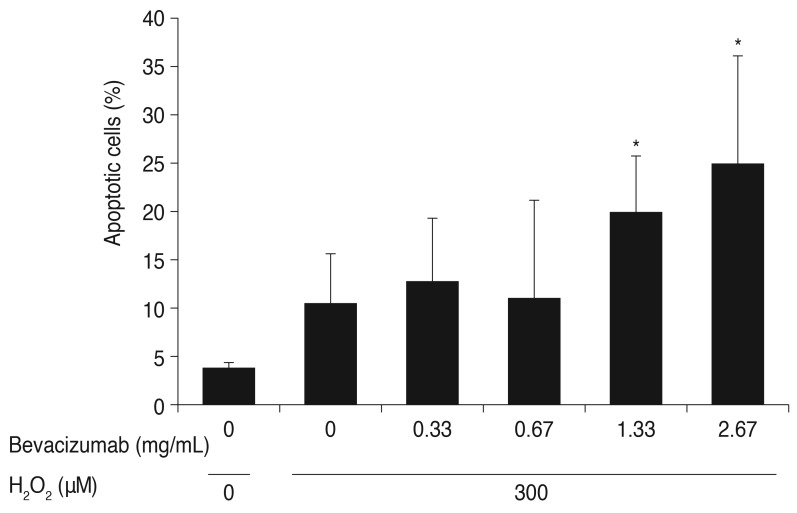
ARPE-19 cells were treated with bevacizumab (0, 0.33, 0.67, 1.33, and 2.67 mg/mL) under high oxidative stress conditions (300 µM H2O2). As mentioned previously, cell apoptosis increased at 300 µM H2O2 (10.42%) compared with the control (3.82%). Cell apoptosis increased to 12.65% and 10.97% at clinical doses of bevacizumab (0.33, 0.67 mg/mL) under high oxidative stress conditions, but there was no statistically significant difference compared to the control. Cell apoptosis did significantly increase to 19.83% and 24.85% at high doses of bevacizumab (1.33, 2.67 mg/mL) under the same oxidative stress. Expression of Bcl-2 mRNA decreased under high oxidative stress conditions (300 µM H2O2), but there was no trend of increase or decrease as the dose of bevacizumab increased (Fig. 5). At 400 µM H2O2, the Bcl-2 mRNA data were not able to be interpreted. We suggest that the reverse transcription-PCR results might be a result of severe cellular apoptosis under high oxidative stress conditions.
Fig. 5. Influence of bevacizumab on apoptosis of retinal pigment epithelial cells under high oxidative stress (300 µM H2O2). The retinal pigment epithelial cells were treated with various concentrations of bevacizumab (0, 0.33, 0.67, 1.33, and 2.67 mg/mL) under high oxidative stress (300 µM of H2O2). As mentioned previously, cell apoptosis increased at 300 µM H2O2 (10.42%) compared with the control (3.82%). Cell apoptosis increased to 12.65% and 10.97% at clinical doses of bevacizumab (0.33, 0.67 mg/mL) under high oxidative stress, but there was no statistically significant difference between the increases. Cell apoptosis significantly increased to 19.83% and 24.85% at high doses of bevacizumab (1.33, 2.67 mg/mL) under the same oxidative stress. Each bar shows the mean ± standard deviation of results of three or more independent experiments. The asterisk indicates a statistically significant difference within the group (*p < 0.05).
Discussion
In the present study, we tested the possible toxicity of bevacizumab on RPE cells under various oxidative stress conditions, and we examined the expression of the anti-apoptotic protein Bcl-2 and the apoptosis of RPE cells. We used H2O2 to induce oxidative stress in order to replicate in vivo conditions that are implicated in the pathogenesis of AMD and the annexin V-FITC apoptosis kit to detect phosphatidylserine externalization as an index of apoptosis.
Intravitreal injections of anti-VEGF agents such as bevacizumab are widely used for the treatment of various retinal disorders associated with new vessels such as AMD or proliferative diabetic retinopathy in order to reduce angiogenesis [13]. Bevacizumab is a full-length, recombinant humanized immunoglobulin G antibody that binds to and inhibits all biologically active forms of VEGF-A isoforms. Despite ongoing clinical trials with intravitreal bevacizumab in many neovascular ocular disorders, its ocular safety remains an issue for research. Hypothetically, repeated injections with bevacizumab could interfere with the neuroprotective and survival actions of VEGF under oxidative stress.
In AMD patients, several studies have reported cases of RPE tears after intravitreal injections of bevacizumab for neovascular AMD [12]. Atrophy of the choriocapillaries or loss of endothelial cell fenestrations impairs nutritional support, which can lead to functional and morphologic damage to the RPE and photoreceptors, with particular adverse effects if the macular lesion is affected [14]. The development of geographic atrophy contributed to visual acuity loss after repeated anti-VEGF injections [15]. In addition, in a recent study in which a retinal neovascularization model was developed, it was suggested that caution is warranted in the treatment of patients with acute or severe retinal neovascularization using anti-VEGF drugs such as bevacizumab in order to prevent capillary nonperfusion and macular ischemia [16].
According to many recent reports, bevacizumab at concentrations at or above the dose normally used in clinical practice is not toxic to RPE cells [17,18,19,20]. Several preclinical experimental toxicity studies have reported the histopathological effects of bevacizumab on retinal cells, retinal neovascular membranes, and capillaries in paraffin-embedded sections [16,21,22] or organotypic culture [23], as well as in ultrastructural evaluations [14,17,21]. Based on in vitro assays, bevacizumab has little toxic effect on ganglion cells, neuroretinal cells, RPE cells, choroidal endothelial cells, or corneal epithelial cells [19,24]. However, there are limited previous preclinical studies associated with RPE under oxidative stress conditions [25].
In our study, RPE cell apoptosis did not show a significant difference at 100 µM H2O2 but decreased at 200 µM H2O2 and increased with higher oxidative stress conditions (300 and 400 µM H2O2) (Fig. 1A). It seems that RPE cells can tolerate up to 200 µM H2O2 due to cell-protective mechanisms, but higher oxidative stress conditions caused in vitro mitochondrial DNA damage and promoted apoptosis. Ballinger et al. [26] and Jin et al. [27] reported that exposure of RPE cells to concentrations of H2O2 that cause in vitro mitochondrial DNA damage also promotes apoptosis, and this correlates well with our results under high oxidative stress conditions.
In different cell types, including vascular smooth muscle cells [28], VEGF expression is triggered by oxidative stress. Other studies of VEGF expression are consistent with our results when H2O2 in concentrations up to 200 µM was added to RPE cells. However, VEGF expression decreased at 300 and 400 µM H2O2, suggesting that VEGF production decreases according to the decrease in live RPE cells [29].
Oxidative stress triggers multiple signaling pathways, including some that are cytoprotective and others that contribute to cell damage and eventually cell death. Among these are the pathways of the Bcl-2 family proteins, which have subcellular locations on the mitochondrial outer membrane, nuclear envelope, and endoplasmic reticulum [30]. Expression of Bcl-2 mRNA decreased as oxidative stress increased, which was consistent with another study [27].
The retina is a highly oxygen-consumptive tissue that always functions under high oxygen tension; RPE cells are therefore exposed to oxidative stress. Chronic oxidative stress can lead to impairment and death of RPE cells, implicating it as a risk factor of AMD. In our study, low to high oxidative stress conditions of RPE cells were implicated in the phase of AMD.
Oxidatively stressed RPE cells undergo apoptosis [27], and Byeon et al. [25] have reported that addition of a high concentration (2.5 mg/mL) of bevacizumab to the culture medium did not affect the survival of either control RPE cells or cells under a low level of oxidative stress (150 µM H2O2). However, under higher oxidative stress levels (200 or 300 µM H2O2), pretreatment with bevacizumab (2.5 mg/mL) induced a significantly higher level of RPE cell death [25].
Addition of clinically applicable (0.33 and 0.67 mg/mL) and high concentrations (1.33 and 2.67 mg/mL) of bevacizumab did not affect the survival of RPE cells under low oxidative stress (100 µM H2O2); nevertheless, Bcl-2 expression decreased in a dose-dependent manner (Fig. 3). Although Bcl-2 is an important survival factor, it is sensitive to oxidative stress, and a decrease in Bcl-2 precedes cell apoptosis.
Under moderate oxidative stress conditions (200 µM H2O2), Bcl-2 expression decreased with increasing concentration of bevacizumab; however, cell apoptosis did not show significant change until 1.33 mg/mL of bevacizumab. It then increased to 10.16% at high doses of bevacizumab (2.67 mg/mL) under the same oxidative stress (Fig. 4).
Under high oxidative stress conditions (300 µM H2O2), RPE cell apoptosis increased at high bevacizumab concentrations (1.33 and 2.67 mg/mL), but this was not correlated with Bcl-2 expression (Fig. 5). This might be due to multiple survival factors in RPE cells or experimental error due to low Bcl-2 expression.
In our experiment, RPE apoptosis did not increase with increased concentration of bevacizumab in the absence of H2O2; however, as the oxidative stress level increased, apoptosis of RPE cells increased. The higher was the oxidative stress, the lower was the concentration of bevacizumab that induced cellular apoptosis. Although cellular apoptosis at clinically applicable levels of bevacizumab (0.33 and 0.67 mg/mL) did not show significant increase, care should be taken since Bcl-2 expression showed a significant decrease even at lower levels of oxidative stress.
To our knowledge, we have shown for the first time that bevacizumab influences Bcl-2 expression and apoptosis of RPE cells under oxidative stress. Our study has some limitations. First, our conclusions are drawn from in vitro studies, which are not the best approximation of in vivo conditions. Moreover, some previous studies have shown that non-confluent ARPE-19 cells behave quite differently in terms of oxidative stress compared to confluent monolayers [31]. Further studies are needed to examine these findings in porcine retina-RPE-choroid cultures or on mouse retinae. Second, it is difficult to determine the degree of H2O2 simulating oxidative stress conditions of human eyes. Third, we could not identify the additional pathway mechanisms of Bcl-2 expression. Further studies are needed to examine the intracellular pathway or cumulative effects at repeated doses. Fourth, we could not determine the additional mechanism of action of bevacizumab in our study. Bevacizumab might bind RPE cell surface receptors in addition to known VEGF receptors.
In our experiment, we demonstrated the possibility that addition of the VEGF inhibitor bevacizumab might block the protective effect of VEGF in our in vitro model under high oxidative stress conditions mimicking those in AMD eyes. Under increased oxidative stress conditions, high doses of bevacizumab might influence RPE cell survival. Furthermore, bevacizumab might affect the expression of anti-apoptotic genes such as Bcl-2 under all oxidative conditions. Since the oxidative stress levels of each patient are unknown, repeated injections of intravitreal bevacizumab, as in eyes with AMD, might influence RPE cell survival. Therefore close monitoring is needed in patients who receive repeated injections of intravitreal bevacizumab.
Acknowledgements
This work was supported by an Inha University research grant.
Footnotes
Parts of this work have been presented and selected as 'hot topics' at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO) at Fort Lauderdale, FL, USA on May 2012.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otani A, Takagi H, Oh H, et al. Vascular endothelial growth factor family and receptor expression in human choroidal neovascular membranes. Microvasc Res. 2002;64:162–169. doi: 10.1006/mvre.2002.2407. [DOI] [PubMed] [Google Scholar]
- 3.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 4.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 5.Hinton DR, He S, Lopez PF. Apoptosis in surgically excised choroidal neovascular membranes in age-related macular degeneration. Arch Ophthalmol. 1998;116:203–209. doi: 10.1001/archopht.116.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, Peairs JJ, Yang P, et al. The importance of Bcl-xL in the survival of human RPE cells. Invest Ophthalmol Vis Sci. 2007;48:3846–3853. doi: 10.1167/iovs.06-1145. [DOI] [PubMed] [Google Scholar]
- 7.Godley BF, Jin GF, Guo YS, Hurst JS. Bcl-2 overexpression increases survival in human retinal pigment epithelial cells exposed to H(2)O(2) Exp Eye Res. 2002;74:663–669. doi: 10.1006/exer.2001.1146. [DOI] [PubMed] [Google Scholar]
- 8.Sorenson CM. Bcl-2 family members and disease. Biochim Biophys Acta. 2004;1644:169–177. doi: 10.1016/j.bbamcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 10.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 11.Gora-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–39. [PubMed] [Google Scholar]
- 12.Gelisken F, Ziemssen F, Voelker M, et al. Retinal pigment epithelial tears after single administration of intravitreal bevacizumab for neovascular age-related macular degeneration. Eye (Lond) 2009;23:694–702. doi: 10.1038/sj.eye.6703098. [DOI] [PubMed] [Google Scholar]
- 13.CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters S, Heiduschka P, Julien S, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143:995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ying GS, Kim BJ, Maguire MG, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol. 2014;132:915–921. doi: 10.1001/jamaophthalmol.2014.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameri H, Chader GJ, Kim JG, et al. The effects of intravitreous bevacizumab on retinal neovascular membrane and normal capillaries in rabbits. Invest Ophthalmol Vis Sci. 2007;48:5708–5715. doi: 10.1167/iovs.07-0731. [DOI] [PubMed] [Google Scholar]
- 17.Inan UU, Avci B, Kusbeci T, et al. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest Ophthalmol Vis Sci. 2007;48:1773–1781. doi: 10.1167/iovs.06-0828. [DOI] [PubMed] [Google Scholar]
- 18.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Luthra S, Narayanan R, Marques LE, et al. Evaluation of in vitro effects of bevacizumab (Avastin) on retinal pigment epithelial, neurosensory retinal, and microvascular endothelial cells. Retina. 2006;26:512–518. doi: 10.1097/01.iae.0000222547.35820.52. [DOI] [PubMed] [Google Scholar]
- 20.Brar VS, Sharma RK, Murthy RK, Chalam KV. Evaluation of differential toxicity of varying doses of bevacizumab on retinal ganglion cells, retinal pigment epithelial cells, and vascular endothelial growth factor-enriched choroidal endothelial cells. J Ocul Pharmacol Ther. 2009;25:507–511. doi: 10.1089/jop.2009.0028. [DOI] [PubMed] [Google Scholar]
- 21.Heiduschka P, Fietz H, Hofmeister S, et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007;48:2814–2823. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- 22.Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–269. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kaempf S, Johnen S, Salz AK, et al. Effects of bevacizumab (Avastin) on retinal cells in organotypic culture. Invest Ophthalmol Vis Sci. 2008;49:3164–3171. doi: 10.1167/iovs.07-1265. [DOI] [PubMed] [Google Scholar]
- 24.Luke M, Warga M, Ziemssen F, et al. Effects of bevacizumab on retinal function in isolated vertebrate retina. Br J Ophthalmol. 2006;90:1178–1182. doi: 10.1136/bjo.2006.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byeon SH, Lee SC, Choi SH, et al. Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci. 2010;51:1190–1197. doi: 10.1167/iovs.09-4144. [DOI] [PubMed] [Google Scholar]
- 26.Ballinger SW, Van Houten B, Jin GF, et al. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- 27.Jin GF, Hurst JS, Godley BF. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr Eye Res. 2001;22:165–173. doi: 10.1076/ceyr.22.3.165.5517. [DOI] [PubMed] [Google Scholar]
- 28.Castilla MA, Caramelo C, Gazapo RM, et al. Role of vascular endothelial growth factor (VEGF) in endothelial cell protection against cytotoxic agents. Life Sci. 2000;67:1003–1013. doi: 10.1016/s0024-3205(00)00693-7. [DOI] [PubMed] [Google Scholar]
- 29.Kannan R, Zhang N, Sreekumar PG, et al. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- 30.Krajewski S, Tanaka S, Takayama S, et al. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 31.Rabin DM, Rabin RL, Blenkinsop TA, et al. Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: a novel culture model for dry AMD. Aging (Albany NY) 2013;5:51–66. doi: 10.18632/aging.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]