Abstract
Aim
Hypothesis 1 of the Surgical Treatment for Ischemic Heart Failure(STICH) trial enrolled 1212 patients with a LVEF of 35% or less and CAD amenable to CABG. Patients were randomized to CABG and optimal medical therapy(MED) or MED alone. The objective was to assess whether or not patients with DM enrolled in the STICH trial would have greater benefit from CABG than patients without DM.
Methods and results
The characteristics and clinical outcomes of patients with and without DM randomized to CABG and MED or MED alone were compared. DM was present in 40%. At baseline, patients with DM had more triple vessel CAD, higher LVEF and smaller left ventricular volumes. In patients with diabetes, the primary outcome of all-cause mortality occurred in 39% of patients in the medical-therapy group and 39% in the CABG group[HR with CABG,0.96(0.73–1.26)]. In patients without diabetes, the primary outcome occurred in 41% of patients in the medical-therapy group and 32% in the CABG group[HR with CABG,0.80(0.63–1.02)]. Whilst numerically it would appear that the treatment effect of CABG is blunted in patients with diabetes, there was no significant interaction between DM and treatment group on formal statistical testing.
Conclusions
Patients with DM enrolled in the STICH trial had more triple vessel disease, smaller hearts and higher LVEF than those without DM. CABG did not exert greater benefit in patients with DM.
Keywords: Diabetes, Heart failure, Ischemic heart disease, Coronary artery bypass graft
Introduction
Diabetes, coronary artery disease (CAD) and heart failure commonly coexist. Diabetes is associated with a 2–4 fold increased risk of CAD and a 4–8 fold increased risk of heart failure.(1–3) The pattern of CAD is often more complex in patients with diabetes: diffuse, small caliber, multi-vessel disease being the main finding. Patients with diabetes are frequently referred for coronary artery bypass grafting (CABG).(4) This practice pattern has been justified by guidelines that until recently were based on subgroup analyses of the Bypass Angioplasty Revascularization Investigation (BARI) study.(5,6)
To investigate the optimal management of CAD in patients with diabetes in the modern era, two recent trials have examined revascularization strategies exclusively in patients with diabetes. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial demonstrated that in patients with diabetes and multivessel CAD, revascularization with CABG reduced cardiovascular events in comparison to medical therapy, a benefit not seen with percutaneous coronary intervention (PCI).(7) In the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, revascularization with CABG was superior to PCI in patients with diabetes and multivessel CAD.(8) Few patients with heart failure were included in these trials. In the BARI 2D study, only 7% of patients had a history of heart failure and only 18% had a left ventricular ejection fraction (LVEF) less than 50%. In FREEDOM less than 3% of patients had a LVEF less than 40%. In patients with CAD and normal LVEF, the presence or absence of diabetes has been used as a major factor in determining the method of revascularization. We do not know if the presence or absence of diabetes should have a similar impact on decision making in patients with low LVEF and heart failure
The Surgical Treatment for Ischemic Heart Failure (STICH) trial enrolled 1212 patients with a LVEF of 35% or less and CAD amenable to CABG.(9) Patients were randomized to CABG and optimal medical therapy (MED) or MED alone. The STICH trial provides a valuable opportunity to compare the characteristics and clinical outcomes of the only large cohort of patients with and without diabetes, CAD and heart failure to be randomized to CABG and MED or MED alone.
Methods
The design of the STICH study has been previously described.(10,11) STICH is a prospective, multicenter, randomized trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) that recruited 2,136 patients with CAD and LVEF ≤ 35% between 2002 and 2007. The trial addressed two primary hypotheses: 1) CABG combined with MED improves survival compared with MED alone (surgical revascularization hypothesis); and 2) surgical ventricular reconstruction added to CABG improves survival free of cardiovascular hospitalization compared with CABG alone in patients with significant anterior wall akinesis (surgical ventricular reconstruction hypothesis). The results of the two primary hypotheses have been reported.(9,12) Only the 1,212 patients in the Hypothesis 1, surgical revascularization hypothesis, were considered for this study. Detailed inclusion and exclusion criteria have been described previously. In brief, patients had to have an LVEF ≤ 35% and have CAD suitable for revascularization in addition to this the patient could not have left main CAD ≥ 50% or angina greater than CCS III angina. The NHLBI and the ethics committee at each recruiting institution approved the study protocol. All patients provided written informed consent. The study complied with the Declaration of Helsinki.
Definitions
Diabetes is defined as a reported history of diabetes or use of diabetic medications at baseline. Chronic renal insufficiency at baseline is defined as patients having a consistent creatinine level >1.5 mg/dL (≥133 µmol/L). Worsening renal insufficiency in the perioperative period is defined as an increase of >2mg/dL and 2X baseline creatinine level or a new requirement for dialysis.
Evaluation of left ventricular function
All patients were required to undergo an echocardiogram to evaluate LV size, volume, and diastolic as well as systolic function within 3 months of enrolment. Baseline radionuclide imaging and/or cardiac magnetic resonance imaging studies were encouraged. Imaging studies were analyzed by Imaging Core Labs. From these imaging studies, best LV volumes and LVEF were obtained based on the hierarchy and the quality of imaging studies.(13) Diastolic function and filling pressure were measured only in patients in sinus rhythm by echocardiography using mitral inflow early diastolic velocity (E), late diastolic velocity (A), E/A ratio, medial mitral annulus early diastolic velocity (e’), and E/e’ ratio.
Endpoints
The five main primary and secondary endpoints from the STICH trial were studied: all-cause death; death from cardiovascular (CV) causes; death or CV hospitalization; death or heart failure hospitalization; and death or all-cause hospitalization.
Statistical analysis
Baseline characteristics of patients with and without diabetes were summarized by the median (interquartile range) for continuous variables and by frequency (percentage) for categorical variables. Baseline differences between the diabetic and non-diabetic patient groups were assessed using the Wilcoxon rank-sum test for the continuous and ordinal variables and the chi-square test or Fisher’s Exact test for categorical variables. Clinical outcomes of patients randomized to MED vs. CABG + MED were statistically compared using Kaplan-Meier analysis and the Cox-proportional hazards model. Outcome differences between the treatment arms were summarized using hazard ratios and 95% confidence intervals generated from the Cox model. The Cox model was also used to test for an interaction between treatment and diabetes status (i.e., to assess whether the effect of CABG compared to MED was statistically different in diabetics compared to non-diabetics). This assessment was also performed adjusting for baseline covariates known from previous analyses of hypothesis 1 patients to be independent risk markers: age, end systolic volume index (ESVI), creatinine, mitral regurgitation, heart rate, and history of stroke. P-values <0.05 were considered to be statistically significant. The primary analyses of the treatment effect of CABG and MED vs MED alone were performed by randomized group (intention to treat). However, supplementary analyses were also performed based on treatment received and the per protocol population as previously described.(9) All calculations were performed using SAS statistical software, version 9.2 (SAS Institute, Cary NC).
Results
At baseline, 40.3% (n=489) of the population had diabetes.
Baseline characteristics
The baseline characteristics of patients with and without diabetes are listed in table 1. Patients with diabetes were older, more likely to be female, had higher body mass index (BMI) and were less likely to be smokers. Patients with diabetes also had higher rates of hypertension, chronic renal insufficiency and hyperlipidemia but lower rates of myocardial infarction (71% v 81%, p<0.001).
Table 1.
Baseline Characteristics of patients in the STICH trial with and without diabetes
| Variable | Diabetes (n=489) |
No Diabetes (n=723) |
P |
|---|---|---|---|
| Demographics | |||
| Age (yrs.) | 60.5 (55.0, 67.6) | 58.9 (52.7, 66.8) | 0.005 |
| Female gender | 17.2% | 8.9% | <0.001 |
| Race-White | 67.9% | 68.5% | 0.834 |
| BMI | 27.4 (24.4, 30.8) | 26.4 (23.7, 29.4) | <0.001 |
| Medical History | |||
| Previous MI | 71.2% | 81.1% | <0.001 |
| Previous stroke | 9.8% | 6.1% | 0.016 |
| Hypertension | 67.3% | 55.2% | <0.001 |
| Hyperlipidemia | 64.2% | 57.7% | 0.023 |
| Peripheral vascular disease |
16.8% | 14.1% | 0.205 |
| Chronic renal insufficiency |
12.5% | 4.6% | <0.001 |
| Atrial fibrillation/flutter |
11.7% | 13.3% | 0.404 |
| Cancer in last 5 yrs. | 1.6% | 0.8% | 0.198 |
| Current smoker | 17.6% | 23.0% | 0.023 |
| Depression | 7.4% | 5.5% | 0.198 |
| Previous CABG | 2.9% | 3.0% | 0.856 |
| Previous PCI | 13.1% | 12.7% | 0.853 |
| ICD | 3.1% | 1.9% | 0.206 |
| Pacemaker for heart rate |
2.5% | 0.8% | 0.022 |
|
Presenting characteristics |
|||
| CCS angina class | 0.059 | ||
| No angina | 41.7% | 32.9% | |
| I | 12.9% | 17.2% | |
| II | 39.1% | 46.2% | |
| III | 4.9% | 3.3% | |
| IV | 1.4% | 0.4% | |
| Current NYHA Class |
0.564 | ||
| I | 12.1% | 11.1% | |
| II | 49.9% | 52.8% | |
| III | 33.9% | 34.0% | |
| IV | 4.1% | 2.1% | |
| Systolic blood pressure (mmHg) |
120 (110, 130) | 120 (110, 130) | 0.189 |
| Pulse (bpm) | 75 (67, 84) | 72 (65, 80) | <0.001 |
| Labs | |||
| Hemoglobin (g/dL) | 13.4 (12.2, 14.6) | 14.1 (13.0, 15.1) | <0.001 |
| Creatinine (mg/dL) | 1.1 (0.9, 1.3) | 1.1 (1.0, 1.2) | 0.642 |
| Sodium (mEg/L) | 139 (136, 141) | 140 (138, 142) | <0.001 |
| BUN (mg/dL) | 23 (16, 36) | 22 (16, 38) | 0.959 |
| Medication use at baseline |
|||
| Beta blocker | 85.1% | 85.8% | 0.741 |
| ACEi or ARB | 90.2% | 89.1% | 0.536 |
| Statin | 82.4% | 80.2% | 0.339 |
| Digoxin | 26.4% | 16.0% | <0.001 |
| Aspirin | 83.0% | 82.4% | 0.789 |
| Warfarin | 10.8% | 10.2% | 0.737 |
| Clopidogrel | 21.9% | 14.0% | <0.001 |
| Loop diuretic | 72.0% | 60.8% | <0.001 |
ACEi – angiotensin converting enzyme inhibitor; ARB – angiotensin receptor blocker BMI – body mass index; BUN – blood urea nitrogen; CABG – coronary artery bypass grafting; CCS – Canadian Classification Score; ICD – implantable cardioverter defibrillator; MI – myocardial infarction; NYHA – New York Heart Association; PCI – percutaneous coronary intervention
Symptoms
There was a trend towards more of the patients with diabetes having no symptoms of angina (42% v 33%), (p=0.059). The distribution of NYHA class was similar in patients with and without diabetes.
Medication use at baseline
Patients with and without diabetes were both well treated with similar rates of angiotensin converting enzyme inhibitor/angiotensin receptor blocker and beta-blocker usage at baseline. Patients with diabetes were more likely to be treated with digoxin, clopidogrel and loop diuretics. At baseline, 40.3% (n=197) of patients with diabetes were treated with insulin.
Baseline LV function and coronary anatomy
Table 2 details the baseline left ventricular function and coronary anatomy in patients with and without diabetes. Triple vessel disease was more common in the diabetic group (67% v 56%, p<0.001), but diabetics and non-diabetics had similar rates of left main stem disease or proximal left anterior descending stenoses. Patients with diabetes had a higher median LVEF [29% in comparison to 27% in patients without diabetes (p=0.015)] and a smaller LVESVI. Diastolic filling pressure was significantly higher in patients with diabetes compared to the patients without diabetes. E velocity (p <0.0001), E/A ratio (p=0.006), and E/e’ (p< 0.001) were significantly higher in patients with diabetes. Patients with diabetes were also less likely to have anterior wall akinesia or dyskinesia (35% v 43%, p=0.002). Rates of mitral regurgitation were relatively similar.
Table 2.
Baseline left ventricular function and coronary anatomy
| Diabetes (n=489) |
No Diabetes (n=723) |
P | |
|---|---|---|---|
| LV Function | |||
| LVEF (%) | 29 (22, 35) | 27 (22, 33) | 0.015 |
| ESVI (mL/m2) | 74 (57, 94) | 82 (64, 106) | <0.001 |
| EDVI (mL/m2) | 105 (85, 128) | 117 (93, 146) | <0.001 |
| E velocity (m/s) (283/475)* | 0.79 (0.60, 1.00) | 0.66 (0.5, 0.8) | <0.0001 |
| A velocity (m/s) (278/463)* | 0.69 (0.50, 0.90) | 0.66 (0.50, 0.80) | 0.067 |
| E/A ratio (277/463)* | 1.46 (0.70, 2.00) | 1.29 (0.64, 1.57) | 0.0061 |
| E/e’ ratio (149/316)* | 20.0 (13.3, 25.0) | 17.3 (10.0, 20.0) | <0.0001 |
| Anterior akinesia or dyskinesia (%) | 35 (10, 56) | 43 (29, 57) | 0.002 |
| Mitral regurgitation | 0.132 | ||
| None or trace | 39.2% | 33.8% | |
| Mild | 42.5% | 48.1% | |
| Moderate | 16.2% | 14.1% | |
| Severe | 2.1% | 4.0% | |
| Coronary Anatomy | |||
| No. vessels with >50% stenosis | <0.001 | ||
| 1 | 5.1% | 12.0% | |
| 2 | 27.8% | 31.9% | |
| 3 | 67.1% | 56.1% | |
| Left main stenosis >50% | 3.1% | 2.4% | 0.448 |
| Proximal LAD stenosis >75% | 66.1% | 69.7% | 0.185 |
EDVI – end diastolic volume index; ESVI – end systolic volume index; LAD – left anterior descending; LV – left ventricle; LVEF – left ventricular ejection fraction;
The numbers in the parenthesis indicate the number of patients in whom the variable was measured (diabetes/no diabetes).
Procedural comparison and perioperative complications
Table 3 compares the procedural details and the perioperative complications in patients with and without diabetes who were randomized to CABG and who received CABG. Both groups had a similar distribution of the number and types of conduits, and the number of distal anastomoses. Patients with diabetes spent a longer time on bypass (97 v 87 minutes, p=0.029), and had a slightly longer cross clamp time (median 57 v 52 minutes, p=0.051). In the perioperative period, the two groups had similar rates of mediastinitis, infection and use of hemodynamic support (intra-aortic balloon pumps (IABP) and inotropes). Patients with diabetes were more likely to develop perioperative AF (23% v 12%, P<0.001) and had more than double the rate of worsening renal insufficiency (9% v 4%, p=0.021) and cardiac arrest requiring CPR (6% v 3%, p=0.037).
Table 3.
Procedural Comparison and Perioperative Complications in patients with and without diabetes. Patients randomized to CABG who received CABG (ie per protocol)
| Diabetes (n=222) |
No Diabetes (n=333) |
P | |
|---|---|---|---|
| Number of conduits | 0.085 | ||
| 1 | 10.4% | 13.8% | |
| 2 | 31.1% | 31.8% | |
| 3 | 41.4% | 43.2% | |
| >4 | 17.2% | 11.1% | |
| Number of arterial conduits | 0.622 | ||
| 0 | 11.3% | 7.5% | |
| 1 | 74.8% | 84.1% | |
| 2 | 11.3% | 6.9% | |
| >3 | 2.7% | 1.5% | |
| Number of distal anastomoses |
0.062 | ||
| 0 | 1.4% | 1.2% | |
| 1 | 9.5% | 12.7% | |
| 2 | 21.6% | 24.1% | |
| 3 | 39.2% | 40.4% | |
| 4 | 20.3% | 16.0% | |
| >5 | 8.2% | 5.7% | |
| Off-pump bypass | 21.6% | 20.4% | 0.733 |
| Time in bypass (mins) | 96.5 (71, 126) | 87.0 (65, 115) | 0.029 |
| Cross-clamp time (mins) | 56.5 (39, 78) | 52.0 (35, 70) | 0.051 |
| ICU length of stay (hrs) | 66.0 (40, 113) | 49.6 (41, 92) | 0.134 |
| Perioperative complications | |||
| Return to operating room | 8.1% | 5.1% | 0.154 |
| Mediastinitis | 1.8% | 2.1% | 0.999 |
| Other infection | 8.1% | 8.4% | 0.900 |
| New onset AF | 23.0% | 11.7% | <0.001 |
| Worsening renal insufficiency |
9.0% | 4.2% | 0.021 |
| IABP | 15.8% | 16.2% | 0.887 |
| Inotropes for low cardiac output |
39.6% | 38.4% | 0.776 |
| Cardiac arrest requiring CPR |
6.3% | 2.7% | 0.037 |
| Pulmonary edema requiring intubation |
3.6% | 1.8% | 0.185 |
AF – atrial fibrillation; CPR – cardiopulmonary arrest; IABP – intra-aortic balloon pump; ICU – intensive care unit; OR – operating room.
Outcomes
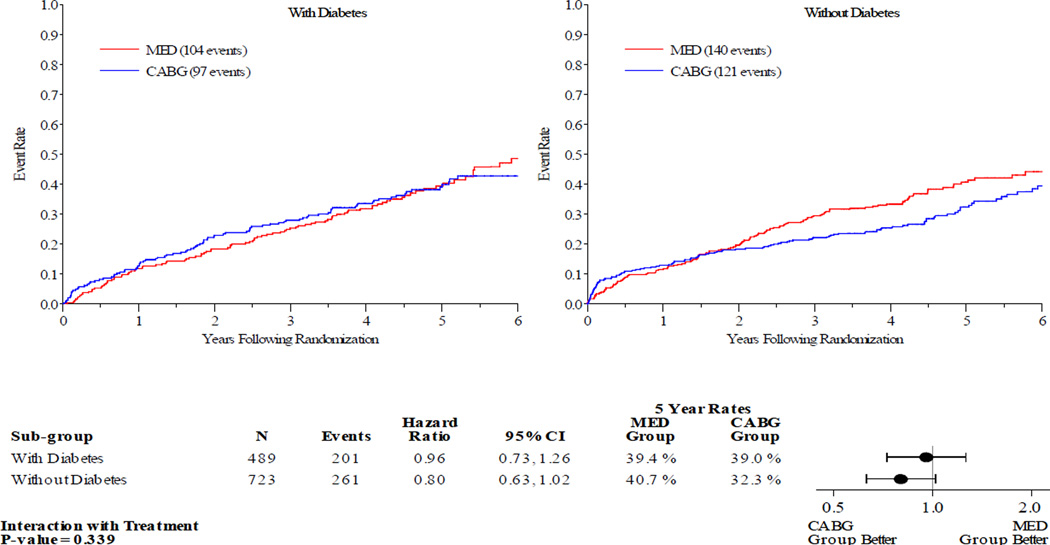
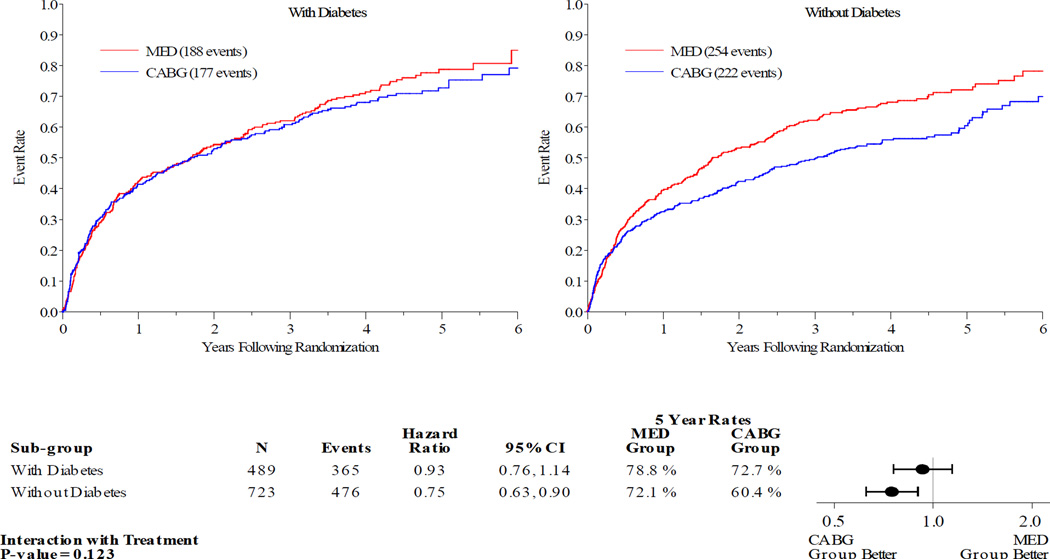
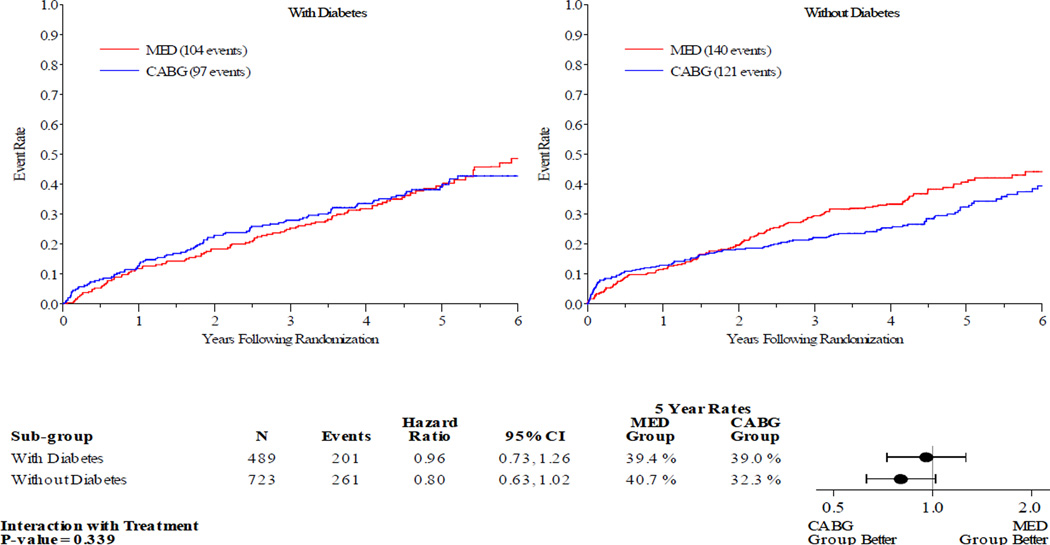
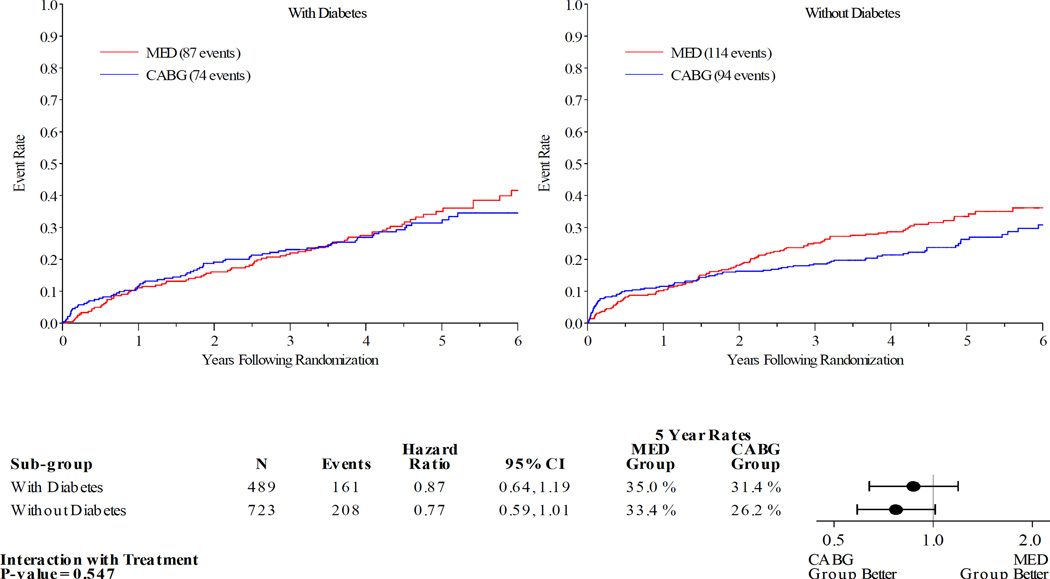
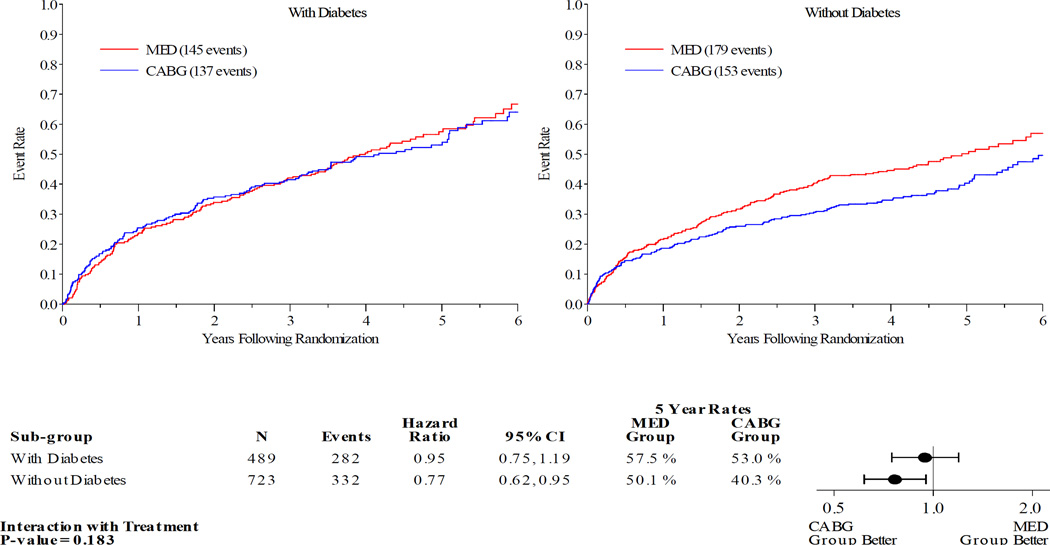
Figures 1–5 display the Kaplan-Meier curves for the intention to treat population, comparing CABG vs. MED in patients with and without diabetes. Each figure also details the corresponding event rates and unadjusted hazard ratios in each group along with a test for interaction between treatment group and diabetic status. Kaplan-Meier curves comparing the outcomes of diabetics with non-diabetics in each treatment arm are available in the online Appendix. Across all the outcomes apart from all-cause death, patients with diabetes had higher event rates than those without. The 5 year rates of all-cause death in the MED group were similar in diabetics and non-diabetics, at 39.4% and 40.7% respectively.
Figure 1.
Kaplan-Meier Estimates of All-Cause Mortality Rates for CABG vs. MED as Randomized (ITT). Red lines represent event rates in patients treated with MED alone. Blue lines represent event rates in patients treated with MED and coronary CABG. CABG – coronary artery bypass grafting; ITT – intention to treat; MED – optimal medical therapy.
Figure 5.
Kaplan-Meier Estimates of Mortality or All-cause Hospitalization Rates for CABG vs. MED as Randomized (ITT) . Red lines represent event rates in patients treated with MED alone. Blue lines represent event rates in patients treated with MED and coronary CABG. CABG – coronary artery bypass grafting; ITT – intention to treat; MED – optimal medical therapy.
Patients with DM had similar clinical outcomes whether randomized to CABG and MED or MED alone (figures 1–5). A statistically significant or near statistically significant improvement in clinical outcomes with CABG compared to MED was documented in patients without DM, but not in patients with DM. However, there was no significant interaction between DM and treatment group on formal statistical testing. Analysis of outcomes based on treatment received and as per-protocol showed similar trends (online Appendix).
Multivariable analysis
Table 4 details the hazard ratios for MED vs. CABG in patients with and without diabetes, before and after adjustment for all independent prognostic covariates. Multivariable adjustment did little to alter the pattern seen in the unadjusted data and the interaction p-values remained non-significant.
Table 4.
Outcomes in the intention to treat population
| 5 Year Event rate (%) | Hazard Ratio (95% CI)1 | Interaction P-value | |||||
|---|---|---|---|---|---|---|---|
| CABG | MED | Unadjusted | Adjusted2 | Unadjusted | Adjusted2 | ||
| All-cause death | Diabetes | 39.0 | 39.4 | 0.96 (0.73–1.26) | 0.88 (0.67, 1.17) | 0.339 | 0.605 |
| No diabetes | 32.3 | 40.7 | 0.80 (0.63–1.02) | 0.80 (0.62, 1.02) | |||
| CV mortality | Diabetes | 31.4 | 35.0 | 0.87 (0.64–1.19) | 0.82 (0.60, 1.12) | 0.547 | 0.731 |
| No diabetes | 26.2 | 33.4 | 0.77 (0.59–1.01) | 0.76 (0.58, 1.00) | |||
| Death or CV hospitalization |
Diabetes | 60.3 | 71.7 | 0.82 (0.66–1.02) | 0.79 (0.63, 0.98) | 0.232 | 0.417 |
| No diabetes | 52.9 | 67.5 | 0.69 (0.57–0.84) | 0.68 (0.56, 0.83) | |||
| Death or HF hospitalization |
Diabetes | 53.0 | 57.5 | 0.95 (0.75–1.19) | 0.86 (0.68, 1.09) | 0.183 | 0.453 |
| No diabetes | 40.3 | 50.1 | 0.77 (0.62–0.95) | 0.75 (0.61, 0.94) | |||
| Death or all- cause hospitalization |
Diabetes | 72.7 | 78.8 | 0.93 (0.76–1.14) | 0.91 (0.74, 1.12) | 0.123 | 0.212 |
| No diabetes | 60.4 | 72.1 | 0.75 (0.63–0.90) | 0.74 (0.62, 0.89) | |||
Hazard ratios are based on totality of data.
Baseline factors adjusted are age, ESVI, creatinine, mitral regurgitation, heart rate, and history of stroke. These are risk factors identified from previous multivariable model analyses among STICH Hypothesis 1 patients.
CABG – coronary artery bypass grafting; CV – cardiovascular; HF – heart failure; MED – optimal medical therapy.
Diabetic treatment at baseline
To assess the effect of baseline diabetic treatment on outcomes we performed Kaplan-Meier analysis of CABG vs. MED in 3 subgroups: diabetics on insulin, diabetics not on insulin and non-diabetics (online Appendix). In both diabetic treatment groups no benefit was seen with CABG over MED.
Myocardial viability
Data on myocardial viability was available for 601 patients out of the 1212 included in this analysis. Of this sub-group, 38% (n=228) had diabetes. When viability was analyzed as a dichotomous (yes/no) variable, patients with diabetes were significantly more likely to have viable myocardium (89% v 76%, p=0.0002). Similarly, when viability was analyzed as a continuous variable (percent of viable segments for each patient), patients with diabetes had an average of 72% viable segments in comparison to an average of 63% viable segments in patients without diabetes (p<0.0001).
Discussion
For the last 2 decades patients with diabetes have been preferentially referred for CABG based on a sub-group analysis of the BARI study.(5) That trial included very few patients with heart failure and even fewer with diabetes and heart failure. More recently, BARI2D and FREEDOM have reported that CABG is superior to PCI and MED in patients with diabetes.(7,8) These trials similarly enrolled very few patients with heart failure and low LVEF, yet their results are often extrapolated to this population. The STICH trial is the first study to randomize a large cohort of patients with heart failure with and without diabetes to CABG or modern MED. Numerically it appears that in patients with heart failure and low LVEF, the group with the most to gain from CABG are the cohort of patients without diabetes. Perhaps this is surprising given the cardiology community’s long-standing and firmly held assumption that results of trials in patients without heart failure could be extrapolated to those with heart failure. We must be cautious not to over interpret this data. The STICH trial was not designed or powered to examine this hypothesis and formal statistical testing did not identify an interaction between diabetes and treatment group. Therefore the most we should formally conclude is that diabetic status has no significant bearing on the treatment effect of CABG in patients with low LVEF. However, the hypothesis that diabetics with low LVEF may derive less benefit from CABG than non-diabetics may warrant further study.
There could be a number of reasons why patients with diabetes and heart failure may derive less benefit from CABG than patients without diabetes. The lower LVESVI and higher LVEF in the diabetic group at baseline may play a role. In the entire STICH population, patients with the highest LVESVI appeared to receive the greatest benefits from CABG.(14) However, even after we adjusted for LVESVI and other prognostic covariates, there is still an apparent divergence of the treatment effect of CABG in patients with and without diabetes.
If there really is no benefit from CABG over MED in patients with diabetes and heart failure perhaps this reflects the particularly severe CAD seen in patients with diabetes and heart failure. Grafts to small, diffusely diseased, diabetic vessels in this population may not confer sufficient benefit to translate into improved clinical outcomes. Similarly, patients without diabetes may have larger, less diseased native vessels that stand to gain more from receiving a graft. Another possibility is that revascularization was less complete in patients with diabetes. This seems unlikely given that patients with and without diabetes received similar numbers of distal anastomoses. It is likely that patients with diabetes also have more severe disease in their branch and distal coronary bed rendering the effect of grafting the major vessels less effective. Perhaps the myocardium of patients with diabetes and heart failure has less to gain from revascularization. Ischemia may not be the main driver of heart failure and outcomes in patients with diabetes with low LVEF, so revascularization may not have the same benefits as in patients without diabetes. As has previously been described, diabetic patients in the current study had higher LVEF and smaller ventricles than patients without diabetes. Diabetics were more likely to be female and hypertensive. This clinical and structural profile suggests a stiffer less compliant ventricle. There are numerous putative pathological processes in diabetes that can lead to small, stiff, fibrotic ventricles e.g. advanced glycation end-product deposition and SERCA2a abnormalities.(15,16) In fact, estimated diastolic filling pressure was significantly higher in patients with diabetes compared to the patients without diabetes. The increased filling pressure can also explain the higher incidence of post-operative atrial fibrillation since it has previously been shown that diastolic dysfunction is a powerful independent predictor for postoperative atrial fibrillation.(17) Diabetes itself predisposes patients to diastolic dysfunction and higher filling pressures which have in turn been shown to be a strong predictor for mortality in patients with ischemic and dilated cardiomyopathy.(18) Of the clinical, laboratory, and imaging variables, E/A ratio (which was higher in diabetic patients) was found to be the third most powerful independent predictor for overall mortality after LVESVI and creatinine in the STICH population (unpublished data). These abnormalities may mitigate the benefit from improved perfusion that is seen in diabetic patients with normal ventricles. Lack of viable myocardium does not appear to be the explanation for lack of benefit in patients with diabetes and heart failure. In the current analysis patients with diabetes had more viable myocardium than those without diabetes.
The baseline characteristics of the patients with diabetes in this analysis suggest that this population is similar to other heart failure populations. The prevalence of diabetes at 40% is in-keeping with that seen in other heart failure studies.(1) As expected, the diabetic patients were older, more likely to be female, had higher BMIs and higher rates of hypertension and hyperlipidemia. Whilst it is often thought that diabetes is associated with less symptoms of angina, so called “silent ischemia” we were only able to demonstrate a trend towards fewer symptoms.
A further novel aspect of this study is our comparison of the procedural and perioperative outcomes of patients with and without diabetes with heart failure, undergoing CABG. Patients with diabetes spent 10 minutes longer on bypass and there was a trend towards them having a longer cross-clamp time. It seems likely that the greater surgical challenge presented by diabetic coronary anatomy and a higher BMI is responsible. Patients with diabetes were more than twice as likely to develop perioperative atrial fibrillation and had more than double the rate of worsening renal insufficiency. These factors could partly relate to the longer bypass times, but also to atrial fibrosis, higher baseline diastolic filling pressure, and pre-existing diabetic nephropathy.
Only separate trials of patients with heart failure with and without diabetes powered to address clinical outcomes will settle the question of how much each population has to gain from surgical revascularization.
We must acknowledge that there are a number of limitations to this study. This is a retrospective subgroup analysis that was not pre-specified and therefore its results must be interpreted as hypothesis generating. The diagnosis of diabetes relied on investigator reported diabetes or baseline treatment for diabetes. It is likely that there will have been patients with undiagnosed diabetes.(19) The speculation that a higher diastolic filling pressure in patients with diabetes at baseline was partly responsible for the lack of evidence of benefit from CABG compared to MED is based on echocardiographic evaluation of diastolic parameters which were only present in a subset of patients with available data in sinus rhythm. The trial was also not blinded and diabetic patients could have been treated differently during initial hospitalization and follow-up than those without diabetes. There is also the possibility of bias during patient selection. For example, did the presence of diabetes make a physician less likely to enroll a patient in the study due to extrapolation of evidence from previous trials conducted in patients without heart failure? This seems unlikely to have been a major issue given that the prevalence of diabetes in the STICH study is similar to other heart failure trials. The STICH trial did not conduct a comprehensive registry which would have answered this question. Not all patients with heart failure were included in the STICH trial. Patients with left main disease and severe angina were excluded. Patients with LVEF>35% were not included. Our conclusions cannot apply to these groups. Lastly, the time course for the effect of CABG on outcomes may differ by diabetes status. The STICH Extension Study will provide an average of 10 years of follow-up on these study patients, which will inform whether these intermediate term results remain stable or are modified over time.
Conclusions
STICH is the only study to date to compare the outcomes of a large number of patients with heart failure with and without diabetes undergoing CABG or MED. Patients with diabetes had higher LVEF, smaller LV volumes, higher diastolic filling pressures, longer time on cardiopulmonary bypass and more perioperative atrial fibrillation and renal dysfunction than those without diabetes. Whilst numerically it would appear that the treatment effect of CABG is blunted in patients with diabetes, there was no significant interaction between diabetes and treatment group on formal statistical testing.
Supplementary Material
Figure 2.
Kaplan-Meier Estimates of All-Cause Mortality or Cardiovascular Hospitalization Rates for CABG vs. MED as Randomized (ITT). Red lines represent event rates in patients treated with MED alone. Blue lines represent event rates in patients treated with MED and coronary CABG. CABG – coronary artery bypass grafting; ITT – intention to treat; MED – optimal medical therapy.
Figure 3.
Kaplan-Meier Estimates of Cardiovascular Mortality Rates for CABG vs. MED as Randomized (ITT) . Red lines represent event rates in patients treated with MED alone. Blue lines represent event rates in patients treated with MED and coronary CABG. CABG – coronary artery bypass grafting; ITT – intention to treat; MED – optimal medical therapy.
Figure 4.
Kaplan-Meier Estimates of All-Cause Mortality or Heart Failure Hospitalization Rates for CABG vs. MED as Randomized (ITT) . Red lines represent event rates in patients treated with MED alone. Blue lines represent event rates in patients treated with MED and coronary CABG. CABG – coronary artery bypass grafting; ITT – intention to treat; MED – optimal medical therapy.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (National Heart, Lung, and Blood Institute grants U01HL69015 and U01HL69013) (NCT00023595). The views expressed in this manuscript do not necessarily reflect those of the NIH or NHLBI.
Abbreviations
- BARI
Bypass Angioplasty Revascularization Investigation
- BMI
Body Mass Index
- CAD
Coronary artery disease
- CABG
Coronary artery bypass graft
- FREEDOM
Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease
- LVEF
Left ventricular ejection fraction
- LVESVI
Left ventricular end systolic volume index
- MED
Optimal medical therapy
- NHLBI
National Heart, Lung, and Blood Institute
- STICH
Surgical Treatment for Ischemic Heart Failure
Footnotes
Conflicts of interest
Dr. Miller: consultant, St. Jude Medical, BioControl, Celadon, Sensible Medical, Respircardia, the National Institutes of Health, Novartis, and Pfizer
Dr. Rouleau: consultant, Novartis
All other authors have reported no relationships to disclose relevant to the contents of this manuscript.
References
- 1.MacDonald MR, Petrie MC, Hawkins NM, Petrie JR, Fisher M, McKelvie R, Aguilar D, Krum H, McMurray JJV. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. 2008;29:1224–1240. doi: 10.1093/eurheartj/ehn156. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, Lee KL, Califf RM, Jones RH. Comparison of coronary artery bypass grafting versus medical therapy on long-term outcome in patients with ischemic cardiomyopathy (a 25-year experience from the Duke Cardiovascular Disease Databank) Am J Cardiol. 2002;90:101–107. doi: 10.1016/s0002-9149(02)02429-3. [DOI] [PubMed] [Google Scholar]
- 5.Influence of Diabetes on 5-Year Mortality and Morbidity in a Randomized Trial Comparing CABG and PTCA in Patients With Multivessel Disease: The Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 1997;96:1761–1769. doi: 10.1161/01.cir.96.6.1761. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Smith SC, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice. Circ. 2012;126:3097–3137. doi: 10.1161/CIR.0b013e3182776f83. [DOI] [PubMed] [Google Scholar]
- 7.A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkouh ME, Domanski M, Sleeper La, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson Ea, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, Bertrand M, Fuster V. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez EJ, Lee KL, Deja Ma, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau J-L. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RH, White H, Velazquez EJ, Shaw LK, Pietrobon R, Panza JA, Bonow RO, Sopko G, O’Connor CM, Rouleau JL. STICH (surgical treatment for ischemic heart failure) trial enrollment. J Am Coll Cardiol. 2010;56:490–498. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velazquez EJ, Lee KL, O’Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O’Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau J-L, Lee KL. Coronary Bypass Surgery with or without Surgical Ventricular Reconstruction. N Engl J Med. Massachusetts Medical Society. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JK, Velazquez EJ, Menicanti L, Pohost GM, Bonow RO, Lin G, Hellkamp AS, Ferrazzi P, Wos S, Rao V, Berman D, Bochenek A, Cherniavsky A, Rogowski J, Rouleau JL, Lee KL Investigators on behalf of the S. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Hear J. 2013;34:39–47. doi: 10.1093/eurheartj/ehs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panza JA, Velazquez EJ, She L, Smith PK, Nicolau JC, Favaloro RR, Gradinac S, Chrzanowski L, Prabhakaran D, Howlett JG, Jasinski M, Hill JA, Szwed H, Larbalestier R, Desvigne-Nickens P, Jones RH, Lee KL, Rouleau JL. Extent of Coronary and Myocardial Disease and Benefit From Surgical Revascularization in LV Dysfunction. J Am Coll Cardiol. 2014;64:553–561. doi: 10.1016/j.jacc.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 16.Heerebeek L Van, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJJ, Schalkwijk CG, Bronzwaer JGF, Diamant M, Borbély A, Velden J Van Der, Stienen GJM, Laarman GJ, Niessen HWM, Paulus WJ. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 17.Melduni RM, Suri RM, Seward JB, Bailey KR, Ammash NM, Oh JK, Schaff HV, Gersh BJ. Diastolic dysfunction in patients undergoing cardiac surgery: a pathophysiological mechanism underlying the initiation of new-onset post-operative atrial fibrillation. J Am Coll Cardiol. 2011;58:953–961. doi: 10.1016/j.jacc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circ. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- 19.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, Rouleau JL, Sigouin C, Solymoss CB, Tsuyuki R, White M, Yusuf S. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21:1368–1375. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.