Abstract
Liver fibrosis occurs in response to any etiology of chronic liver injury including hepatitis B and C, alcohol consumption, fatty liver disease, cholestasis, and autoimmune hepatitis. Hepatic stellate cells (HSCs) are the primary source of activated myofibroblasts that produce extracellular matrix (ECM) in the liver. Various inflammatory and fibrogenic pathways contribute to the activation of HSCs. Recent studies also discovered that liver fibrosis is reversible and activated HSCs can revert to quiescent HSCs when causative agents are removed. Although the basic research for liver fibrosis has progressed remarkably, sensitive and specific biomarkers as non-invasive diagnostic tools, and effective anti-fibrotic agents have not been developed yet. This review highlights the recent advances in cellular and molecular mechanisms of liver fibrosis, especially focusing on origin of myofibroblasts, inflammatory signaling, autophagy, cellular senescence, HSC inactivation, angiogenesis, and reversibility of liver fibrosis.
Keywords: Alcoholic liver disease, Angiogenesis, Autophagy, Hepatic stellate cells, IL-17, IL-22, IL-33, Liver cirrhosis, Reversal, Senescence
Introduction
Fibrosis is a wound healing response that produces and deposits extracellular matrix (ECM) proteins including collagen fibers, causing tissue scarring [1, 2]. Liver usually regenerates after liver injury. However, when liver injury and inflammation are persistent and progressive, liver cannot regenerate normally and causes fibrosis. Progressive liver fibrosis results in cirrhosis where liver cells cannot function properly due to the formation of fibrous scar and regenerative nodules and the decreased blood supply to the liver [1, 2]. A variety of etiologies, such as hepatitis B and C infection, chronic alcohol abuse, non-alcoholic steatohepatitis (NASH), cholestasis, and autoimmune hepatitis, ultimately progress to liver cirrhosis. Although removal of causative agents of liver fibrosis will regress liver tissue scarring, it is difficult to treat advanced cirrhosis [3]. To date, there is no approved anti-fibrotic drug. Liver transplantation is the only curative therapy for liver cirrhosis. However, due to insufficient number of donor livers, the development of effective anti-fibrotic drugs is needed. Hepatic stellate cells (HSCs) are the major precursor of activated myofibroblasts, the cell type that produces ECM proteins during liver fibrosis. Quiescent HSCs store Vitamin A-containing lipid droplets, and HSCs lose lipid droplets when they are activated. Transforming growth factor (TGF)-β and platelet-derived growth factor (PDGF) are two major cytokines that contribute to HSC activation and proliferation, resulting in activation into myofibroblasts [4]. Many other cytokines, intracellular signaling, and transcription factors are involved in this process [4]. Controlling the activation process of HSCs would be an ideal therapeutic strategy for liver fibrosis. Therefore, the understanding of molecular mechanisms underlying HSC activation is crucial. This review highlights the recent advancement of molecular mechanisms of liver fibrosis.
Origin of activated myofibroblasts
Although it is believed that HSCs are the major precursor of myofibroblasts, other cell types, such as endogenous portal fibroblasts and myofibroblasts derived from liver parenchymal cells undergoing epithelial-mesenchymal transition (EMT) are also suggested to contribute to the myofibroblast pool [5]. The contribution of different sources of cells to the myofibroblast pool may be determined by the different etiology of liver fibrosis. The study done by Iwaisako et al. used phenotypic analysis to identify two collagen-producing cell populations: Vitamin A positive HSCs and Vitamin A negative portal fibroblasts using collagen promoter-driven green fluorescent protein (GFP) transgenic mice [5]. They demonstrated that myofibroblasts are mainly differentiated from HSCs in hepatotoxin (carbon tetrachloride [CCl4])-induced liver fibrosis. In early cholestatic liver disease, portal fibroblasts are the major source of the myofibroblast pool, while in later cholestatic injury HSCs predominate [5]. Intriguingly, Asahina and colleagues demonstrated that the differentiation of mesothelial cells contribute to the HSC pool upon liver injury [6].Mesothelial cells have the capacity to differentiate into both HSCs and myofibroblasts in CCl4-induced liver injury, whereas cholestatic liver injury induces the differentiation of mesothelial cells only into HSCs, but not myofibroblasts, suggesting that cells other than HSCs, such as portal fibroblasts, contribute to the myofibroblast pool in cholestatic liver injury [6]. Schwabe and colleagues also attempted to answer the same question. They newly generated Cre transgenic mice under control of the promoter of Lecithin retinol acyltransferase (Lrat), an enzyme required for Vitamin A metabolism, which is predominantly expressed in HSCs [7]. The study determined that HSCs are the primary cells to transdifferentiate into myofibroblasts in all mouse models of liver fibrosis, under conditions of more extensive fibrosis (toxic, cholestatic, and fatty liver disease) [7]. They also demonstrated that Lrat positive HSCs are not derived from bone marrow and does not differentiate into hepatocytes and cholangiocytes when liver regenerates [7]. It should be pointed out that the definitive study has not yet been performed in which each proposed myofibroblast precursor (i.e. HSCs or portal fibroblasts) are genetically labeled with a cell-specific inducible Cre so that a classic pulse-chase experiment could be performed in a fibrosis model to follow a discrete cell population into the activated myofibroblast population (Fig. 1).
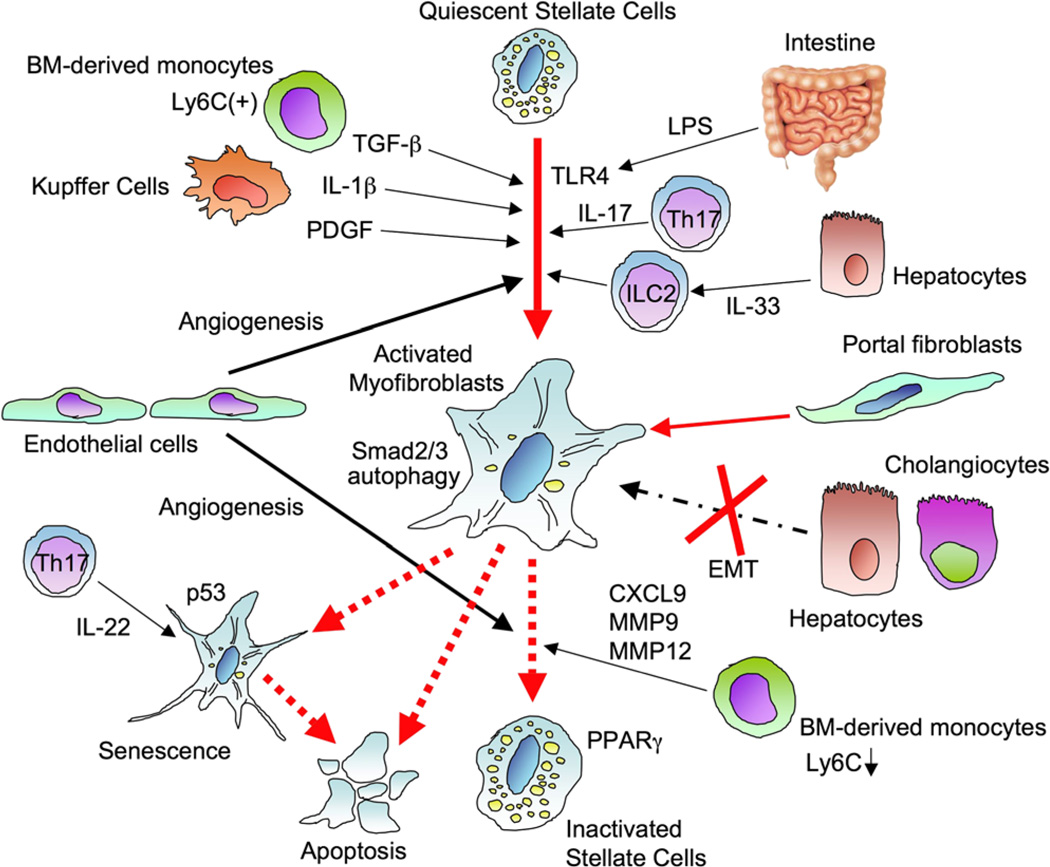
Fig. 1.
Activation and regression of hepatic stellate cells. Quiescent hepatic stellate cells (HSCs) store Vitamin A containing lipid droplets and lose Vitamin A when the cells are activated. Hepatic epithelial injury, such as death of hepatocytes and biliary epithelial cells, induces activation of HSCs directly or through cytokines released from immune cells including Kupffer cells, bone marrow-derived monocytes, Th17 cells, and innate lymphoid cells (ILC). Transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), interleukin-1β (IL-1β), IL-17, and intestine-derived lipopolysaccharide (LPS) promote HSC activation. IL-33 promotes HSC activation through ILC2. Autophagy in HSCs is associated with HSC activation. The activated myofibroblast pool is mainly constituted by activated HSCs, but biliary injury induces differentiation of portal fibroblasts to activated myofibroblasts. However, there is no evidence of epithelial-mesenchymal transition for constituting the myofibroblast pool. After the cessation of causative liver injury, fibrosis starts regression, and activated HSCs induce apoptosis or revert into a quiescent state. Peroxisome proliferator-activated receptor γ (PPARγ) expression in HSCs is associated with HSC reversal. Some activated HSCs become senescent, resulting in loss of profibrogenic property in which p53 plays a role. Moreover, angiogenesis contributes to both fibrosis development and regression
In addition, several other studies using genetic cell fate mapping concluded that EMT does not contribute to the myofibroblast pool and liver fibrosis in mice. The studies labeled hepatocytes, cholangiocytes, and both hepatocytes and cholangiocytes using the Cre-loxP technology (Albumin-Cre, CK19-Cre and AFP-Cre, respectively) and traced these cells during the development of liver fibrosis [8–10]. The studies found that myofibroblasts originate from neither hepatocytes nor cholangiocytes. Although we have to be aware that mouse study cannot recapitulate all human diseases, currently available mouse studies suggest that: (1) EMT does not occur; (2) HSCs are the major source of myofibroblasts in hepatotoxic liver fibrosis; (3) portal fibroblasts are important contributors to themyofibroblast population in early cholestatic liver injury; and (4) mesothelial cells have potential to differentiate into both HSCs and myofibroblasts upon liver injury.
TLR4 and intestinal microbiome
Plasma and portal endotoxin (also known as lipopolysaccharide [LPS], Gram negative bacterial cell wall component) levels are elevated in cirrhotic patients. Since gut leakiness, bacterial overgrowth, dysbiosis are seen in patients with liver cirrhosis, it is conceivable that translocated microbial products and TLR4, an endogenous sensor for LPS, may contribute to liver disease progression [11]. Indeed, mice deficient in TLR4, CD14, and LPS-binding protein are resistant to mouse model of alcoholic liver disease [12]. Similarly, TLR4 mutant mice and mice with gut sterilization fail to develop liver fibrosis [4, 13]. Notably, TLR4 mutant mice show similar elevation of blood LPS levels with WT mice during liver fibrosis, suggesting that TLR4 primarily functions in the liver [13]. Although immune cells including Kupffer cells express TLR4, HSCs are the primary cells for TLR4-mediated liver fibrosis (Fig. 1) [13]. TLR4-stimulated HSCs produce a variety of chemokines (e.g. CCL2, CCL3, CXCL2, CXCL10) and express adhesion molecules (e.g. E-selectin, VCAM-1, ICAM-1) that promote inflammatory cell infiltration into the liver. TLR4 signaling also enhances TGF-β signaling in HSCs by downregulating BMP and activin membrane bound inhibitor (BAMBI), a decoy receptor for TGF-β receptor, promoting fibrogenic response [13]. In HSCs, nuclear factor (NF)-κ Bp50:p50 and HDAC1 transcriptionally regulates BAMBI expression [14]. Moreover, TLR4-mediated fibronectin production in HSCs drives angiogenesis, promoting liver fibrosis and portal hypertension [15]. TLR4 signaling also regulates HSC activation and liver fibrosis through inhibiting miR-29 expression [16]. Importantly, TLR4 SNPs are associated with the degrees of liver fibrosis in HCV patients [17], demonstrating the clinically relevant role of TLR4 in human liver fibrosis. In contrast to TLR4 that functions within the liver, TLR2, a receptor for Gram-positive bacterial components, can maintain the intestinal barrier function to prevent bacterial translocation in liver fibrosis. TLR2−/− mice show reduced liver fibrosis by inhibiting translocation of LPS into the liver [18]. Intestinal microbial environment may be affected by different etiologies of liver disease. Bile duct ligation (BDL) induces bacterial overgrowth in early stage but does not alter the composition of intestinal bacteria whereas chronic CCl4 treatment changes in the composition of intestinal bacteria only after liver fibrosis has developed [19]. Dysbiosis in CCl4-treated mice represents increased Firmicutes and Actinobacteria [19]. The difference between quantitative changes (bacterial overgrowth) after BDL and qualitative changes (dysbiosis) by CCl4 treatment may be due to the direct effect of decreased intestinal bile acids and the secondary effect of CCl4-induced hepatoxicity, respectively. Although BDL alone does not induce dysbiosis, the high fat diet (HFD) feeding condition significantly alters the composition of intestinal bacteria (increased ratio of Gram-positive bacteria and reduced ratio between Bacteroidetes and Firmicutes) after BDL [20].Accordingly, cholestasis-induced liver fibrosis is augmented in HFD-fed mice compared with control chow-fed animals [20]. The study further purified “fibrogenic” Gram-negative bacteria from HFD + BDL mice and confirmed that these “fibrogenic” bacteria significantly augment liver fibrosis [20]. HFD feeding is also associated with the increased composition of Clostridium cluster XI, the bacteria that metabolite primary bile acids to deoxycholic acid (DCA) [21]. Intestine-derived DCA further induces DNA damage and production of reactive oxygen species to promote hepatocellular carcinoma (HCC) development [21]. In fibrosis-associated hepatocarcinogenesis model (DEN + CCl4), the composition of intestinal microbiome is similar between WT and TLR4 mutant mice, suggesting that TLR4 does not play a role in intestinal dysbiosis during chronic liver injury [22]. The study also confirmed that non-absorbable long-term antibiotics treatment and germ-free condition suppress the growth of fibrosis-associated HCC [22]. However, a recent report studying liver fibrosis using germ-free animals demonstrated that the germ-free mice are more susceptible to hepatotoxin-induced liver fibrosis, suggesting beneficial bacteria existed in the intestine that prevents liver fibrosis, in addition to harmful bacteria that increase in chronic liver disease [23]. The discrepancy between the studies done by Dapito et al. and Mazagova et al. may be explained by the duration of toxin exposure, or with or without DEN treatment. The different husbandry and mouse house environment of control animals between the studies may also be considered.
Inflammatory cytokines
Recent studies demonstrated the importance of IL-17, IL-22, and IL-33 in liver fibrosis (Fig. 1). IL-17 is mainly produced from Th17 cells and upregulated in hepatitis B and C, alcoholic liver disease, and autoimmune hepatitis [24]. IL-17 is a proinflammatory and profibrogenic cytokine that activates NF-κB and STAT3 in Kupffer cells and HSCs. IL-17-stimulated HSCs upregulate levels of collagen α1(I), α-smooth muscle actin (αSMA), and TGF-β, promoting liver fibrosis [25]. Mice deficient in IL-17A or IL-17RA are resistant to cholestasis and toxin-induced liver fibrosis [25]. Interestingly, the anti-fibrotic effect of endocannabinoid CB2 receptor signaling is mediated through inhibiting IL-17 production [26].
Th17 cells also produce IL-22. In the liver, IL-22R is expressed on hepatocytes and HSCs, but not immune cells. IL-22 induces its biological functions, such as cell proliferation, tissue repair, and wound healing response through STAT3 [27]. Blood IL-22 levels are elevated in cirrhotic patients, and elevated IL-22 levels correlate with severity of liver cirrhosis, and complications and mortality rate [28]. Although IL-22 is procarcinogenic [29], IL-22 is protective against alcoholic liver disease, T cell-mediated hepatitis model, and acetaminophen-induced liver injury [30–32]. Moreover, IL-22 has an anti-fibrotic effect and IL-22 treatment inhibits liver fibrosis through induction of HSC senescence via STAT3 and p53 [33]. However, some studies show that IL-22 is pathogenic and promotes liver inflammation and fibrosis through Th17 cells and liver progenitor cells in hepatitis B patients and hepatitis B virus transgenic mice [34–36]. While the hepatoprotective effect of IL-22 is well-documented, the detrimental effect of IL-22 is seen in hepatitis B and HCC.
In cirrhotic patients, the levels of IL-33 and its receptor ST2 are elevated [37]. IL-33 expression is also upregulated in mouse liver fibrosis induced by exposure to CCl4 and TAA, and infection of Schistosoma mansoni [37]. IL-33 is released from injured hepatocytes as a danger-alerting molecule. IL-33 induces IL-13 production in liver resident innate lymphoid cells type II (ILC2). IL-13 signaling then enhances TGF-β signaling through IL-4Rα and STAT6 in HSCs, promoting liver fibrosis [37]. IL-33−/− mice, mice treated with soluble ST2, or mice with depletion of ILC by anti-Thy1.2 antibody are resistant to liver fibrosis [37]. IL-33 is also involved in the development of primary biliary cirrhosis, biliary repair, and carcinogenesis through ILC2 and IL-13 [38, 39].
TGF-β and liver fibrosis
Transforming growth factor-β plays a central role in fibrotic diseases including liver fibrosis [40]. In the liver, liver macrophages including Kupffer cells are the main producers of TGF-β, while HSCs also produce TGF-β. TGF-β is produced as the latent form that requires processing to be active. An αv integrin contributes to liver fibrogenesis via activation of TGF-β [41]. Binding of bioactive TGF-β to TGF-β receptor type II phosphorylates TGF-β type I receptor that activates Smad- and non-Smad pathways [40]. In HSCs, TGF-β-mediated Smad2/3 activation induces the transcription of type I and III collagen, promoting liver fibrosis (Fig. 1). Smad signaling also induces Smad7 transcription, negatively regulating TGF-β signaling [40]. Another TGF-β negative regulator BAMBI interacts with TGF-β type I receptor and Smad7 to inhibit TGF-β signaling [42]. A new report demonstrated the role of Vitamin D nuclear receptor (VDR) in modulation of TGF-β-Smad signaling. Activation of VDR antagonizes Smad binding to the promoter region of profibrogenic genes in HSCs [43]. Accordingly, VDR-deficiency promotes and Vitamin D treatment attenuates liver fibrosis in mice [43].
In primary culture hepatocytes, TGF-β induces EMT-like phenotypical changes that express type I collagen. Unlike in vitro observations, the TGF-β-mediated EMT-like changes are not observed in liver fibrosis in vivo [8, 10]. Instead, TGF-β signaling mediates hepatocyte death in lipid-laden hepatocytes, which secondarily activates HSCs to promote liver fibrosis [44]. TGF-β signaling also induces connective tissue growth factor in hepatocytes, promoting liver fibrosis [45].
HSC senescence in liver fibrosis and HCC
Senescent HSCs are often observed in cirrhotic livers. Senescent activated HSCs lose their proliferative and collagen-producing capacity and have increased inflammatory property to produce inflammatory cytokines compared with replicating activated HSCs [46]. p53 is associated with cellular senescence through p21 induction (Fig. 1). HSCs isolated from p53−/− mice are resistant to undergo senescence and have more proliferative ability than WT HSCs [47]. Accordingly, p53−/− mice exhibit more severe liver fibrosis than WT mice, implying that p53-mediated cellular senescence restricts the development of liver fibrosis [47]. Moreover, senescent HSCs upregulate expression of inflammatory cytokines and are prone to apoptosis through NK cell-mediated killing, which limits fibrosis progression [47]. The follow-up study further demonstrated p53 expression in senescent HSCs to be associated with the polarization of liver macrophages to M1-state through their senescence-associated secretory phonotype (SASP), resulting in inhibiting the development of hepatocellular carcinoma (HCC) [48]. Consistently, p53−/− HSCs induce the polarization of macrophages to M2 phenotype that promote HCC proliferation through affecting tumor microenvironment [48]. Intriguingly, IL-22 also induces HSC senescence through the STAT3-p53 axis, limiting liver fibrosis. IL-22-mediated induction of HSC senescence may be a new interventional strategy for liver fibrosis [33]. In contrast to the aforementioned studies, there is a report showing that SASP phenotypes in HSCs promote obesity-associated HCC development [21]. Interestingly, in this study obesity-mediated HSC senescence and SASP phenotype are not associated with liver fibrosis [21]. Thus, the role of senescence of HSCs in liver fibrosis is still unresolved and requires further experiments using cell-specific genetic modifications to HSCs in experimental models of liver fibrosis in vivo.
Autophagy in liver fibrosis
Autophagy is the process to maintain cellular homeostasis by degrading and recycling protein aggregates or damaged organelles (e.g. mitochondria). Autophagy flux is observed during HSC activation, and inhibition of autophagy suppresses HSC activation and proliferation [49]. Therefore, mice with HSCs lacking autophagy have reduced HSC activation and liver fibrosis. Since autophagy is associated with lipid degradation, HSCs lacking autophagy fail to lose lipid droplets and maintain cells in a quiescent state, indicating the requirement of autophagy for HSC activation (Fig. 1) [49]. α1 anti-trypsin (AT) deficiency is a common genetic disease that causes liver disease by accumulating mutant Z protein within endoplasmic reticulum of hepatocytes [50, 51]. Mice harboring α1 AT Z mutation recapitulate many features of human α1 AT deficiency including liver fibrosis [50, 51]. In patients with α1 AT deficiency and mice with mutant Z protein, autophagy in the hepatocyte is activated and autophagic vacuoles contain α1 AT mutant Z protein. Treatment with autophagy-inducing drugs, carbamazepine or rapamycin, suppresses hepatic accumulation of globules containing α1 AT mutant Z protein and liver fibrosis [50, 51]. Thus, while autophagy is required for HSC activation, autophagy induction in hepatocytes is beneficial for many other liver diseases, including α1 AT deficiency, alcoholic liver disease (ALD), and NASH.
Angiogenesis and liver fibrogenesis
Hepatic stellate cells are the hepatic pericytes and their contractility regulates sinusoid contraction associated with intrahepatic resistance of blood flow and portal hypertension [52]. Endothelin-1 and angiotensin II control the contractility of HSCs [52]. HSCs also contribute to angiogenesis through production of vascular endothelial growth factor (VEGF) and angiopoietin-1 [53, 54]. Inhibition of angiogenesis by blocking VEGF or angiopoietin-1 inhibits liver fibrosis, implying an important role of angiogenesis in liver fibrogenesis (Fig. 1) [54, 55].
The liver regenerates after acute liver injury while liver induces fibrosis instead of normal regeneration during chronic liver injury. Rafii and colleagues demonstrated that chemokine receptors CXCR4 and CXCR7 regulate switching between regeneration and fibrogenesis [56]. Acute liver injury induced by CCl4 or acetaminophen upregulates CXCR7 in liver sinusoidal endothelial cells (LSECs). CXCR7 signaling induces production of hepatocyte growth factor (HGF) and Wnt2 through inhibitor of DNA binding 1 (Id1) in LSECs, promoting liver regeneration [56]. In contrast, chronic liver injury induced by BDL or chronic CCl4 treatment upregulates CXCR4 through fibroblast growth factor receptor 1 (FGFR1). The FGFR1-CXCR4 axis in LSECs inhibits the CXCR7-Id1 pathway to inhibit normal liver regeneration but activate HSCs, shifting aberrant regenerative response, fibrosis [56].
Reversibility of liver fibrosis
Fibrosis has been believed to be irreversible for a long time. However, many researchers also predicted reversibility of fibrosis because fibrosis can regress when the causative conditions including alcohol, hepatitis viruses, chemicals, biliary obstruction, and obesity are removed both in patients and in rodent models. It has been reported that activated HSCs undergo apoptosis during fibrosis resolution (Fig. 1) [57]. On the other hand, in culture experiment activated HSCs can revert to a quiescent condition if peroxisome proliferator-activated receptor γ (PPARγ) is overexpressed or the cells are treated with a PPARγ ligand [58]. Recently, two independent studies using cell fate tracking demonstrated that approximately 40–50% of activated HSCs reverted to a quiescent state in vivo [59, 60]. These “previously activated” or inactivated HSCs were more sensitive to the second fibrogenic stimuli than “never-activated” or quiescent HSCs [59, 60]. In addition, “previously activated” HSCs differentiated into neither hepatocytes nor cholangiocytes, and inactivated HSCs did not originate from bone marrow [59]. However, it is still unclear whether all activated HSCs can inactivate or whether there are fully activated HSCs that have reached a “point of no return” and cannot reverse.
While liver macrophages are required for liver fibrosis development, monocyte/ macrophage lineage also contributes to resolution of liver fibrosis by producing matrix metalloproteases (MMPs) that degrade ECM. When liver inflammation ceases, bone marrow-derived inflammatory Ly6C-expressing monocytes differentiate into restorative macrophages with low expression of Ly6C that produce MMP9 and MMP12, inducing liver fibrosis regression [61].
Angiogenesis also plays a key role in liver fibrosis resolution. Inhibition of VEGF inhibits fibrosis resolution [44]. Liver macrophages contribute to fibrosis resolution through production of CXCL9 and MMP-13 [44]. Accordingly, overexpression of CXCL9 and VEGF accelerate fibrosis resolution [44].
Conclusion
This review highlighted new insight into the cellular and molecular mechanisms of liver fibrosis including the regression of liver fibrosis. Because multiple liver cells contribute to fibrosis progression, identifying the responsible cells to differentiate into myofibroblasts and understanding the HSC biology including its activation and inactivation are noteworthy. Inflammation and fibrosis are tightly connected and regulated. Therefore, more sensitive and specific biomarkers for liver fibrosis by measuring blood levels of inflammatory mediators are feasible. Moreover, controlling inflammatory pathway could be an attractive therapeutic strategy for liver fibrosis. Recent studies have pointed out the connection between intestinal microbiota and hepatic immune system. Modulation of intestinal microbiota has potential to prevent liver fibrosis progression. On the other hand, removal of causative factors is the most realistic therapeutic strategy, which enhances the reversibility of liver. Simultaneously, we have to tackle to treat cirrhosis that reached a “point of no return”. A recent clinical trial of farnesoid X receptor agonist for NASH patients successfully reduced liver fibrosis [62]. Future and ongoing clinical trials will validate effectiveness, specificity, and safety for novel therapeutic strategies including combination therapies for liver fibrosis.
Acknowledgments
This manuscript was supported by NIH grants R01AA02017204, R01DK085252 and P42ES010337.
Footnotes
Conflict of interest None declared.
Contributor Information
Ekihiro Seki, Email: Ekihiro.Seki@cshs.org, Division of Gastroenterology, Department of Medicine, Cedars-Sinai Medical Center, 8700 Beverly Blvd., DAVIS, Suite D2099, Los Angeles, CA 90048, USA.
David A. Brenner, School of Medicine, University of California San Diego, La Jolla, CA, USA
References
- 1.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DA. Reversibility of liver fibrosis. Gastroenterology & Hepatology. 2013;9:737–739. [PMC free article] [PubMed] [Google Scholar]
- 4.Seki E, Schwabe RF. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111:E3297–E3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci U S A. 2013;110:2324–2329. doi: 10.1073/pnas.1214136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholten D, Weiskirchen R. Questioning the challenging role of epithelial-to-mesenchymal transition in liver injury. Hepatology. 2011;53:1048–1051. doi: 10.1002/hep.24191. [DOI] [PubMed] [Google Scholar]
- 10.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Chen X, Yang L, Kisseleva T, Brenner DA, Seki E. Transcriptional repression of the transforming growth factor beta (TGF-beta) Pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by Nuclear Factor kappaB (NF-kappaB) p50 enhances TGF-beta signaling in hepatic stellate cells. J Biol Chem. 2014;289:7082–7091. doi: 10.1074/jbc.M113.543769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Zou L, Jagavelu K, Simonetto DA, Huebert RC, Jiang ZD, et al. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol. 2012;56:893–899. doi: 10.1016/j.jhep.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–1340. e1331. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 22.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29:1043–1055. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. e761–e763. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology. 2014;59:296–306. doi: 10.1002/hep.26598. [DOI] [PubMed] [Google Scholar]
- 27.Cobleigh MA, Robek MD. Protective and pathological properties of IL-22 in liver disease: implications for viral hepatitis. Am J Pathol. 2013;182:21–28. doi: 10.1016/j.ajpath.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Kronenberger B, Rudloff I, Bachmann M, Brunner F, Kapper L, Filmann N, et al. Interleukin-22 predicts severity and death in advanced liver cirrhosis: a prospective cohort study. BMC Med. 2012;10:102. doi: 10.1186/1741-7015-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–909. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- 30.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 31.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV, et al. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;193:2512–2518. doi: 10.4049/jimmunol.1400588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology. 2014;59:1331–1342. doi: 10.1002/hep.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–1906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188–198. e187. doi: 10.1053/j.gastro.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Zhang JY, Lv S, Wang H, Gong M, Du N, et al. Interleukin-33 promotes disease progression in patients with primary biliary cirrhosis. Tohoku J Exp Med. 2014;234:255–261. doi: 10.1620/tjem.234.255. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–3251. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning Y, et al. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem. 2009;284:30097–30104. doi: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–1350. e1331. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng HL, Ciuclan L, Liu Y, Hamzavi J, Godoy P, Gaitantzi H, et al. Profibrogenic transforming growth factor-beta/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes. Hepatology. 2007;46:1257–1270. doi: 10.1002/hep.21806. [DOI] [PubMed] [Google Scholar]
- 46.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 47.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaushal S, Annamali M, Blomenkamp K, Rudnick D, Halloran D, Brunt EM, et al. Rapamycin reduces intrahepatic alpha-1-antitrypsin mutant Z protein polymers and liver injury in a mouse model. Exp Biol Med. 2010;235:700–709. doi: 10.1258/ebm.2010.009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 52.Rockey DC. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis. 2001;21:337–349. doi: 10.1055/s-2001-17551. [DOI] [PubMed] [Google Scholar]
- 53.Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 54.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 55.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347–1354. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280:4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 59.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]