Abstract
Purpose
Resistance to chemotherapy-induced apoptosis represents a major obstacle to cancer control. Overexpression of Bcl-2 is seen in multiple tumor types and targeting Bcl-2 may provide therapeutic benefit. A phase I study of navitoclax, a novel inhibitor of Bcl-2 family proteins, was conducted to evaluate safety, pharmacokinetics, and preliminary efficacy in patients with solid tumors.
Patients and Methods
Patients enrolled to intermittent dosing cohorts received navitoclax on day −3, followed by dosing on days 1 to 14 of a 21-day cycle. Patients on continuous dosing received a 1-week lead-in dose of 150 mg followed by continuous daily administration. Blood samples were collected for pharmacokinetic analyses, biomarker analyses, and platelet monitoring.
Results
Forty-seven patients, including 29 with small-cell lung cancer (SCLC) or pulmonary carcinoid, were enrolled between 2007 and 2008, 35 on intermittent and 12 on continuous dosing cohorts. Primary toxicities included diarrhea (40%), nausea (34%), vomiting (36%), and fatigue (34%); most were grade 1 or 2. Dose- and schedule-dependent thrombocytopenia was seen in all patients. One patient with SCLC had a confirmed partial response lasting longer than 2 years, and eight patients with SCLC or carcinoid had stable disease (one remained on study for 13 months). Pro-gastrin releasing peptide (pro-GRP) was identified as a surrogate marker of Bcl-2 amplification and changes correlated with changes in tumor volume.
Conclusion
Navitoclax is safe and well tolerated, with dose-dependent thrombocytopenia as the major adverse effect. Preliminary efficacy data are encouraging in SCLC. Efficacy in SCLC and the utility of pro-GRP as a marker of treatment response will be further evaluated in phase II studies.
INTRODUCTION
The efficacy of many chemotherapeutic agents is dependent on activation of intrinsic apoptosis after DNA damage. Bcl-2 family proteins are central regulators of intrinsic apoptosis and overexpression of Bcl-2 in multiple solid tumor types has been hypothesized to play a role both in tumor cell survival as well as resistance to chemotherapy.1–3 Bcl-2 overexpression is particularly frequent in small-cell lung cancer (SCLC),4–9 where chemotherapy resistance represents a major obstacle to effective therapy. Bcl-2 upregulation has been seen in SCLC cell lines selected for chemotherapy resistance,10 and has been implicated in the mechanism of fibroblast growth factor 2- and Etz-mediated chemotherapy resistance in SCLC.11,12
Navitoclax is a potent and highly selective inhibitor of antiapoptotic members of the Bcl-2 family, with nanomolar affinity for Bcl-2, Bcl-xL, and Bcl-w. The potency and specificity of this agent are related to its unique mechanism of action, as a BH3 domain mimetic, to block the interaction of antiapoptotic family members with BH3 domain-containing pro-apoptotic proteins. Navitoclax is an orally available analog of ABT-737, first described to cause mechanism-based killing of multiple SCLC cell lines and regression of tumor xenografts.13,14 These effects were also seen with navitoclax15,16 with significant tumor growth inhibition in nine of 11 tumor models and prolonged complete regression in some cases. Recent detailed mechanistic studies demonstrate that the cytotoxicity of ABT-737, unlike that of other small molecule inhibitors of Bcl-2 in clinical development including obatoclax, requires an intact intrinsic apoptotic pathway.17 Preclinical data in animal models demonstrated marked and immediate thrombocytopenia with navitoclax that resolved on cessation of the drug. This is a mechanism-based toxicity induced by inhibition of Bcl-xL in circulating platelets,13,18 which is required for platelet survival.19,20 Here we describe a phase I dose-escalation study to evaluate the safety (with particular attention to platelet dynamics), pharmacokinetics, and preliminary efficacy of navitoclax administered on intermittent and continuous dosing schedules in patients with relapsed or refractory SCLC and other solid tumors.
In addition, potential biomarkers of response were evaluated, including amplification of Bcl-2 in circulating tumor cells (CTCs), since amplification of a region of 18q that contains Bcl-2 has recently been demonstrated to correlate with SCLC cell line sensitivity to ABT-737 in vitro.21 Notably, this region contains not only Bcl-2, but also the gene for another potential marker of SCLC, pro-gastrin releasing peptide (pro-GRP). Pro-GRP is a tumor cell-secreted peptide that has previously been studied as a potential biomarker of disease progression and response to therapy in SCLC. Therefore, we also determined levels of circulating pro-GRP pre- and post-treatment, and correlated pretreatment levels with Bcl-2 amplification, and change in levels during treatment with changes in tumor volume. Finally, caspase-mediated cleavage of the epithelial marker CK18, assessed with an antibody specific for the cleaved product, M30, was studied as another biomarker of navitoclax-induced apoptosis.
PATIENTS AND METHODS
Eligibility
Patients had histologically documented SCLC or other nonhematologic malignancies, age ≥ 18, Eastern Cooperative Oncology Group performance status of ≤ 2, measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST version 1.0), and had received at least one prior chemotherapy regimen with documented progression. Patients with brain metastases were included if they had surgery and/or radiation therapy followed by 21 days of stable neurologic function and stable disease by imaging before the first dose of study drug. Additional inclusion criteria included adequate bone marrow, renal and hepatic function per local laboratory reference range, nonpregnant status, and a life expectancy of ≥ 90 days.
Patients were excluded if they had an underlying predisposing condition to or active bleeding; recent history of thrombocytopenia-associated bleeding; active immune thrombocytopenic purpura, autoimmune hemolytic anemia, or peptic ulcer disease; refractoriness to platelet transfusions within 1 year; need for full-dose anticoagulation, steroid, or aspirin therapy within 7 days.
The protocol was approved and monitored by all local institutional review boards; all patients provided written informed consent.
Drug Administration and Definition of Dose-Limiting Toxicity
Patients enrolled in the intermittent dosing cohort received navitoclax on day −3 (lead-in dose), followed by dosing on days 1 to 14 of a 21-day cycle. In continuous dosing cohorts, patients received a 1-week lead-in dose of 150 mg, followed by 21 of 21 days of once daily dosing. Dose-limiting toxicity (DLT) was defined as any grade 3, 4, or 5 adverse event (AE) in cycle 1 considered possibly or probably related to navitoclax, with the following exceptions: grade 3 or 4 afebrile neutropenia, leukopenia, or lymphopenia; alopecia; grade 3 nausea, vomiting, or diarrhea unless unresponsive to treatment; or grade 3 tumor lysis syndrome without clinical symptoms. Grade 2 or higher bleeding associated with thrombocytopenia of any grade and any unexpected grade 2 toxicity resulting in dose modification or delay of longer than 1 week were also considered DLTs. Any DLT required an interruption of dosing until the toxicity grade returned to ≤ grade 1 or to baseline. After a DLT of thrombocytopenia, ABT-263 could be reintroduced only if the platelet count rose to ≥ 50,000/μL (≤ grade 2). In those situations, the dose level was determined on an individual basis by the investigator and the Abbott Medical Monitor.
Safety and Efficacy Assessments
Patients were evaluated with history, physical exam, vital signs, CBC, chemistries, urinalysis, and Eastern Cooperative Oncology Group performance status weekly through cycle 2 and on day 1 of each subsequent cycle. Incidence and severity of AEs were collected at each study visit and graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events version 3. Radiographic tumor assessments were performed at baseline, after the second and fourth cycles, and after every 3 cycles thereafter. Response was measured according to standard RECIST. While all patients were considered evaluable for safety, patients with lower than 80% dosing compliance during cycle 1 were considered unevaluable for response. Additional clinical parameters were measured as described in Appendix (online only).
Pharmacokinetics
For patients on 14 of 21-day dosing cohorts, blood samples were collected on day −3 before cycle 1 for pharmacokinetic monitoring at time 0, 2, 3, 4, 6, 8, 24, 48, and 72 hours. For patients on 21 of 21-day dosing cohorts, blood samples were collected on cycle 1, day 1 at time 0, 2, 3, 4, 6, 8, and 24 hours. For all patients, collections were also performed on cycle 1 day 14 (blood samples at 0, 2, 3, 4, 6, 8 hours). In addition, predose samples were collected beyond cycle 1 for all patients. Plasma concentration measurements are detailed in the Appendix (online only).
Biomarker Assessment
Blood samples for CTCs and the plasma marker Pro-GRP were collected as described in Appendix (online only). CTCs were analyzed via the CellSearch system (Veridex, Raritan, NJ). Blood (10 mL) collected in CellSearch tubes was processed within 72 hours after collection. Fluorescent in situ hybridization (FISH) was performed as previously described21 using a Vysis LSI Bcl-2 (orange) probe and chromosome 18 probe (green) developed by Abbott Molecular. Similar probes were used to assess Bcl-2 amplification in tumor biopsies. Pro-GRP was measured using ARCHITECT ELISA kits (Abbott Diagnostics, Abbott Park, IL). Serum samples were analyzed for M30 and M65 (Peviva, Bromma, Sweden) using previously described assays validated to good clinical laboratory practice.22,23
Statistical Analyses
This study was designed using an adaptation of the continuous reassessment method (CRM) for dose escalation24–26 (further described in online-only Appendix). Two stages of dose escalation were planned. In stage I, dosing began at 10 mg with a cohort of three patients, with a plan thereafter for single patient cohorts and doubling of the dose in subsequent cohorts until a DLT was observed. Once a DLT was observed, stage II began with cohort sizes of three patients and CRM was employed to select the next dose, but with the constraint that escalation will not exceed the greater of 100 mg or 40%. Descriptive statistics summarized the demographics, safety data, and pharmacokinetics. Correlations between median Bcl-2 copy number, pro-GRP, M30, dose, and best tumor response were made using the JMP 8.0 statistical software (JMP, Cary, NC).
RESULTS
Dose Escalation
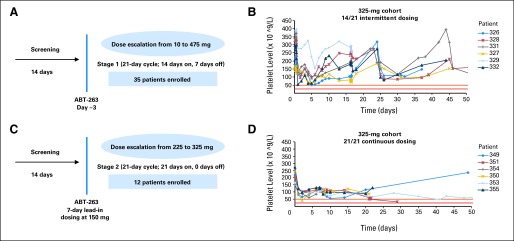
Forty-seven patients were enrolled in this study from April 2007 to May 2008. Table 1 presents the baseline characteristics of this patient population, more than 60% of whom had SCLC or atypical pulmonary carcinoid. The median duration on study was 38 days (range, 17 to 882 days). Nine patients were considered unevaluable for response due to lower than 80% dosing compliance in cycle 1, which was primarily related to missed doses secondary to nausea, vomiting, or diarrhea. The most common reasons for study discontinuation were radiographic progressive disease, declining performance status, and withdrawal of consent. Thirty-five patients were treated on intermittent dosing cohorts ranging from 10 to 475 mg, with a day −3 lead-in dose, followed by dosing on days 1 through 14 of a 21-day cycle (Fig 1A). The kinetics of thrombocytopenia paralleled that seen in animal models. Platelet counts for all patients dropped to a nadir within 24 to 72 hours of dosing on day −3, consistent with peripheral destruction on Bcl-xL inhibition. However, with marrow compensation, platelet counts subsequently showed partial recovery during ongoing dosing and then recovered close to baseline during the week off drug (Fig 1B). A recurrent variable decline in platelet count occurred when drug was restarted.
Table 1.
Baseline Patient Demographics and Clinical Characteristics (N = 47)
| Characteristic | No. | % |
|---|---|---|
| Median age, years | 62 | |
| Sex | ||
| Male | 27 | 57 |
| Female | 20 | 43 |
| ECOG performance status | ||
| 0 | 16 | 35 |
| 1 | 26 | 56 |
| 2 | 27 | 56 |
| No. of prior therapies | ||
| 1-2 | 24 | 51 |
| ≥ 3 | 23 | 49 |
| Median time since last therapy, days | 49 | |
| Range | 12-445 | |
| Tumor type | ||
| SCLC | 26 | 55 |
| Atypical pulmonary carcinoid | 3 | 6 |
| Other | 18 | 38 |
| Dosing schedule | ||
| 14/21 | 35 | |
| SCLC | 17 | |
| Atypical pulmonary carcinoid | 3 | |
| 21/21 | 12 | |
| SCLC | 4 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; SCLC, small-cell lung cancer.
Fig 1.
Platelet dynamics with different dosing schedules. (A) Dosing schema for intermittent dosing. (B) Platelet levels over time in six representative patients on intermittent dosing cohorts. Day 0 represented in this figure is study day −3. Patient 326 was a 60-year-old man with non–small-cell lung cancer (NSCLC); patient 327 was an 80-year-old man with pancreatic cancer; patient 328 was a 68-year-old man with NSCLC; patient 329 was a 64-year-old man with small-cell lung cancer (SCLC); patient 331 was a 60-year-old woman with neuroendocrine carcinoid; patient 332 was a 62-year-old man with SCLC. (C) Dosing schema for continuous dosing. (D) Platelet levels over time in six representative patients on continuous dosing cohorts. Day 0 represented in this figure is the lead-in period day 1. Red and orange lines represent the boundaries of a dose-limiting toxicity (grade 3, 25,000 to 50,000); 325 mg was chosen as a representative dose for comparing the two different dosing schedules. Patient 349 was a 65-year-old man with SCLC; patient 350 was a 67-year-old man with SCLC; patient 351 was a 57-year-old woman with SCLC; patient 353 was a 63-year-old man with SCLC; patient 354 was a 65-year-old woman with SCLC; patient 355 was a 60-year-old man with SCLC.
Given the kinetics of thrombocytopenia and platelet recovery, with recovery even during continued dosing, the protocol was amended to evaluate whether a low lead-in dose for 7 days before therapeutic dosing could prime the marrow to upregulate platelet production, to allow for higher continuous dosing and to minimize subsequent platelet variability. A lead-in dose of 150 mg was chosen, as this was the dose level at which the mean maximal platelet drop of approximately 60% from baseline was expected based on observations in other concurrent studies of navitoclax as a single agent.
Based on a maximum-tolerated dose estimation of 350 mg using intermittent dosing, the daily equivalent dosing of 225 mg was the starting point for the dose escalation in continuous dosing cohorts (Fig 1C), where less variability in platelet dynamics over time were observed (Fig 1D). Twelve additional patients were treated on continuous dosing cohorts before an estimated maximum-tolerated dose of 325 mg was reached.
Pharmacokinetics
Table 2 presents the pharmacokinetic profile of navitoclax exposure after a single lead-in dose (day −3) or after the day 14 dose on the 14 of 21-day dosing schedule, demonstrating that exposure was dose-proportional. Peak concentrations (Cmax) were observed approximately 7 hours postdose with a half-life of approximately 15 hours. Peak-to-trough plasma concentration ratio was close to two-fold at steady-state and overall the interpatient variability in exposure was 40%. Dosage at or above 225 mg met the minimum plasma exposure predicted to be in the therapeutic range based on animal models.
Table 2.
Pharmacokinetic Parameters of Navitoclax After Oral Administration (14/21-day dosing schedule)
| Study Day by Dose (mg) | No. | Tmax (hours) |
Cmax (μg/mL) |
AUC0-24 (μg × hr/mL) |
AUC0-inf (μg × hr/mL) |
CL/F (L/h) |
t1/2* (hours) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 10 | |||||||||||||
| −3 | 4 | 6.0 | 1.6 | 0.13 | 0.04 | 1.4 | 0.4 | 2.0 | 0.6† | 5.3 | 1.4† | 15.3 | 2.2† |
| 14 | 4 | 6.0 | 0.0 | 0.12 | 0.07 | — | — | — | — | — | — | — | — |
| 20 | |||||||||||||
| −3 | 4 | 6.3 | 2.4 | 0.38 | 0.05 | 4.9 | 0.9 | 7.1 | 1.9 | 3.0 | 0.8 | 13.5 | 6.6 |
| 14 | 4 | 7.0 | 1.2 | 0.46 | 0.14 | — | — | — | — | — | — | — | — |
| 30 | |||||||||||||
| −3 | 6 | 6.0 | 1.3 | 0.67 | 0.29 | 7.3 | 3.4 | 10.1 | 4.6 | 5.0 | 5.7 | 14.1 | 10.4 |
| 14 | 5 | 4.4 | 2.1 | 0.81 | 0.21 | — | — | — | — | — | — | — | — |
| 130 | |||||||||||||
| −3 | 4 | 11.0 | 8.7 | 1.84 | 1.22 | 25.4 | 12.8 | 42.0 | 12.4 | 3.3 | 0.8 | 16.9 | 1.2 |
| 14 | 4 | 6.0 | 1.6 | 2.81 | 1.75 | — | — | — | — | — | — | — | — |
| 225 | |||||||||||||
| −3 | 3 | 5.7 | 2.5 | 2.97 | 1.24 | 39.5 | 18.8 | 53.0 | 24.6 | 5.3 | 3.3 | 12.8 | 0.8 |
| 14 | 4 | 7.5 | 1.0 | 5.10 | 0.85 | — | — | — | — | — | — | — | — |
| 325 | |||||||||||||
| −3 | 6 | 10.0 | 6.9 | 2.55 | 1.46 | 40.9 | 25.8 | 80.5 | 44.3‡ | 5.9 | 4.7† | 15.8 | 3.1† |
| 14 | 8 | 5.8 | 2.3 | 4.31 | 2.14 | — | — | — | — | — | — | — | — |
| 425 | |||||||||||||
| −3 | 5 | 5.0 | 2.0 | 3.43 | 1.75 | 53.7 | 30.8 | 99.2 | 69.5 | 7.5 | 6.4 | 15.0 | 3.4 |
| 14 | 3 | 3.7 | 4.0 | 2.87 | 0.78 | — | — | — | — | — | — | — | — |
| 475 | |||||||||||||
| −3 | 3 | 12.0 | 10.4 | 3.76 | 1.71 | 66.8 | 35.6 | 81.0 | 29.7§ | 6.3 | 2.3§ | 15.7 | 0.2§ |
| 14 | 1 | 6.0 | 5.59 | — | — | — | — | — | — | — | — | ||
NOTE. All doses were taken under nonfasting condition.
Abbreviations: Tmax, time to maximum observed plasma concentration; Cmax, maximum plasma concentration; AUC, area under the curve; inf, infinity; CL/F, apparent oral clearance; t1/2, terminal half-life; SD, standard deviation.
Harmonic mean ± pseudo SD.
N = 3.
N = 5.
N = 2.
Safety
The most frequent AEs excluding thrombocytopenia were diarrhea (40%), vomiting (36%), nausea (34%), and fatigue (34%), as detailed in Table 3. The majority of these were grade 1 or 2 (Appendix Table A1, online only). While all patients experienced some degree of thrombocytopenia, only 15% met criteria for AEs. In addition, three patients were removed from study for severe AEs that were deemed to be possibly or probably related to study drug. These included fatal respiratory failure in one patient that occurred at the lowest dosing level during cycle 1; left ventricular systolic dysfunction with a drop from 55% to 70% at baseline to 40% during cycle 1 and resolution to 55% 1 week after stopping drug; and asymptomatic lipase elevation from a baseline of grade 2 to grade 3 with normalization to baseline within 1 week off drug.
Table 3.
Most Common AEs
| AE | Patients for All Grades(N = 47) |
14/21-Day Dosing Cohorts by Grade (mg) |
21/21-Day Dosing by Grade (mg) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-30 (n = 16) |
130 (n = 4) |
225 (n = 3) |
325 (n = 6) |
425 (n = 5) |
475 (n = 3) |
225 (n = 6) |
325 (n = 6) |
|||||||||||
| No. | % | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | |
| Lymphopenia | 2 | 4.3 | 1 (3) | 1 | ||||||||||||||
| Neutropenia | 1 | 2.1 | 1 (3) | 1 | ||||||||||||||
| Thrombocytopenia | 7 | 14.9 | 1 (4) | 1 (4) | 1 (4) | 1 | 1 (3) | 1 | 1 (3) | |||||||||
| Diarrhea | 19 | 40.4 | 2 | 1 | 4 | 4 | 2 | 2 | 4 | |||||||||
| Enteritis | 1 | 2.1 | 1 | |||||||||||||||
| Nausea | 16 | 34 | 2 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 4 | |||||||
| Vomiting | 17 | 36.2 | 3 | 1 | 3 | 2 | 1 | 1 | 2 | 4 | ||||||||
| Fatigue | 16 | 34 | 2 | 2 (3) | 3 | 2 | 2 (3) | 1 | 3 | 1 | ||||||||
| Decreased appetite | 9 | 19 | 2 | 1 | 2 | 3 | 1 | |||||||||||
| Weight decrease | 2 | 4.3 | 1 | 1 (3) | ||||||||||||||
| Hypophosphataemia | 1 | 2.1 | 1 (3) | |||||||||||||||
| Hemoptysis | 1 | 2.1 | 1 (3) | |||||||||||||||
| Respiratory failure | 1 | 2.1 | 1 (5) | |||||||||||||||
NOTE. All AEs are reported if > 10% or if grade 3, 4, or 5. If grade 3, 4, or 5, the specific grade is reported in parentheses.
Abbreviation: AE, adverse event.
Antitumor Activity
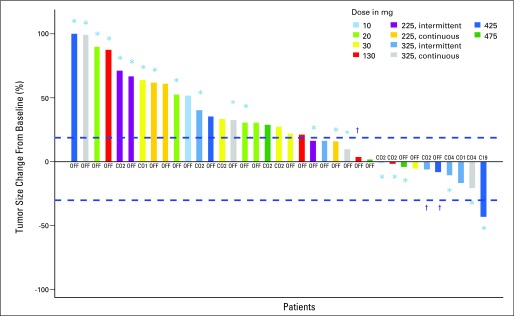
Of the 38 patients who were evaluable for response (23 with SCLC or pulmonary carcinoid), eight had stable disease (five SCLC and three atypical pulmonary carcinoid) and one patient with SCLC remained on study for 13 months. One patient with SCLC had a partial response that has been sustained for longer than 35 months and remains on study; this patient had a localized recurrence after first-line treatment with progressive disease on second-line therapy before study entry. Overall, among patients with disease control, the median number of prior therapies was three (range, one to five therapies). The majority of patients with disease control were those treated at the highest dose levels (Fig 2) and the median duration of disease control was 5 months (range, 2 to 35 months). Dosing schedule details for patients with disease control are presented in Appendix Table A2 (online only).
Fig 2.
Best overall percentage change from baseline in target lesion measurement by RECIST (Response Evaluation Criteria in Solid Tumors) guidelines for patients at different dose levels. The best tumor percent change is defined as the maximum reduction/minimum increase from baseline in tumor load. For patients who have the best percent change from baseline at multiple cycles, the earliest cycle is labeled. Best tumor percent change from baseline for the leftmost patient is truncated at 100%. Note that for some patients, a reduction in target lesions did not translate to response based on growth of new lesions. (*) Patients with small-cell carcinoma. (†) Patients with pulmonary carcinoid tumors.
Biomarker Analyses
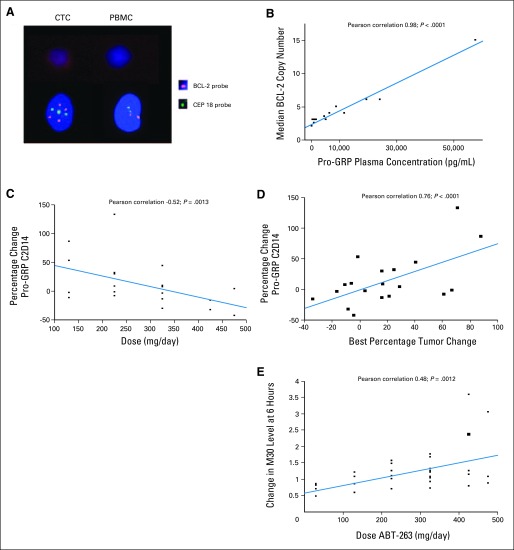
To determine whether Bcl-2 amplification was associated with response, Bcl-2 copy number was assessed in blood samples from 46 patients when feasible. Twenty-one patients had eight or more CTCs detected and in these, FISH for Bcl-2 was performed. Figure 3A shows an example of a patient with amplification of Bcl-2 in CTCs, but not in peripheral blood mononuclear cells (PBMC). In patients where Bcl-2 amplification was performed, Bcl-2 copy number was compared to circulating pro-GRP levels. There was a direct correlation between median Bcl-2 copy number and circulating pro-GRP (R2 = 0.98), consistent with previous studies demonstrating coamplification of these two genes21 (Fig 3B). When only patients with SCLC or neuroendocrine tumors were analyzed, the same correlation was seen (R2 = 0.95, data not shown). Overall, pro-GRP levels declined or stabilized with increasing navitoclax dose (Fig 3C), but more importantly, the change in pro-GRP levels correlated with best percentage tumor change (Fig 3D; R2 = 0.76).
Fig 3.
Biomarkers of navitoclax activity and tumor response. (A) Fluorescent in situ hybridization analysis of Bcl-2 in a patient with small-cell lung cancer demonstrating amplified signal (four copies) of Bcl-2 (orange) and three copies of CEP 18 (green) in circulating tumor cells (CTCs), but no amplification (two copies of both) in peripheral blood mononuclear cells (PBMC). (B) Pro-gastrin releasing peptide (pro-GRP) plasma concentration (pg/mL) plotted against mean Bcl-2 copy number. (C) Relative change in pro-GRP plasma concentration with different dose levels. (D) The relationship between best tumor percentage change is plotted against percentage change of pro-GRP from baseline to cycle 2 day 14. (E) Changes in circulating M30 levels with increasing dose as measured in cycle 1, 6 hours after first exposure.
A dose-dependent transient increase in circulating M30, a serum marker for tumor cell apoptosis, was also observed in dose cohorts ≥ 130 mg (R2 = 0.48; P = .0012; Fig 3E). In most instances, the rise in M30 was rapid, occurring within 6 hours after the first dose. Evidence of apoptosis was sustained through 14 days of oral dosing, mirroring the biomarker behavior in a SCLC human xenograft preclinical model (data not shown).27
DISCUSSION
Deregulation of apoptosis is a hallmark of cancer and several studies have suggested that antiapoptotic gene activity is critical for continued survival and proliferation.12,28 Bcl-2 is attractive therapeutic target in SCLC and other tumors, but, to date, anti-Bcl-2 therapies have not demonstrated significant clinical efficacy. Trials of an antisense oligonucleotide to Bcl-2 mRNA were largely unsuccessful, but downregulation of Bcl-2 in clinical specimens was limited and did not correlate with response.29,30 In addition, the Bcl-2 family contains both antiapoptotic members (ie, Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and Bcl-A2), pro-apoptotic multidomain members (ie, Bak, Bax), and BH3-only domain members (ie, Bim, Bid, Puma, Bad, Bik, Noxa, and Bmf), and many agents that target Bcl-2 do so with a lack of specificity among this group.31
Navitoclax has a unique mechanism of action that disrupts the regulatory step that prevents pro-apoptotic Bcl-2 family members from initiating apoptotic pathways. Although this study was open to all solid tumors, given the level of preclinical activity seen in SCLC models, enrollment was enriched for patients with small cell or other neuroendocrine tumors.
Navitoclax was overall well-tolerated and the majority of treatment-related AEs (ie, diarrhea, nausea, vomiting, and fatigue) other than thrombocytopenia were grade 1 or 2 and manageable. Thrombocytopenia was experienced by all patients on this study, as expected for a drug that inhibits Bcl-xL function. Consistent with a peripheral apoptotic process that did not affect platelet production or function, a relative increase in large platelet forms was noted among the study population. Although intermittent dosing was associated with significant intracycle variability of platelets, changing to a continuous dosing schedule after a priming lead-in dose effectively mitigated the variability in platelet counts. Thrombocytopenia developed and resolved in a predictable fashion without clinical sequelae and, based on dose- and schedule dependence, behaves as a pharmacodynamic marker of on-target drug activity.
In order to evaluate the basis for response, ongoing evaluations of Bcl-2 in tumor tissue and blood are underway. Importantly, we were able to demonstrate that CTCs were readily detectable, that Bcl-2 amplification was present in CTCs and that Bcl-2 gene copy number correlated directly with plasma pro-GRP levels. The data indicate that the easily identifiable plasma protein pro-GRP can serve as an accurate surrogate for Bcl-2 amplification. Furthermore, the direct correlation with best tumor response seen in patients treated at sufficient dose levels indicates that pro-GRP can serve as a pharmacodynamically relevant biomarker of response to anti-Bcl-2 therapy. Measures of epithelial apoptosis, such as M30, also show promise as pharmacodynamic markers of drug activity that merit further evaluation.
Given the large set of both antiapoptotic and pro-apoptotic Bcl-2 family members, Bcl-2 and pro-GRP are clearly not the only biomarkers of relevance to response. Preclinical studies of navitoclax and ABT-737 have already identified markers of insensitivity, such as Mcl-1, an antiapoptotic protein not targeted by these drugs.13,16 Mcl-1 overexpression was identified in many SCLC cell lines resistant to these two drugs32–34 and downregulation or neutralization of Mcl-1 appears to be sufficient to restore sensitivity in vitro.33,35 Combinations of navitoclax and agents that downregulate Mcl-1, including several standard chemotherapy drugs, may provide a potent therapeutic strategy against a broad spectrum of small-cell carcinomas.
Despite known resistance mechanisms, surprisingly durable single-agent activity was seen in this phase I dose-escalation trial in patients with heavily pretreated SCLC, a tumor type that has no demonstrated effective therapy in this setting. The majority of disease control was seen in patients with SCLC or pulmonary carcinoid tumors, consistent with previous data suggesting high levels of Bcl-2 expression in these tumors, as well as potential dependence on Bcl-2 for both survival and chemotherapy resistance. The two patients who remained on study for longer than 1 year sustained 22% and 35% reductions in tumor burden, respectively. These results suggest that in some instances, Bcl-2 inhibition alone is sufficient to cause apoptosis. In these cancers, cells may be primed for death via Bcl-2 inhibition,12 since survival is maintained only by overexpression of Bcl-2. Notably, minor regressions in some lesions were seen in patients with pulmonary carcinoid, suggesting that the poor response rates of these tumors to chemotherapy may be due to antiapoptotic mechanisms of resistance rather than simply a more indolent growth rate.
Currently, both single-agent and combination studies with chemotherapy are underway in SCLC to further define the role of navitoclax in this disease. These studies are using the continuous dosing schedule defined here to minimize platelet variability. The biomarkers of response developed here may prove informative in defining a subpopulation most likely to benefit from this targeted approach.
Acknowledgment
We thank Michael Dawson for operational support, Emma Dean for biomarker analyses, Di Li, Joseph Beason for statistical analyses, and Ai Lockard for manuscript editorial assistance. This study was funded by Abbott Laboratories.
Presented in part at the 101st Annual Meeting of the American Association for Cancer Research, April 17-21, 2010, Washington, DC; International Association for the Study of Lung Cancer: World Conference on Lung Cancer 2009, July 13-August 4, 2009, San Francisco, CA; 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL; 100th Annual Meeting of the American Association for Cancer Research, April 18-22, 2009, Denver, CO; Chicago Multidisciplinary Symposium in Thoracic Oncology, November 13-15, 2008, Chicago, IL; European Organisation for Research and Treatment of Cancer–National Cancer Institute–American Association for Cancer Research Symposium: Molecular Targets and Cancer Therapeutics, October 21-24, 2008, Geneva, Switzerland; European Society of Medical Oncology, September 12-16, 2008, Stockholm, Sweden; and 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Appendix
Safety assessments.
ECG and two-dimensional ECHO were obtained on cycle 1 day 3, cycle 1 day 14, cycle 3 day 1, cycle 3 day 14, and end of study. Platelet counts were obtained on cycle 1 day 3 through cycle 1 day 14, and weekly through each subsequent cycle. Lymphocyte enumeration was performed at screening, cycle 1 day 14, at the end of cycle 4, and at the end of every third cycle thereafter.
Pharmacokinetics.
Plasma concentrations of navitoclax were determined using a validated liquid chromatographic method with tandem mass spectrometric detection. The pharmacokinetic parameter values of navitoclax were estimated using noncompartmental methods with WINNonlin (Scientific Consultant, Apex, NC) professional version 5.2.1 (Pharsight Corporation, Mountain View, CA).
Biomarker assessment.
Blood samples for CTCs and the plasma marker Pro-GRP were collected at screening, cycle 1 day 14, cycle 2 day 14, and at the end of study treatment. Samples for serum marker M30 were collected at screening, predose, 6 and 24 hours postdose on cycle 1 day 3 (for 14 of 21-day dosing) or lead-in day 2 (for 21 of 21-day dosing). Samples were also collected on cycle 1 day 14; predose and 6 hours postdose on cycle 2 day 1; cycle 2 day 14; end of cycle 4; and every third cycle thereafter and at the final visit for all patients.
Statistical considerations.
Continuous reassessment method was a model-guided adaptive design. After completion of each dose level, the current data along with the previous information were incorporated cumulatively into a dose-response curve, based on the logistic regression analysis. The model estimated the maximum-tolerated dose (MTD) in real time and allocated the next cohort of patients to the estimated MTD based on a dose-response model. The process was updated and reassessed for each dose level until MTD was reached. In this study, the target MTD corresponded to the 30th percentile on the curve. The 30% was used to approximate an accepted MTD determination, which corresponded to the 1/3 and 2/6 rule from the conventional 3+3 design. Under continuous reassessment method modeling, there is an increased likelihood of receiving treatment at the near efficacious dose levels.
Table A1.
Grading of Most Common Adverse Events
| Adverse Event1 | No. of Patients | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Diarrhea | 19 | 12 | 63 | 2 | 37 | 0 | 0 | 0 | 0 |
| Nausea | 16 | 7 | 44 | 8 | 50 | 1 | 6 | 0 | 0 |
| Vomiting | 17 | 11 | 65 | 5 | 29 | 1 | 6 | 0 | 0 |
| Fatigue | 16 | 5 | 31 | 7 | 44 | 1 | 6 | 0 | 0 |
Table A2.
Dose Exposure for Subjects With Disease Control
| Subject No. | Cancer Type | Best Response | Durability of Response (cycles) | Assigned Dose Level (mg) | Dose Schedule | Exposure per Cycle (mg) |
|---|---|---|---|---|---|---|
| 301 | SCLC | SD | 4 | 10 | 14/21 | 140 |
| 317 | Carcinoid | SD | 6 | 130 | 14/21 | 1,820 |
| 320 | SCLC | SD | 2 | 130 | 14/21 | 1,820 |
| 331 | Carcinoid | SD | 8 | 325 | 14/21 | 4,550 |
| 332 | SCLC | SD | 2 | 425 | 14/21 | 5,950 |
| 335 | Carcinoid | SD | 2 | 325 | 14/21 | 4,550 |
| 338 | SCLC | PR | 35 | 425 | 14/21 | 5,950 |
| 348 | SCLC | SD | 2 | 225 | 21/21 | 4,725 |
| 353 | SCLC | SD | 17 | 325 | 21/21 | 6,825 |
Abbreviations: SCLC, small-cell lung cancer; SD, stable disease; PR, partial response.
Footnotes
Supported by Abbott Laboratories and Genentech; and in part by the Alice and Steve Cutler Fund for Young Investigators at the Dana-Farber Cancer Institute (L.G.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00445198.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Evelyn M. McKeegan, Abbott Laboratories (C); Philip M. Hemken, Abbott Laboratories (C); Andrew P. Krivoshik, Abbott Laboratories (C); Sari H Enschede, Abbott Laboratories (C); Cathy Nolan, Abbott Laboratories (C); Yi-Lin Chiu, Abbott Laboratories (C); Todd Busman, Abbott Laboratories (C); Hao Xiong, Abbott Laboratories (C); Rod Humerickhouse, Abbott Laboratories (C) Consultant or Advisory Role: Philip Bonomi, Abbott Laboratories (C); David Gandara, Amgen (C), Biodesix (C), Boehringer Ingelheim (C), Bristol-Myers Squibb/ImClone (C), GlaxoSmithKline (C), Genentech (C), Merck (C), Novartis (C), sanofi-aventis (C), Response Genetics (C), AstraZeneca (C), Eli Lilly (U); Charles M. Rudin, Syndax Pharmaceuticals (C), OSI Pharmaceuticals (C), Genentech (C) Stock Ownership: Evelyn M. McKeegan, Abbott Laboratories; Philip M. Hemken, Abbott Laboratories; Andrew P. Krivoshik, Abbott Laboratories; Sari H Enschede, Abbott Laboratories; Yi-Lin Chiu, Abbott Laboratories; Todd Busman, Abbott Laboratories; Hao Xiong, Abbott Laboratories; Rod Humerickhouse, Abbott Laboratories Honoraria: Philip Bonomi, Abbott Laboratories Research Funding: Philip Bonomi, Abbott Laboratories; David Gandara, Abbott Laboratories, Bristol-Myers Squibb/ImClone, Genetech, Eli Lilly, Merck, Novartis, Pfizer; Divis Khaira, Abbott Laboratories, Schering-Plough; Caroline Dive, Abbott Laboratories Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Leena Gandhi, Christine L. Hann, Philip M. Hemken, Caroline Dive, Andrew P. Krivoshik, Sari H. Enschede, Cathy Nolan, Yi-Lin Chiu, Hao Xiong, Rod Humerickhouse, Geoffrey I. Shapiro, Charles M. Rudin
Financial support: Andrew P. Krivoshik, Rod Humerickhouse
Administrative support: Cathy Nolan
Provision of study materials or patients: Leena Gandhi, D. Ross Camidge, Moacyr Ribeiro de Oliveira, Philip Bonomi, Divis Khaira, Christine L. Hann, Andrew P. Krivoshik, Rod Humerickhouse, Geoffrey I. Shapiro, Charles M. Rudin
Collection and assembly of data: Leena Gandhi, Moacyr Ribeiro de Oliveira, Divis Khaira, Evelyn M. McKeegan, Elizabeth Litvinovich, Caroline Dive, Andrew P. Krivoshik, Cathy Nolan, Todd Busman, Rod Humerickhouse, Charles M. Rudin
Data analysis and interpretation: Leena Gandhi, D. Ross Camidge, Moacyr Ribeiro de Oliveira, David Gandara, Divis Khaira, Evelyn M. McKeegan, Elizabeth Litvinovich, Andrew P. Krivoshik, Sari H. Enschede, Cathy Nolan, Yi-Lin Chiu, Todd Busman, Hao Xiong, Rod Humerickhouse, Geoffrey I. Shapiro, Charles M. Rudin
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Reed JC. Bcl-2: Prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995;9:451–473. [PubMed] [Google Scholar]
- 2.Ohmori T, Podack ER, Nishio K, et al. Apoptosis of lung cancer cells caused by some anti-cancer agents (MMC, CPT-11, ADM) is inhibited by bcl-2. Biochem Biophys Res Commun. 1993;192:30–36. doi: 10.1006/bbrc.1993.1377. [DOI] [PubMed] [Google Scholar]
- 3.Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biochim Biophys Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ezra JM, Kornstein MJ, Grimes MM, et al. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol. 1994;145:1036–1040. [PMC free article] [PubMed] [Google Scholar]
- 5.Eerola AK, Tormanen U, Rainio P, et al. Apoptosis in operated small cell lung carcinoma is inversely related to tumour necrosis and p53 immunoreactivity. J Pathol. 1997;181:172–177. doi: 10.1002/(SICI)1096-9896(199702)181:2<172::AID-PATH715>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Brambilla E, Negoescu A, Gazzeri S, et al. Apoptosis-related factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am J Pathol. 1996;149:1941–1952. [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser U, Schilli M, Haag U, et al. Expression of bcl-2–protein in small cell lung cancer. Lung Cancer. 1996;15:31–40. doi: 10.1016/0169-5002(96)00568-5. [DOI] [PubMed] [Google Scholar]
- 8.Breton C, Story MD, Meyn RE. Bcl-2 expression correlates with apoptosis induction but not loss of clonogenic survival in small cell lung cancer cell lines treated with etoposide. Anticancer Drugs. 1998;9:751–757. doi: 10.1097/00001813-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Jiang SX, Sato Y, Kuwao S, et al. Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol. 1995;177:135–138. doi: 10.1002/path.1711770206. [DOI] [PubMed] [Google Scholar]
- 10.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 11.Pardo OE, Arcaro A, Salerno G, et al. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: Correlation with resistance to etoposide-induced apoptosis. J Biol Chem. 2002;277:12040–12046. doi: 10.1074/jbc.M109006200. [DOI] [PubMed] [Google Scholar]
- 12.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.Hann CL, Daniel VC, Sugar EA, et al. Therapeutic efficacy of ABT- 737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–3277. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 16.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 17.Vogler M, Weber K, Dinsdale D, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 18.Roberts AW, Wilson WH, Gandhi L, et al. Ongoing phase 1 studies of ABT-263: Mitigating Bcl-XL induced thrombocytopenia with lead-in and continuous dosing. J Clin Oncol. 2009;27(suppl):147s. abstr 3505. [Google Scholar]
- 19.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 21.Olejniczak ET, Van Sant C, Anderson MG, et al. Integrative genomic analysis of small-cell lung carcinoma reveals correlates of sensitivity to bcl-2 antagonists and uncovers novel chromosomal gains. Mol Cancer Res. 2007;5:331–339. doi: 10.1158/1541-7786.MCR-06-0367. [DOI] [PubMed] [Google Scholar]
- 22.Cummings J, Ward TH, LaCasse E, et al. Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2005;92:532–538. doi: 10.1038/sj.bjc.6602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings J, Ranson M, Lacasse E, et al. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2006;95:42–48. doi: 10.1038/sj.bjc.6603220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol. 1998;41:429–436. doi: 10.1007/s002800050763. [DOI] [PubMed] [Google Scholar]
- 25.O'Quigley J, Shen LZ. Continual reassessment method: A likelihood approach. Biometrics. 1996;52:673–684. [PubMed] [Google Scholar]
- 26.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 27.Micha D, Cummings J, Shoemaker A, et al. Circulating biomarkers of cell death after treatment with the BH-3 mimetic ABT-737 in a preclinical model of small-cell lung cancer. Clin Cancer Res. 2008;14:7304–7310. doi: 10.1158/1078-0432.CCR-08-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Gaizo Moore V, Letai A. Rational design of therapeutics targeting the BCL-2 family: Are some cancer cells primed for death but waiting for a final push? Adv Exp Med Biol. 2008;615:159–175. doi: 10.1007/978-1-4020-6554-5_8. [DOI] [PubMed] [Google Scholar]
- 29.Tolcher AW, Chi K, Kuhn J, et al. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:3854–3861. doi: 10.1158/1078-0432.CCR-04-2145. [DOI] [PubMed] [Google Scholar]
- 30.Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26:870–876. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogler M, Dinsdale D, Dyer MJ, et al. Bcl-2 inhibitors: Small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–367. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 32.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 33.Lin X, Morgan-Lappe S, Huang X, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small- molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 34.Hauck P, Chao BH, Litz J, et al. Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT- 737. Mol Cancer Ther. 2009;8:883–892. doi: 10.1158/1535-7163.MCT-08-1118. [DOI] [PubMed] [Google Scholar]
- 35.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]