Abstract
Iron contributes to c-Jun N-terminal kinases (JNK) activation in young rats and white matter injury in piglets after intracerebral hemorrhage (ICH). In the present study, we examined the effect of deferoxamine on ICH-induced white matter injury and JNK activation and in aged rats. Male Fischer 344 rats (18 months old) had either an intracaudate injection of 100 µl of autologous blood or a needle insertion (sham). The rats were treated with deferoxamine or vehicle with different regimen (dosage, duration and time window). White matter injury and activation of JNK were examined. We found that a dose of DFX should at more than 10 mg/kg for a therapeutic duration more than 2 days with a therapeutic time window of 12 hours to reduce ICH-induced white matter loss at 2 months. ICH-induced white matter injury was associated with JNK activation. The protein levels of phosphorylated-JNK (P-JNK) were upregulated at day-1 after ICH and then gradually decreased. P-JNK immunoreactivity was mostly located in white matter bundles. ICH-induced JNK activation was reduced by DFX treatment. This study demonstrated that DFX can reduce ICH-induced JNK activation and white matter damage.
Keywords: intracerebral hemorrhage, c-Jun N-terminal kinases, deferoxamine, white matter injury
Introduction
Intracerebral hemorrhage (ICH) is a common and often fatal subtype of stroke1. Survivors often experience progressive deterioration in their neurological conditions due to secondary brain injury via toxic blood components, such as iron2–6.
White matter injury is common in ICH patients. Clinical data based on volumetric magnetic images showed more extensive white matter damage detected in patients with ICH than those with ischemic stroke or other cerebral small vessel pathologies7. ICH-induced white matter injury reflects the vulnerability of brain to further insults and predicts poor outcome after ICH8. Iron also plays a role in white matter injury after ICH. Our recent study showed systemic treatment with deferoxamine (DFX), an iron chelator, attenuated white matter edema after ICH in piglets9.
c-Jun N-terminal kinases (JNK) are important stress-responsive kinases that are activated in response to various forms of brain insults, such as cerebral ischemia and subarachnoid hemorrhage10–12. Activated JNK phosphorylates serine on a variety of cellular targets and leads to cell death13, 14. Our previous study has demonstrated that both ICH and intracerebral infusion of ferrous iron activated JNK signaling pathway and resulted in neurological deficits. DFX suppressed ICH induced JNK activation and improved neurological outcomes in young rats15.
ICH is mostly disease of the elderly. Age is considered as a significant factor in determining brain injury. In this study, we examined optimal dose, optimal duration and therapeutic time windows of DFX for ICH-induced white matter damage in aged rats. We also investigated the effects of DFX on JNK activation in the aged rat model of ICH.
Materials and Methods
Animal preparation and intracerebral injection
All animal procedures were approved by the University Committee on Use and Care of Animals, University of Michigan, and all studies were conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals. A total of 230 of male Fisher 344 rats at age of 18-month (body weight at 380–450g from National Institutes of Health, Bethesda) were used for study. Rats were anesthetized with pentobarbital (45 mg/kg, i.p.) and the right femoral artery was catheterized to sample blood and monitor arterial blood pressure, blood pH, PaO2, PaCO2, hematocrit, and glucose levels. Body temperature was maintained at 37.5 °C by a feedback-controlled heating pad. Rats were then positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA, U.S.A.) and a cranial burr hole (1 mm) was drilled near the right coronal suture 3.5 mm lateral to the midline. A 26-gauge needle was inserted into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, and 3.5 mm lateral to the bregma). Autologous arterial blood (100µL) was infused at the rate of 10µl/min using a microinfusion pump (World Precision Instruments). After that, the needle was remained in position for 10 minutes for the blood clotting and then gently removed. The burr hole was filled with bone wax, and the skin incision was closed with suture after infusion.
Experimental groups
There were 5 parts of experiments in this study.
Part 1: ICH rats were treated with DFX (10, 50 or 100 mg/kg administered intramuscularly; n=9 for each dose) or vehicle (n=12) at 2 and 6 hours after ICH and then every 12 hours for 7 days. Sham rats were treated with 100mg/kg DFX (n=5) or vehicle (n=5). Rats were euthanized for histologic examination at 56 days after surgery.
Part 2: Rats were treated with 50 mg/kg DFX 2 and 6 hours after ICH, then every 12 hours for either 2, 5, 7, or 14 days (n=9 for each duration) or treated with vehicle (n=3) for 14 days. Sham rats were treated with DFX (n=3) or vehicle (n=3) for 14 days. Rats were then euthanized for histologic examination at 56 days after surgery.
Part 3: ICH rats were treated with DFX starting at 2, 4, 12, 24, or 48 hours (n=9 for each) or administered vehicle at 48 hours (n=3), and then a second injection was administered 4 hours after the first injection, then followed by an injection every 12 hours for 7 days. Rats were killed for histologic examination at 56 days after ICH.
Part 4: ICH or sham rats were treated with DFX (100mg/kg administered intramuscularly; 2h after blood infusion and then at 12h intervals for up to 7 days) or vehicle (the same amount of saline) treatment. The ICH rats were euthanized at 1, 3 and 7 days later (n=5 each group, each time points), and the sham control rats were killed at 7 days (n=3 each group), for histological examination.
Part 5: Rats received an intracerebral infusion of 100 µL autologous blood, and then received either DFX (100mg/kg administered intramuscularly; 2h after blood infusion and then at 12h intervals for up to 7 days) or vehicle (the same amount of saline) treatment. Rats were euthanized at days 1, 3 and 7 (n=4 each group) for Western blot analysis.
Immunohistochemistry staining
Immunohistochemistry staining was performed as described previously16. Briefly, Rats were euthanized (pentobarbital, 60mg/kg i.p.) and perfused with 4% paraformaldehyde in 0.1mM phosphate-buffered saline (pH 7.4). Brains were harvested and kept in 4% paraformaldehyde for 24 hours and immersed in 30% sucrose for 3–4 days under 4°C. After that, the brain samples were embedded in a mixture of 30% sucrose and optimal cutting temperature compound (Sakura Finetek, Inc., Torrance, USA) and sectioned to 18µ-thick slices on a cryostat. Immunohistochemistry studies were performed with avidin-biotin complex technique as previously described17. The primary antibody was rabbit anti-phosphorylated-JNK antibody (Cell signaling, 1:200 dilution). The second antibody was biotinylated goat anti-rabbit IgG (Bio-Rad Laboratories, 1:400 dilution). For immunofluorescence, the primary antibody was rabbit anti-phosphorylated-JNK antibody (Cell signaling, 1:200 dilution) and mouse anti-myelin basic protein (MBP, AbD Serotec, 1:100 dilution). The secondary antibodies were Alexa Fluor 488-conjugated donkey anti-rabbit mAb (Invitrogen, 1:500 dilution) and Alexa Fluor 594-conjugated donkey anti-mouse mAb (Invitrogen, 1:500 dilution). The double labeling was analyzed using a fluorescence microscope (Olympus, BX51).
Luxol fast blue staining
Luxol fast blue staining was performed using Luxol fast blue-cresyl echt violet stain kit (American Mastertech). Brain sections were dehydrated with alcohols, and then incubated in in Luxol fast blue stain solution at 60°C overnight, followed by washing with distilled water. Sections were then quickly dipped in 0.05% lithium carbonate and 70% regent alcohol for gray and white matter differentiation. Then, the slices were incubated in cresyl echt violet stain for 10 minutes, dipped in 70% regent alcohol for 5–10 times, dehydrated through 3 changes of absolute alcohol and finally mounted with Permount (Fisher Scientific). The white matter bundles were examined by light microscopy. The quantitative analysis for Luxol fast blue stained section was performed as described in the previous literature18. The area of reduced Luxol fast blue staining in the ipsilateral basal ganglia was measured and expressed as a percentage of the contralateral side.
Western blotting
Western blot analysis was performed as previously described19. Briefly, brain tissue was perfused with 0.1mM phosphate-buffered saline (pH 7.4) after euthanasia and bilateral basal ganglia were sampled. Then, each sample was immersed in western sample buffer and sonicated. Protein concentration was determined by Bio-Rad protein assay kit, and 50µg protein from each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a Hybond-C pure nitrocellulose membrane (Amersham). The primary antibodies were rabbit anti-JNK antibody (Cell signaling, 1:1000 dilution) and rabbit anti-phosphorylated-JNK antibody (Cell signaling, 1:1000 dilution). The secondary antibodies were goat anti rabbit IgG (1:2000 dilution). The antigen–antibody complexes were visualized with the ECL chemiluminescence system (Amersham) and exposed to Kodak X-OMAT film. The relative densities of bands were analyzed with NIH Image J.
Statistical analysis
All the data in this study are presented as mean ± SD. Data were analyzed by Student t test for single comparisons or ANOVA with by post hoc Bonferroni-Dunn correction for multiple comparisons. P<0.05 was considered statistically significant.
Results
Physiological Variables
All physiological variables were measured before intracerebral infusion. Mean arterial blood pressure, blood pH, PaO2 and PaCO2, hematocrit, and blood glucose were within normal ranges (mean arterial blood pressure, 80–120mmHg; blood pH, 7.30–7.50; PaO2, 80–120mmHg; PaCO2, 35–45mmHg; hematocrit, 35–55%; glucose, 80–130mg/dL).
Mortality
Mortality was low in this study. A total of 10 animals died after the experiments, including 3 in the first part, 2 in the second part, 3 in the third part and 2 in the four part of experiment.
Dose response of DFX
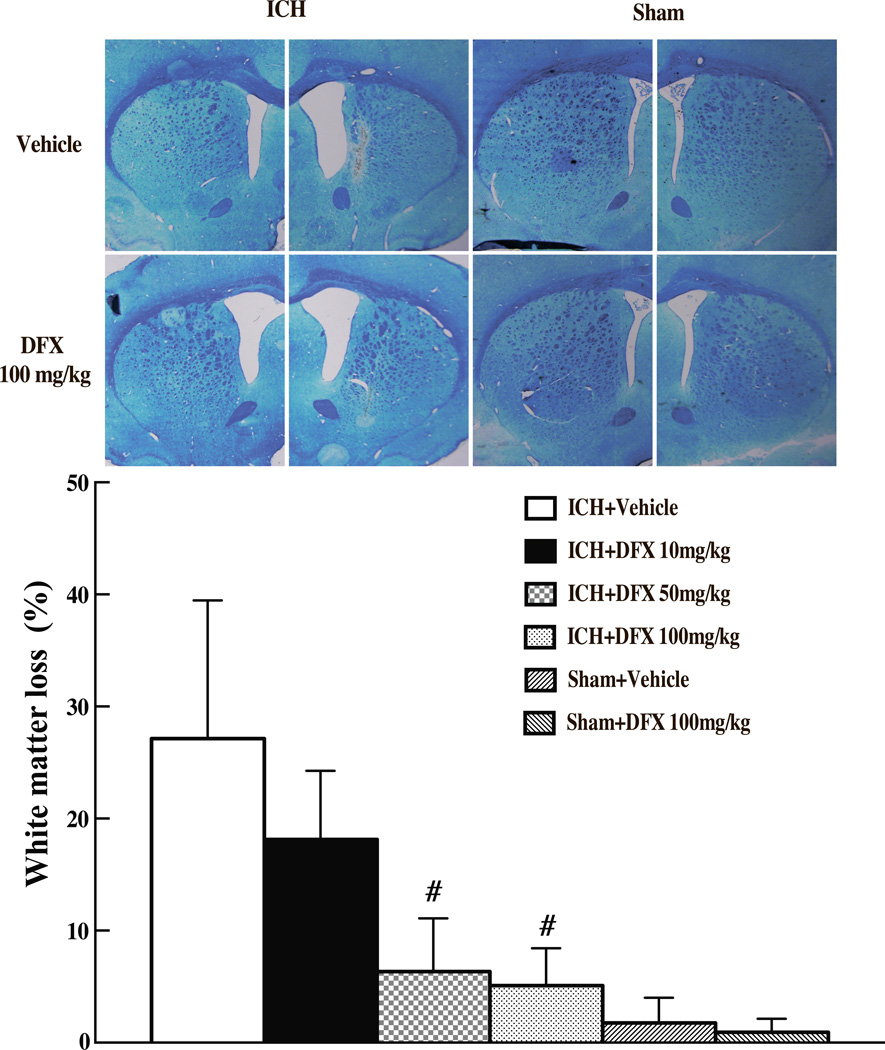
Luxol fast blue stained showed that ICH caused white matter loss in the basal ganglia after ICH. The percentage of white matter loss was calculated as follows: (contralateral striatal bundle area-ipsilateral striatal bundle area)/contralateral striatal bundle area × 100%. ICH caused significant white matter loss (27.2 ±12.3 vs. 1.8 ± 2.2% in sham group, p<0.01, Fig 1). DFX treatments at 50 mg/kg and 100 mg/kg, but not at 10 mg/kg resulted in less white matter loss (6.3 ± 4.8% and 5.1 ± 3.3% vs. 27.2 ± 12.3% in vehicle-treated group, p<0.01) at 2 months after ICH (Fig 1).
Figure 1.
Luxol fast blue stained white matter in the ipsilateral and contralateral basal ganglia and percentage of ipsilateral white matter reduction of aged rats treated with different doses of DFX (10mg/kg, 50mg/kg and 100mg/kg, started at 2 hours after ICH and lasted for 7 days) at 2 months after ICH or sham operation. Values are mean ± SD, #p<0.01 vs. with vehicle group.
Therapeutic duration of DFX
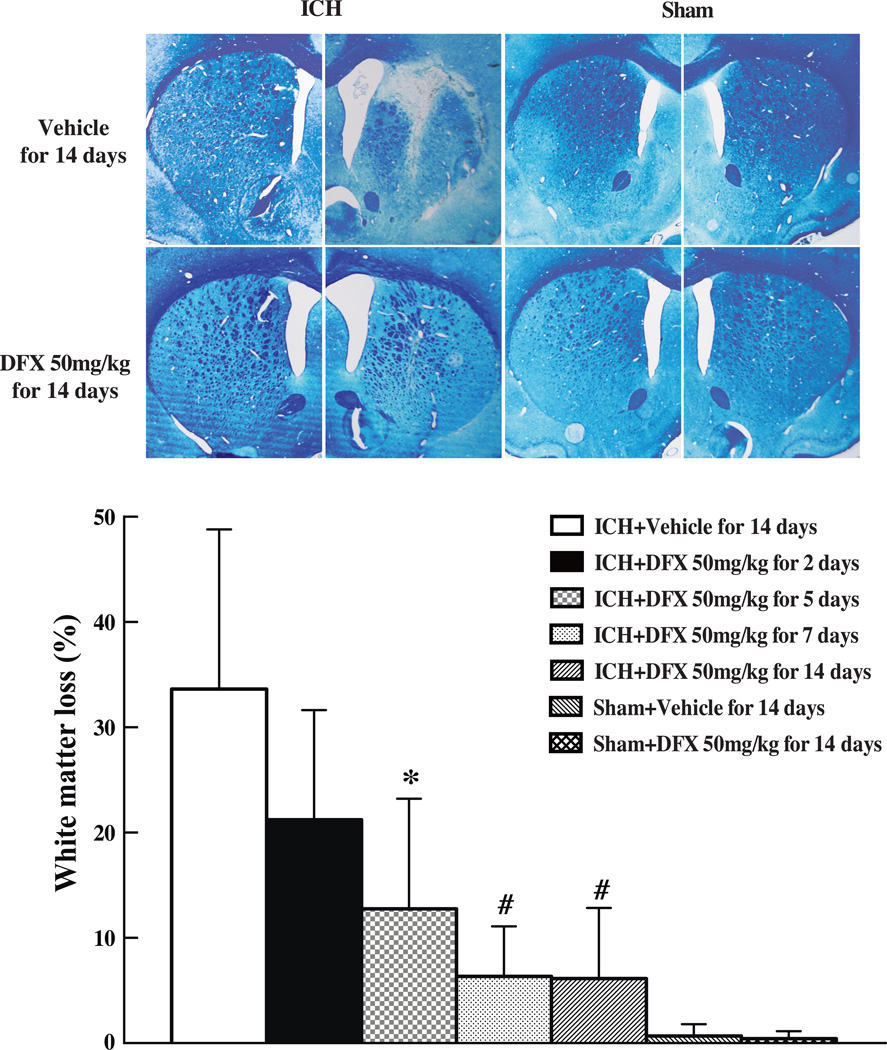
To determine the effect of therapeutic duration of DFX in white matter injury after ICH, DFX at 50mg/kg was given at 2 hours after ICH for 2, 5, 7 or 14 days. We found that DFX treatments for 5, 7 and 14 days significantly attenuated white matter loss at 2 months after ICH (For 7 days treatment: 6.3 ± 4.8% vs. 33.6 ± 15.2% in vehicle-treated group, p<0.01, Fig 2). DFX treatment for 2 days failed to attenuate white matter loss (21.2 ± 10.4% vs. 33.6 ± 15.2% in vehicle treated group, p>0.05, Fig 2).
Figure 2.
Luxol fast blue stained white matter and the ratio of ipsilateral white matter reduction in aged rats at 2 months after ICH or sham operation treated with DFX (50mg/kg, started at 2 hours and lasted for 2, 5, 7, 14 days after ICH). Values are mean ± SD, *p<0.05, #p<0.01 vs. vehicle group.
Therapeutic time window of DFX
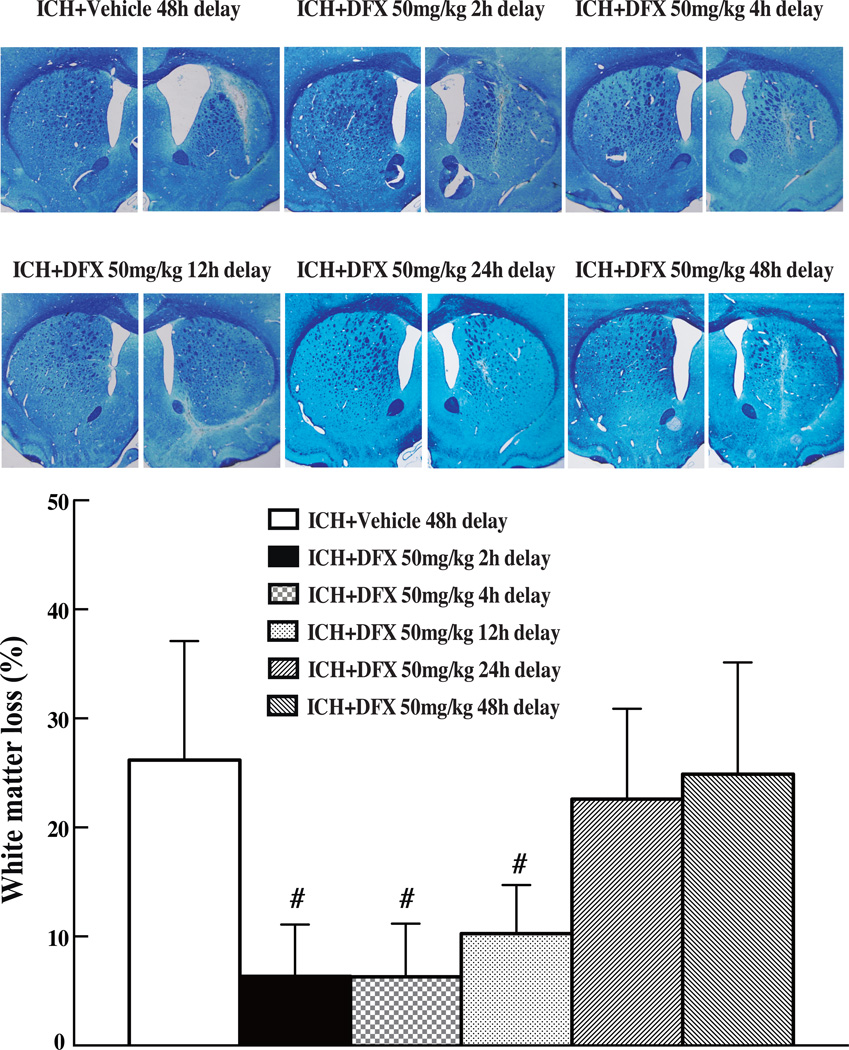
To determine therapeutic time window of DFX, rats were treated with DFX (50mg/kg) starting at 2, 4, 12, 24 or 48 hours after ICH. We found that the therapeutic time window of DFX for ICH-induced white matter injury is 12 hours (Fig 3).
Figure 3.
Luxol fast blue stained white matter and the ratio of ipsilateral white matter reduction in the aged rats at 2 months after ICH and with the treatment of DFX (50mg/kg) initiated at 2, 4, 12, 24 and 48 hours after ICH and lasted for 7 days. Values are mean ± SD, #p<0.01 vs. vehicle group.
JNK activation in the brain after ICH
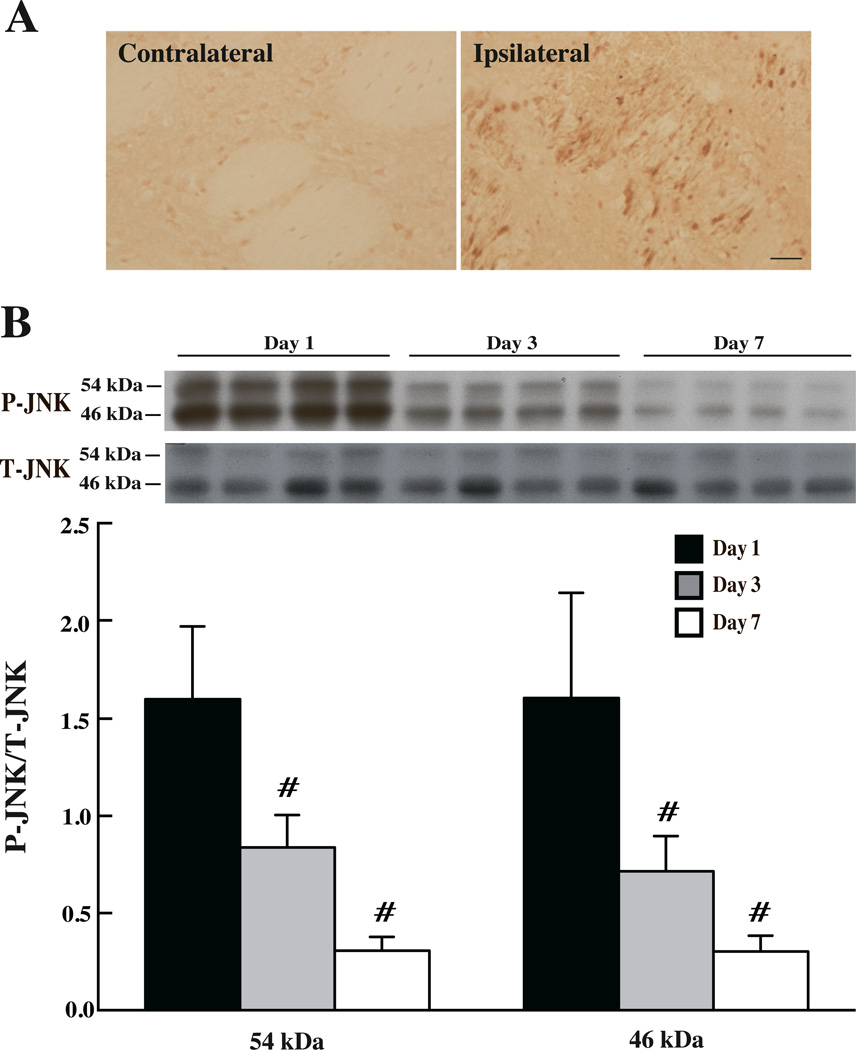
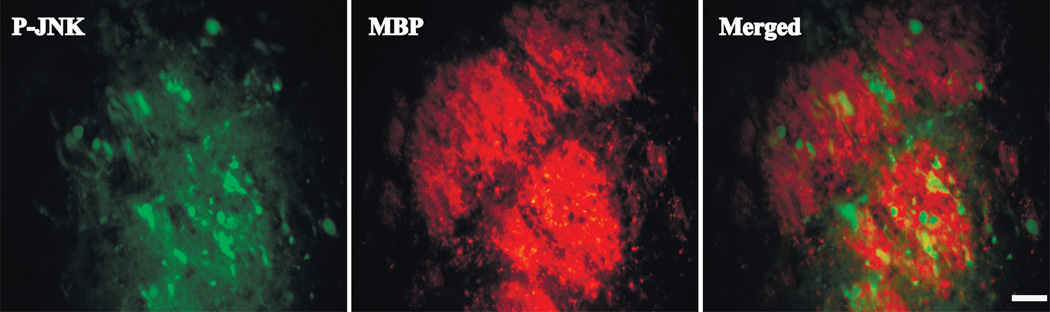
Phosphorylated-JNK (P-JNK) positive cells were detected by immunohistochemistry in ipsilateral basal ganglia after ICH, but not after sham operation. These positive cells were abundant at day 1 after ICH and gradually decreased at day 3 and 7 (Fig 4). Western blot assay confirmed that the protein level of P-JNK (Fig 4) peaked at day 1 (P-JNK/T-JNK, 46 kDa: 1.61±0.54 and 54kDa: 1.6 ± 0.37) and subsided at day 3 (P-JNK/T-JNK, 46 kDa: 0.72±0.18 and 54kDa: 0.84±0.17, p<0.01 vs. day 1) and then day 7 (P-JNK/T-JNK, 46 kDa: 0.30±0.08 and 54kDa: 0.31±0.07, p<0.01 vs. day 1). Immunofluorescence double labeling showed that P-JNK positive staining was mostly located in the white matter bundles after ICH (Fig 5).
Figure 4.
(A) The immunoreactivity of phosphorylated-JNK in the basal ganglia of aged rats at 24 hours after 100µl autologous blood injected into the right caudate, scale bar=20µm (B) JNK protein levels in the ipsilateral basal ganglia at days 1, 3 and 7 after ICH. Values are mean ± SD; n=4, #p<0.01, vs. day 1.
Figure 5.
Double labeling of P-JNK and myelin basic protein (MBP) in the ipsilateral basal ganglia of aged rats at 24 hours after ICH, scale bar=20µm.
DFX reduced ICH-induced JNK activation
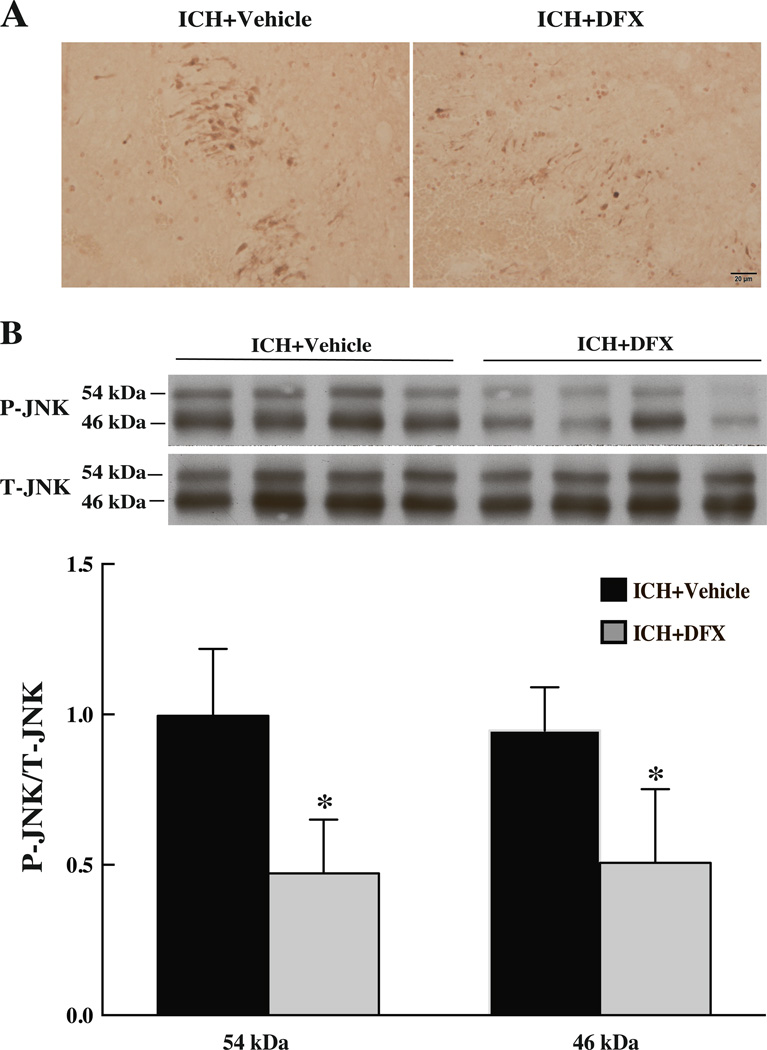
DFX treatment (100mg/kg, i.m. at 2h after ICH and then at 12h intervals) reduced P-JNK immunoreactivity in the ipsilateral basal ganglia at 24 hours after ICH (Fig 6). Western blots showed lower levels of P-JNK in the DFX-treated group compare with those in the vehicle-treated group (P-JNK/T-JNK, 46kDa: 0.51 ± 0.25 vs. 0.95 ± 0.14; 54kDa: 0.47± 0.18 vs. 0.99 ± 0.22, p<0.05; Fig 6).
Figure 6.
Immunoreactivity (A) and protein levels (B) of JNK in the ipsilateral basal ganglia of aged rats treated with deferoxamine (100mg/kg administered intramuscularly; 2h after blood infusion and then at 12h intervals) at 24 hours after ICH. Scale bar=20µm, values are mean ± SD; n=4, *p<0.05 vs. vehicle group.
Discussion
There were several important findings in the current study: (1) to reduce white matter injury after ICH, the dose of DFX should be more than 10 mg/kg, therapeutic duration should be more than 2 days and the treatment should be started within 12 hours after ICH; (2) ICH causes JNK activation in white matter bundles and white matter loss; and (2) DFX treatment reduces ICH-induced JNK activation and white matter loss.
Although white matter injury is known as a frequent complication of ICH, the underlying pathophysiology remains poorly understood. In addition, some studies have shown an increase of vulnerability of white matter in aged animals20, 21, indicating the significance of studying on white matter injury in experimental ICH models, especially in aged animals. The present study characterizes the relation among iron chelation, JNK activation and white matter injury after ICH in aged rat.
In the present study, DFX reduced white matter loss in the aged rat after ICH. In the previous studies, we demonstrated that DFX reduced ICH-induced caudate atrophy and neurological deficits in aged rat22, 23. In the first part of our study, we used DFX at doses of 10, 50 and 100mg/kg to observe long-term white matter damage and found systemic DFX treatment at 50 or 100mg/kg significantly attenuated white matter loss in the ipsilateral striatum. This dosage conformed to our previous report that >10mg/kg DFX was the optimal dose to reduce ICH-induced brain edema, neurological deficits, and brain atrophy22.
In the optimal duration studies, we found systemic treatment of 50mg/kg DFX (initiated 2 hours after ICH) for 5, 7 days or 14 days reduced ICH-induced white matter loss. This also coincided with the duration that was proved to be effective in attenuating ICH-induced brain atrophy. The duration of 7 days is recommended optimal for the reason previously mentioned that DFX treatment for 14 days causes significant body weight loss in aged rat23. This might be related with DFX-induced digestive side effects including abdominal discomfort and nausea.
In therapeutic time window studies, we demonstrated that early DFX (50mg/kg for 7 days) administration within 12 hours after ICH induction attenuated the white matter injury after ICH. This therapeutic window is narrower than the time window in brain atrophy and neurological deficits studies, suggesting white matter might be more sensitive to iron-induced damage.
Iron overload occurs and contributes to the brain injury after ICH24. JNK pathway is a potent mediator of inflammation and cell death25, 26. Our previous study indicated administration of DFX after ICH reduced free iron contents, suppressed JNK activation and improved ICH induced neurological deficits in young rats.15 In the present study, we found that DFX reduces white matter injury and JNK activation in aged rats. Studies suggest a link between iron and JNK activation. In an in vitro study, both mRNA and protein expression of JNK were significantly increased upon Fe2+ stimulation in microglia27. JNK signaling pathway was reported the shared pathway linking neuroinflammation, blood-brain barrier disruption and oligodendroglial apoptosis in the white matter injury after lipopolysaccharide-sensitized hypoxic ischemia. Pharmacological inhibition of JNK protected against white matter injury by reducing microglia activation, vascular endothelial cell damage and oligodendrocyte progenitor apoptosis28. In addition, activation of JNK signaling pathway leads to the production of pro-inflammatory cytokines, such as TNF, which can induce apoptosis in oligodendrocytes and demyelination in the white matter29. In the current study, we found P-JNK positive cells were mainly expressed in the white matter bundles after ICH in aged rat. DFX was effective in reducing the number of P-JNK positive cells in the early phase and attenuating chronic white matter loss in the ipsilateral basal ganglia after ICH. These findings indicate that iron-induced JNK activation may play a role in white matter injury after ICH.
In summary, ICH leads to acute JNK activation and chronic white matter injury in aged rats. Systemic administration of DFX after ICH suppresses JNK activation and attenuates white matter loss.
Acknowledgement
This study was supported by grants NS-052510, NS-073595, NS-079157, NS-084049 and NS-091545 from the National Institutes of Health (NIH) and 973 Program-2014CB541600.
Footnotes
Disclosure: We declare that we have no conflict of interest.
Reference
- 1.Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke; a journal of cerebral circulation. 2009;40:394–399. doi: 10.1161/STROKEAHA.108.523209. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi G, Strahle J, Hua Y, Keep RF. Progress in translational research on intracerebral hemorrhage: Is there an end in sight? Progress in neurobiology. 2014;115:45–63. doi: 10.1016/j.pneurobio.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey AS, Xi G. Intracerebral hemorrhage: A multimodality approach to improving outcome. Translational stroke research. 2014;5:313–315. doi: 10.1007/s12975-014-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: From mechanisms to clinical translation. Progress in neurobiology. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Rost NS, Rahman RM, Biffi A, Smith EE, Kanakis A, Fitzpatrick K, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75:1670–1677. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Kim BJ, Ryu WS, Kim CK, Kim N, Park BJ, et al. White matter lesions and poor outcome after intracerebral hemorrhage a nationwide cohort study. Neurology. 2010;74:1502–1510. doi: 10.1212/WNL.0b013e3181dd425a. [DOI] [PubMed] [Google Scholar]
- 9.Xie Q, Gu YX, Hua Y, Liu WQ, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke; a journal of cerebral circulation. 2014;45:290–292. doi: 10.1161/STROKEAHA.113.003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuno S, Saito A, Hayashi T, Chan PH. The c-jun n-terminal protein kinase signaling pathway mediates bax activation and subsequent neuronal apoptosis through interaction with bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yatsushige H, Yamaguchi M, Zhou CM, Calvert JW, Zhang JH. Role of c-jun n-terminal kinase in cerebral vasospasm after experimental subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2005;36:1538–1543. doi: 10.1161/01.STR.0000170713.22011.c8. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Progress in neurobiology. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: An apoptosis junkie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 14.Guan QH, Pei DS, Liu XM, Wang XT, Xu TL, Zhang GY. Neuroprotection against ischemic brain injury by sp600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain research. 2006;1092:36–46. doi: 10.1016/j.brainres.2006.03.086. [DOI] [PubMed] [Google Scholar]
- 15.Wan S, Zhan RY, Zheng SS, Hua Y, Xi G. Activation of c-jun-n-terminal kinase in a rat model of intracerebral hemorrhage: The role of iron. Neurosci Res. 2009;63:100–105. doi: 10.1016/j.neures.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin H, Xi G, Keep RF, Wu J, Hua Y. Darpp-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Translational stroke research. 2013;4:130–134. doi: 10.1007/s12975-012-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 18.Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropath Appl Neuro. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 19.Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Translational stroke research. 2013;4:546–553. doi: 10.1007/s12975-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popa-Wagner A, Schroder E, Walker LC, Kessler C. Beta-amyloid precursor protein and ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral artery occlusion: Effect of age. Stroke; a journal of cerebral circulation. 1998;29:2196–2202. doi: 10.1161/01.str.29.10.2196. [DOI] [PubMed] [Google Scholar]
- 21.Badan I, Dinca I, Buchhold B, Suofu Y, Walker L, Gratz M, et al. Accelerated accumulation of n- and c-terminal beta app fragments and delayed recovery of microtubule-associated protein 1b expression following stroke in aged rats. Eur J Neurosci. 2004;19:2270–2280. doi: 10.1111/j.0953-816X.2004.03323.x. [DOI] [PubMed] [Google Scholar]
- 22.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke; a journal of cerebral circulation. 2009;40:1858–1863. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: Therapeutic time window and optimal duration. Stroke; a journal of cerebral circulation. 2010;41:375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain research. 2002;953:45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- 25.Mielke K, Herdegen T. Jnk and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Progress in neurobiology. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, et al. Neuroprotection against focal ischemic brain injury by inhibition of c-jun n-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Yan ZF, Gao JH, Sun L, Huang XY, Liu Z, et al. Role and mechanism of microglial activation in iron-induced selective and progressive dopaminergic neurodegeneration. Molecular neurobiology. 2014;49:1153–1165. doi: 10.1007/s12035-013-8586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LW, Tu YF, Huang CC, Ho CJ. Jnk signaling is the shared pathway linking neuroinflammation, blood-brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. Journal of neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage invitro. Annals of neurology. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]