Abstract
STAT3 is both a transcription activator and an oncogene that is tightly regulated under normal physiological conditions. However, abundant evidence indicates that STAT3 is persistently activated in several cancers, with a crucial position in tumor onset and progression. In addition to its traditional role in cancer cell proliferation, invasion, and migration, STAT3 also promotes cancer through altering gene expression via epigenetic modification, inducing epithelial–mesenchymal transition (EMT) phenotypes in cancer cells, regulating the tumor microenvironment, and promoting cancer stem cells (CSCs) self-renewal and differentiation. STAT3 is regulated not only by the canonical cytokines and growth factors, but also by the G-protein-coupled receptors, cadherin engagement, Toll-like receptors (TLRs), and microRNA (miRNA). Despite the presence of diverse regulators and pivotal biological functions in cancer, no effective therapeutic inventions are available for inhibiting STAT3 and acquiring potent antitumor effects in the clinic. An improved understanding of the complex roles of STAT3 in cancer is required to achieve optimal therapeutic effects.
Cancer progression is a multistep and complex process that begins with abnormal cells with malignant potential or neoplastic characteristics and continues with tumor growth, stromal invasion, and metastasis. This event not only relies on tumor-intrinsic effects, but also the tumor microenvironment which includes surrounding and supportive stroma, humoral factors, different effectors of immune system, and vasculature. As a transcription activator and an oncogene, STAT3 which is frequently detected with persistent activation in most human cancer cell lines and tumor tissues, is crucial in tumor cell proliferation, invasion, and migration, and is capable of inducing epithelial–mesenchymal transition (EMT), regulating the tumor microenvironment and promoting CSCs self-renewal and differentiation which all benefit the progression of cancer. Recent studies illustrated that STAT3 can also regulate gene expression through epigenetic modification, such as regulating the chromatin organization by unphosphorylated STAT31 and contributing to the silencing of tumor-suppressor genes via DNA methylation by acetylated STAT32,3. Much evidence has revealed the central cancer-promoting role of STAT3, thereby making it an ideal target for cancer therapy. However, despite the numerous regulators and pivotal biological functions in cancer, effective therapeutic inventions to inhibit STAT3 and to achieve potent antitumor effects in the clinic have not been identified and still need to be explored further. Therefore, a comprehensive exploration of the complicated biological behaviors of STAT3 in cancer is direly needed to inhibit the STAT3 signaling pathway.
Novel insights into the regulation of STAT3
Cytokine receptors, receptor tyrosine kinases, and non-receptor tyrosine kinases
Cytokine receptors which function as receptors for the interleukin-6 (IL-6) family cytokines are the most well-known traditional activators of STAT3. The interleukin-6 (IL-6) family cytokines function as ligands bind to a corresponding receptor to induce the homodimerization or the heterodimerization of gp130. After dimerization of the gp130 receptor complex, Janus kinases (JAK) are catalytically activated and transphosphorylate tyrosine residues in the gp130 receptor intracellular domain. Subsequently, the gp130 receptor complex recruits STAT3 to docks to the phosphorylated residues of the receptor via the SH2 domain of STAT3. After docking, JAK activity induces the tyrosine phosphorylation of STAT3. The phosphorylated STAT3 proteins finally result in a series of changes of cell biology.
Receptor tyrosine kinases (RTKs) can catalyze the phosphorylation of STAT3 via its intrinsic tyrosine kinase activity in the receptor. The more common receptors include EGFR, VEGFR, PDGFR, and colony stimulating factor-1. Similar to RTKs, non-receptor tyrosine kinases (nRTKs) can also directly phosphorylate STAT3 through transferring a phosphate group from ATP to the tyrosine residue of STAT3. The well-known nRTKs include SFKs and ABl. These two types of kinase both can induce STAT3 to undergo activation, dimerization, transportation to the nuclear and then regulate the corresponding target genes.
G-protein-coupled receptors/ Rho GTPase family /cadherin engagement
GPCRs are the largest family of membrane proteins that mediate in the signal transduction from the extracellular to intracellular space. GPCRs transmit signals not only via secondary messengers but also transcription factors. Recently, JAKs and STATs have been identified as novel downstream effectors of different heterotrimeric G proteins. Several GPCRs, such as angiotensin II (Ang II)4 and S1PR1/25, mediate STAT3 activation by JAKs.
Recent studies suggest that Rac1 which belongs to the Rho GTPase family has an essential role in STAT3 tyrosine phosphorylation. As an effector of Rac1, the evolutionarily conserved male germ cell RacGAP (MgcRacGAP) binds to the DNA-binding domain of STAT3 via its cysteine-rich and GAP domains; the MgcRacGAP–STAT3 association with the IL-6R/gp130 complex mediates the phosphorylation of STAT3 induced by IL-66,7. In addition to tyr705 phosphorylation, the Rac1/MgcRacGAP complex may also be involved in STAT3 translocation to the nucleus.
Recently, cadherin engagement has been revealed as a new pathway to activate STAT3. Work from several laboratories indicated that the cell density can cause a sharp increase in STAT3 phosphorylation in breast carcinoma, head and neck squamous cell carcinoma, and normal epithelial cells. After cadherin engagement, Rac1/Cdc42 (another member of the Rho GTPase family) is dramatically activated, and then trans-activates NF-κB and increases the expression of IL6, which is responsible for the observed STAT3 activation8,9.
Toll-like receptors
STAT3 is also directly activated by TLR stimulation during the production of IgG by human B cell10 and TLR mediated STAT3 activation is required for antibody production and IL-10 production10. As a classical activator of TLR4, the lipopolysaccharide (LPS) can remarkably increase the level of phosphorylated STAT3 in the human bladder cancer T24 cell line, suggesting the activation of STAT3 by TLR4 signaling11.
The activation of TLR3 during oxidative stress protects photoreceptor survival and visual function. In this TLR3 protection during injury, STAT3 is activated and has a critical role12,13. STAT3 activation is also correlated with the high expression of TLR2 in tumor tissue14. In addition, TLR7 ligation can also induce STAT3 activation and interface with Notch, as well as the canonical NF-κB and MAP kinase pathways15.
In addition to cytokines and growth factors, CpG can directly activate STAT3 within minutes via TLR9. This finding reveals a second mechanism by which STAT3 mediates immunosuppression16 while creating a potent checkpoint or inhibitor of antitumor immune response17. The mechanism by which TLR9 activates STAT3 was recently demonstrated. JAK2 is recruited by Frizzled 4 (FZD4) and then is activated depending on the TLR9 engagement with CpG oligodeoxynucleotidesve (ODNs) and this links CpG–TLR9–FZD4 signaling with subsequent STAT3 tyrosine phosphorylation18.
miRNAs
Recent studies have indicated that miRNAs are critical regulators of STAT3 signaling in the pathogenesis of cancer. MiR-519d functions as a tumor suppressor in breast cancer by suppressing STAT3 expression19. The low expression level of miR-20a, a negative regulator of STAT3, can enhance de-repressed STAT3 expression and activation and boost proliferation pathways in hepatocellular and this suggest miR-20a may represent a novel potential therapeutic target and biomarker for survival of cancer patients20.
Let-7 miRNA family members are widely considered to be tumor suppressors. Let-7 re-expression in poorly differentiated PDAC cell lines can enhance the cytoplasmic expression of suppressor of cytokine signaling 3 (SOCS3), which blocks STAT3 activation by JAK2, and reduce the phosphorylation of STAT3 and its downstream signaling events, thereby reduce the growth and migration of PDAC cells21. Iliopoulos and colleagues revealed that Src activation triggers a nuclear factor (NF)-κB-mediated inflammatory response that directly activates LIN28 transcription, which leads to let-7 inhibition and causes a high expression of IL-6 coupled with the activation of STAT3. Their study demonstrated that the interaction of let-7 and IL-6–STAT3 completes a negative-feedback loop in cellular transformation and first describes the importance of epigenetic regulation in promoting inflammation and cancer22. Additionally, downregulation of miR-200 and let-7 via STAT3 can induce the EMT phenomenon in breast cancer; conversely, inactivation of STAT3 or re-expression of both miRNAs proved sufficient to induce mesenchymal-to-epithelial transition (MET) in mesenchymal breast cancer23.
Tyrosine phosphatases
Tyrosine phosphorylation catalyzed by tyrosine kinases (PTKs) is critical for STAT3 activation. By contrast, the dephosphorylation of STAT3 by PTPs including SHP2, SHP1, CD45, PTP1B, PTP2B and PTPRT, are essential to ensure proper amplitudes and kinetics of STAT3 activation24. SHP2 negatively regulating STAT3 was observed in melanoma cells and glioma cells25,26, and morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP127. In addition, adiponectin significantly inhibits leptin-induced JAK2 activation and STAT3 transcriptional activity via increasing PTP1B protein and activity in oesophageal cancer cells28.
PIAS protein family
The protein inhibitor of activated STAT (PIAS) proteins which vary between 507 (PIASy) and 650 (PIAS1) amino acid residues are encoded by four genes, namely, PIAS1, PIASx (PIAS2), PIAS3, and PIASy (PIAS4). PIAS proteins regulate transcription through several mechanisms, including blocking the DNA-binding activityof transcription factors, recruiting transcriptional co-repressors, and promoting protein SUMOylation. Recent studies showed that PIAS proteins can deregulate the activity of STAT329,30,31.
SOCS protein family
SOCS (Suppressor of cytokine signaling) contain eight members (CIS, SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SOCS6, and SOCS732). The SOCS proteins negatively regulate the JAK-STAT3 signaling pathway through three mechanisms: first, by inhibiting JAK kinase or target JAKs for degradation by proteasome; second, by shielding the STAT3 binding sites on the cytokine receptor; third, by targeting proteins for proteasomal degradation via ubiquitination. Among those proteins, SOCS1 and SOCS3 are the best characterized so far. A recent study showed that SOCS1 and SOCS3 can promote myogenic differentiation by inhibiting JAK1 and gp130, respectively30. Platelet factor 4 (PF4) inhibits the IL-17/STAT3 pathway by upregulating the expression of SOCS333.
Other regulation patterns of STAT3
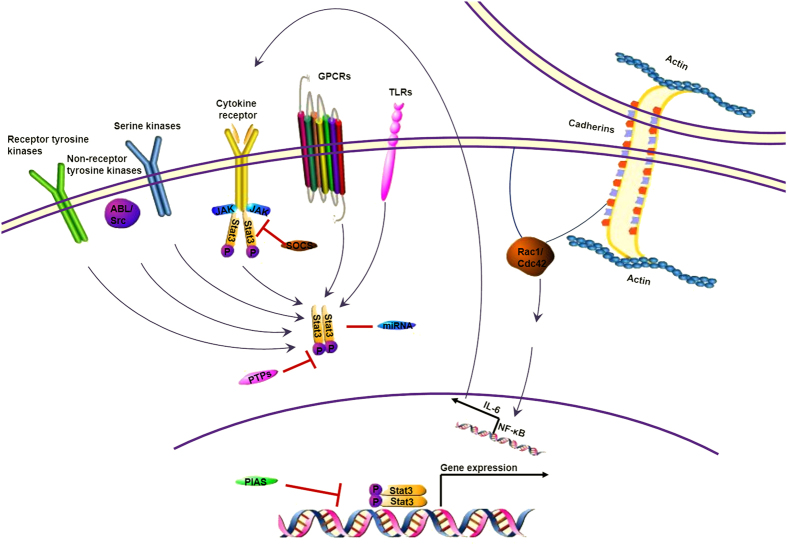
Apart from the phosphorylation at Tyr705, STAT3 can also be activated by the phosphorylation of Ser727. Various serine kinases, such as MAPK (p38MAPK, ERK, and JNK), PKCδ, mTOR, NLK, and an H-7–sensitive kinase, have been reported to phosphorylate STAT3 at the serine 727, which is required for STAT3 maximal transcriptional activity34,35,36,37,38,39. NF-κB activation is a well-known player that promotes the production of IL-6, which stimulates STAT3 activation. Interestingly, a recent study indicated that STAT3 was responsible for eliciting constitutive NF-κB activity in human melanoma and prostate cancer cells40. This finding reveals a STAT3 → NF-κB → IL-6 feed-forward signaling loop in carcinogenesis; meanwhile, the molecular mechanism linking inflammation to cancer was gradually clarified41,42,43. Other factors such as UV radiation or sun light, carcinogen, stress, smoke, and infection are also known to have a significant role in STAT3 activation. (Fig. 1.)
Figure 1. Multiple regulation pathways of STAT3 in cancer.
Cytokine receptors, especially receptors for IL-6 family cytokines, are the most well-known traditional activators of STAT3. Receptor tyrosine kinases, non-Receptor tyrosine kinases and some serine kinases can also regulate the STAT3 activity. Recently, studies found that GPCRs and TLRs are involved in the regulation of STAT3. Cadherin engagement accompanied with the high level of Rac1/Cdc42 also dramatically regulates STAT3 through activating the NF-κB signal pathway. SOCS inhibits STAT3 signaling via blockade of upstream signaling through interactions with gp130 and JAK family members. Various miRNAs either restrict or enhance STAT3 signaling. PTPases dephosphorylate STAT3 and prevents dimer formation. PIAS proteins directly compete with STAT3 for either binding opportunities with the activating receptor or for dimerization and translocation into the nucleus.
STAT3 regulates gene expression through epigenetic modification during cancer progression
Although the persistent phosphorylated form of STAT3 has been found in several cancers and leads to gene expression promoting cell proliferation and resistance to apoptosis, as well as tumor angiogenesis, invasion, and migration, unphosphorylated STAT3 also acts as a weak but potentially biologically relevant transcription factor that can activate a series of STAT3 target genes44,45 through direct binding to a responsive GAS promoter and promotes the development of cancer. The mechanism of unphosphorylated STAT3 target DNA binding is by regulating chromatin organization and binding to AT-rich DNA sequences, which play important roles in regulation of gene expression and/or chromatin organization because of their special structure with a narrow minor groove that can be recognized by proteins46. Unphosphorylated STAT92E proteins can maintain heterochromatin via the regulation of histone H3 Lys-9trimethylation (H3K9me3) in Drosophila47,48. The epigenetic modification function of unphosphorylated STAT3 was also identified by Timofeeva1 who suggested that cancer cells such as DU145 and MCF-7 cells have a more open or, at least, more accessible chromatin conformation than the non-transformed MCF-10A cells, thereby allowing for unphosphorylated STAT3 binding, suppression of CHOP expression, and subsequent inhibition of the apoptosis of cancer cells with the involvement of the N-terminal domain of STAT3.
The epigenetic gene silencing effect of gene promoter region mediated through the CpG methylation by DNA methyltransferase 1 (DNMT1) and other members of the DNMT family have key roles in the inhibition of tumor-suppressor gene expression in cancer cells. STAT3 acetylation, another activation form of STAT3, can also contribute to regulate DNMT1 binding to several tumor-suppressor gene promoters and promote the promoter methylation of relative genes and the development of cancer. STAT3 is acetylated on a single lysine residue, Lys685, by its co-activator p300/CREB-binding protein (CBP) in response to cytokine treatment, such as IL-6, LIF, and OSM49,50,51. Acetylated STAT3 induces promoter gene methylation of a major tumor-suppressor gene, ARHI, and thereby leads to the low expression level of ARHI and promotes cancer cell proliferation in ovarian cancer2. Several other tumor-suppressor genes, including CDKN2 cell A, DLEC1, STAT1, and PTPN6, can also be induced promoter methylation by acetylated STAT3 in cancer cell lines3.
Pivotal biological functions of STAT3 in cancer
STAT3 is involved in EMT promoting cancer invasion and metastasis
As a phenotypic switch, EMT is characterized by cells losing the epithelial polarity and acquiring mesenchymal characteristic resulting in the decrease of cell-cell junction and promoting the invasion and metastasis ability of cells. Enough evidences show that the EMT phenotypes plays a significant role in promoting progression of many cancers, such as non-small cell lung cancer (NSCLC)52, ovarian carcinomas53, hepatocellular carcinomas(HCC)54, breast cancer55, nasopharyngeal cancer56. Various studies demonstrated that STAT3 can modulate the expression of EMT-related transcription factors (Twist, Snail, ZEB1, etc.) and thereby influence the EMT phenotypes. For instance, in HCC cells, STAT3 was revealed to bind to the promoter of Twist, mediate its transcriptional activity, and then promote the EMT process and increase the cells invasion and migration ability for the first time57. In bresat cancer, STAT3 activation by EGF treatment induced higher Snail expression and the high expression level of snail was reversed by N-myc downstream-regulated gene 2 (NDRG2) through inhibits STAT3 binding to the Snail promoter and subsequently inhibit the EMT process and cancer progression58. Additionally, prolonged activation of STAT3 leads to low expression of let-7 and miR-200 coupled with the upregulation of ZEB1 in OSM-triggered EMT, which contributes to the acquisition of the mesenchymal phenotype and invasive capability as well as promotion of breast cancer progression23. These findings suggest that STAT3 responds to the integrating signals from multiple extracellular stimuli that influence the EMT phenotype, regulates the level of EMT-related transcription factors, and enhances the cancer cell abilities of invasion, metastasis. Therefore, targeting STAT3 may provide a means to reverse the EMT phenotypes and prevent cancer invasion and metastasis.
STAT3 in tumor microenvironment
As a major regulator of tumorigenesis, tyrosine phosphorylated STAT3 has been detected and is mainly distributed on the leading edge of tumors in association with stromal, immune, and endothelial cells59. This result effectively suggests that STAT3 has a critical role in the communication between cancer cells and their microenvironment which has the following aspects. (A) Production of humoral factors. For instance, the paracrine sources of IL-6 from cancer-associated fibroblasts, adipocytes, or myeloid cells on the edge of the tumors and the autocrine production of IL-6 can both activate the STAT3. The pSTAT3 in turn promotes the expression of IL-6, thus forming amplification loops of the production of IL-6, which can induce the vast expression of autocrine and paracrine cytokines and growth factors, including IL-8, CCL5, CCL2, CCL3, IL1-β, GM-CSF, VEGF, and MCP-1, which are highly expressed and play an important role in the generation and development of cancer. (B) Interaction with fibroblasts, adipocytes and macrophages. Cancer-associated fibroblasts (CAFs) can promote cancer progression via remodeling the ECM, induction of angiogenesis, recruitment of inflammatory cells, and directly stimulating cancer cell proliferation via the secretion of growth factors and mesenchymal–epithelial cell interactions, which are mainly regulated by the IL-6–STAT3–Twist signaling pathway by upregulating the expression of CXCL1260, a Twist target gene associated with the regulation of the CAF phenotype. When cancer-associated adipocytes separated from the breast cancer patients co-culture with MCF7 and MDA-MB-231 breast cancer cell lines, adipocytes revert to an immature and proliferative phenotype, and promote cancer cell migration via the high expression of IL-661. Due to the protumoral functions, tumor-associated macrophages have received much attention as novel cancer target cells. Researchers shown that suppressing STAT3 activation by triterpenoid compounds can inhibited macrophage polarization to M2 phenotype which are involved in tumor development and poor clinical prognosis62. (C) Promotion of immune suppression. Cancer cells regulate their immunological environment, recruit immune cells, subvert their functions to their own advantage, prevent them from mounting an effective immune response and instead promote cancer progression. STAT3 is an established molecular hub of immune suppression. STAT3-regulated genes encoding VEGF and IL-10 mediate crosstalk between cancer cells and contribute to a state of immunosuppression63. STAT3 promotes myeloid-derived suppressor cells (MDSCs) expansion and immune suppression in lung cancer64 and exosomal Hsp70 mediates immune suppression activity of MDSCs via p-STAT365. Blocking the STAT3 activity is in favor of reversing the hepatocellular carcinoma-induced immune suppression and enhancing the NK cell functions66. (D) Linking inflammation to cancer. STAT3 and NF-κB are two important transcription factors; both function as critical regulators in inflammation and cancer development, with vital roles to control the communication between cancer cells and inflammatory cells. They cooperatively bind at a subset of gene promoters and synergistically induce their target gene expression and function in the process of inflammation and tumorigenesis. Pro-inflammation cytokines induced by NF-κB or STAT3 can positively feedback to induce STAT3 and NF-κB activation42 and promote cancer progression. The collaborative functions of STAT3 and NF-κB make a link from inflammation to cancer and the mechanism inflammatory responses have a decisive role at different stages of tumor development becomes gradually clear. This may provide a new treatment strategy for cancer. (E) Tumor angiogenesis. Growing evidence indicates that activated STAT3 participates in angiogenesis regulation, with a critical role67. VEGF and bFGF are known to be involved in endothelial cell proliferation, extracellular matrix degradation, endothelial cell migration, and modulation of junctional adhesion molecules; both have been described as the leading mediators of angiogenesis that can be upregulated by activated STAT3 in glioblastoma stem cells68, papillary thyroid cancer69, and colorectal cancer70, thereby promoting the formation of new blood vessels and development of cancer. STAT3 is involved in various aspects of tumor microenvironment to cultivate a favorable environment for cancer development. Targeting STAT3 present a feasible stategy to weaken the supporting function of tumor microenvironment and improving the therapeutic effect for cancer.
STAT3 regulates CSCs
Given its important role in sustaining the self-renewal and differentiation of Embryonic Stem CellS (ESCs)71,72,73, STAT3 is also evidently essential for regulating CSCs of cancers such as ovarian cancer74, HCC75, breast cancer76 colorectal cancer77, glioblastoma78, lung cancer79, and prostate cancer80. The STAT3 regulatory mechanism of stem cell self-renewal and differentiation is mainly focused on the ESC-specific roles of LIF. When LIF binds to LIFR and gp130, the heterodimerized compound can activate STAT3 and then, birdged by Bcl3 to the Oct4 signaling and maintain pluripotency of ESCs71,81. Other IL-6 family members, such as OSM, CNTF, CTF-1, and CLC, which form heterodimerization of gp130 with the LIF receptor, can also maintain the self-renewal and differentiation of stem cells because of their shared signaling mechanisms that converge on STAT3.
As a multifunctional cytokine, IL-6 has been implicated in the maintenance of stem cancer cells through the IL-6/gp130/STAT3 signaling pathway. In gene expression profiles of CD44+/CD24– breast CSCs, IL-6 has been demonstrated to be upregulated15. Liu79 showed that IL-6/JAK2/STAT3 pathway upregulates DNMT1 and enhances cancer initiation and lung CSC proliferation via the downregulation of p53 and p21, which results from DNA hypermethylation. In addition, IL-6/STAT3/NF-κB signaling pathways are both activated in CSCs and its microenvironment82,83. Activation of these pathways stimulates further cytokine production and generates positive feedback loops that in turn drive CSC self-renewal. Furthermore, the constitutive activation of STAT3/NF-κB signaling can regulate the Notch pathway, which appears to play a key role in CSCs in a variety of cancers and controls cell fate determination, survival, proliferation, and the maintenance of stem cells84.
Although the activation of STAT3 via IL-6 has been identified as necessary for promoting CSC-like phenotypes, other STAT3 activators are also involved in the regulation of CSCs. Conti85 first showed the role of TLR2 in mammary CSC self-renewal through binding to its receptor HMGB1, increasing the secretion of IL-6 and subsequently activating the STAT3 signaling pathway. Downregulation of miR-1181 can promote CSC-like phenotypes in human pancreatic cancer by promoting the STAT3 signaling pathway and the activation of the CSC transcription factor SOX286. RhoC expression is found to be correlated to CSC formation in head and neck squamous cell carcinoma (HNSCC). RhoC elevates the expression level of IL-6 and then promotes the phosphorylation of STAT3ser727and STAT3tyr705 as well as the high expression of Nanog, oct3/4, and sox2 in HNSCC87. In addition, a novel EGFR/STAT3/Sox-2 paracrine signaling pathway which is required for macrophage-induced upregulation of Sox-2 and CSC phenotypes in tumor cells is identified88.
STAT3 inhibitors
STAT3 is considered as an ideal molecular target of cancer therapy because this target plays a pivotal role in tumorigenesis and cancer cell biology. As such, great efforts have been devoted to the discovery of potent and selective inhibitors that target STAT3. STAT3 inhibitors are divided into two types depending on whether the activity of STAT3 can be inhibited indirectly or directly. Indirect inhibitors block upstream effectors, such as cytokine and kinases, involved in STAT3 activation. For instance, ALD518, a humanized anti-IL-6 antibody, helps NSCLC patients obtain therapeutic benefits against cachexia, anemia, and drug resistance89. WP1066, a JAK2 inhibitor, suppresses ovarian cancer growth, migration, and invasion; this inhibitor also enhances the chemosensitivity of ovarian cancer cells and decreases the rate of STAT3 phosphorylation90. Direct inhibitors directly block the SH2, DNA-binding, and N-terminal domains of STAT3 to suppress protein dimerization, to inhibit DNA binding, and to prevent nuclear translocation, respectively. Among these domains, the SH2 domain has been considered as the most commonly investigated site because of its critical involvement in STAT3 activation; furthermore, inhibitors targeting the SH2 domain constitute the largest class of direct inhibitors.
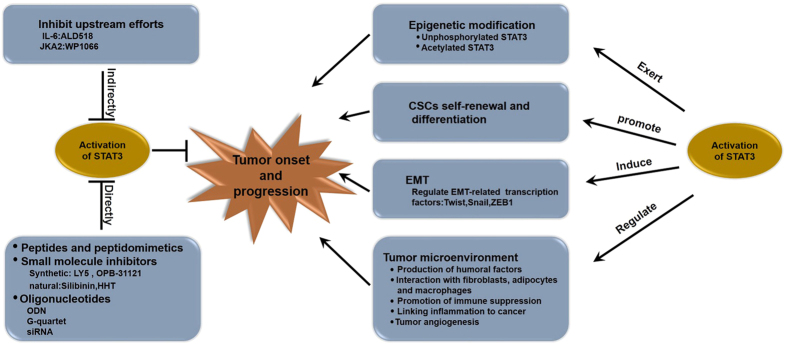
Inhibitors are also divided into three classes of compounds on the basis of structure. (A) One of these classes includes peptides and peptidomimetics. Although peptides and peptidomimetics can directly disrupt the dimerization of STAT3 and effectively inhibit its transcriptional activity, these inhibitors present several challenges related to low cell permeability and stability. (B) Another class of these compounds comprises small molecule inhibitors. With advances in medicinal chemistry and structural applications based on high-throughput virtual screening and site-directed computational fragment-based drug design approach in silico, small-molecule STAT3 inhibitors, which overcome problems related to cell permeability, show much feasibility to inhibit the STAT3 activity. Novel synthetic or natural small-molecule STAT3 inhibitors have been evaluated by using preclinical models. LY5, a novel non-peptide, cell-permeable small molecule inhibitor of STAT3 dimerization, blocks STAT3 activation with low IC50 values (0.5–1.4 M) and strong binding affinity to the STAT3 SH2 domain. LY5 selectively inhibits persistent STAT3 activation and induces the apoptosis of medulloblastoma cells and becomes a promising therapeutic drug candidate for human medulloblastoma by inhibiting STAT3 signaling91. OPB-31121, an inhibitor assessed through active clinical trials, interacts and exhibits a high affinity to the SH2 domain of STAT392 and elicits a significant antitumor effect on leukemia93 and gastric cancer94. Silibinin, a natural polyphenolic flavonoid extracted from the seeds of milk thistle (Silybum marianum), is an optimum inhibitor of pSTAT3 in gastric95, breast cancer96, prostate97 in preclinical studies and clinical trials related to this flavonoid have also been conducted. However, therapeutic effects on cancer patients remain unsatisfactory because the bioavailability of its flavonolignan structure is low98. Homoharringtonine (HHT), another natural compound extracted from Cephalotaxus harringtonia, significantly inhibits the STAT3 activity by suppressing the IL-6/JAK1/STAT3 signaling pathway and induces the apoptosis of Gefiinib-resistant lung cancer cells. In vivo, HHT remarkably suppresses the tumor growth but Gefiinib does not exhibit comparative effect in nude mice injected H1975 cells and this identifies the HTT as a novel potential natural inhibitors for patients with NSCLC in a EGFR-independent manner99. (C) Oligonucleotides. Because of the application of advanced molecular techniques, oligonucleotides inhibitors targeting STAT3 seem viable to selectively inhibit STAT3 activity. Decoy Oligonucleotide (ODN) usually is a double stranded 10–20 base pair DNA containing a TF’s consensus and selectively inhibits STAT3 activity by competitively binding to the DNA binding domain of STAT3; thus, specific gene expression is effectively attenuated. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors has been recently assessed. With improvements in cyclization, STAT3-targeting ODN seems more amenable to systemic administration and can yield optimum effects by downregulating STAT3 target genes and by suppressing tumor growth100. G-quartet oligonucleotides are G-rich oligodeoxynucleotides that form four-stranded potassium-dependent intramolecular G-quartet structures and occupy sites within the SH2 domains of STAT3. These oligonucleotides effectively inhibit Stat3 activation and tumor growth in head and neck cancer101, NSCLC102, Prostate Cancer103. Nonetheless, their large size and potassium dependence limit their cellular delivery and possibility to be assessed in clinical trials. Small interfering RNA (siRNA) is a natural post-transcriptional gene-silencing mechanism to turn off unwanted genes. Targeting STAT3 using siRNA represent a useful approach for the treatment of breast cancer104, lung adenocarcinoma105. However, further studies should be conducted regarding STAT3 silencing for cancer therapy. (Fig. 2.)
Figure 2. Pivotal biologiccal functions in cancer and inhibitors of STAT3.
STAT3 plays a pivotal role in tumor onset and progression through altering gene expression via epigenetic modification, inducing EMT phenotypes in cancer cells, regulating the tumor microenvironment, and promoting CSCs self-renewal and differentiation. As an ideal target for cancer therapy, lots of indirect or direct inhibitors for STAT3 have been developed recently.
Conclusion and future directions
Although, STAT3 is an ideal target of cancer therapy because of its multiple regulatory pathways and pivotal biological functions in cancer; furthermore, various inhibitors targeting STAT3 have been developed for cancer therapy, no candidate compounds are potent enough to provide beneficial therapeutic effects for cancer patients. As such, new directions for cancer therapy by targeting STAT3 should be explored. For instance, small-molecule inhibitors of GPCR, TLR, and miRNAs related to STAT3 regulation can be applied to treat cancer. Current anticancer-targeted therapeutics mainly focuses on inhibiting the tyrosine phosphorylation of STAT3, however, epigenetic modification function of STAT3 may present a novel and powerful therapeutic approach for cancer treatment. Therefore, further studies should be conducted to address the questions regarding STAT3 in cancer and to find the best efficient strategies that can inhibit the STAT3 activity to gain optimum therapeutic effects.
Additional Information
How to cite this article: Yuan, J. et al. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci. Rep. 5, 17663; doi: 10.1038/srep17663 (2015).
Acknowledgments
This review was supported by grants from the National Natural Science Foundation of China (Nos. 81372844 and 81472474), Tianjin Municipal Science and Technology Commission (No. 12JCZDJC24500 and 12JCQNJC07000), Changjiang Scholars and Innovative Research Team (IRT_14R40), 863 Project (2012AA020206-5), Specialized Research Fund for the Doctoral Program of Higher Education ((20131202110002).
Footnotes
Author Contributions J.Y. conceived and wrote the manuscript and prepared figures; R.N. and F.Z. provided expert comments and edits. All authors reviewed the manuscript.
References
- Timofeeva O. A. et al. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci USA 110, 1267–1272, doi: 10.1073/pnas.1211805110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. STAT3 acetylation-induced promoter methylation is associated with downregulation of the ARHI tumor-suppressor gene in ovarian cancer. Oncol Rep 30, 165–170, doi: 10.3892/or.2013.2414 (2013). [DOI] [PubMed] [Google Scholar]
- Lee H. et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc Natl Acad Sci USA 109, 7765–7769, doi: 10.1073/pnas.1205132109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L. et al. Angiotensin II in atrial structural remodeling: the role of Ang II/JAK/STAT3 signaling pathway. Am J Transl Res 7, 1021–1031 (2015). [PMC free article] [PubMed] [Google Scholar]
- Smith G. S., Kumar A. & Saba J. D. Sphingosine Phosphate Lyase Regulates Murine Embryonic Stem Cell Proliferation and Pluripotency through an S1P/STAT3 Signaling Pathway. Biomolecules 3, 351–368, doi: 10.3390/biom3030351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonozuka Y. et al. A GTPase-activating protein binds STAT3 and is required for IL-6-induced STAT3 activation and for differentiation of a leukemic cell line. Blood 104, 3550–3557, doi: 10.1182/blood-2004-03-1066 (2004). [DOI] [PubMed] [Google Scholar]
- Matsuura A. & Lee H. H. Crystal structure of GTPase-activating domain from human MgcRacGAP. Biochem Biophys Res Commun 435, 367–372, doi: 10.1016/j.bbrc.2013.04.094 (2013). [DOI] [PubMed] [Google Scholar]
- Raptis L., Arulanandam R., Geletu M. & Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res 317, 1787–1795, doi: 10.1016/j.yexcr.2011.05.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geletu M. et al. Classical cadherins control survival through the gp130/Stat3 axis. Biochim Biophys Acta 1833, 1947–1959, doi: 10.1016/j.bbamcr.2013.03.014 (2013). [DOI] [PubMed] [Google Scholar]
- Liu B. S., Cao Y., Huizinga T. W., Hafler D. A. & Toes R. E. TLR-mediated STAT3 and ERK activation controls IL-10 secretion by human B cells. Eur J Immunol 44, 2121–2129, doi: 10.1002/eji.201344341 (2014). [DOI] [PubMed] [Google Scholar]
- Ying H. et al. TLR4 mediates MAPK-STAT3 axis activation in bladder epithelial cells. Inflammation 36, 1064–1074, doi: 10.1007/s10753-013-9638-7 (2013). [DOI] [PubMed] [Google Scholar]
- Patel A. K. & Hackam A. S. A novel protective role for the innate immunity Toll-Like Receptor 3 (TLR3) in the retina via Stat3. Mol Cell Neurosci 63, 38–48, doi: 10.1016/j.mcn.2014.09.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. K. & Hackam A. S. Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol 54, 122–131, doi: 10.1016/j.molimm.2012.11.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye H. et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell 22, 466–478, doi: 10.1016/j.ccr.2012.08.010 (2012). [DOI] [PubMed] [Google Scholar]
- Ochi A. et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Invest 122, 4118–4129, doi: 10.1172/JCI63606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M. et al. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res 69, 2497–2505, doi: 10.1158/0008-5472.CAN-08-3031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C. A. et al. Toll-like Receptors in Regulatory T Cells of Patients With Head and Neck Cancer. Arch Otolaryngol Head Neck Surg 136, 1253–1259, doi: 10.1001/archoto.2010.195 (2010). [DOI] [PubMed] [Google Scholar]
- Herrmann A. et al. TLR9 is critical for glioma stem cell maintenance and targeting. Cancer Res 74, 5218–5228, doi: 10.1158/0008-5472.CAN-14-1151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Zhao Y. & Wang B. miR-519d-mediated downregulation of STAT3 suppresses breast cancer progression. Oncol Rep, doi: 10.3892/or.2015.4160 (2015). [DOI] [PubMed] [Google Scholar]
- Fan M. Q. et al. Decrease expression of microRNA-20a promotes cancer cell proliferation and predicts poor survival of hepatocellular carcinoma. J Exp Clin Cancer Res 32, 21, doi: 10.1186/1756-9966-32-21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. et al. MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression. Cancer Lett 347, 54–64, doi: 10.1016/j.canlet.2014.01.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D., Hirsch H. A. & Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706, doi: 10.1016/j.cell.2009.10.014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. et al. Stat3-coordinated Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene 32, 5272–5282, doi: 10.1038/onc.2012.573 (2013). [DOI] [PubMed] [Google Scholar]
- Lim W. A. & Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell 142, 661–667, doi: 10.1016/j.cell.2010.08.023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T. et al. Glucose-6-phosphate dehydrogenase and NADPH oxidase 4 control STAT3 activity in melanoma cells through a pathway involving reactive oxygen species, c-SRC and SHP2. Am J Cancer Res 5, 1610–1620 (2015). [PMC free article] [PubMed] [Google Scholar]
- Furcht C. M. et al. Multivariate signaling regulation by SHP2 differentially controls proliferation and therapeutic response in glioma cells. J Cell Sci 127, 3555–3567, doi: 10.1242/jcs.150862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C., Phromnoi K. & Aggarwal B. B. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem Pharmacol 85, 898–912, doi: 10.1016/j.bcp.2012.12.018 (2013). [DOI] [PubMed] [Google Scholar]
- Beales I. L., Garcia-Morales C., Ogunwobi O. O. & Mutungi G. Adiponectin inhibits leptin-induced oncogenic signalling in oesophageal cancer cells by activation of PTP1B. Mol Cell Endocrinol 382, 150–158, doi: 10.1016/j.mce.2013.08.013 (2014). [DOI] [PubMed] [Google Scholar]
- Dabir S. et al. PIAS3 activates the intrinsic apoptotic pathway in non-small cell lung cancer cells independent of p53 status. Int J Cancer 134, 1045–1054, doi: 10.1002/ijc.28448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y., Wang X. & Wu Z. SOCS1, SOCS3, and PIAS1 promote myogenic differentiation by inhibiting the leukemia inhibitory factor-induced JAK1/STAT1/STAT3 pathway. Mol Cell Biol 29, 5084–5093, doi: 10.1128/MCB.00267-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Kim C., Sethi G. & Ahn K. S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 6, 6386–6405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H. M. SOCS Proteins in Macrophage Polarization and Function. Front Immunol 5, 357, doi: 10.3389/fimmu.2014.00357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S. et al. Platelet factor 4 inhibits IL-17/Stat3 pathway via upregulation of SOCS3 expression in melanoma. Inflammation 37, 1744–1750, doi: 10.1007/s10753-014-9903-4 (2014). [DOI] [PubMed] [Google Scholar]
- Stark G. R. & Darnell J. E. Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514, doi: 10.1016/j.immuni.2012.03.013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S., Renaud S. J., Schleussner E., Graham C. H. & Markert U. R. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Exp Cell Res 315, 1724–1733, doi: 10.1016/j.yexcr.2009.01.026 (2009). [DOI] [PubMed] [Google Scholar]
- Frank D. A. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett 251, 199–210, doi: 10.1016/j.canlet.2006.10.017 (2007). [DOI] [PubMed] [Google Scholar]
- Kojima H. et al. STAT3 regulates Nemo-like kinase by mediating its interaction with IL-6-stimulated TGFbeta-activated kinase 1 for STAT3 Ser-727 phosphorylation. Proc Natl Acad Sci USA 102, 4524–4529, doi: 10.1073/pnas.0500679102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T. & Kovarik P. Serine phosphorylation of STATs. Oncogene 19, 2628–2637, doi: 10.1038/sj.onc.1203481 (2000). [DOI] [PubMed] [Google Scholar]
- Jain N., Zhang T., Kee W. H., Li W. & Cao X. Protein Kinase C Associates with and Phosphorylates Stat3 in an Interleukin-6-dependent Manner. Journal of Biological Chemistry 274, 24392–24400, doi: 10.1074/jbc.274.34.24392 (1999). [DOI] [PubMed] [Google Scholar]
- Lee H. et al. Persistently Activated Stat3 Maintains Constitutive NF-κB Activity in Tumors. Cancer Cell 15, 283–293, doi: 10.1016/j.ccr.2009.02.015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Mao R. & Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 4, 176–185, doi: 10.1007/s13238-013-2084-3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss A. L. Sphingosine-1-phosphate: Driver of NFkappaB and STAT3 persistent activation in chronic intestinal inflammation and colitis-associated cancer. JAKSTAT 2, e24150, doi: 10.4161/jkst.24150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G. & Karin M. NF-κB and STAT3 – key players in liver inflammation and cancer. Cell Res 21, 159–168, doi: 10.1038/cr.2010.183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto A. et al. JAB1 regulates unphosphorylated STAT3 DNA-binding activity through protein-protein interaction in human colon cancer cells. Biochem Biophys Res Commun 438, 513–518, doi: 10.1016/j.bbrc.2013.07.105 (2013). [DOI] [PubMed] [Google Scholar]
- Yang J. et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res 65, 939–947 (2005). [PubMed] [Google Scholar]
- Timofeeva O. A. et al. Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J Biol Chem 287, 14192–14200, doi: 10.1074/jbc.M111.323899 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. et al. Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat Cell Biol 10, 489–496, doi: 10.1038/ncb1713 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. et al. JAK signaling globally counteracts heterochromatic gene silencing. Nat Genet 38, 1071–1076, doi: 10.1038/ng1860 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. L., Guan Y. J., Chatterjee D. & Chin Y. E. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307, 269–273, doi: 10.1126/science.1105166 (2005). [DOI] [PubMed] [Google Scholar]
- Wang R., Cherukuri P. & Luo J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J Biol Chem 280, 11528–11534, doi: 10.1074/jbc.M413930200 (2005). [DOI] [PubMed] [Google Scholar]
- Ohbayashi N. et al. LIF- and IL-6-induced acetylation of STAT3 at Lys-685 through PI3K/Akt activation. Biol Pharm Bull 30, 1860–1864 (2007). [DOI] [PubMed] [Google Scholar]
- Jefri M., Huang Y. N., Huang W. C., Tai C. S. & Chen W. L. YKL-40 regulated epithelial-mesenchymal transition and migration/invasion enhancement in non-small cell lung cancer. BMC Cancer 15, 590, doi: 10.1186/s12885-015-1592-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L. et al. Snail promotes epithelial-mesenchymal transition and invasiveness in human ovarian cancer cells. Int J Clin Exp Med 8, 7388–7393 (2015). [PMC free article] [PubMed] [Google Scholar]
- Liu Z. et al. Highmobility group box 1 has a prognostic role and contributes to epithelial mesenchymal transition in human hepatocellular carcinoma. Mol Med Rep, doi: 10.3892/mmr.2015.4182 (2015). [DOI] [PubMed] [Google Scholar]
- Lee Y., Jung W. H. & Koo J. S. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat, doi: 10.1007/s10549-015-3550-9 (2015). [DOI] [PubMed] [Google Scholar]
- Lin Z. et al. S100A4 hypomethylation affects epithelial-mesenchymal transition partially induced by LMP2A in nasopharyngeal carcinoma. Mol Carcinog, doi: 10.1002/mc.22389 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang C., Guo F., Xu G., Ma J. & Shao F. STAT3 cooperates with Twist to mediate epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Rep 33, 1872–1882, doi: 10.3892/or.2015.3783 (2015). [DOI] [PubMed] [Google Scholar]
- Kim M. J., Lim J., Yang Y., Lee M. S. & Lim J. S. N-myc downstream-regulated gene 2 (NDRG2) suppresses the epithelial-mesenchymal transition (EMT) in breast cancer cells via STAT3/Snail signaling. Cancer Lett 354, 33–42, doi: 10.1016/j.canlet.2014.06.023 (2014). [DOI] [PubMed] [Google Scholar]
- Azare J. et al. Stat3 mediates expression of autotaxin in breast cancer. PLoS One 6, e27851, doi: 10.1371/journal.pone.0027851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Yeo S., Sung C. O. & Kim S. Twist1 Is a Key Regulator of Cancer-Associated Fibroblasts. Cancer Res, doi: 10.1158/0008-5472.CAN-14-0350 (2014). [DOI] [PubMed] [Google Scholar]
- Fujisaki K. et al. Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res Treat 150, 255–263, doi: 10.1007/s10549-015-3318-2 (2015). [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Takeya M. & Komohara Y. A novel strategy for inducing the antitumor effects of triterpenoid compounds: blocking the protumoral functions of tumor-associated macrophages via STAT3 inhibition. Biomed Res Int 2014, 348539, doi: 10.1155/2014/348539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 11, 1314–1321, doi: 10.1038/nm1325 (2005). [DOI] [PubMed] [Google Scholar]
- Wu L., Du H., Li Y., Qu P. & Yan C. Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am J Pathol 179, 2131–2141, doi: 10.1016/j.ajpath.2011.06.028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J. et al. Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med Oncol 32, 453, doi: 10.1007/s12032-014-0453-2 (2015). [DOI] [PubMed] [Google Scholar]
- Sun X., Sui Q., Zhang C., Tian Z. & Zhang J. Targeting blockage of STAT3 in hepatocellular carcinoma cells augments NK cell functions via reverse hepatocellular carcinoma-induced immune suppression. Mol Cancer Ther 12, 2885–2896, doi: 10.1158/1535-7163.MCT-12-1087 (2013). [DOI] [PubMed] [Google Scholar]
- Brown M. E. et al. Characterization of STAT3 expression, signaling and inhibition in feline oral squamous cell carcinoma. BMC Vet Res 11, 206, doi: 10.1186/s12917-015-0505-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N. et al. Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumour Biol, doi: 10.1007/s13277-015-3673-y (2015). [DOI] [PubMed] [Google Scholar]
- Yan L. I. et al. Expression of signal transducer and activator of transcription 3 and its phosphorylated form is significantly upregulated in patients with papillary thyroid cancer. Exp Ther Med 9, 2195–2201, doi: 10.3892/etm.2015.2409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju G. P. et al. Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1alpha and STAT-3. Angiogenesis 16, 903–917, doi: 10.1007/s10456-013-9364-7 (2013). [DOI] [PubMed] [Google Scholar]
- Onishi K. & Zandstra P. W. LIF signaling in stem cells and development. Development 142, 2230–2236, doi: 10.1242/dev.117598 (2015). [DOI] [PubMed] [Google Scholar]
- Ohtsuka S. & Niwa H. The differential activation of intracellular signaling pathways confers the permissiveness of embryonic stem cell derivation from different mouse strains. Development 142, 431–437, doi: 10.1242/dev.112375 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. et al. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat Commun 6, 7095, doi: 10.1038/ncomms8095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubaker K. et al. Targeted Disruption of the JAK2/STAT3 Pathway in Combination with Systemic Administration of Paclitaxel Inhibits the Priming of Ovarian Cancer Stem Cells Leading to a Reduced Tumor Burden. Front Oncol 4, doi: 10.3389/fonc.2014.00075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motawi T. K., El-Boghdady N. A., El-Sayed A. M. & Helmy H. S. Comparative study of the effects of PEGylated interferon-alpha2a versus 5-fluorouracil on cancer stem cells in a rat model of hepatocellular carcinoma. Tumour Biol, doi: 10.1007/s13277-015-3920-2 (2015). [DOI] [PubMed] [Google Scholar]
- Thakur R., Trivedi R., Rastogi N., Singh M. & Mishra D. P. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep 5, 10194, doi: 10.1038/srep10194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J. et al. The effectiveness of an anti-human IL-6 receptor monoclonal antibody combined with chemotherapy to target colon cancer stem-like cells. Int J Oncol 46, 1551–1559, doi: 10.3892/ijo.2015.2851 (2015). [DOI] [PubMed] [Google Scholar]
- Song J. PI3K/Akt and Stat3 signaling regulated by PTEN control of the cancer stem cell population, proliferation and senescence in a glioblastoma cell line. Int J Oncol, doi: 10.3892/ijo.2013.1765 (2013). [DOI] [PubMed] [Google Scholar]
- Liu C.-C. et al. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulatorsviaDNMT1 upregulation. International Journal of Cancer, n/a-n/a, doi: 10.1002/ijc.29033 (2014). [DOI] [PubMed] [Google Scholar]
- Schroeder A. et al. Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res 74, 1227–1237, doi: 10.1158/0008-5472.CAN-13-0594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y. et al. Bcl3 Bridges LIF-STAT3 to Oct4 Signaling in the Maintenance of Naive Pluripotency. Stem Cells, doi: 10.1002/stem.2201 (2015). [DOI] [PubMed] [Google Scholar]
- Lin C. et al. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling pathways. J Cell Biochem 114, 2061–2070, doi: 10.1002/jcb.24553 (2013). [DOI] [PubMed] [Google Scholar]
- Korkaya H., Liu S. & Wicha M. S. Regulation of Cancer Stem Cells by Cytokine Networks: Attacking Cancer’s Inflammatory Roots. Clinical Cancer Research 17, 6125–6129, doi: 10.1158/1078-0432.ccr-10-2743 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J. M. et al. Constitutive Activation of Signal Transducer and Activator of Transcription 3 (STAT3) and Nuclear Factor B Signaling in Glioblastoma Cancer Stem Cells Regulates the Notch Pathway. Journal of Biological Chemistry 288, 26167–26176, doi: 10.1074/jbc.M113.477950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L. et al. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J 27, 4731–4744, doi: 10.1096/fj.13-230201 (2013). [DOI] [PubMed] [Google Scholar]
- Jiang J. et al. MiR-1181 inhibits stem cell-like phenotypes and suppresses SOX2 and STAT3 in human pancreatic cancer. Cancer Lett 356, 962–970, doi: 10.1016/j.canlet.2014.11.007 (2015). [DOI] [PubMed] [Google Scholar]
- Hoque M. O., Islam M., Sharma S. & Teknos T. N. RhoC Regulates Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma by Overexpressing IL-6 and Phosphorylation of STAT3. PLoS One 9, e88527, doi: 10.1371/journal.pone.0088527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. Tumor-Associated Macrophages Regulate Murine Breast Cancer Stem Cells Through a Novel Paracrine EGFR/Stat3/Sox-2 Signaling Pathway. Stem Cells 31, 248–258, doi: 10.1002/stem.1281 (2013). [DOI] [PubMed] [Google Scholar]
- Bayliss T. J., Smith J. T., Schuster M., Dragnev K. H. & Rigas J. R. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 11, 1663–1668, doi: 10.1517/14712598.2011.627850 (2011). [DOI] [PubMed] [Google Scholar]
- Tang Y. J. et al. Inhibitor of signal transducer and activator of transcription 3 (STAT3) suppresses ovarian cancer growth, migration and invasion and enhances the effect of cisplatin in vitro. Genet Mol Res 14, 2450–2460, doi: 10.4238/2015.March.30.3 (2015). [DOI] [PubMed] [Google Scholar]
- Xiao H. et al. A novel small molecular STAT3 inhibitor, LY5, inhibits cell viability, cell migration, and angiogenesis in medulloblastoma cells. J Biol Chem 290, 3418–3429, doi: 10.1074/jbc.M114.616748 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla L. et al. Hitting the right spot: Mechanism of action of OPB-31121, a novel and potent inhibitor of the Signal Transducer and Activator of Transcription 3 (STAT3). Mol Oncol 9, 1194–1206, doi: 10.1016/j.molonc.2015.02.012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa F. et al. A novel STAT inhibitor, OPB-31121, has a significant antitumor effect on leukemia with STAT-addictive oncokinases. Blood Cancer J 3, e166, doi: 10.1038/bcj.2013.63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J. et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett 335, 145–152, doi: 10.1016/j.canlet.2013.02.010 (2013). [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Cai H., Jiang G., Zhou T. B. & Wu H. Silibinin inhibits proliferation, induces apoptosis and causes cell cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway inhibition. Asian Pac J Cancer Prev 15, 6791–6798 (2014). [DOI] [PubMed] [Google Scholar]
- Kim S. et al. Induction of fibronectin in response to epidermal growth factor is suppressed by silibinin through the inhibition of STAT3 in triple negative breast cancer cells. Oncol Rep 32, 2230–2236, doi: 10.3892/or.2014.3450 (2014). [DOI] [PubMed] [Google Scholar]
- Agarwal C., Tyagi A., Kaur M. & Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis 28, 1463–1470, doi: 10.1093/carcin/bgm042 (2007). [DOI] [PubMed] [Google Scholar]
- Bosch-Barrera J. & Menendez J. A. Silibinin and STAT3: A natural way of targeting transcription factors for cancer therapy. Cancer Treat Rev 41, 540–546, doi: 10.1016/j.ctrv.2015.04.008 (2015). [DOI] [PubMed] [Google Scholar]
- Cao W. et al. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer cells. Sci Rep 5, 8477, doi: 10.1038/srep08477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M. et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov 2, 694–705, doi: 10.1158/2159-8290.CD-12-0191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing N. et al. Targeting signal transducer and activator of transcription 3 with G-quartet oligonucleotides: a potential novel therapy for head and neck cancer. Mol Cancer Ther 5, 279–286, doi: 10.1158/1535-7163.mct-05-0302 (2006). [DOI] [PubMed] [Google Scholar]
- Weerasinghe P. et al. Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. Int J Oncol 31, 129–136 (2007). [PubMed] [Google Scholar]
- Reddy K. R., Guan Y., Qin G., Zhou Z. & Jing N. Combined treatment targeting HIF-1alpha and Stat3 is a potent strategy for prostate cancer therapy. Prostate 71, 1796–1809, doi: 10.1002/pros.21397 (2011). [DOI] [PubMed] [Google Scholar]
- Cheng J. Q. et al. Design and In Vitro Evaluation of Layer by Layer siRNA Nanovectors Targeting Breast Tumor Initiating Cells. PLoS One 9, e91986, doi: 10.1371/journal.pone.0091986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleriu M. G. et al. A new strategy in the treatment of chemoresistant lung adenocarcinoma via specific siRNA transfection of SRF, E2F1, Survivin, HIF and STAT3. Eur J Cardiothorac Surg 46, 877–886, doi: 10.1093/ejcts/ezu087 (2014). [DOI] [PubMed] [Google Scholar]