Abstract
More and more studies have investigated the effects of Ezrin expression level on the prognostic role in various tumors. However, the results remain controversial rather than conclusive. Here, we performed a systematic review and meta-analysis to evaluate the correlation of Ezrin expression with the prognosis in various tumors. the pooled hazard ratios (HR) with the corresponding 95% confidence intervals (95% CI) were calculated to evaluate the degree of the association. The overall results of fifty-five studies with 6675 patients showed that elevated Ezrin expression was associated with a worse prognosis in patients with cancers, with the pooled HRs of 1.86 (95% CI: 1.51–2.31, P < 0.001) for over survival (OS), 2.55 (95% CI: 2.14–3.05, P < 0.001) for disease-specific survival (DFS) and 2.02 (95% CI: 1.13–3.63, P = 0.018) for disease-specific survival (DSS)/metastasis-free survival (MFS) by the random, fixed and random effect model respectively. Similar results were also observed in the stratified analyses by tumor types, ethnicity background and sample source. This meta-analysis suggests that Ezrin may be a potential prognostic marker in cancer patients. High Ezrin is associated with a poor prognosis in a variety of solid tumors.
Ezrin is an important member of the ERM (Ezrin-radixin-moesin) cytoskeleton-associated proteins family, which started to look like a transit protein between membrane proteins and actin filaments1,2. Nevertheless, recent studies have revealed that Ezrin is an important signaling molecule that is well-documented to be associated with many cellular processes, including cell proliferation, cell adhesion, cell motility, signal transduction and so on3,4,5,6, all of those processes play a vital role in tumorigenesis, development, invasion and metastasis in a variety of human malignancies7,8,9,10,11,12,13,14.
Ever since the first report about the prognosis effect of Ezrin on uveal malignant melanoma in 200115, numerous studies have been considered on investigating the prognostic effects of Ezrin expression in various tumors, such as bladder cancer, non-small cell lung cancer (NSCLC), breast cancer, squamous cell carcinoma of the head and neck (HNSCC), soft tissue sarcomas(STS), Gastric cancer, Osteosarcoma Hepatocellular carcinoma, ovarian carcinoma and so on16,17,18,19,20,21,22,23,24,25,26,27,28,29, most of which revealed that a poor prognostic outcome stemed from those cancer patients with high Ezrin expression15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46. However, because of insignificant or opposite results47,48,49,50,51,52,53,54, the reliability of Ezrin acting as a prognostic biomarker in various malignancies has not been reached consensus. Therefore, the prognostic value of Ezrin in cancer patients remains controversial. In terms of the limits of the single study, as well as in order to better understanding the significance of Ezrin expression in the prognosis of cancer patients, performing a comprehensive meta-analysis to evaluate the published studies is necessary.
In the present meta-analysis, the aim is to assess the correlation between Ezrin expression and the survival outcomes in cancer patients via collecting global related literatures to carry out a systematic analysis.
Results
Study characteristics
As shown in Supplementary Figure S1, a total of 299 articles were initially retrieved using the search strategy. After the manual evaluation of title and abstract, 236 articles were excluded because of being irrelevant or duplicate. Among the remaining 63 articles, 19 were further removed due to lack of the essential data about survival outcome. In addition, There were one article47 investigated in two different types of intrahepatic cholangiocarcinoma and another one50 investigated in two independent patient cohorts, so we considered the data from these studies as an individual separately. Finally, a total of 44 articles including 55 studies were included in the meta-analysis.
The main characteristics of the eligible studies are summarized in Table 1. All of the 55 studies were retrospective in design. The studies enrolled 6,675 cases (ranged from 19 to 487 per study) from the United States, Sweden, China, the United Kingdom, Italy, Spain, Korea, Brazil, Finland, France, Germany and Japan, which evaluated a wide range of carcinomas, including 14 for digestive cancer, 6 for osteosarcoma, 5 for squamous cell carcinoma of the head, 5 for gynecologic cancer, 5 for bladder cancer, 3 for hepatobiliary cancer , 2 for lung cancer, 3 for soft tissue sarcomas and 10 for “other cancers”. Thirty-six studies comprising 5,456 cases reported HRs for OS, 10 studies comprising 1,709 cases for DFS and 9 studies comprising 1,416 cases for DSS/MFS. Tissue samples with formalin-fixed and paraffin-embedded (FFPE) tissues were used in 37 studies, while 18 studies used tissue microarray (TMA). Immunohistochemical method was used in all studies. In addition, the standard of the cut-off values was no uniform in each study, with the values ranged from at least positive to >80% value.
Table 1. Main characteristics of the eligible studies included in the meta-analysis.
| Author | Year | Origin of population | No. of patients | Type | Sample source | Assay | Positive(n) | Cut-off | Survival analysis | HR estimation | HR(95%) | follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wennersten | 2014 | Sweden | 263 | Bladder cancer | TMA | IHC | 112 | ≥10% | OS | SC | 0.43(0.24–1.32) | NA |
| Andersson | 2014 | Sweden | 100 | Urothelial bladder cancer | TMA | IHC | 59 | ﹥17.5% | OS | SC | 0.44(0.19–1.71) | 71.04(0.36–98.5) |
| Andersson | 2014 | Sweden | 342 | Urothelial bladder cancer | TMA | IHC | 120 | ﹥27.5% | OS | SC | 0.50(0.35–1.93) | ≥60 |
| TMA | IHC | 136 | ﹥12.5% | DSS | SC | 0.29(0.14–0.96) | ||||||
| Piao | 2014 | China | 106 | PDAC | FFPE tissues | IHC | 73 | ﹥25% | OS | Reported | 2.16(1.38–3.39) | NA |
| Jin | 2014 | China | 108 | NSCLC | FFPE tissues | IHC | 71 | ≥25% | OS | SC | 2.17(0.92–4.09) | >60 |
| Wang | 2014 | China | 60 | LSCC | FFPE tissues | IHC | 45 | ≥50% | OS | SC | 2.27(1.65–4.93) | 58.1(26–83) |
| Wang | 2014 | China | 63 | TSCC | FFPE tissues | IHC | 34 | ﹥30% | OS | SC | 3.56(1.44–6082) | NA |
| Lin | 2013 | China | 186 | CRA | FFPE tissues | IHC | 114 | at least moderate | OS | Reported | 0.56(0.40–0.78) | 60 |
| Mao | 2013 | China | 107 | brain astrocytomas | FFPE tissues | IHC | 96 | ≥50% | DFS | SC | 4.03(2.49–8.32) | 2–56 |
| Arumugam | 2013 | UK and Italy | 76 | CAV | FFPE tissues | IHC | 42 | at least positive | OS | Reported | 15.22(1.98–117.03) | median 20 m |
| Kong | 2013 | China | 51 | Early–stage cervical cancer | FFPE tissues | IHC | 34 | ﹥25% | OS | SC | 3.42(1.23–5.31) | |
| Pinilla | 2013 | Spain | 117 | PTCLs | TMA | IHC | 92 | ﹥80% | OS | SC | 0.23(0.19–0.93) | 23.44(0–150) |
| Ma | 2013 | China | 487 | Breast cancer | FFPE tissues | IHC | 74 | ≥75% | OS | Reported | 2.42(1.36–3.92) | 64.8 |
| FFPE tissues | IHC | ≥75% | DFS | Reported | 2.55(2.13–2.99) | |||||||
| Schlecht | 2012 | USA | 130 | HNSCC | FFPE tissues | IHC | 34 | ≥10% | OS | Reported | 4.10(1.40–12.60) | 52.4 |
| FFPE tissues | IHC | ≥10% | DSS | SC | 3.96(1.57–7.03) | |||||||
| Lee | 2012 | Korea | 112 | NSCLC | FFPE tissues | IHC | 33 | at least positive | OS | Reported | 1.85(1.05–3.62) | 23(1–153) |
| Gao | 2012 | China | 216 | LSCC | FFPE tissues | IHC | 129 | ≥50% | OS | Reported | 3.58(1.45–8.87) | 65(4–126) |
| Carneiro | 2011 | Sweden | 227 | STS | TMA | IHC | 110 | at least positive | MFS | Reported | 1.80(0.90–3.70) | 48(12–228) |
| Lam | 2011 | HongKong | 150 | Gastric cancer | TMA | IHC | 117 | at least moderate | OS | SC | 2.64(1.27–4.19) | NA |
| Aishima | 2011 | Japan | 41 | ICC–Perihilar | FFPE tissues | IHC | 20 | ﹥11% | OS | SC | 1.37(0.57–2.26) | 37.56 |
| Aishima | 2011 | Japan | 69 | ICC–Peripheral | FFPE tissues | IHC | 14 | ﹥11% | OS | SC | 2.13(0.88–3.58) | 37.56 |
| Wang | 2011 | China | 200 | nasopharyngeal carcinoma | FFPE tissues | IHC | 134 | at least moderate | OS | SC | 3.43(1.99–6.37) | 76.8(10.3–117.5) |
| Wang | 2011 | China | 75 | SACC | FFPE tissues | IHC | 23 | at least intense | OS | SC | 2.90(1.44–5.85) | 99.37(52–138) |
| Patara | 2011 | Brazil | 250 | CRA | TMA | IHC | 21 | at least moderate | OS | SC | 1.76(1.26–2.44) | NA |
| Li | 2011 | China | 436 | Gastric cancer | TMA | IHC | 236 | at least moderate | OS | SC | 2.56(2.14–4.18) | ﹥60 |
| Korkeila | 2011 | Finland | 76 | Rectal cancer | FFPE tissues | IHC | 33 | at least moderate | DFS | SC | 3.95(1.20–5.41) | 40(2–113) |
| FFPE tissues | IHC | at least moderate | DSS | SC | 3.07(2.48–6.55) | |||||||
| Xie | 2011 | China | 307 | ESCC | TMA | IHC | 240 | at least moderate | OS | Reported | 1.62(1.12–2.34) | NA |
| Boldrini | 2010 | Brazil | 34 | osteosarcomas | FFPE tissues | IHC | 26 | ≥50% | OS | AP/ED | 2.45(0.79–3.11) | 27.4(9–69) |
| Huang | 2010 | Taiwan | 74 | Myxofibrosarcomas | TMA | IHC | 35 | at least moderate | DSS | SC | 3.89(2.04–7.85) | 53.7 |
| TMA | IHC | at least moderate | MFS | SC | 2.11(1.36–3.02) | |||||||
| Kang | 2010 | Korea | 100 | Hepatocellular carcinoma | FFPE tissues | IHC | 28 | ﹥10% | OS | Reported | 1.91(1.16–3.13) | 82(41–162) |
| FFPE tissues | IHC | ﹥10% | DFS | Reported | 1.47(0.91–2.38) | |||||||
| Wei | 2009 | Taiwan | 347 | GISTs | TMA | IHC | 229 | ≥50% | DFS | Reported | 2.36(1.25–4.45) | 36.6(1–235) |
| Palou | 2009 | Spain | 92 | Bladder tumors | TMA | IHC | 12 | ﹥20% | DSS | SC | 0.27(0.11–0.89) | 90.5(3–173) |
| Kim | 2009 | Korea | 70 | osteosarcoma | FFPE tissues | IHC | 39 | ﹥10% | OS | SC | 2.52(1.19–4.41) | 59.9 |
| FFPE tissues | IHC | ﹥10% | DFS | SC | 2014(1.12–4.09) | |||||||
| Gao | 2009 | China | 193 | ESCC | FFPE tissues | IHC | 90 | ≥50% | OS | SC | 1.83(1.01–3.33) | 65(4–126) |
| Elzagheid | 2008 | Finland | 74 | Colorectal cancer | FFPE tissues | IHC | 61 | at least moderate | DSS | SC | 2.93(1.10–4.98) | 30.8(4.7–149.8) |
| Ferrari | 2008 | Italy | 95 | osteosarcomas | FFPE tissues | IHC | 76 | at least positive | DFS | SC | 2.95(1.24–6.55) | 47(10–115) |
| Fauceglia | 2007 | USA | 108 | HNSCC | TMA | IHC | 93 | DFS | AP/DE | 3.04(0.83–5.88) | ||
| Kim | 2007 | Korea | 64 | osteosarcomas | FFPE tissues | IHC | 33 | at least positive | OS | Reported | 30.30(4.00–228.30) | 78.2(12–137) |
| FFPE tissues | at least positive | MFS | Reported | 35.90(4.80–268.50) | ||||||||
| Salas | 2007 | France | 37 | osteosarcomas | FFPE tissues | IHC | 23 | ﹥1% | OS | SC | 3.23(2.28–5.93) | 54(10–150) |
| FFPE tissues | IHC | ﹥1% | EFS | SC | 2.24(1.35–4.22) | |||||||
| Madan | 2006 | USA | 40 | HNSCC | FFPE tissues | IHC | 19 | ≥10% | OS | Reported | 1.82(1.00–3.20) | 41.2(1–128) |
| Köbel | 2006 | Germany | 164 | Endormetrioid carcinomas | FFPE tissues | IHC | 83 | at the median | OS | SC | 2.23(1.04–4.28) | 57.4(0.13–93.4) |
| Köbel | 2006 | Germany | 105 | ovarian carcinoma | FFPE tissues | IHC | 51 | at least moderate | OS | SC | 1.97(1.19–3.42) | 37.3(1.13–96.5) |
| Weng | 2005 | Sweden | 50 | STS | FFPE tissues | IHC | 25 | ﹥1% | OS | SC | 2.59(1.52–4.23) | 90(50–134) |
| Yeh | 2005 | Taiwan | 84 | Pancreatic cancer | FFPE tissues | IHC | 49 | at least moderate | OS | SC | 2.17(1.18–3.96) | NA |
| Khanna | 2004 | USA | 19 | osteosarcomas | TMA | IHC | 9 | DFS | SC | 3.92(1.84–8.27) | NA | |
| Moilanen | 2003 | Finland | 440 | ovarian carcinoma | TMA | IHC | 318 | ≥10% | OS | SC | 0.58(0.44–1.87) | 152.4 |
| Mäkitie | 2001 | Finland | 130 | Uveal Malignant Melanoma | FFPE tissues | IHC | 83 | at least positive | OS | Reported | 1.71(0.90–3.23) | 264(216–312) |
TSCC: tongue squamous cell carcinoma; CRA: colorectal adenocarcinoma; SACC: Salivary gland adenoid cystic carcinoma; CAV: cancer of the ampulla of Vater; PDAC: pancreatic ductal adenocarcinoma; NSCLC: nonsmall cell lung cancer; STS: soft tissue sarcomas; LSCC: laryngeal Squamous Cell Carcinoma; TSCC: tTongue squamous cell carcinoma; CRA: colorectal adenocarcinoma; CAV: cancer of the ampulla of Vater; PTCLs: peripheral T-cell lymphomas; HNSCC: squamous cell carcinoma of the head and neck; ICC: intrahepaticcholangiocarcinoma; SACC: salivary gland adenoid cystic carcinoma; ESCC: esophageal Squamous Cell Carcinoma; GISTs: gastrointestinal stromal tumors; FFPE: formalin-fixed, paraffin-embedded; TMA: tissue microarray; IHC: immunohistochemistry; HR: hazard ratio; OS: overall survival; DFS: disease-free survival; DSS: disease-specific survival; MFS: metastasis-free survival; SC: survival curve; AP:author provided; DE: data-extrapolated; NA: not available.95% CI: 95% confidence interval;
Mata-analysis Results
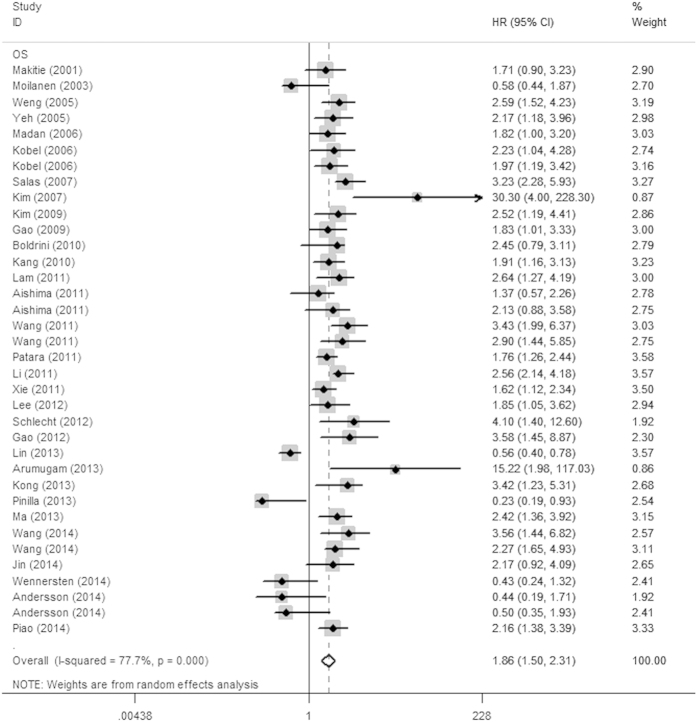
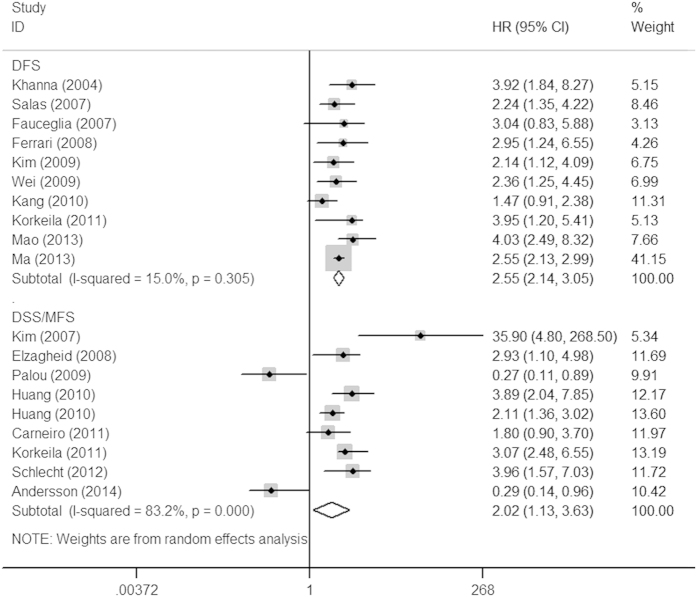
The association between Ezrin expression and various cancers prognosis is illustrated in Fig. 1 and Fig. 2. Overall, elevated Ezrin expression had a worse outcome in cancer patients, with the pooled HRs of 1.86 (95% CI: 1.51–2.31, P < 0.001) for OS and 2.02 (95% CI: 1.13–3.63, P = 0.018) for DSS/MFS with a random model because of the significant heterogeneity (I2 = 77.7%, P < 0.001; I2 = 76.7%, P < 0.001, respectively). Additionally, high Ezrin expression was also correlated with DFS, with the pooled HR of 2.55 (95% CI: 2.14–3.05, P < 0.001) calculated by a fixed model because of the absence of heterogeneity (I2 = 15%, P = 0.305).
Figure 1. Forrest plots of studies evaluating HRs of Ezrin expression for OS.
The squares and horizontal lines correspond to the study-specific HR and 95% CI. The area of the squares reflects the study-specific weight (inverse of the variance). The diamonds represent the pooled HR and 95% CI.
Figure 2. Forrest plots of studies evaluating HRs of Ezrin expression for DFS and DSS/MFS.
The squares and horizontal lines correspond to the study-specific HR and 95% CI. The area of the squares reflects the study-specific weight (inverse of the variance). The diamonds represent the pooled HR and 95% CI.
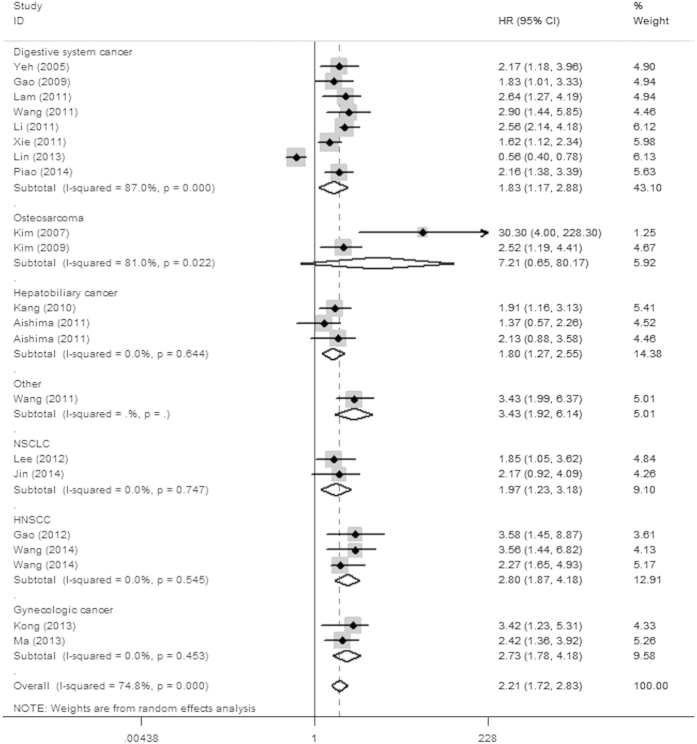
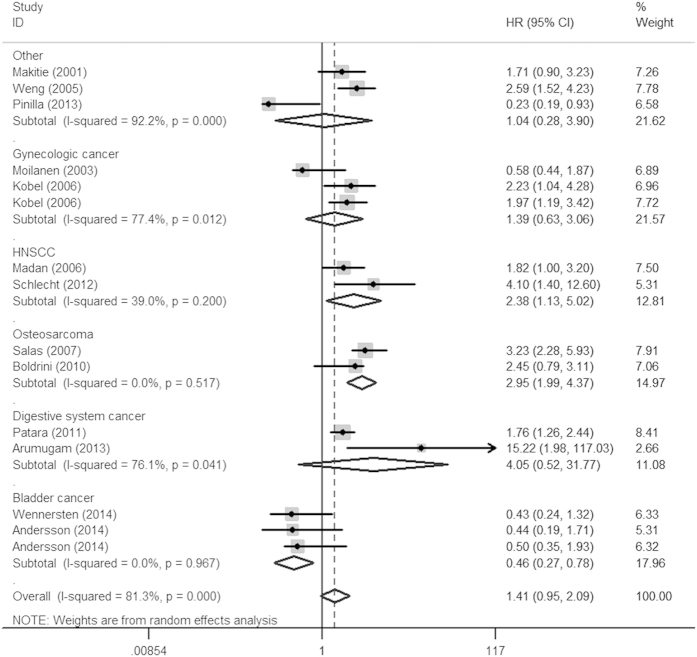
To explore the sources of heterogeneity, sub-group analysis for OS and DSS/MFS were conducted by the ethnicity, sample source and cancer types. The main results of this subgroup analysis for prognostic role of Ezrin in various tumors are shown in Table 2. In the ethnicity subgroup analyses, considerable heterogeneity was observed no matter the cancer patients were Asian or Caucasian for OS and DSS/MFS, the results showed that Ezrin over-expression reduced significantly the OS (HR = 2.21, 95% CI:1.72–2.83, P < 0.001) and DSS/MFS (HR = 4.18, 95%CI:1.60–10.95, P = 0.004) in Asian cancer patients, but not in Caucasian ones (HR = 1.41, 95%CI: 0.95–2.09, P = 0.092; HR = 1.40, 95%CI: 0.61–3.19, P = 0.426, respectively).
Table 2. Results of meta-analysis for Ezrin on prognostic effect in cancer patients.
| Outcome | Variables | No. of studies | Model | Pooled HR(95%) | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2(%) | Pvalue | |||||
| OS | 36 | Random | 1.86(1.51–2.31) | 77.70% | 0.000 | |
| Cancer type | ||||||
| Digestive system cancer | 10 | Random | 1.93(1.31–2.85) | 84.70% | 0.000 | |
| HNSCC | 5 | Fixed | 2.54(1.85–3.49) | 0% | 0.489 | |
| Gynecologic cancer | 5 | Random | 1.86(1.10–3.15) | 71.10% | 0.000 | |
| Osteosarcoma | 4 | Random | 3.16(1.90–5.26) | 47.60% | 0.026 | |
| Hepatobiliary cancer | 3 | Fixed | 1.80(1.27–2.56) | 0% | 0.644 | |
| Bladder cancer | 3 | Fixed | 0.49(0.27–0.78) | 0% | 0.967 | |
| NSCLC | 2 | Fixed | 1.97(1.23–3.18) | 0% | 0.747 | |
| Other | 4 | Random | 1.41(0.51–3.91) | 90.80% | 0.000 | |
| Ethnicity | ||||||
| Caucasian | 15 | Random | 1.41(0.95–2.09) | 81.30% | 0.000 | |
| Asian | 21 | Random | 2.21(1.72–2.83) | 74.80% | 0.000 | |
| Sample source | ||||||
| FFPE | 26 | Random | 2.32(1.84–2.92) | 71.20% | 0.000 | |
| TMA | 10 | Random | 1.02(0.64–1.61) | 85.50% | 0.000 | |
| DFS | 10 | Fixed | 2.55(2.14–3.05) | 15.00% | 0.305 | |
| Cancer type | ||||||
| Osteosarcoma | 4 | Fixed | 2.60(1.90–3.65) | 0% | 0.605 | |
| Digestive system cancer | 2 | Fixed | 2.92(1.80–4.75) | 4.80% | 0.305 | |
| Other | 4 | Random | 2.48(1.70–3.60) | 58.90% | 0.063 | |
| Ethnicity | ||||||
| Caucasian | 5 | Fixed | 3.02(2.17–4.20) | 0% | 0.734 | |
| Asian | 5 | Random | 2.37(2.14–3.05) | 45.60% | 0.119 | |
| Sample source | ||||||
| FFPE | 7 | Random | 2.49(1.97–3.15) | 33.90% | 0.169 | |
| TMA | 3 | Fixed | 2.94(1.90–4.54) | 0% | 0.598 | |
| DSS/MFS | 9 | Random | 2.02(1.13–3.63) | 83.20% | 0.000 | |
| Cancer type | ||||||
| Digestive system cancer | 2 | Fixed | 3.03(2.01–4.56) | 0% | 0.919 | |
| Bladder cancer | 2 | Random | 0.73(0.11–4.65) | 88.50% | 0.003 | |
| Soft tissue sarcomas | 3 | Random | 1.43(0.45–4.57) | 89.60% | 0.000 | |
| Other | 2 | Random | 9.71(1.16–81.04) | 75.30% | 0.044 | |
| Ethnicity | ||||||
| Caucasian | 6 | Random | 1.40(0.61–3.19) | 86.40% | 0.000 | |
| Asian | 3 | Random | 4.18(1.60–10.95) | 77.60% | 0.000 | |
| Sample source | ||||||
| FFPE | 4 | Random | 3.82(2.20–6.64) | 47.70% | 0.125 | |
| TMA | 5 | Random | 1.12(0.46–2.70) | 87.40% | 0.000 | |
Random-effects model was used when p-value for heterogeneity test < 0.05; otherwise, fixed-model was used. I2 the percentage of variability in HR attributable to heterogeneity. Abbreviations: HNSCC: squamous cell carcinoma of the head and neck; NSCLC: nonsmall cell lung cancer; FFPE: formalin-fixed, paraffin-embedded; TMA: tissue microarray.
In the sub-group analyses based on sample source, the results demonstrated that high Ezrin expression had a worse prognosis for OS (HR = 2.32, 95% CI:1.84–2.92, P < 0.001) and DSS/MFS (HR = 3.82, 95% CI: 2.20–6.64, P < 0.001) from FFPE samples, but not those from TMA ones (HR = 1.02, 95%CI: 0.64–1.61, P = 0.947; HR = 1.12, 95%CI: 0.46–2.70, P = 0806, respectively). However, we founded that there were a significant heterogeneity between the two kinds of samples whether they were for OS or for DSS/MFS.
In the stratified analyses according to cancer type, over-expression of Ezrin yielded a worse OS in digestive system cancers (HR = 1.93, 95% CI: 1.31–2.85, P = 0.001), HNSCC (HR = 2.54, 95% CI: 1.85–3.49, P < 0.001), gynecologic cancer (HR = 1.86, 95%CI: 1.10–3.15, P = 0.021), osteosarcoma (HR = 3.16, 95% CI: 1.90–5.26, P < 0.001), hepatobiliary cancer (HR = 1.80, 95% CI: 1.27–2.56, P = 0.001), NSCLC (HR = 1.97, 95% CI: 1.23–3.18, P = 0.005) and a worse DSS/MFS in digestive cancers (HR = 3.03, 95% CI: 3.01–4.56, P < 0.001). However, positive Ezrin expression was a predictor of good prognosis in bladder cancer for OS (HR = 0.49, 95% CI: 0.27–0.78, P = 0.004). Furthermore, we also performed sub-group analysis restricted to cancer type in different ethnicities for OS (Table 3), the results showed that Ezrin positive expression was associated with a poor prognosis of various tumors, especially HNSCC (HR = 2.80, 95% CI: 1.87–4.18, P < 0.001) and gynecologic cancer (HR = 2.73, 95% CI: 1.78–4.18, P < 0.001) among Asians (Fig. 3), with the exception of osteosarcoma (HR = 7.21, 95% CI: 0.65–80.17, P = 0.108). However, individuals elevating Ezrin expression had a significantly improved survival of bladder cancer (HR = 0.46, 95% CI: 0.27–0.78, P = 0.004) among Caucasians (Fig. 4).
Table 3. Stratified analyses of Ezrin on overall survival in cancer patients among Asians and Caucasians.
| OS | No. of studies | Model | Pooled HR(95%) | Heterogeneity | |
|---|---|---|---|---|---|
| I2(%) | Pvalue | ||||
| Asian | 21 | Random | 2.21(1.72–2.83) | 74.80% | 0.000 |
| Digestive system cancer | 8 | Random | 1.83(1.17–2.88) | 87.0% | 0.000 |
| HNSCC | 3 | Fixed | 2.80(1.87–4.18) | 0% | 0.545 |
| Gynecologic cancer | 2 | Fixed | 2.73(1.78–4.18) | 0% | 0.453 |
| Osteosarcoma | 2 | Random | 7.21(0.65–80.17) | 81.0% | 0.022 |
| Hepatobiliary cancer | 3 | Fixed | 1.80(1.27–2.56) | 0% | 0.644 |
| NSCLC | 2 | Fixed | 1.97(1.23–3.18) | 0% | 0.747 |
| Other | 1 | — | 3.43(1.92–6.14) | — | — |
| Caucasian | 15 | Random | 1.41(0.95–2.09) | 81.30% | 0.000 |
| Digestive cancer | 2 | Random | 4.05(0.52–31.77) | 76.10% | 0.041 |
| HNSCC | 2 | Fixed | 2.38(1.13–5.02) | 39.00% | 0.200 |
| Gynecologic cancer | 3 | Random | 1.39(0.63–3.06) | 77.40% | 0.012 |
| Osteosarcoma | 2 | Fixed | 2.95(1.99–4.37) | 0% | 0.517 |
| Bladder cancer | 3 | Fixed | 0.46(0.27–0.78) | 0% | 0.967 |
| Other | 3 | Random | 1.04(0.28–3.90) | 92.20% | 0.000 |
Random-effects model was used when p-value for heterogeneity test < 0.05; otherwise, fixed-model was used.I2 the percentage of variability in HR attributable to heterogeneity. Abbreviations: HNSCC: squamous cell carcinoma of the head and neck; NSCLC: nonsmall cell lung cancer.
Figure 3. Forest plot of overall survival associated with Ezrin in cancer patients among Asians.
Figure 4. Forest plot of overall survival associated with Ezrin in cancer patients among Caucasians.
Publication bias and sensitivity analysis
Both Begg’s funnel plot and the Egger’s test were performed to evaluate the publication bias of the inclusion studies. As shown in Fig. 5a–c, the shape of the funnel plots revealed no obvious asymmetry. And the P values of Egger’s test for OS, DFS and DSS/MFS were 0.389, 0.597 and 0.743, respectively, indicating that there was no significant publication bias in the meta-analysis. Meanwhile, the sensitivity analysis was performed to measure the effects of each individual study on the pooled HRs for the OS, DFS or DSS/MFS by omitting studies, respectively. The results demonstrated that no individual study significant influenced the overall HR, as shown in Supplementary Figure S2a, Figure S2b and Figure S2c. This suggested that the results of the present meta-analysis are credible.
Figure 5.
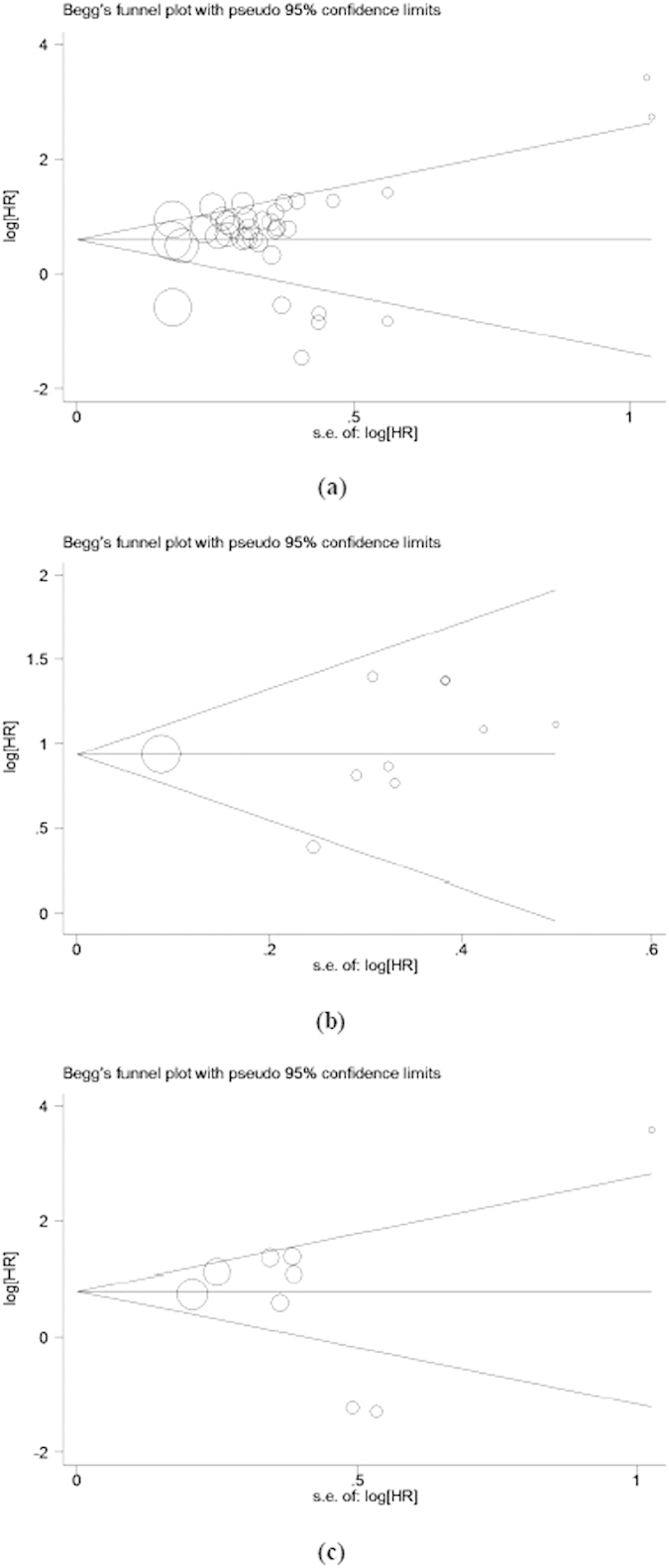
Begg’s funnel plots for publication bias test of OS (a), DFS (b) and DSS/MFS (c).
Discussion
Ezrin, the most important member of the Ezrin/radixin/moesin (ERM) family, is mainly expressed in a variety of malignant tissues which originate from epithelial or non-epithelial cells55. Generally, Ezrin is mainly distributed in the cytoplasm with an inactive form, Once activated by threonine and tyrosine phosphorylation, Ezrin would transform into a special active form56. The basic biological function of Ezrin is to link transmembrane proteins to actin cytoskeleton57,58. In addition to acting as a cross-linker, Ezrin is involved in transmission of signals in response to extracellular cues59,60. The biological pathways associated with Ezrin include protein kinase C, Rho-kinase, NF-kB, PI3 kinase/Akt and so on61. Moreover, as a metastasis-related oncogene, Ezrin also participate in modulating multiple cellular processes62, including the formation of microvilli63, maintenance of cell shape64, cell-cell adhesion65, cell motility and invasion66. Hence, it seems that Ezrin might play an important role in the development of cancer. There is growing evidence that Ezrin expression level is associated with tumor progression and dissemination67. Numerous epidemiological studies have also assessed the correlation of high Ezrin expression and poor outcome in cancer patients so far, such as digestive system cancer16,17,18,19,20,21,22,23,24,25, osteosarcoma26,27,28,29,30,31,79,80, HNSCC32,33,34,35,36, gynecologic cancer37,38,39, hepatobiliary cancer43 and so on. However, the results about the prognostic value of Ezrin expression in cancer patients remain inconsistent. Some studies reported that up-regulated Ezrin was a negative prognostic factor for survival for cancer patients15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46, However, other studies showed an opposite result48,50,51,52,78. To resolve the conflicting issues, we performed a systematic review and meta-analysis on the association between Ezrin expression and prognostic value in cancer patients.
As the first qualitative analysis of Ezrin expression related to survival outcome of various tumors, Han et al.68 retrieved 29 studies and found that over-expression of Ezrin might be associated with worse prognosis. However, the number of inclusion studies in the analysis was not relatively enough and at least 26 eligible studies were not included in the above meta-analysis, of which 8 studies about osteosarcomas were absolutely not included. Furthermore, the data reported by Han et al.68 for the study by Jörgren et al.69 were inconsistent with the data and the conclusion provided by Jörgren et al.69 in their original article. The HR value reported by Han et al.68 for OS is 1.89 (95% CI = 1.16–3.10), this suggested that high Ezrin expression was associated with worse prognosis in rectal cancer patients. But after carefully studying the data presented by Jörgren et al.69, we found Jörgren et al.69 just provided HR value about LR (local recurrence), not about OS. Moreover, the conclusion by Jörgren et al.69 showed that Ezrin expression had no impact on overall survival of patients with rectal cancer. Therefore, the conclusion by Han et al.68 was still being debated and uncertain. In view of this, we performed this updated meta-analysis including 44 articles with 55 studies and elucidated that the high Ezrin expression was significantly associated with poor OS, DFS and DSS/MFS in cancer patients.
This meta-analysis was performed according to the guidelines and recommendations for improving the quality of reporting of medical research such as REMARK70 and PRISMA71. Estimation of HR using multivariate proportional hazards model was used to evaluate the prognostic significance between ezrin expression and survival outcomes in each study, variables entered into the multivariate analysis mainly included Age, Gender, Tumor size, Tumor grade, TNM tumor stage, Lymph node metastasis, Ezrin expression. These positive factors contributed to the strengths of this meta-analysis.
The evidence included in the present meta-analysis indicated Ezrin expression as a poor prognostic marker in a variety of tumors. However, it should be noted that there are some limitations to the analyses presented here. First, because the number of prognostic studies dealing with each type of cancers was ≤5, the results of the particular carcinomas might be less powerful. Second, English articles were only recruited, and language bias might exist. Third, some HRs were calculated indirectly by the data extracted from the literature, however, these data were less reliable than direct data from the original literature. Fourth, different cutoffs used to assess high Ezrin level in the studies might also have contributed to the heterogeneity, because there is not a standard cutoff value of Ezrin level for increased survival risk. Fifth, significant heterogeneity existed in between studies, even though we calculated the pooled subgroup data with random-effects models. The heterogeneity in these studies could be attributed to the differences by different population characteristics or study designs. In addition, different sample types could also explained the heterogeneity, because tissue microarray (TMA) probably obtained more false-negative cases than the whole section. Finally, some inevitable publication bias might exist in the literature-based analysis, because more positive results tended to be published, thus potentially exaggerating the association between Ezrin expression and poor outcomes. Moreover, because all of the included studies were retrospective, which may have also introduced reporting bias. Therefore, our findings should be interpreted with caution.
In summary, our meta-analysis has demonstrated that the high Ezrin expression is significantly associated with poor survival in cancer patients. However, our results should be also considered cautiously for the above reasons. Further multicenter prospective studies and large clinical investigations should be conducted to validate the prognostic value of Ezrin in various tumors.
Methods
Search strategy
Guided by the guidelines of the Meta-analysis of Observational Studies in Epidemiology group (MOOSE), we carried out the meta-analysis72. A comprehensive search for all relevant articles published until 31 January 2015 that assessed on the prognostic value of Ezrin in various cancers was performed. The PubMed and EMBASE databases were retrieved with the following search terms or keywords:“Ezrin”, “prognosis OR prognostic OR survival OR outcome” and “cancer OR tumor OR carcinoma OR neoplasm”. Human studies were only restricted in this search. In addition, we also manually reviewed the references of relevant articles to obtain additional findings.
Inclusion and Exclusion Criteria
In this meta-analysis, the candidate studies were recruited according to the following criteria: (i) studied the patients who suffering from any type of cancers; (ii) evaluated Ezrin expression using Immunohistochemical method; (iii) assesed the correlation between Ezrin expression level and clinical outcome; and (iv) English articles. Articles were excluded based on any of the following criteria: (i) reviews, letters, comments, conference abstracts, or laboratory articles; (ii) articles not in English; (iii) absence of key information, such as HR, 95% CI, and P value, or useful data for calculation established by Parmar, Williamson, and Tierney73,74,75; and (iv) overlapping studies. The most recent or complete studies were selected if the same patient cohort was utilized in different articles. Full manuscript was available after examining the abstract if any doubt of suitability remained as well.
Quality Assessment
According to a critical review checklist of the Dutch Cochrane Centre proposed by MOOSE, we strictly assessed the quality of all the studies included72: (i) a detailed description about study population and origin of country; (ii) a definite description of the study design; (iii) a definite type of carcinoma; (iv) a definite description of outcome assessment; (v) a definite measurement method of Ezrin and (vi) a definite cut-off of Ezrin. Otherwise, We would exclude the studies in order to ensure the quality of the meta-analysis.
Data Extraction and Conversion
Two reviewers extracted the required information from all eligible studies independently. The extracted data included the following elements: the first author’s name, publication year, country of origin, sample size, tumor type, Ezrin measurement method, cut-off value, follow-up duration, the HRs of Ezrin for OS, DFS or DSS/MFS, as well as their 95% CIs and P values. Multivariate Cox proportional hazards regression analysis was used in the present analysis. If the HR and its 95% CI were not available directly, they were calculated from the corresponding data or Kaplan-Meier curves provided in the articles using the method reported previously75.
Statistical analysis
All these HRs and the corresponding 95% CIs were calculated to combine the pooled data following Tierney’s method75. A test of heterogeneity of combined HRs was performed using Cochran’s Q test and Higgin’sI2 statistics76. A P value < 0.05 and/or I2 > 50% indicated significant heterogeneity, a random-effect model was used to calculate the pooled HR; otherwise, the fixed-effect model was used. Generally, pooled HR of >1 was assumed to indicate a significant association with worse prognosis and was interpreted as statistically significant if the 95% CI for the pooled HR did not overlap one. Sensitivity analysis was carried out by removing each study at a time to evaluate the stability of the results. Publication bias was analyzed by performing funnel plots qualitatively, and estimated by Begg’s and Egger’s test quantitatively. Two sided P < 0.05 was considered statistically significant77. All analyses used in the meta-analysis were performed by SPSS version 13.0 and STATA version 12.0 (Stata Corp., College Station, TX, USA).
Additional Information
How to cite this article: Li, J. et al. Prognostic Value of Ezrin in Various Cancers: A Systematic Review and Updated Meta-analysis. Sci. Rep. 5, 17903; doi: 10.1038/srep17903 (2015).
Supplementary Material
Acknowledgments
This study was supported by the Science and Technology Planning Project of Guangdong Province (Grant No. 2011B060300004).
Footnotes
Author Contributions Conceived and designed the study: K.H.W. and J.W.L.; Performed the experiments: H.L.Y., G.W. and B.Y.; Contributed material/analysis tools: J.W.L., H.L.Y., D.J., G.W. and B.Y.; Analyzed the data: J.W.L., K.H.W. and H.L.Y.; Statistical analyses: D.J., G.W. and B.Y.; Writing of manuscript: J.W.L. and K.H.W.; Preparation of tables and figures: H.L.Y., D.J., G.W. and B.Y.; All authors reviewed the manuscript.
References
- Bretscher A., Reczek D. & Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sc 110, 3011–3018 (1997). [DOI] [PubMed] [Google Scholar]
- Bretscher A., Edwards K. & Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol.Cell Bio l3, 586–599 (2002). [DOI] [PubMed] [Google Scholar]
- Gautreau A., Louvard D. & Arpin M. ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr. Opin. Cell Bio 14, 104–109 (2002). [DOI] [PubMed] [Google Scholar]
- Saotome I., Curto M. & McClatchey A. I. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 6, 855–864 (2004). [DOI] [PubMed] [Google Scholar]
- Sarrió D. et al. Abnormal Ezrin localization is associated with clinicopathological features in invasive breast carcinomas. Breast Cancer Res. Trea 98, 71–79 (2006). [DOI] [PubMed] [Google Scholar]
- Srivastava J., Elliott B. E., Louvard D. & Arpin M. Src-dependent Ezrin phosphorylation in adhesion-mediated signaling. Mol. Biol. Cell 16, 1481–1490 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. et al. Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and Ezrin translocation are stimulated by interleukin 1alpha and epidermal growth factor. Cancer 92, 3068–3075 (2001). [DOI] [PubMed] [Google Scholar]
- Deng X. et al. Parathyroid hormone-related protein and Ezrin are upregulated in human lung cancer bone metastases. Clin. Exp. Metastasis 24, 107–119 (2007). [DOI] [PubMed] [Google Scholar]
- Xie J. J. et al. Roles of Ezrin in the growth and invasiveness of esophageal squamous carcinoma cells. Int. J. Cancer 124, 2549–2558 (2009). [DOI] [PubMed] [Google Scholar]
- Morales F. C., Molina J. R., Hayashi Y. & Georgescu M. M. Overexpression of Ezrin inactivates NF2 tumor suppressor in glioblastoma. NeuroOncol 12, 528–539(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W. H., Ahlén J., Aström K., Lui W. O. & Larsson C. Prognostic impact of immunohistochemical expression of Ezrin in highly malignant soft tissue sarcomas. Clin. Cancer Res 11, 6198–6204 (2005). [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P., Dulguerov P., Beck A., Bonet M. & Allal A. S. Value of Ezrin, maspin and nm23-H1 protein expressions in predicting outcome of patients with head and neck squamous-cell carcinoma treated with radical radiotherapy. J. Clin. Pathol 60, 185–189 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzagheid A. et al. Intense cytoplasmic Ezrinimmunoreactivity predicts poor survival in colorectal cancer. Hum. Pathol 39, 1737–1743 (2008). [DOI] [PubMed] [Google Scholar]
- Wang L., Lin G. N., Jiang X. L. & Lu Y. Expression of Ezrin correlates with poor prognosis of nasopharyngeal carcinoma. Tumour. Biol 32, 707–712 (2011). [DOI] [PubMed] [Google Scholar]
- Mäkitie T., Carpén O., Vaheri A. & Kivelä T. Ezrin as a prognostic indicator and its relationship to tumor characteristics in uveal malignant melanoma. Invest Ophthalmol Vis Sci 42, 2442–2449 (2001). [PubMed] [Google Scholar]
- Yeh T. S. et al. Significance of cellular distribution of Ezrin in pancreatic cystic neoplasms and ductal adenocarcinoma. Arch Surg 140, 1184–1190 (2005). [DOI] [PubMed] [Google Scholar]
- Gao S. Y. et al. Sp1 and AP-1 regulate expression of the human gene VIL2 in esophageal carcinoma cells. J BiolChem 284, 7995–8004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E. K. et al. A microRNA contribution to aberrant Ras activation in gastric cancer. Am J Transl Res 3, 209–218 (2011). [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y. et al. Expression of the membrane-cytoskeletal linker Ezrin in salivary gland adenoid cystic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112, 96–104 (2011). [DOI] [PubMed] [Google Scholar]
- Patara M. et al. Ezrin expression as a prognostic marker in colorectal adenocarcinoma. Pathol Oncol Res 17, 827–833 (2011). [DOI] [PubMed] [Google Scholar]
- Li L., Wang Y. Y., Zhao Z. S. & Ma J. Ezrin is associated with gastric cancer progression and prognosis. Pathol Oncol Res 17, 909–915 (2011). [DOI] [PubMed] [Google Scholar]
- Xie J. J. et al. Prognostic implication of Ezrin expression in esophageal squamous cell carcinoma. J SurgOncol 104, 538–543 (2011). [DOI] [PubMed] [Google Scholar]
- Lin L. J. & Chen L. T. Association between Ezrin protein expression and the prognosis of colorectal adenocarcinoma. Mol Med Rep 8, 61–66 (2013). [DOI] [PubMed] [Google Scholar]
- Arumugam P. et al. Ezrin expression is an independent prognostic factor in gastro-intestinal cancers. J Gastrointesturg 17, 2082–2091 (2013). [DOI] [PubMed] [Google Scholar]
- Piao J. et al. Ezrin protein overexpression predicts the poor prognosis of pancreatic ductal adenocarcinomas. ExpMolPathol 98, 1–6 (2004). [DOI] [PubMed] [Google Scholar]
- Salas S. et al. Ezrin and alpha-smooth muscle actin are immunohistochemical prognostic markers in conventional osteosarcomas. Virchows Arch 451, 999–1007 (2007). [DOI] [PubMed] [Google Scholar]
- Kim M. S., Song W. S., Cho W. H., Lee S. Y. & Jeon D. G. Ezrin expression predicts survival in stage IIB osteosarcomas. ClinOrthopRelat Res 459, 229–36 (2007). [DOI] [PubMed] [Google Scholar]
- Kim C. et al. Clinical value of Ezrin expression in primary osteosarcoma. Cancer Res Treat 41, 138–144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna C. et al. The membrane-cytoskeleton linker Ezrin is necessary for osteosarcoma metastasis. Nat Med 10, 182–186 (2004). [DOI] [PubMed] [Google Scholar]
- Ferrari S. et al. Prognostic significance of immunohistochemical expression of Ezrin in non-metastatic high-grade osteosarcoma. Pediatr Blood Cancer 50, 752–756 (2008). [DOI] [PubMed] [Google Scholar]
- Boldrini E., Peres S. V., Morini S. & Camargo B. Immunoexpression of Ezrin and CD44 in patients with osteosarcoma. J PediatrHematolOncol 32, e213–e217 (2010). [DOI] [PubMed] [Google Scholar]
- Madan R. et al. Differential tissue and subcellular expressionof ERM proteins in normal and malignant tissues: cytoplasmic Ezrin expression has prognostic signficance for head and neck squamous cell carcinoma. Head Neck 28, 1018–1027 (2006). [DOI] [PubMed] [Google Scholar]
- Gao W. et al. Fascin-1, Ezrin and paxillin contribute to the malignant progression and are predictors of clinical prognosis in laryngeal squamous cell carcinoma. PLoS One 7, e50710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu M. & Zhao C. Y. Expression of Ezrin and moesin related to invasion, metastasis and prognosis of laryngeal squamous cell carcinoma. Genet Mol Res 13, 8002–8013 (2014). [DOI] [PubMed] [Google Scholar]
- Schlecht N. F. et al. Cytoplasmic Ezrin and moesin correlate with poor survival in head and neck squamous cell carcinoma. Head Neck Pathol 6, 232–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. Akt/Ezrin Tyr353/NF-κB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer 110, 695–705 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köbel M. et al. Ezrin expression is related to poor prognosis in FIGO stage Iendometrioidcarcinomas. Mod Pathol 19, 581–597 (2006). [DOI] [PubMed] [Google Scholar]
- Köbel M. et al. Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J GynecolPathol 25, 121–130 (2006). [DOI] [PubMed] [Google Scholar]
- Ma L., Liu Y. P., Zhang X. H., Geng C. Z. & Li Z. H. Relationship of RhoA signaling activity with Ezrin expression and its significance in the prognosis for breast cancer patients. Chin Med J (Engl) 126, 242–247 (2013). [PubMed] [Google Scholar]
- Lee H. W., Kim E. H. & Oh M. H. Clinicopathologic implication of Ezrin expression in non-small cell lung cancer. Korean J Pathol 46, 470–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Yuan X. R., Xu S. S., Jiang X. C. & Zhao X. T. Expression and functional significance of Ezrin in human brain astrocytoma. Cell BiochemBiophys 67, 1507–1511 (2013). [DOI] [PubMed] [Google Scholar]
- Carneiro A. et al. Ezrin expression predicts local recurrence and development of metastases in soft tissue sarcomas. J ClinPathol 64, 689–694 (2011). [DOI] [PubMed] [Google Scholar]
- Kang Y. K., Hong S. W., Lee H. & Kim W. H. Prognostic implications of Ezrin expression in human hepatocellular carcinoma. Mol Carcinog 49, 798–804 (2010). [DOI] [PubMed] [Google Scholar]
- Wei Y. C. et al. Ezrin overexpression in gastrointestinal stromal tumors: an independent adverse prognosticator associated with the non-gastric location. Mod Pathol 22, 1351–1360 (2009). [DOI] [PubMed] [Google Scholar]
- Korkeila E. A. et al. Preoperative radiotherapy modulates Ezrin expression and its value as a predictive marker in patients with rectal cancer. Hum Pathol 42, 384–392 (2011). [DOI] [PubMed] [Google Scholar]
- Jin T. et al. Prognostic implications of Ezrin and phosphorylated Ezrin expression in non-small cell lung cancer. BMC Cancer 14, 191(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aishima S. et al. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J SurgPathol 35, 590–598 (2011). [DOI] [PubMed] [Google Scholar]
- Moilanen J. et al. Ezrinimmunoreactivity in relation to survival in serous ovarian carcinoma patients. GynecolOncol 90, 273–281 (2003). [DOI] [PubMed] [Google Scholar]
- Kong J. et al. High expression of Ezrin predicts poor prognosis in uterine cervical cancer. BMC Cancer 13, 520 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G. et al. Reduced expression of Ezrin in urothelial bladder cancer signifies more advanced tumours and an impaired survival: validatory study of two independent patient cohorts. BMC Urol 14, 36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennersten C. et al. Incident urothelial cancer in the Malmö Diet and Cancer Study: cohort characteristics and further validation of Ezrin as a prognostic biomarker. DiagnPathol 9, 189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou J. et al. Protein expression patterns of Ezrin are predictors of progression in T1G3 bladder tumours treated with nonmaintenance bacillus Calmette-Guérin. EurUrol 56, 829–836 (2009). [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pinilla S. M. et al. Loss of TCR-beta F1 and/or EZRIN expression is associated with unfavorable prognosis in nodal peripheral T-cell lymphomas. Blood Cancer J 3, e111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. Y. et al. Prognostic implication of Ezrin overexpression in myxofibrosarcomas. Ann SurgOncol 17, 3212–3219 (2010). [DOI] [PubMed] [Google Scholar]
- Vaheri A. et al. The Ezrin protein family: membrane-cytoskeleton interactions and disease associations. CurrOpin Cell Biol 9, 659–666 (1997). [DOI] [PubMed] [Google Scholar]
- Turunen O., Wahlström T. & Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the Ezrin protein family. J Cell Biol 126, 1445–1453 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S. et al. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126, 391–401(1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiska L. et al. Binding of the cytoplasmic domain of intercellular adhesion molecule-2 (ICAM-2) to alpha-actinin. J BiolChem 271, 26214–26219 (1996). [DOI] [PubMed] [Google Scholar]
- Arpin M., Chirivino D., Naba A. & Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell AdhesMigr 5, 199–206 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet-Valle´e S. ERM proteins from cellular architecture to cell signaling. Biol Cell 92, 305–316 (2000). [DOI] [PubMed] [Google Scholar]
- Brambilla D. & Fais S. The Janus-faced role of Ezrin in “linking” cells to either normal or metastatic phenotype. Int J Cancer 125, 2239–2245 (2009). [DOI] [PubMed] [Google Scholar]
- Chiang Y., Chou C., Hsu K., Huang Y. & Shen M. EGF upregulatesNaþ/Hþ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol 214, 810–819 (2008). [DOI] [PubMed] [Google Scholar]
- Baumgartner M. et al. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. ProcNatlAcadSci 103, 13391–13396 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava J., Elliott B., Louvard D. & Arpin M. Src-dependent Ezrin phosphorylation in adhesion-mediated signaling. MolBiol Cell 16, 1481–1490 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker J.-M. et al. 17b-Estradiol enhances breast cancer cell motility and invasion via extra-nuclear activation of actin-binding protein Ezrin. PLoS One 6, e22439 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuan Y. C. et al. Ezrin mediates c-Myc actions in prostate cancer cell invasion. Oncogene 29, 1531–1542 (2009). [DOI] [PubMed] [Google Scholar]
- Yu Y. et al. Expression profiling identifies the cytoskeletal organizer Ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med 10, 175–181 (2004). [DOI] [PubMed] [Google Scholar]
- Han K. et al. Prognostic value of Ezrin in solid tumors: a meta-analysis of the literature. PLoS One 8, e68527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörgren F., Nilbert M., Rambech E., Bendahl P. O. & Lindmark G. Ezrin expression in rectal cancer predicts time to development of local recurrence. Int J Colorectal Dis 27, 893–899 (2012). [DOI] [PubMed] [Google Scholar]
- McShane L. M. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93, 387–391 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Parmar M. K., Torri V. & Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17, 2815–2834 (1998). [DOI] [PubMed] [Google Scholar]
- Williamson P. R., Smith C. T., Hutton J. L. & Marson A. G. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 21, 3337–3351 (2002). [DOI] [PubMed] [Google Scholar]
- Tierney J. F., Stewart L. A., Ghersi D., Burdett S. & Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16(2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101(1994). [PubMed] [Google Scholar]
- Palmerini E. et al. Prognostic and predictive role of CXCR4, IGF-1R and Ezrin expression in localized synovial sarcoma: is chemotaxis important to tumor response? Orphanet J Rare Dis 10, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Shen J. N., Xie X. B., Wang J. & Huang G. Expression change of Ezrin as a prognostic factor in primary osteosarcoma. Med Oncol 28, S636–S643 (2011). [DOI] [PubMed] [Google Scholar]
- Mu Y., Zhang H., Che L. & Li K. Clinical significance of microRNA-183/Ezrin axis in judging the prognosis of patients with osteosarcoma. Med Onco . 31, 821 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.