Abstract
Direct amination of heteroarenes and arenes has been achieved in a one-pot C–H zincation/copper-catalyzed electrophilic amination procedure. This amination method provides an efficient and rapid approach to access a diverse range of heteroaromatic and aromatic amines including those previously inaccessible using C–H amination methods. The mild reaction conditions and good functional-group compatibility demonstrate its great potential for the synthesis of important and complex amines.
Keywords: amination, C–H functionalization, copper, heterocycles, synthetic methods
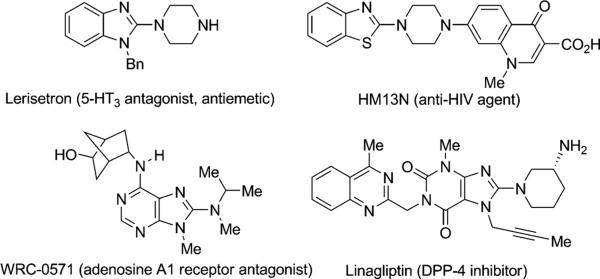
Heteroaromatic amines, especially 2-aminoazoles, are key structural motifs which are widely found in biologically important molecules and medicines.[1] For example, the 2-piperazinylbenzimidazole derivative lerisetron is currently in clinical trials for the treatment of nausea associated with cancer chemotherapy (Figure 1).[1a] Other classes of heteroaryl amines, such as anti-HIVagent HM13N,[1d] WRC-0571,[1b] and linagliptin,[1c] are also extensively used in drug discovery. Therefore, efficient methods for the synthesis of these important heteroaryl amines are highly valuable.
Figure 1.
Selected examples of bioactive heteroaromatic amines.
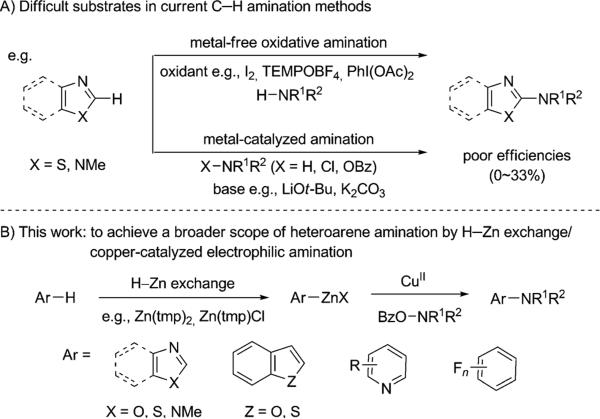
Complementary to the Buchwald–Hartwig amination from aryl halides,[2] direct C–H amination provides a new and potentially more effective C–N bond-formation approach for the synthesis of heteroaromatic amines. Several metal-free C–H/N–H coupling methods have been developed by an oxidative rearomatization pathway for the synthesis of 2-aminooxazoles.[3] Simultaneously, effective metal-catalyzed C–H aminations have also been achieved by various transition metals.[4,5] Despite these significant advances, the current methods often suffer from a limited arene scope and poor efficiencies, particularly on important skeletons including benzimidazoles, benzothiazoles, and thiazoles (Scheme 1A). This limitation is largely due to the challenging metalation step associated with the inherently high dissociation energy of sp2 C–H bonds, and also leads to the common requirement of harsh reaction conditions (high temperatures, strong oxidants, and acidic or basic additives). A more general C–H amination method is needed to access those highly valuable aminated heteroarenes (Figure 1). Mild reaction conditions are desirable to broaden the scope of its applications.[6]
Scheme 1.
Direct C–H amination to access heteroaromatic amines. Bz= benzoyl, TEMPO= 2,2,6,6-tetramethyl-1-piperidinyloxy.
Herein we report a C–H zincation and copper-catalyzed electrophilic amination as a modular and facile amination approach to rapidly access a broad scope of important heteroaromatic and aromatic amines (Scheme 1B). Our approach is built on the use of strong and non-nucleophilic zinc bases,[7] such as Zn(tmp)2 (tmp = 2,2,6,6-tetramethylpiperidide, pKa of the conjugate acid = 37),[8] to generate the corresponding organozincates from a wide range of heteroarenes. Importantly, the resulting organozinc intermediates can serve as a more reactive surrogate of C–H bonds toward amination.[9] Thus, this strategy will overcome the narrow substrate scope and harsh reaction conditions of the previous C–H amination methods. In comparison to organozinc intermediates prepared from the heteroaryl halides and Grignard reagents,[10] our approach using the direct H–Zn exchange will make use of various arenes and heteroarenes as more convenient starting materials, and offer a better functional-group compatibility.[11] Based on this hypothesis, we have developed an operationally convenient one-step C–H amination procedure and examined its efficiency on a wide scope of heteroarenes and arenes, including both electron-rich and electron-deficient substrates. Particularly useful is its high efficacy for those substrates, such as benzimidazoles, pyridines, benzothiophenes, and less acidic C–H bonds, which were isolated in poor yields under previous amination conditions.[4b–k,12] The application of our chemistry has been demonstrated in the preparation of a diverse range of important heteroaromatic amines, including the potent antiemetic lerisetron.
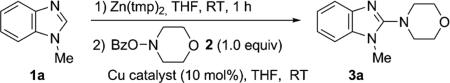
Our studies began with the amination of N-methyl benzimidazole (1a) with the hydroxylamine 2 via the formation of its organozinc intermediate using Zn(tmp)2 (Table 1). We focused on O-acylhydroxylamines as the electrophilic nitrogen source because of their easy availability and previous use in the electrophilic amination of different organometallic reagents.[9a–c,13] To our delight, the aminated product 3a was formed upon treating the diarylzinc intermediate with 2 and a copper catalyst, among which Cu(OAc)2 was most effective (entries 2–6). Without a copper catalyst, no aminated product was observed (entry 1). Furthermore, a stoichiometric amount of the diarylzinc intermediate was needed to fully convert 2 into 3a, thus suggesting that the resulting monoarylzinc benzoate was ineffective for the amination under these reaction conditions (entries 7 and 8).[14]
Table 1.
Optimization studies for copper-catalyzed amination of benz-imidazole (1a) and 2.[a]
| |||||
|---|---|---|---|---|---|
| Entry | 1a (equiv) | Zn(tmp)2 (equiv) | Copper catalyst | t[a] [h] | Yield [%][b] |
| 1 | 2.1 | 1.0 | - | 24[c] | 0 |
| 2 | 2.1 | 1.0 | CuCl | 4 | 88 |
| 3 | 2.1 | 1.0 | CuOTf·tol | 4 | 71 |
| 4 | 2.1 | 1.0 | CuCl2 | 3.5 | 89 |
| 5 | 2.1 | 1.0 | Cu(OTf)2 | 5 | 76 |
| 6 | 2.1 | 1.0 | Cu(OAc)2 | 5 | 99 |
| 7 | 1.4 | 0.6 | Cu(OAc)2 | 72[c] | 56 |
| 8 | 1.05 | 0.5 | Cu(OAc)2 | 72[c] | 40 |
Time required for complete consumption of 2 in step 2.
Yields determined by 1H NMR spectroscopy with CH2Br2 as a quantitative internal standard.
2 not fully consumed after 72 h. THF = tetra-hydrofuran.
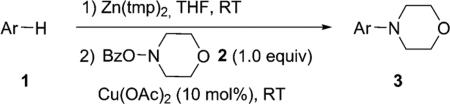
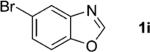
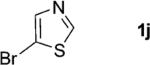
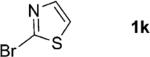
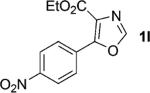
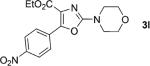
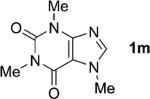
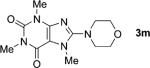
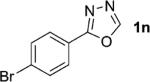
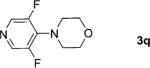
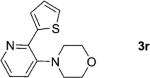
One of the important attributes of this new amination approach is its potential to directly access a broad array of heteroaromatic amines, including those inaccessible from other C–H amination methods.[4b–k,12] Toward this end, we examined the amination reactions of different heteroarenes and arenes using 2 (Table 2). We first looked into simple heteroarenes, including both the electron-deficient benzothiazole 1b and benzoxazole 1c, and electron-rich benzofuran 1d and benzothiophene 1e. All the reactions successfully provided the aminated products (entries 2–5). Analogous reactions with the imidazole 1 f, oxazole 1g, and thiazole 1h also occurred in excellent yields (entries 6–8). Next we examined the amination of functionalized arenes (entries 9–14), including the bromobenzoxazole 1i, bromothiazoles 1j–k, disubstituted oxazole 1l, caffeine 1m, and 1,3,4-oxadiazole 1n. These reactions gave the corresponding aminated azoles in 82–96% yields. In the examination of this method on pyridinyl C–H bonds, the reactions of the pyridines 1o–r all proceeded smoothly, thus affording the aminated products 3o–r, albeit at an elevated amination temperature (50 °C; entries 15–18). Lastly, the amination of the arene 1s also proceeded smoothly (entry 19). The high regioselectivity observed in these aminated products is presumably derived from the selective zinc metalation. It is noteworthy that many functionalities were well tolerated, and include halide, ester, nitro, and nitrile groups. Many of these groups would be incompatible with the amination conditions by C–H lithiation to form organozinc intermediates.[13] Such a broad scope demonstrates the value of our amination protocol, which proceeds by C–H zinc metalation in comparison to other amination strategies.
Table 2.
Amination of heteroarenes and arenes.[a]
| ||||
|---|---|---|---|---|
| Entry | 1 | t [h][b] | 3 | Yield [%][c] |
| 1 |
|
1; 5 |
|
96 |
| 2 | 1; 5 | 93 | ||
| 3 | 1; 5 | 95 | ||
| 4 |
|
1;[d] 5 |
|
71 |
| 5 | 1;[d] 5 | 70 | ||
| 6 |
|
1; 12 |
|
82 |
| 7 | 1; 12 | 92 | ||
| 8 | 1; 12 | 95 | ||
| 9 |
|
1; 24 |
|
96 |
| 10 |
|
1; 5 |
|
85 |
| 11 |
|
1; 5 |
|
90 |
| 12 |
|
1; 4 |
|
89 |
| 13 |
|
1;[e] 24 |
|
82 |
| 14 |
|
1; 4 |
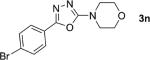
|
91 |
| 15 |
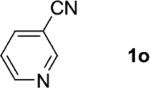
|
2; 12[f] |
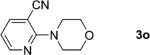
|
81 |
| 16 |

|
2; 12[f] |
|
79 |
| 17 |
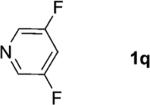
|
2; 12[f] |
|
91 |
| 18 |
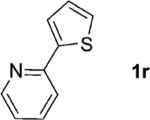
|
2; 12[f] |
|
74 |
| 19 |
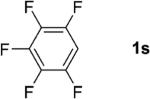
|
1; 12 |
|
75 |
Reactions typically run on 0.2 mmol scale. 1 (2.1 equiv), 2 (1.0 equiv, 0.08 m), Zn(tmp)2 (1.0 equiv).
Reaction time for deprotonation and amination step respectively.
Yield of isolated product.
Zn-(tmp)2·LiCl·MgCl2[4g] was used as base to form stable zincate intermediates.
Deprotonation step run in CH2Cl2 because of the poor solubility of 1m in THF.
Amination step run at 50°C.
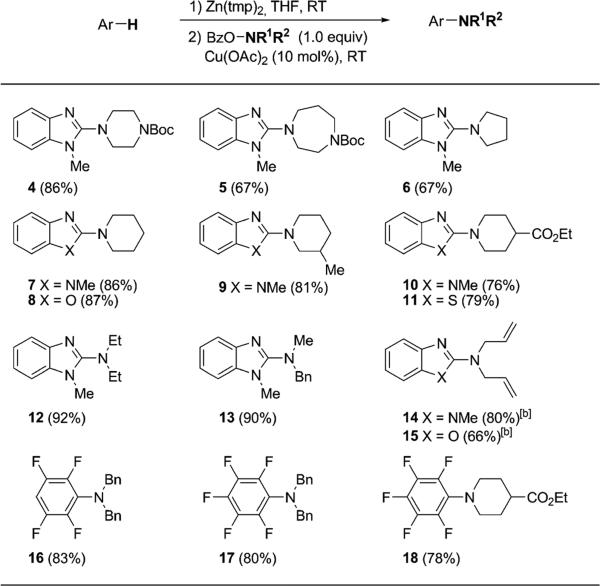
The scope of the amines is also crucial for extensive utility of this amination method. Next we examined different O-benzoyl hydroxylamines derived from simple dialkylamines in the amination reactions with representative heteroarenes and arenes (Table 3). We were pleased to find that all the reactions proceeded smoothly in modest to excellent yields (67–97%), thus allowing the introduction of a variety of cyclic and acyclic alkylamino groups. Notably, the cleavage of the benzyl group or allyl group can additionally afford either a secondary amine or a primary amine (e.g., 13–17).
Table 3.
Scope of O-benzoyl hydroxylamines.[a]
Reactions run on 0.2 mmol scale. Conditions: Ar-H (2.1 equiv), Zn(tmp)2 (1.0 equiv), RT, 1 h; BzONR1R2 (1.0 equiv, 0.08 m), Cu(OAc)2 (10 mol%), RT, 5 h. Yields given are those of the isolated products.
[b] Amination step run for 24 h. Boc=tert-butoxycarbonyl.
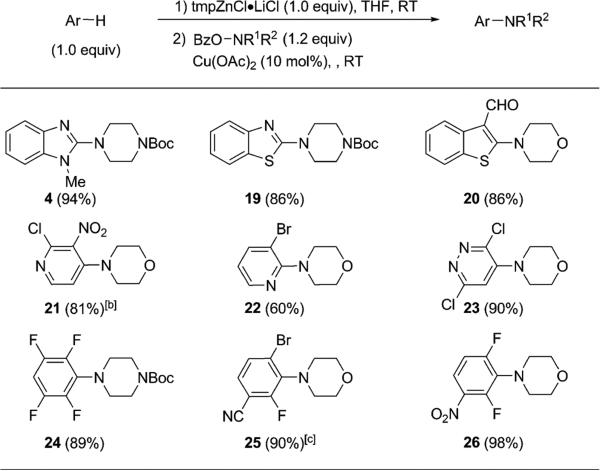
Recognizing that the use of Zn(tmp)2 would require the sacrifice of an additional equivalent of the arene moiety, we next exploited the amination of an alternative monoarylzinc intermediate using arenes as the limiting reagent and tmpZnCl·LiCl[15] in the C–H metalation (Table 4). Encouragingly, these reactions from both electron-deficient and electron-rich substrates all provided the aminated products in excellent yields, and included the azoles 4 and 19, benzothiophene 20, pyridines 21–22, pyridazine 23, and arenes 24–26. These preliminary results suggest that arylzinc chloride was equally effective as a diarylzinc for electrophilic amination under the current reaction conditions and extend the synthetic utility of this amination with the flexibility of using heteroarenes as the limiting reagent.
Table 4.
Direct amination using a tmpZnCl·LiCl-mediated metalation.[a]
Reactions typically run on 0.2 mmol scale. Yields given are those of the isolated products.
[b] Amination step run at 50 °C.
[c] Deprotonation run at 65 °C.
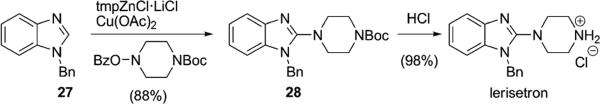
Given the broad generality and operational simplicity of this amination reaction, its utility for the synthesis of medicinally valuable agents was demonstrated by the rapid synthesis of lerisetron (Scheme 2). This 5-HT3 receptor antagonist was readily prepared from the simple benzimidazole 27 by using the standard amination conditions.
Scheme 2.
A rapid synthesis of lerisetron.
In summary, we have developed a direct and facile amination reaction of heteroarenes and arenes, including both electron-poor and electron-rich substrates. This transformation was achieved by a one-pot C–H zincation and copper-catalyzed electrophilic amination using O-acylhydroxylamines. It features broad substrate scope, high efficiency, mild reaction conditions, and good functional-group compatibility. It demonstrates great potential as a rapid and powerful way to access a variety of highly functionalized complex heteroaromatic amines which are of broad interest in organic synthesis and drug discovery. Furthermore, this work also provides insight into developing a modular C–H zincation/amination transformation for effective amination of sp3 C–H bonds.
Supplementary Material
Footnotes
We acknowledge financial support from Duke University and fellowships from the Burroughs Wellcome Endowment (S.L.M.) and the Duke University Pharmacological Sciences Training Program (C.E.H.).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201311029.
References
- 1.a Orjales A, Mosquera R, Labeaga L, Rodes R. J. Med. Chem. 1997;40:586. doi: 10.1021/jm960442e. [DOI] [PubMed] [Google Scholar]; b Jin CY, Burgess JP, Rehder KS, Brine GA. Synthesis. 2007:219. [Google Scholar]; c Eckhardt M, Langkopf E, Mark M, Tadayyon M, Thomas L, Nar H, Pfrengle W, Guth B, Lotz R, Sieger P, Fuchs H, Himmelsbach F. J. Med. Chem. 2007;50:6450. doi: 10.1021/jm701280z. [DOI] [PubMed] [Google Scholar]; d Massari S, Daelemans D, Barreca ML, Knezevich A, Sabatini S, Cecchetti V, Marcello A, Pannecouque C, Tabarrini O. J. Med. Chem. 2010;53:641. doi: 10.1021/jm901211d. [DOI] [PubMed] [Google Scholar]; e Gallardo-Godoy A, Gever J, Fife KL, Silber BM, Prusiner SB, Renslo AR. J. Med. Chem. 2011;54:1010. doi: 10.1021/jm101250y. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zhang TZ, Yan ZH, Sromek A, Knapp BI, Scrimale T, Bidlack JM, Neumeyer JL. J. Med. Chem. 2011;54:1903. doi: 10.1021/jm101542c. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Bansal Y, Silakari O. Bioorg. Med. Chem. 2012;20:6208. doi: 10.1016/j.bmc.2012.09.013. [DOI] [PubMed] [Google Scholar]; h Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M, Hahn SA, Triola G, Wittinghofer A, Bastiaens PI, Waldmann H. Nature. 2013;497:638. doi: 10.1038/nature12205. [DOI] [PubMed] [Google Scholar]
- 2.a Paul F, Patt J, Hartwig JF. J. Am. Chem. Soc. 1994;116:5969. [Google Scholar]; b Guram AS, Buchwald SL. J. Am. Chem. Soc. 1994;116:7901. [Google Scholar]; c Hooper MW, Utsunomiya M, Hartwig JF. J. Org. Chem. 2003;68:2861. doi: 10.1021/jo0266339. [DOI] [PubMed] [Google Scholar]; d Charles MD, Schultz P, Buchwald SL. Org. Lett. 2005;7:3965. doi: 10.1021/ol0514754. [DOI] [PubMed] [Google Scholar]
- 3.a Kim HJ, Kim J, Cho SH, Chang S. J. Am. Chem. Soc. 2011;133:16382. doi: 10.1021/ja207296y. [DOI] [PubMed] [Google Scholar]; b Lamani M, Prabhu KR. J. Org. Chem. 2011;76:9552. doi: 10.1021/jo201402a. [DOI] [PubMed] [Google Scholar]; c Wertz S, Kodama S, Studer A. Angew. Chem. 2011;123:11713. doi: 10.1002/anie.201104735. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50:11511. [Google Scholar]; d Yotphan S, Beukeaw D, Reutrakul V. Synthesis. 2013;45:936. [Google Scholar]
- 4.a Armstrong A, Collins JC. Angew. Chem. 2010;122:2332. doi: 10.1002/anie.200906750. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:2282. [Google Scholar]; b Monguchi D, Fujiwara T, Furukawa H, Mori A. Org. Lett. 2009;11:1607. doi: 10.1021/ol900298e. [DOI] [PubMed] [Google Scholar]; c Wang Q, Schreiber SL. Org. Lett. 2009;11:5178. doi: 10.1021/ol902079g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kawano T, Hirano K, Satoh T, Miura M. J. Am. Chem. Soc. 2010;132:6900. doi: 10.1021/ja101939r. [DOI] [PubMed] [Google Scholar]; e Matsuda N, Hirano K, Satoh T, Miura M. Org. Lett. 2011;13:2860. doi: 10.1021/ol200855t. [DOI] [PubMed] [Google Scholar]; f Li Y, Xie Y, Zhang R, Jin K, Wang X, Duan C. J. Org. Chem. 2011;76:5444. doi: 10.1021/jo200447x. [DOI] [PubMed] [Google Scholar]; g Kienle M, Wagner AJ, Dunst C, Knochel P. Chem. Asian J. 2011;6:517. doi: 10.1002/asia.201000367. [DOI] [PubMed] [Google Scholar]; h Mitsuda S, Fujiwara T, Kimigafukuro K, Monguchi D, Mori A. Tetrahedron. 2012;68:3585. [Google Scholar]; i Wagh YS, Bhanage BM. Tetrahedron Lett. 2012;53:6500. [Google Scholar]; j Xu J, Li JR, Wei Z, Zhang Q, Shi DX. RSC Adv. 2013;3:9622. [Google Scholar]; k Boudet N, Dubbaka SR, Knochel P. Org. Lett. 2008;10:1715. doi: 10.1021/ol800353s. [DOI] [PubMed] [Google Scholar]
- 5.a Yoo EJ, Ma S, Mei T-S, Chan KSL, Yu J-Q. J. Am. Chem. Soc. 2011;133:7652. doi: 10.1021/ja202563w. [DOI] [PubMed] [Google Scholar]; b Ng KH, Chan ASC, Yu WY. J. Am. Chem. Soc. 2010;132:12862. doi: 10.1021/ja106364r. [DOI] [PubMed] [Google Scholar]; c Ng KH, Ng FN, Yu WY. Chem. Commun. 2012;48:11680. doi: 10.1039/c2cc36502b. [DOI] [PubMed] [Google Scholar]; d Dong Z, Dong G. J. Am. Chem. Soc. 2013;135:18350. doi: 10.1021/ja410823e. [DOI] [PubMed] [Google Scholar]; e Cho SH, Kim JY, Lee SY, Chang S. Angew. Chem. 2009;121:9291. doi: 10.1002/anie.200903957. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:9127. [Google Scholar]; f Grohmann C, Wang HG, Glorius F. Org. Lett. 2012;14:656. doi: 10.1021/ol203353a. [DOI] [PubMed] [Google Scholar]; g Ng K-H, Zhou Z, Yu W-Y. Org. Lett. 2012;14:272. doi: 10.1021/ol203046n. [DOI] [PubMed] [Google Scholar]; h Kim JY, Park SH, Ryu J, Hwan S, Kim SH, Chang S. J. Am. Chem. Soc. 2012;134:9110. doi: 10.1021/ja303527m. [DOI] [PubMed] [Google Scholar]; i Grohmann C, Wang H, Glorius F. Org. Lett. 2013;15:3014. doi: 10.1021/ol401209f. [DOI] [PubMed] [Google Scholar]; j Shin K, Baek Y, Chang S. Angew. Chem. 2013;125:8189. doi: 10.1002/anie.201302784. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52:8031. [Google Scholar]; k Tang RJ, Luo CP, Yang L, Li CJ. Adv. Synth. Catal. 2013;355:869. [Google Scholar]; l Yu S, Wan B, Li X. Org. Lett. 2013;15:3706. doi: 10.1021/ol401569u. [DOI] [PubMed] [Google Scholar]; m Thirunavukkarasu VS, Raghuvanshi K, Ackermann L. Org. Lett. 2013;15:3286. doi: 10.1021/ol401321q. [DOI] [PubMed] [Google Scholar]; n Wang JA, Hou JT, Wen J, Zhang J, Yu XQ. Chem. Commun. 2011;47:3652. doi: 10.1039/c0cc05811d. [DOI] [PubMed] [Google Scholar]
- 6.Wencel-Delord J, Droge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]
- 7.a Kondo Y, Shilai M, Uchiyama M, Sakamoto T. J. Am. Chem. Soc. 1999;121:3539. [Google Scholar]; b Haag B, Mosrin M, Ila H, Malakhov V, Knochel P. Angew. Chem. 2011;123:9968. [Google Scholar]; Angew. Chem. Int. Ed. 2011;50:9794. [Google Scholar]; c Nguyen TT, Marquise N, Chevallier F, Mongin F. Chem. Eur. J. 2011;17:10405. doi: 10.1002/chem.201100990. [DOI] [PubMed] [Google Scholar]; d Clegg W, Dale SH, Harrington RW, Hevia E, Honeyman GW, Mulvey RE. Angew. Chem. 2006;118:2434. doi: 10.1002/anie.200503213. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:2374. [Google Scholar]; e Clegg W, Dale SH, Hevia E, Honeyman GW, Mulvey RE. Angew. Chem. 2006;118:2430. doi: 10.1002/anie.200503202. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:2370. [Google Scholar]
- 8.a Rees WS, Just O, Schumann H, Weimann R. Polyhedron. 1998;17:1001. [Google Scholar]; b Hlavinka ML, Hagadorn JR. Organometallics. 2007;26:4105. [Google Scholar]; c Wunderlich SH, Knochel P. Angew. Chem. 2007;119:7829. doi: 10.1002/anie.200701984. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:7685. [Google Scholar]; d Balloch L, Kennedy AR, Mulvey RE, Rantanen T, Robertson SD, Snieckus V. Organometallics. 2011;30:145. [Google Scholar]
- 9.a Berman AM, Johnson JS. J. Org. Chem. 2005;70:364. doi: 10.1021/jo048168g. [DOI] [PubMed] [Google Scholar]; b Berman AM, Johnson JS. J. Am. Chem. Soc. 2004;126:5680. doi: 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]; c Berman AM, Johnson JS. Synthesis. 2005;11:1799. [Google Scholar]; d Barker TJ, Jarvo ER. J. Am. Chem. Soc. 2009;131:15598. doi: 10.1021/ja907038b. [DOI] [PubMed] [Google Scholar]
- 10.a Iwao M, Reed JN, Snieckus V. J. Am. Chem. Soc. 1982;104:5531. [Google Scholar]; b Berman AM, Johnson JS. J. Org. Chem. 2006;71:219. doi: 10.1021/jo051999h. [DOI] [PubMed] [Google Scholar]
- 11.a Knochel P, Singer RD. Chem. Rev. 1993;93:2117. [Google Scholar]; b Knochel P, Perea JJA, Jones P. Tetrahedron. 1998;54:8275. [Google Scholar]; c Netherton MR, Fu GC. Adv. Synth. Catal. 2004;346:1525. [Google Scholar]; d Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. doi: 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a Tran LD, Roane J, Daugulis O. Angew. Chem. 2013;125:6159. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52:6043. [Google Scholar]; b Thu HY, Yu WY, Che CM. J. Am. Chem. Soc. 2006;128:9048. doi: 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]; c Sun K, Li Y, Xiong T, Zhang JP, Zhang QA. J. Am. Chem. Soc. 2011;133:1694. doi: 10.1021/ja1101695. [DOI] [PubMed] [Google Scholar]; d Xiao B, Gong TJ, Xu J, Liu ZJ, Liu L. J. Am. Chem. Soc. 2011;133:1466. doi: 10.1021/ja108450m. [DOI] [PubMed] [Google Scholar]; e del Amo V, Dubbaka SR, Krasovskiy A, Knochel P. Angew. Chem. 2006;118:8002. doi: 10.1002/anie.200603089. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:7838. [Google Scholar]
- 13.a Liu S, Yu Y, Liebeskind LS. Org. Lett. 2007;9:1947. doi: 10.1021/ol070561w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tan Y, Hartwig JF. J. Am. Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. [DOI] [PubMed] [Google Scholar]; c Yan XY, Chen C, Zhou YQ, Xi CJ. Org. Lett. 2012;14:4750. doi: 10.1021/ol302004t. [DOI] [PubMed] [Google Scholar]; d Xiao Q, Tian LM, Tan RC, Xia Y, Qiu D, Zhang Y, Wang JB. Org. Lett. 2012;14:4230. doi: 10.1021/ol301912a. [DOI] [PubMed] [Google Scholar]; e Rucker RP, Whittaker AM, Dang H, Lalic G. Angew. Chem. 2012;124:4019. doi: 10.1002/anie.201200480. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51:3953. [Google Scholar]; f Matsuda N, Hirano K, Satoh T, Miura M. Angew. Chem. 2012;124:3702. doi: 10.1002/anie.201108773. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51:3642. [Google Scholar]; g Miki Y, Hirano K, Satoh T, Miura M. Org. Lett. 2013;15:172. doi: 10.1021/ol303222s. [DOI] [PubMed] [Google Scholar]; h Dumas AM, Molander GA, Bode JW. Angew. Chem. 2012;124:5781. doi: 10.1002/anie.201201077. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51:5683. [Google Scholar]; i Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ArZnOBz was formed as a byproduct in the amination reaction of diarylzinc and BzONR1R2. See the Supporting Information for a proposed reaction pathway.
- 15.a Unsinn A, Ford MJ, Knochel P. Org. Lett. 2013;15:1128. doi: 10.1021/ol400248e. [DOI] [PubMed] [Google Scholar]; b Haas D, Mosrin M, Knochel P. Org. Lett. 2013;15:6162. doi: 10.1021/ol403019c. [DOI] [PubMed] [Google Scholar]; c Bresser T, Monzon G, Mosrin M, Knochel P. Org. Process Res. Dev. 2010;14:1299. doi: 10.1021/jo100884u. [DOI] [PubMed] [Google Scholar]; d Bresser T, Mosrin M, Monzon G, Knochel P. J. Org. Chem. 2010;75:4686. doi: 10.1021/jo100884u. [DOI] [PubMed] [Google Scholar]; e Mosrin M, Knochel P. Org. Lett. 2009;11:1837. doi: 10.1021/ol900342a. [DOI] [PubMed] [Google Scholar]; f Mosrin M, Bresser T, Knochel P. Org. Lett. 2009;11:3406. doi: 10.1021/ol901275n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.