Abstract
Cytometry by time-of-flight (CyTOF) is a novel technology for the real-time analysis of single cells. CyTOF is a significant advance in fields including immunology, hematology, and oncology. It resolves multiple metal-conjugated probes per cell with minimal signal overlap, which maximizes the information obtained from each individual sample. CyTOF provides the ability to phenotypically and functionally profile cells from normal and diseased states. Single cell technologies enable researchers to measure the effects of a drug at the single cell level and better understand its mechanism of action. Here, we discuss novel instruments for the analysis of individual biological cells, the impact of recent innovations in support of drug development, and the important roles of CyTOF in drug profiling.
History of single cell measurements
The study of cells began approximately 350 years ago in 1665 (http://www.smithlifescience.com/celltheory.htm), when Antonie van Leeuwenhoek invented the microscope and used it to describe the cells in a drop of pond water. In 1833, Robert Brown discovered the nucleus in plant cells and, in 1838, Matthias Jakob Schleiden concluded that all plant tissues comprise cells and that an embryonic plant arose from a single cell. In 1839, Theodor Schwann reached the same conclusion as Schleiden in relation to animal tissue. In 1840, Albrecht von Roelliker realized that sperm and eggs are also cells and, in 1845, Carl Heinrich Braun was the first to identify cells as the basic unit of life. Rudolf Virchow went on to demonstrate that the cell theory applied to diseased tissue as well as to healthy tissue. Since the original discovery of cells, biologists have depended on advancements in technology for their research. Mack Fulwyler was the inventor of the forerunner to today's flow cytometers, particularly the cell sorter. He developed this in 1965, followed by a publication in Science [1]. The first fluorescence-based flow cytometry device was developed in 1968 by Wolfgang Göhde from the University of Münster, filed for patent on December 18, 1968 [2].
Flow cytometry fluorescence-activated cell sorting (FACS), invented during the 1970s, is now widely used for single cell measurements in medicine and biology [3]. Cells are suspended in a narrow liquid stream such that they pass single file through the path of multiple laser beams, each of a different wavelength. Optical detectors convert fluorescent light emitted from each cell into an electrical signal. Often the cells are labeled with fluorescent antibodies to specific membrane proteins. Based on the intensity of signal emitted at different wavelengths, the cells can be analyzed one by one for various properties, such as size, granularity, and expression of membrane-bound proteins. Flow cytometers and cell sorters can process thousands of single cells per hour and can analyze up to 18 protein markers at a time. Over the past 30 years, FACS has enabled the identification and purification of a variety of cell types, including stem cells in tissues and tumors. Recent advances include phosphospecific antibodies that enable measurement of phosphorylation states in multiple proteins, thus making it possible to monitor signaling networks in thousands of single cells [4]. A recent breakthrough, CyTOF, combines flow cytometry and mass spectrometry (MS) [5–12]. The principle of CyTOF is described below and in Fig. 1. Table 1 compares mass cytometry, flow cytometry, liquid chromatography (LC)–MS, and single cell sequencing technologies. The type of study and the information required determine the type of instrument that can be used.
Figure 1.
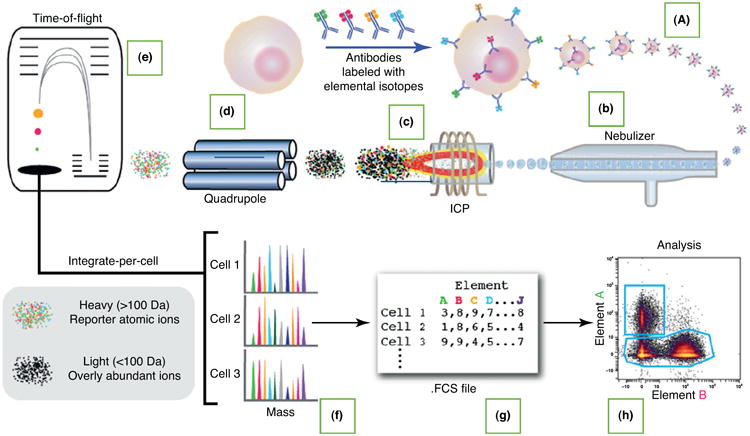
Workflow of mass cytometry analysis. A liquid sample containing cells labeled with heavy metal isotope-conjugated probes (ICPs) (a) is introduced into the nebulizer (b), where it is aerosolized. The aerosol droplets are directed into the ICP torch (c), where the cells are vaporized, atomized, and ionized. Low-mass ions are removed in the radiofrequency (RF) quadrupole ion guide (d), resulting in a cloud of ions enriched for the probe isotopes. The ion cloud then enters the time-of-flight (TOF) chamber (e), where the ions are separated on the basis of their mass:charge ratio as they accelerate toward the detector. Thus, the time-resolved detector measures a mass spectrum (f) that represents the identity and quantity of each isotope on a per cell basis. Data are generated in .fcs format (g) and analyzed using the cloud-based Cytobank platform (h). Reproduced from [40].
Table 1. Comparison between mass cytometry, flow cytometry, LC–MS and single cell sequencing techniquesa.
| Feature | Technology | |||
|---|---|---|---|---|
| CyTOF | Flow cytometry | LC–MS (e.g. FTMS or Orbitrap) | Single cell sequencing | |
| Application | Biomarkers, immunogenicity, PD | Biomarkers, immunogenicity, PD | Biomarkers, PK/PD | Biomarkers, immunogenicity |
| Workflow | Isolate cells; immunostain; determine protein expression or modifications by amount of metal ions observed | Isolate cells; immunostain; compensate fluorophores; determine protein expression or modifications by intensity of fluorescence observed | Extract proteins from cells or tissue; reduce complexity of mixture by HPLC segmentation; determine protein identity or modifications by peptides recovered | Isolate cells; extract DNA; whole-genome amplification; next-generation sequencing |
| Multiplex capacity | ∼120 | ∼20 | ∼1000+ | ∼1000+ |
| Sample barcoding | Ye s | Ye s | Ye s | Yes |
| Detection limit | ∼300 molecules/single cell | 100–300 molecules/single cell (varies by fluorophore, without compensation) | ∼100,000 molecules | ∼100 molecules/single cell |
| Data analysis complexity | Moderate–high | Low–high (deconvolution for high-parameter flow) | High | High |
| Information content per sample | High | Moderate | High | High |
| Single cell resolution | Ye s | Ye s | No | Yes |
| Throughput | ∼1000 cells/s | ∼40,000 cells/s | ∼20 samples/day | ∼100 cells/week |
| Analytical efficiency | ∼30% | ∼90% | ∼90% | ∼30% |
| Live cell analysis | Not possible | Possible | Not possible | Not possible |
| Cost/sample | ∼US$100 | US$10 | ∼US$150 | ∼US$1000 |
| Instrument cost | ∼US$650,000 | US$500,000 | ∼US$850,000 | ∼US$250,000 |
| Vendor(s) | Single, Fluidigm | Multiple | Multiple | Multiple |
| Impact | Multiplex capacity, specificity, small sample size | Sensitivity, downstream live cell analysis | Sensitivity, throughput and automated, specificity, small sample size | Sensitivity, multiplex capacity, specificity, small sample size |
Abbreviations: FTMS, Fourier transform ion cyclotron resonance mass spectrometry; HPLC, high performance liquid chromatography.
CyTOF instruments
Principle and technology
CyTOF technology is novel in that it analyzes individual cells labeled with stable heavy metal isotopes using TOF inductively coupled plasma mass spectrometry (TOF ICPMS) technology [5–8]. This can expand the number of measureable markers by overcoming the limitations that arise from the spectral overlap between signals from different fluorescent labels, and represents an exciting new avenue of research. However, the current practice of flow cytometry is still limited by the need to have antibodies to the target of interest [6]. With over 120 detection channels available, CyTOF has the ability to concurrently resolve multiple elemental probes per cell at high acquisition rates without the need for compensation, thereby maximizing the per cell information obtained from a single sample. Current CyTOF techniques enable the detection of 40 parameters at the single cell level; this capacity will increase as more isotopes become available. These capabilities provide researchers with a previously unattainable ability to generate high-resolution phenotypic and functional profiles of cells in both normal and diseased states.
Figure 1 shows the process through sample introduction (Fig. 1a), ionization (Fig. 1b,c), mass analysis (Fig. 1d,e), and data acquisition (Fig. 1f–h). After cells stained with metal-conjugated isotopes in a single cell suspension are introduced into the CyTOF, a multistep process within the instrument produces a file that records the identity and amount of each isotope on each cell.
The sample introduction system desolvates the liquid sample suspension and introduces cells one at a time into the isotope-conjugated probe (ICP) source for ionization. The liquid sample is introduced into a nebulizer, where it is aerosolized into a heated spray chamber. Within the spray chamber, the high temperature partially vaporizes the aerosol, and gas flows direct the aerosolized cells to the ICP source. For liquid sample analysis, it is crucial to remove as much water as possible from the sample so that it can be efficiently ionized in the plasma. Liquid cell suspension is aerosolized by nebulizer gas as it exits the nebulizer. Make-up gas carries the aerosol through the heated spray chamber, where it is partially desolvated and delivered to the ICP torch.
Ionization
The mixture of single cell aerosol droplets and argon that exits the spray chamber is transmitted to the ICP source, where it is vaporized, atomized, and ionized in the plasma for subsequent mass analysis. The plasma is created within the plasma torch by induction using a radiofrequency (RF)-generated electromagnetic field. The torch comprises the torch body (a fused assembly of two concentric quartz tubes) and a quartz sample injector tube, which is inserted inside the torch body. When assembled, the torch comprises three concentric chambers. The outermost chamber (between the torch body tubes) contains argon ‘plasma’ gas flowing at 17 L/min, which is ignited to form the plasma. The central chamber (between the inner torch body tube and the sample injector) contains argon ‘auxiliary’ gas flowing at approximately 1 L/min, which is used to change the position of the base of the plasma relative to the sample injector. The innermost chamber inside the sample injector transmits the argon stream and sample aerosol from the spray chamber directly into the center of the plasma. The torch assembly is mounted inside an induction load coil that is supplied with a RF-generated current that creates an electromagnetic field.
Formation of the ICP discharge and ionization of the sample
Plasma is formed by collision-induced ionization of argon gas within an intense electromagnetic field. First, argon plasma gas flows tangentially from the outer chamber of the torch body. RF power supplied to the load coil produces an oscillating current (40 MHz), creating a strong electromagnetic field precisely at the point that the plasma gas exits the outer chamber. A high voltage spark strips away free electrons from the exiting argon atoms. These free electrons accelerate dramatically in the electromagnetic field and collide with sufficient energy to ionize the argon gas into plasma. Temperatures within the plasma typically range from 5000 to 10,000 K. When the aerosolized sample is introduced through the injector into the base of the plasma, the water droplets are rapidly vaporized. The desolvated individual cells are then broken down into a cloud of ground-state atoms. Subsequent electron collisions result in ionization of the cell. Thus, the argon ionic beam that exits the plasma contains bursts of ionic clouds corresponding to individual cells that were introduced into the torch in aerosol form.
Mass analysis
The ion beam leaving the torch enters the low-pressure ion optical chamber. High-mass ions leaving the quadropole ion guide are directed to the TOF chamber, where they are separated on the basis of their mass:charge ratio and directed to the detector. The quadrupole ion guide removes unwanted low-molecular-weight argon and endogenous cellular ions from the beam that emerges from the quadrupole ion deflector, transmitting clouds that contain isotopic probe ions (>80 amu) to the TOF analyzer. The ions are pushed into the TOF chamber at 13-ms intervals. Ionic clouds are subjected to an electrostatic force that orthogonally accelerates the incoming ions toward the detector. As a result, the ions separate based on their mass:charge ratio, with lighter elements reaching the detector first.
Considerations during sample preparation
To perform single-cell analysis in human diseases or their animal models, the cells of interest must first be separated from the population. After separation, cells are stained in suspension with a panel of metal conjugates directed against targets of interest. Cells are stained with an iridiumbased DNA intercalator to distinguish single cells from doublets or aggregates [9]. In addition, cells are stained with cisplatin to identify the dead cells in the samples [10]. One consideration here is that the best results are achieved when the cells are incubated for 1 min with cisplatin at 50 μM (final concentration); the rate of cell degradation and/or death increases significantly with increases in concentrations or incubation times. Additionally, incubation times between 12 hours and 24 hours work well with an iridium intercalator. Another important consideration is the sample stability in water. Although the stained cells may be stored for up to 6 months in buffer, once they are washed and prepared for analysis, the best results are obtained when samples are processed within 24 hours. Figure 2 is a comparison of the stability of CD4 and CD8 cells over time; because the antibodies are unstable in water, the pattern is significantly disrupted after 1 day and even more so after 7 days. This suggests that, once the cells are suspended in water, the analysis should be performed as expeditiously as possible, preferably within the first day.
Figure 2.
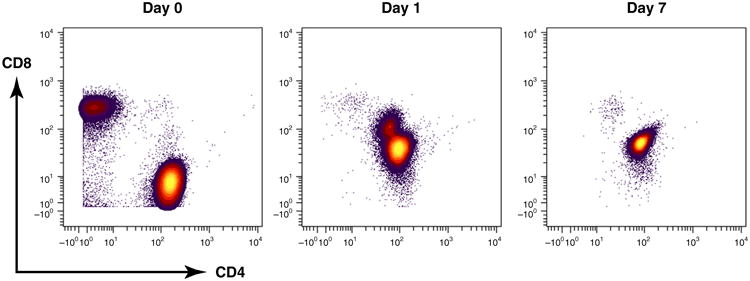
Representative antibody surface-staining results after the sample has been stored in de-ionized water for Day 0, 1, and 7 using mass cytometry of fixed peripheral blood mononuclear cells (PBMCs).
Following staining, the cells are then introduced into an ICP, where they are individually atomized and ionized. The cloud of atomic ions for each single cell is extracted into the ion optics and TOF regions of the mass cytometer, where the ions are separated by mass. After the ions are separated in the TOF chamber, they are detected using a discrete dynode electron multiplier. When an ion strikes the first dynode of the detector, several secondary electrons are liberated. These electrons strike the next dynode, where they generate more electrons. This process is repeated at each dynode, resulting in an electron pulse that is captured by the anode of the detector. The output analog signal is amplified and converted to digital values at 1-ns sampling intervals. In recording the data, the intensity of the signal detected in each channel is directly proportional to the number of antibodies tagged with the isotope of the corresponding channel bound to that cell. These data are formatted as .FCS files.
Data analysis
Cytobank is a cloud-based platform for cytometry data analysis, collaboration, storage, and data management. Cytobank has the ability to analyze fluorescence and mass cytometry data, including standard and advanced analysis tools and visualizations. The data can be plotted in a single dimension, in 2D dot plots or even in 3D. The regions on these plots can be sequentially separated, based on ion intensity from mass cytometry, by creating a series of subset gates. These include: raw data-plotting tools, such as histograms (and histogram overlays), dot plots, and gating; statistical representations of multiple samples, such as heatmaps and dose–response curve; and 2D space (SPADE and viSNE) [11]. viSNE is a new high-dimensional cytometry analysis tool based on the t-distributed stochastic neighbor embedding (t-SNE) algorithm [12]. viSNE plots cells in 2D based on marker expression, placing related cells closer than unrelated cells in a biaxial plot. By combining state-of-the-art tools, such as SPADE and viSNE, we can visualize and characterize cell populations of interest. There are specific gating protocols for diagnostic and clinical samples that, when combined with computational methods, offer a significant improvement in the ability to find rare and hidden populations. Once the data are collected, the analysis is performed on a separate computer. Figure 3 shows a SPADE diagram and viSNE maps for peripheral blood mononuclear cells analyzed by CyTOF mass cytometry.
Figure 3.
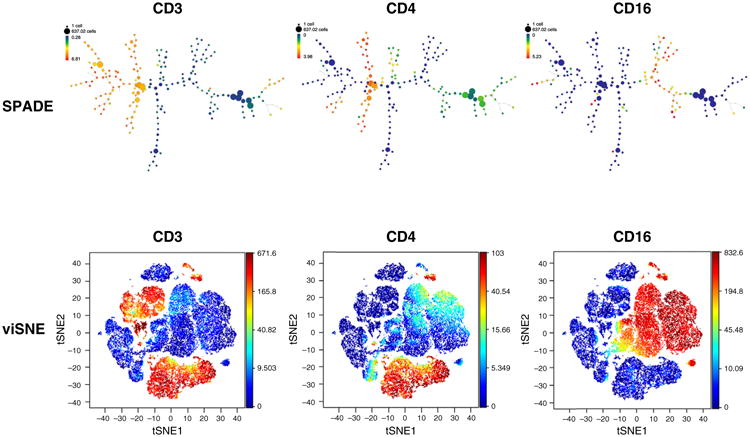
Representative antibody surface-staining results of fixed peripheral blood mononuclear cell (PBMC) analysis by SPADE and viSNE. Node size represents the number of cells and the color indicates expression of CD3 (cluster of differentiation 3), CD4 and CD16.
Applications of CyTOF in drug development
In drug discovery, CyTOF technology canbeused to screen drug candidates for changes in immunophenotype or cell function using ex vivo or in vivo designs. During the preclinical phase, CyTOF can be used for assessing the immunotoxicology of a candidate drug by evaluating the immunophenotype of various cell populations in whole blood, tissue, or other matrices. In addition, it can be used to assess pharmacodynamic (PD) markers of interest for further elucidation of on- or off-target effects. In a clinical setting, CyTOF can be used for safety, receptor occupancy, and PD assessments, and to investigate the mechanisms of action of toxicity.
There is increasing interest in single cell experimentation methods from a variety of disciplines, such as cancer research [13–16], cardiovascular research [17,18], embryonic stem cells and development [19–21], gene expression profiling [22–24], hematopoietic stem cells and progenitors [24–27], immunity and/or infectious disease [28,29], induced pluripotent stem cells [30], neural research [31,32], and RNA SEQ [33,34]. For tissue-specific autoimmune diseases, including type 1 diabetes mellitus, multiple sclerosis, or rheumatoid arthritis, CyTOF can make a significant contribution to the development of more-effective drugs. In these diseases, a variety of immune cell populations, such as T cells, B cells, and macrophages, as well as non-immune cells, interact with each other to evoke site-directed chronic inflammation. In the diseases or disease models, CyTOF can qualify and quantify these populations and their activation status with its ability to detect multiplexed information from single cells. Additionally, samples with a limited size, such as a biopsy of the affected tissue, can be subjected to the analysis. Hence, CyTOF will be a useful tool for identifying novel therapeutic targets or evaluating newly developed drugs.
Individual cells can differ dramatically in size, protein levels, and expressed RNA transcripts. These variations open new avenues to address previously irresolvable questions in cancer research, stem cell biology, immunology, developmental biology, and neurology. Single cell experiments provide a wealth of information that reaches over and above cell population-averaged measurements and enables access to dynamic cell phenotypes at the cellular and subcellular levels. CyTOF provides a powerful tool for drug development. Immunofluorescence can be used on tissue sections, cultured cell lines, or individual cells, and can be used to analyze the distribution of proteins, glycans, and small biological and nonbiological molecules. The CyTOF method is expected to have important clinical applications in cancer research. CyTOF and immunofluorescence are complementary to each other. In many disease states, inflammatory responses result in detectable changes in cytokine levels that can be measured in biological fluids, such as serum and plasma, making cytokines valuable biomarkers. Excessive or diminished cytokine levels are associated with many clinical conditions and disease states. Table 2 shows the cytokine biomarkers and target cells of various diseases.
Table 2. Cytokine biomarkers and target cells of various diseases.
| Asthma/airway inflammation | Cardiovascular disease | Cancer and malignancy | Rheumatoid arthritis |
|---|---|---|---|
| Cytokine biomarkers | |||
| Eotaxin; GM-CSF; IFNγ; IL-4, -5, -8, -10, -12, -13, -17A, -17F, -18; TNFα | CCL2 (MCP-1); CCL3 (MIP-1α); CCL4 (MIP-1β); CRP; CSF; CXCL16; erythropoietin; FGF; fractalkine (CX3CL1); G-CSF; GM-CSF; IFNγ; IL-1, -2, -5, -6, -8, -8 (CXCL8), -10, -15, -18; M-CSF; PDGF; RANTES (CCL5); TNFα; VEGF | Eotaxin; GM-CSF; IFNγ; IL-4, -5, -8, -10, -12, -13, -17A, -18; TNFα | IL-1β, -1RA, -6,-7,-10–12,-15,-17, -18,-23; MIP-3α; TGFβ; TNFα |
|
| |||
| Target cells | |||
| TH2, B cells, dendritic cells, mast cells, basophils, eosinophils, airway epithelial cells | Monocytes and/or macrophages, T cells, vascular endothelial cells | T cells, regulatory T cells, natural killer cells, dendritic cells, macrophages, cancer stem cells | TH1, TH17, B cells, neutrophils, monocytes and/or macrophages, synoviocytes |
Pharmacokinetics/pharmacodynamics
Pharmacokinetics (PK) investigates the fate of substances administered externally to a living organism and provides insight into how drugs distribute throughout the body, but cannot explain how drugs work at the cellular level. PD is the study of the biochemical and physiological effects resulting from a certain drug concentration. However, technical restrictions imposed by the minute amounts of chemicals involved have limited our ability to determine how a single cell is affected by a specific drug after administration. With the fast development of single cell detection and handling techniques, it is becoming possible to study single cell PK. Thurber et al. used real-time in vivo single cell PK imaging of a poly(ADP ribose) polymerase 1 (PARP1) inhibitor to show that the lack of clinical effect was probably not due to tumor cells receiving insufficient concentrations of a PARP1 inhibitor [36]. Drug inefficiency was likely related to proteomic heterogeneity or the insensitivity of cancer cells to DNA repair inhibition. The authors showed that the drug conjugates retained their target binding and PK properties. This suggests that single cell PK imaging and derived modeling will improve understanding of drug action at a single cell resolution in vivo [35].
Dieterlen et al. used flow cytometry to study the pharmacologic effects on immune cells after organ transplantation. Therapeutic drug monitoring requires the application of reliable and effective methods to study PD variability by direct measurements of drug effects on immune cell functions. Although flow cytometry was used, CyTOF would be agood choice for this type of study [37]. Another study showed that single cell PK imaging reveals a therapeutic strategy to overcome drug resistance to the microtubule inhibitor eribulin. Eribulin mesylate was developed as a potent microtubule-targeting cytotoxic agent to treat taxane-resistant cancers, but recent clinical trials have shown that it eventually fails in many patient subpopulations for unclear reasons. Using intravital imaging and automated tracking of cellular dynamics, it was found that resistance to eribulin and the fluorescent analog depended directly on multidrug resistance protein 1 (MDR1) [38].
Highly multiplexed, single cell technologies could be crucial for identifying correlates of disease or immunological interventions, as well as for elucidating the underlying mechanisms of immunity. The complex heterogeneity of cells and their interconnectedness with each other are major challenges in drug development. Accurate characterization of samples with high cellular heterogeneity in fields including hematology, stem cell biology, tissue engineering, and cancer biology, might only be achieved by analyzing single cells. Single cell research is vital because rare cells of interest might be in the minority, with their behavior masked by the majority, or because the dynamics of the populations of interest are offset in time.
Concluding remarks
Recent innovation in CyTOF brings the capability for simultaneous detection of many proteins to single cell biology and facilitates greater understanding of both cell type and function at the single cell level. Single cell molecular analysis has provided new insight into diverse research areas, such as immunology and oncology. The development of CyTOF technology has an important role in accelerating advances in diagnostics and treatment of a range of diseases. CyTOF techniques in cancer research have shown great promise in improving our understanding of this complex family of diseases. Exciting new developments in the field provide great insights into rare tumor cells, revealing their genetic and transcriptomic relations to the major populations of differentiated tumor cells. Given that drug companies are becoming increasingly focused on the mechanism of drug–target interactions and their resulting pharmacology to have good understanding of the mechanism of action, single cell analysis will have a key role in drug development. The ability to concurrently measure 40 separate biomarkers at the single cell level with no compensation has the potential to identify rare diseased cells at an early stage, which could help to prevent or minimize disease progress. It might also provide nonaggressive, personalized therapy, and diagnostics through treatment, remission, and relapse. The capability to measure multiple parallel translational pathway responses to agonist and/or antagonist intervention using CyTOF will greatly optimize the drug discovery process by providing a wealth of information during the analysis.
In the near future, there is a need to develop methods for analyzing individual cells that could be used to predict alterations in cell behavior over time [39]. The hope is that these tools will enable time-dependent measurements at the single cell level in complex tissue environments. Such technologies could provide information about the health status of a given cell, and guide treatments related to specific disease states. In addition, there is a need to develop more sensitive and selective tools to identify rare cells in a mixed population, such as cells that are potentially cancerous, becoming drug resistant, or infected with a pathogenic virus.
Acknowledgments
The authors would like to thank Ruth Montgomery for her review and insightful comments on the manuscript.
References
- 1.Fulwyler M. Electronic separation of biological cells by volume. Science. 1965;150:910–911. doi: 10.1126/science.150.3698.910. [DOI] [PubMed] [Google Scholar]
- 2.Dittrich W, Ghde W. Flow-through chamber for photometers to measure and count particles in a dispersion medium, DE 1815352 [Google Scholar]
- 3.Shapiro HM. Practical Flow Cytometry. 4th. Wiley-Liss; 2003. [Google Scholar]
- 4.Irish JM, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Bendall SC, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandura DR, et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2009;81:6813–6822. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- 7.Cheung RK, Utz PJ. Screening: CyTOF – the next generation of cell detection. Nat Rev Rheumatol. 2011;7:502–503. doi: 10.1038/nrrheum.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raj A, et al. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ornatsky OI, et al. Study of cell antigens and intracellular DNA by identification of element-containing labels and metallointercalators using inductively coupled plasma mass spectrometry. Anal Chem. 2008;80:2539–2547. doi: 10.1021/ac702128m. [DOI] [PubMed] [Google Scholar]
- 10.Fienberg HG, et al. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry A. 2012;81:467–475. doi: 10.1002/cyto.a.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amir el AD, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–2605. [Google Scholar]
- 13.Saadatpour A, et al. Characterizing heterogeneity in leukemic cells using single-cell gene expression analysis. Genome Biol. 2014;15:525. doi: 10.1186/s13059-014-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccirillo S, et al. Genetic and functional diversity of propagating cells in glioblastoma. Stem Cell Rep. 2014;4:7–15. doi: 10.1016/j.stemcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landau D, et al. Locally disordered methylation forms the basis of intratumor methylome variation in chronic lymphocytic leukemia. Cancer Cell. 2014;26:813–825. doi: 10.1016/j.ccell.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melchor L, et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia. 2014;28:1705–1715. doi: 10.1038/leu.2014.13. [DOI] [PubMed] [Google Scholar]
- 17.Chini V, et al. Micro-RNAs and next generation sequencing: new perspectives in heart failure. Clin Chim Acta. 2014;443:114–119. doi: 10.1016/j.cca.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Fu J, et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens A, et al. Sox7 is regulated by Etv2 during cardiovascular development. Stem Cells Dev. 2014;23:2004–2013. doi: 10.1089/scd.2013.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavez SL, et al. Comparison of epigenetic mediator expression and function in mouse and human embryonic blastomeres. Hum Mol Genet. 2014;23:4970–4984. doi: 10.1093/hmg/ddu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jasnos L, et al. Determining cell division symmetry through the dissection of dividing cells using single-cell expression analysis. Nat Protoc. 2014;9:505–516. doi: 10.1038/nprot.2014.032. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, et al. A bead-based microfluidic approach to integrated single-cell gene expression analysis by quantitative RT-PCR. J R Soc Chem. 2014;5:4886–4893. doi: 10.1039/C4RA13356K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorrell C, et al. The organoid-initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Rep. 2014;2:275–283. doi: 10.1016/j.scr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett R, et al. Laser microdissection of the alveolar duct enables single-cell genomic analysis. Front Oncol. 2014;4:260. doi: 10.3389/fonc.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hough SR, et al. Single-cell gene expression profiles define selfrenewing, pluripotent, and lineage primed states of human pluripotent stem cells. Stem Cell Rep. 2014;2:881–895. doi: 10.1016/j.stemcr.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becher B, et al. High-dimensional analysis of the murinemyeloid cell system. Nat Immunol. 2014;12:1181–1189. doi: 10.1038/ni.3006. [DOI] [PubMed] [Google Scholar]
- 27.Shao L, et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014;123:3105–3115. doi: 10.1182/blood-2013-07-515619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss-Albee DM, et al. Coordinated regulation of NK receptor expression in the maturing human immune system. J Immunol. 2014;193:4871–4879. doi: 10.4049/jimmunol.1401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swadling L, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horowitz A, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victor M, et al. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84:311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothwell PE, et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi S, et al. MicroRNA-9 and microRNA-326 regulate human dopamine D2 receptor expression and the microRNA-mediated expression regulation is altered by a genetic variant. J Biol Chem. 2014;289:13434–13444. doi: 10.1074/jbc.M113.535203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treutlein B, et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurber GM, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun. 2013;4:1504. doi: 10.1038/ncomms2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurber GM, et al. Effect of small-molecule modification on single-cell pharmacokinetics of PARP inhibitors. Mol Cancer Ther. 2014;13:986–9895. doi: 10.1158/1535-7163.MCT-13-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dieterlen MT, et al. Flow cytometry-based pharmacodynamic monitoring after organ transplantation. Methods Cell Biol. 2011;103:267–284. doi: 10.1016/B978-0-12-385493-3.00011-5. [DOI] [PubMed] [Google Scholar]
- 38.Ashley M, et al. Single-cell pharmacokinetic imaging reveals a therapeutic strategy to overcome drug resistance to the microtubule inhibitor eribulin. Sci Transl Med. 2014;6:261ra152. doi: 10.1126/scitranslmed.3009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brice Gaudillière B, et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;24:255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendall SC, et al. Trends Immunol. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


