Abstract
Glucagon-like peptide 1 (GLP-1) analogues are used for the treatment of type 2 diabetes. The ability of the GLP-1 system to decrease food intake in rodents has been well described and parallels results from clinical trials. GLP-1 receptors are expressed in the brain, including within the ventral tegmental area (VTA) and the nucleus accumbens (NAc). Dopaminergic neurons in the VTA project to the NAc, and these neurons play a pivotal role in the rewarding effects of drugs of abuse.
Based on the anatomical distribution of GLP-1 receptors in the brain and the well-established effects of GLP-1 on food reward, we decided to investigate the effect of the GLP-1 analogue Exendin-4 on cocaine- and dopamine D1-receptor agonist-induced hyperlocomotion, on acute and chronic cocaine self-administration, on cocaine-induced striatal dopamine release in mice and on cocaine-induced c-fos activation. Here, we report that GLP-1 receptor stimulation reduces acute and chronic cocaine self-administration and attenuates cocaine-induced hyperlocomotion. In addition, we show that peripheral administration of Exendin-4 reduces cocaine-induced elevation of striatal dopamine levels and striatal c-fos expression implicating central GLP-1 receptors in these responses. The present results demonstrate that the GLP-1 system modulates cocaine's effects on behavior and dopamine homeostasis, indicating that the GLP-1 receptor may be a novel target for the pharmacological treatment of drug addiction.
Keywords: GLP-1, Exendin-4, self-administration, cocaine, c-fos, dopamine, addiction
1. Introduction
Drug abuse and drug dependence are major health problems, and effective pharmacological interventions to treat them are lacking. To develop novel treatment strategies, a better understanding of the complex neurobiology involved in drug abuse and drug dependence is needed. Midbrain dopaminergic neurons projecting to the nucleus accumbens (NAc) are believed to mediate the reinforcing effects of drugs of abuse [1,2], which play a pivotal role in the development of drug abuse and drug dependence. Therefore, research efforts have focused on the development of candidates that interact with the dopamine reward system.
Glucagon-like peptide 1 (GLP-1) is a peptide produced in enteroendocrine L-cells of the intestinal mucosa [3] and secreted from the intestinal tract in response to food intake [4]. However, GLP-1 is also localized in the neurons of the brainstem nucleus of the solitary tract (NTS), which has widespread projections within the central nervous system (CNS) [5]. GLP-1 receptors are expressed in the ventral tegmental area (VTA) and NAc [6] and it has recently been shown that GLP-1-producing NTS neurons project directly to the VTA and the shell and core regions of the NAc [7].
Selective GLP-1 receptor agonists, e.g., liraglutide (Victoza©) and exenatide (Byetta©), increase insulin secretion, decrease glucagon secretion and gastric emptying and are used in the clinic to treat type 2 diabetes [8]. GLP-1 is also known to reduce food intake and body weight in rodents, most likely mediated via a central effect [9,10], and similar effects have also been reported in humans [11].
Exendin-4 (Ex-4) is a selective GLP-1 agonist. The native hormone is produced in the gut of the Gila monster Heloderma suspectum, a desert reptile, and found at a high concentration in its saliva. Ex-4 is able to cross the blood-brain barrier [12,13] and has been shown to regulate food reward in rats by a central mode of action [14]. Emerging data suggest that GLP-1R signaling may modulate hedonic behaviors [15]. For instance, Ex-4 attenuates d-amphetamine-induced hyperactivity [16] as well as cocaine-induced place preference [17] and self-administration of alcohol in mice [18].
Considering the anatomical and functional relationship between GLP-1 receptors and the brain reward system, we decided to further investigate whether GLP-1 receptor stimulation modulates the addictive properties of the indirect dopamine receptor agonist cocaine. To this end, we studied the effects of Ex-4 on acute and chronic cocaine self-administration and show for the first time, that Ex-4 attenuates these effects. In addition we show that Ex-4 can inhibit dopamine D1 receptor- and cocaine-induced hyperlocomotion as well as cocaine-induced striatal dopamine release and striatal c-fos expression in mice.
2. Methods
2.1 Animals
Male NMRI mice (Taconic, Denmark) that weighed 28-35g were used for microdialysis and locomotor activity experiments, male NMRI mice that weighed 20-22g were used for the acute self-administration experiment and male C57Bl/6 weighing 20-24g were used for chronic self-administration experiments. All mice were housed in Makrolon cages (20 × 35 × 15 cm) enriched with cardboard housing and nesting material. The animals were kept at room temperature (22°C ± 2) in a 12-hour light/dark cycle (lights on at 6:00 A.M.) with free access to food and water. All experiments were performed during the light cycle between 9:00 A.M. and 4:00 P.M. The mice were allowed to acclimatize to the animal facility for 6-9 days prior to initiation of the experiments. All experiments were approved by the Danish Experimental Animal Inspectorate and were in accordance with the directives of the “Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985) and the council of the European Communities (86/809/EEC).
2.2 Drugs
Cocaine hydrochloride was obtained from the Copenhagen University Hospital Pharmacy, Denmark. Ex-4 was purchased from Tocris Bioscience, UK, and SKF-82,958 hydrobromide was purchased from Sigma-Aldrich. Ex-4 (0.3-100 μg/kg), cocaine and SKF-82,958 were dissolved in 0.9% saline solution (10 ml/kg). In all experiments, Ex-4 was administered intraperitoneally (i.p.) 90-100 min prior to cocaine, which was administered subcutaneously (s.c.) or intravenously (i.v.).
2.3 Locomotor activity measurements
The locomotor activity cages were equipped with 5×8 infrared light sources plus photocells [19]. The light beams crossed the cage 1.8 cm above the bottom of the cage. During the test session, locomotor activity was recorded as crossings of infrared light beams. All experiments were conducted in a clean cage with a scant lining of bedding material. In the first experiment, NMRI mice were injected i.p. with Ex-4 (0.3-30 μg/kg) or saline, placed into the activity cages, injected s.c. with cocaine (30 mg/kg) or saline 90 minutes later and replaced into the activity cages for an additional 60 minutes. In the second experiment, NMRI mice were injected i.p. with Ex-4 (30 μg/kg) or saline, placed into the activity cages, injected s.c. with SKF-82,958 (0.01, 0.1, and 1 mg/kg) or saline 90 minutes later and replaced into the activity cages for an additional 60 minutes.
2.4 Acute cocaine self-administration
The self-administration procedure and apparatus was described previously [19]. The self-administration apparatus consisted of transparent plastic boxes (8 × 8 × 8 cm) with a centered frontal nose-poke hole (12 mm diameter) 1 cm above the floor and a centered posterior vertical opening (width 5 mm) through which the tail extended. Dual photocells projected an infrared beam 1 mm in front of the nose-poke hole. Immediately before being placed in the test boxes, the mice were left for approximately 3 min 30-35 cm below a 150 W infrared light bulb to induce vasodilatation, thus facilitating the insertion of the infusion needle (27 G, 0.4 × 40 mm, Sterican®, B. Braun) into their tail veins. A fixed ratio 1 (FR-1) schedule was used, with no delay between nose-poke and infusion, so that each nose-poke induced the i.v. infusion of 1.4 μl cocaine or vehicle. After placing the mouse in the self-administration box for a 10-min habituation period during which nose-poking did not induce infusions, one priming infusion was given by the experimenter immediately before starting a 30-min session. Immediately after the session, the correct placement of the infusion needle was verified by manual infusion of the tested drug by an experimenter blind to the number of nose-pokes produced, and the animals were quickly sacrificed. Mice were excluded from further analysis if they had not produced at least five nose-pokes during the self-administration session or correct placement of the infusion needle could not be verified.
Two acute cocaine self-administration experiments were performed. In the first experiment, NMRI mice received i.p. administration of the GLP-1 agonist Ex-4 at doses of 10 (n=12), 30 (n=14), or 100 (n=12) μg/kg or the corresponding vehicle (nsaline=11, ncocaine=13). Ninety minutes later, the animals were subjected to the self-administration procedure described above with access to i.v. administration of saline or cocaine at 0.03 mg/kg/infusion, previously shown to be the optimal dose for self-administration [19]. In the second experiment, NMRI mice received i.p. administration of 30 μg/kg Ex-4 or saline. 90 minutes later, the animals were subjected to the self-administration procedure described above with access to i.v. administration of cocaine doses of 0.01 (nvehicle=11, nEx-4=12), 0.03 (nvehicle=14, nEx-4=12), 0.1 (nvehicle=12, nEx-4=14), or 0.3 (nvehicle=11, nEx-4=11) mg/kg/infusion or saline (nvehicle=15, nEx-4=12).
2.5 Chronic cocaine self-administration
Equipment and training procedures were previously described [20]. Operant chambers (Med Associates, USA) contained two nose-poke holes 10 mm above the grid floor, both equipped with photocells and a discriminative cue light, positioned on either side of a small dish-shaped plate into which liquid food could be delivered. Responding in the right hole resulted in delivery of a reinforcer and illumination of the cue light for 20 s, during which additional responses were counted but had no scheduled consequences (i.e., post-reinforcer timeout). Under oxygen/isoflurane vapor anesthesia, a catheter (SILASTIC tubing; 0.18-mm inner diameter, 0.41-mm outer diameter) was inserted 1.2 cm into the right or left jugular vein and anchored to the vein with sutures. The catheter ran subcutaneously to the base located above the midscapular region. During the subsequent 7 days of postsurgical recovery, 0.02 ml of 0.9% saline containing heparin (30 U/ml; SAD, Denmark) and antibiotic (cefazolin, 50 mg/ml; Hexal, Germany) was infused daily through the catheter to prevent clotting and infection. Catheter patency was confirmed 7 days after surgery and after completion of each experimental phase by the loss of muscle tone and clear signs of anesthesia within 3 s after infusion of 0.02– 0.03 ml ketamine (15 mg/ml; Pfizer, Denmark) plus midazolam (0.75 mg/ml; Matrix Pharmaceuticals, Denmark) in saline. If catheters failed during the first 10 acquisition sessions (about 15%), the mice were excluded from further experiments.
2.6 Cocaine self-administration under a FR1 schedule
Cocaine (1.0 mg/kg/infusion) was available in daily 3-h sessions until baseline criteria were met (≥20 reinforcers earned, with ≤20% variation over two consecutive sessions and ≥70% responses in the active hole). In consecutive sessions, saline was substituted for cocaine until extinction criteria were met (<50% of the baseline responding for cocaine self-administration). Cocaine (1.0 mg/kg/infusion) was then made available again until the previously established baseline criteria were met again or until a new baseline was established with similar requirements as above. Subsequently, the effect of Ex-4 (10μg/mg) or vehicle on a cocaine dose–effect functions (saline, 0.03, 0.1, 0.3, 1.0, 3.2 mg/kg/infusion of cocaine) was determined for each mouse (n=7) according to a Latin-square design. Each dose of cocaine (0.03, 0.1, 0.32, and 1.0 mg/kg/infusion) was tested two days in a row followed by saline twice and then 3.2 mg/kg/infusion cocaine twice. The order of pretreatment with Ex-4 (10 μg/mg) or vehicle i.p. 60 minutes prior to testing was determined at random for each dose of cocaine tested. To prevent overdosing, total drug intake was limited to 30 mg/kg per session.
2.7 In vivo microdialysis
Twenty to 30 minutes prior to surgery, the mice were treated with analgesic (Metacam, 5 mg/kg, s.c., Boehringer Ingelheim), deeply anaesthetized with sevoflurane (Baxter) and subsequently placed in a stereotaxic frame. Intracerebral guide cannulae (CMA Microdialysis AB, Solna, Sweden) were stereotaxically implanted into the brain to allow positioning of the dialysis probe in the striatum (AP: +1 mm, ML: +1.1 mm relative to bregma and DV: -3.0 mm relative to the skull surface [21]) and fixed in place with one anchor screw and dental cement. After surgery, the animals were housed in individual cages and left to recover for at least 24 hours. Correct placement of the probes was verified histologically at the end of the experiment. The 2 mm microdialysis probe (CMA/7) was perfused at a rate of 1.8 μl/min with an artificial cerebrospinal fluid solution (147 mM NaCl, 4 mM KCl and 2.3 mM CaCl2, adjusted to pH 6.5). A swivel setup and a bowl (Instech Laboratories, Inc., Plymouth Meeting, PA, USA) were used, allowing the animals to move freely during dialysis. The first five 20-min fractions were discarded to obtain stable basal values. After the first five fractions were discarded, three 20-min fractions were collected to establish dopamine (DA) baseline levels. Subsequently, mice were injected with Ex-4 (30 μg/kg, n=6) or vehicle (n=6), and five 20-min fractions were collected. The mice were then injected with cocaine (30 mg/kg s.c.), giving rise to two treatment groups, vehicle-cocaine and Ex-4 (30 μg/kg)-cocaine, and six 20-min fractions were collected. All fractions were assayed immediately after collection using high-performance liquid chromatography (HPLC) with electrochemical detection [22].
2.8 DA measurement
The concentrations of DA were determined by HPLC with electrochemical detection. The column was a Prodigy 3 μ ODS (3) C18 (2 mm × 100 mm, particle size 3 μm, Phenomenex). The mobile phase consisted of 55 mM sodium acetate, 1 mM octanesulfonic acid, 0.1 mM Na2EDTA and 8% Acetonitrile, adjusted to pH 3.2 with 0.1 M acetic acid, and was degassed with an on-line degasser. 20 microliters of the sample was injected, and the flow rate was 0.15 mL/min. The electrochemical detection was accomplished using an amperometric detector (Antec Decade from Antec, Leiden, The Netherlands) with a glassy carbon electrode set at 0.8 V, with an Ag/AgCl reference electrode. The output was recorded by the program “LC solution” (Simatzhu), which was also used to calculate the peak areas. Basal extracellular levels of DA in all groups were calculated as the mean of three fractions collected prior to drug or vehicle administration and were set to 100%. All other values were expressed on a relative scale (mean ± S.E.M.) as a percentage of the basal (control) levels.
2.9 C-fos expression
Mice were pretreated with Ex-4 (30μg/kg, i.p., n=8) or saline (n=16). Ninety minutes later half the mice of the saline pretreated group and all the Ex-4 pretreated mice received an injection of cocaine (30mg/kg, s.c.) and the remaining mice of the saline pretreated group received another injection of saline. Thirty minutes later all mice were quickly decapitated and the brains were frozen on dry ice and stored at −80°C until further processing. The procedure for c-fos in situ hybridization was essentially as described previously [19]. Briefly, coronal sections (15μm) were cut through the striatum (+1.42 to +0.62 mm relative to bregma [21] on a cryostat and mounted on Superfrost™ Plus microscope slides (Menzel, Germany). Sections were hybridized to a synthetic oligonucleotide DNA probe (5′-CGG GCA GTG GCA CGT CTG GAT GCC GGC TGC CTT GCC TTC TCT GAC TGC-3′, DNATechnology, Aarhus, Denmark) that had been end labeled with [α-35S]-dATP (1,250 Ci/mmol, PerkinElmer, Denmark), using terminal deoxynucleotidyl transferase (Roche Diagnostics, Germany). To assess the sequence specificity of the hybridisation signal, a 100-fold excess of unlabeled oligonucleotide was added to the in situ hybridisation buffer which completely abolished the hybridisation signal (data not shown). Slides were exposed to Kodak BioMax MR film (Amersham Biosciences, Sunnyvale, CA) together with [14 C]-microscales and the film was developed 10 days later. For semiquantitative determination of mRNA levels, computer-assisted image analysis was performed (Scion Image; National Institutes of Health) by an observer blinded to treatment. Optical densities (nCi/g) based on calibration curves were obtained using [14C]-microscales. Measurements of optical densities were performed bilaterally in the dorsal striatum, as defined in the area marked in gray in figure 3, in three to four adjacent sections per animal. The striatal area was selected by manual delineation of the structure from its border to the corpus callosum, lateral ventricle, and ∼0.5mm dorsal to the anterior commisure. Background measurements, immediately adjacent to each brain section, were subtracted from each measurement before calculations. Values were averaged for each animal before statistical analysis.
Figure 3.
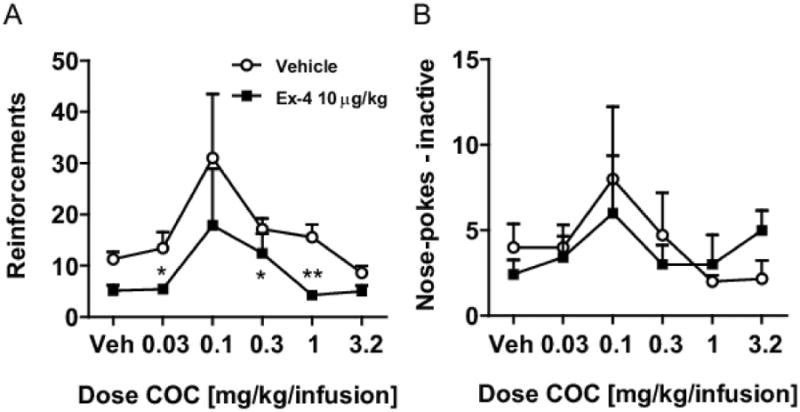
(A) Pre-treatment with Ex-4 at 10μg/kg i.p. significantly decreased the number of reinforcements earned following a chronic cocaine administration setup at cocaine doses of 0.03, 0,32, and 1.0 mg/kg/infusion ** p<0,01, * p<0.05 vs. vehicle i.p.but not (B) inactive responses.
2.10 Data analysis
The data are presented as the means ± standard errors of the means (SEM) in all experiments. Data were analyzed using GraphPad Prism (GraphPad Software, Inc) or SPSS (version 20.0.0). In the acute self-administration experiment, three extreme values, defined as values more than three inter-quartile ranges below the 25th or above 75th percentile, were excluded from analysis: two from the 30 μg/kg Ex-4/0.01 mg/kg/infusion cocaine group and one from the 30 μg/kg Ex-4/0.03 mg/kg/infusion cocaine group. The reported results were all significant before exclusion of these extreme values, and we only excluded them to provide a more representative view of the data. A high variance was observed at effective cocaine doses (0.03, 0.1 mg/kg/infusion) compared with saline and non-effective cocaine doses. The higher variance at effective cocaine self-administration doses was most likely because some animals failed to learn the self-administration paradigm during the single session. To counter this variance in homogeneity, the number of nose pokes was square root transformed before statistical analyses.
All data were examined with one-way or two-way ANOVA followed by Bonferroni-corrected comparisons except for the chronic intravenous cocaine self-administration experiment which was analyzed using non-parametric statistics. The effect of cocaine on active, inactive, and time out nose-poke responses was analyzed by Kruskal-Wallis Analysis of Variance. The effect of exendin-4 pretreatment on the number of active, inactive, and time-out nose-poke responses was assessed by Mann-Whitney U-tests.
A p value <0.05 was considered statistically significant for all experiments.
3. Results
3.1 Exendin-4 inhibits basal and cocaine-induced locomotor activity
First, we measured cocaine-induced hyperactivity, as total beam breaks during the time interval 90-150 minutes, in the vehicle/COC group. This observed increase in cocaine-induced activity was attenuated in the groups pretreated with Ex-4 at doses of 3 and 30 μg/kg, but not at 0.3 μg/kg, although this dose appeared marginally effective as well (One-way ANOVA, F(4,49)=3.65; p<0.05, nvehicle/vehicle=13, nvehicle/COC=14, nEx-4/COC=8-10, Fig. 1b). We also measured basal locomotion as total beam breaks during the first 90 minutes of the test. Ex-4 pretreatment decreased locomotor activity at all doses tested (One-way ANOVA, F(4,80)=4.44; p<0.01, nvehicle=15, nEx-4=15-20, Fig. 1a). Thus, Ex-4 pretreatment decreased the initial novelty-induced locomotion as well as cocaine-induced hyperactivity.
Figure 1.
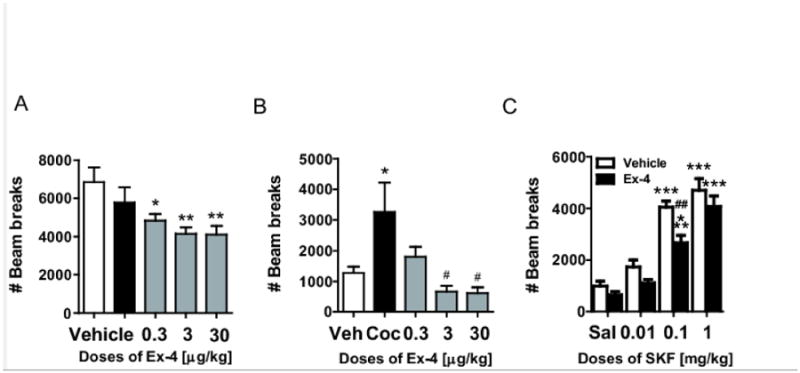
(A) Basal locomotion after saline or Ex-4 treatment. Locomotion was evaluated as the total beam breaks during 90 minutes. ** p<0.01, * p<0.05. vs vehicle. (B) Cocaine-induced hyperactivity after 90 minutes of basal locomotion. Locomotor activity is presented as the total beam breaks during 90-150 minutes, * p<0.05 vs. vehicle, # p<0.05 vs. COC. (C) SKF-82,958-induced hyperactivity after 90 minutes of basal locomotion. Locomotor activity is presented as the total beam breaks during 90-150 minutes, *** p<0.0001 vs. the corresponding saline group, ## p<0.001 vs. the corresponding vehicle group.
3.2 Exendin-4 decreases dopamine D1 receptor agonist-induced locomotor activity
Next, we determined the involvement of the post-synaptic dopamine system in the regulation of locomotion by Ex-4. The dopamine D1 receptor agonist SKF-82,958 induced hyperactivity in both saline and Ex-4 pretreated mice at dosages of 0.1 and 1 mg/kg (p<0.001 vs. the corresponding saline group, Bonferroni post-hoc t-tests after significant one-way ANOVA, F(3,35)=33.7; p<0.0001; F(3,35)=31.3; p<0.0001, respectively; n=8-10, Fig. 1c). Importantly, SKF-82,958-induced hyperactivity was attenuated by Ex-4 pretreatment, particularly at the intermediate dose of 0.1 mg/kg (Bonferroni post-hoc t-test after significant treatment effect in two-way ANOVA, (F(1,64)=13.12, p<0.001, Fig. 1b). Thus, Ex-4 pretreatment regulates dopamine D1 receptor activation of locomotor behavior.
3.3 Exendin-4 inhibits acute self-administration of cocaine in mice
To further determine the ability of Ex-4 to regulate cocaine actions, we first performed acute self-administration experiments. In the first experiment (Fig. 2a), i.p. administration of the two highest doses of Ex-4 (30 and 100 μg/kg) decreased self-administration of the peak effective cocaine dose (see Fig. 2b) in mice (30 μg/kg/infusion; p<0.01, p<0.05, respectively, Bonferroni post-hoc test after significant one-way ANOVA, F(4,61)=4.8, p=0.002, n=14 and 12, respectively). Nose-poking remained elevated at the lowest dose of Ex-4 (0.01 mg/kg), similar to vehicle-pretreated mice receiving cocaine i.v. In the second experiment (Fig. 2b), two-way ANOVA revealed a significant treatment effect of Ex-4 (F(1,114)=11.45, p=0.001) and cocaine (F(4,114)=3.7, p<0.01). Similar to the treatment effect of Ex-4 in the first experiment, the 30 μg/kg Ex-4 dose, used in the second experiment, significantly reduced the nose-poking frequency for i.v.-administered cocaine at the peak effective dose of cocaine (Bonferroni post-hoc test after significant two-way ANOVA, t=2.64, p<0.05) (Fig. 2). Additionally, Ex-4 i.p., in comparison with vehicle, did not significantly decrease nose-poking when saline was present as the i.v. control reinforcer, indicating that Ex-4 affected cocaine-induced responses, not basal responding. Furthermore, in vehicle pretreated mice, cocaine doses of both 0.03 and 0.1 mg/kg/infusion increased self-administration significantly compared to saline i.v. (p<0.01 and p<0.05, respectively, Bonferroni post-hoc test after one-way ANOVA, F(4,62)=3.89, p<0.007), while similar analysis after Ex-4 i.p.-administration revealed no significant changes in self-administration rates compared to saline i.v. (Fig. 2b).
Figure 2.
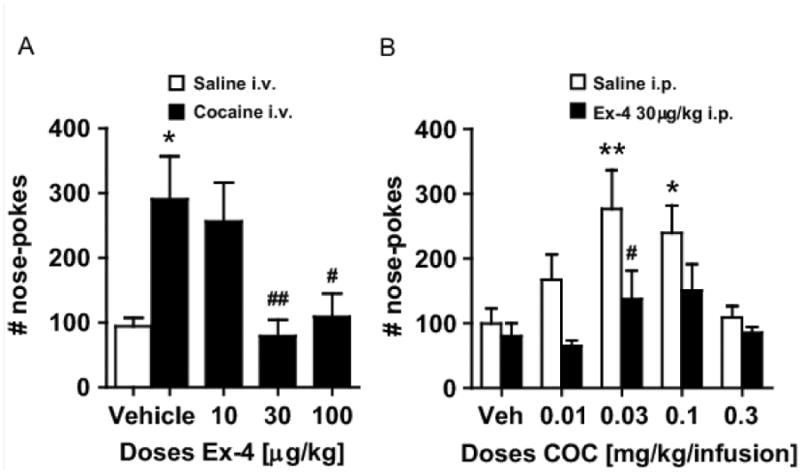
(A) Pre-treatment with Ex-4 at 30 and 100μg/kg i.p. significantly decreased nose-poking for cocaine at the peak effective self-administration dose for cocaine (0.03 mg/kg/infusion). * p<0.05 vs. vehicle i.p./saline i.v.; ## p<0.01, # p<0.05 vs. vehicle i.p./cocaine i.v. (B) Dose-effect curves after acute cocaine self-administration: effects of Ex-4. Cocaine was self-administered i.v. according to an inverted U-shaped curve in NMRI mice. Pre-treatment with Ex-4 at 30μg/kg i.p. shifted cocaine's dose-effect curve downward. ** p<0.01, * p<0.05, vs. the corresponding saline i.v. group; # p<0.05 vs. the corresponding vehicle-pretreated group.
3.4 Exendin-4 inhibits chronic self-administration of cocaine in mice
To further investigate the effects of GLP-1 receptor stimulation on the reinforcing properties of cocaine, we investigated the influence of Ex-4 on chronic self-administration of cocaine. Kruskal-Wallis Analysis of Variance showed that vehicle pretreated animals self-administered cocaine at a significant rate (H5=12.97; p<0.05), while nose-pokes at the inactive hole or during the time-out were not affected by the dose of cocaine available. Pretreatment with 10μg/kg Ex-4 abolished responding for cocaine (H5=7.22; p=0.21) (Fig. 3a). Also in Ex-4 pretreated animals, cocaine had no effect on nose-pokes emitted at the inactive hole (Fig. 3b) or during the time-out. Post-hoc Mann-Whitney U-tests showed that Ex-4 pretreatment inhibited responding at cocaine doses of 0.03 (p<0.05); 0.32; (p<0.05) and 1.0 μg/kg/infusion (p<0.01). Pretreatment with Ex-4 (10μg/kg) also affected nose pokes emitted at the active hole when saline was available (p<0.05), but not nose pokes emitted at the inactive hole or during the time-out for any dose of cocaine available.
3.5 Exendin-4 inhibits cocaine-induced c-fos expression in striatum
We next evaluated the ability of Ex-4 to regulate neuronal activity induced by cocaine. Cocaine, as compared to saline, caused significant increases in c-fos expression in the striatum (p<0.001, Bonferonni post-hoc test following significant one-way ANOVA, F(2,21)=23.46, p<0.001) in saline pretreated NMRI mice (Fig. 4). Pretreatment with Ex-4 fully inhibited the cocaine-induced striatal c-fos expression (Fig. 4).
Figure 4.
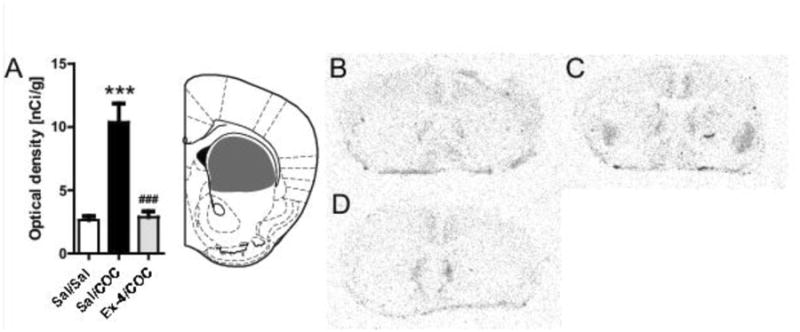
Ex-4 attenuates cocaine-induced c-fos expression in the striatum. (A) Optical density analysis of the autoradiograms (***p<0.0001 vs. saline/saline, ###p<0.001 vs. saline/COC, Bonferonni post-hoc t-tests after significant One-way ANOVA) (B) C-fos expression in striatum determined by radioactive in situ hybridization in brain coronal slices in saline/saline group, (C) saline/COC (D) Ex-4/COC. The grey area in (B) indicates the area measured. Diagram adopted from Franklin and Paxinos [21].
3.6 Exendin-4 attenuates cocaine-induced dopamine increase in the striatum
To probe whether Ex-4 regulation of cocaine behaviors might stem from its ability to reduce cocaine-induced increase in extracellular striatal dopamine, we performed microdialysis experiments. Cocaine induced an increase in striatal dopamine in the saline and Ex-4 pretreated groups (One-way ANOVA, F(11,60)=4.62, and F(11,60)=2.65, p<0.001, and p<0.01, respectively) (Fig. 5). In the saline pretreated group, dopamine levels increased up to 550% after 60 min, after that time, they gradually decreased to 350% of basal at the end of the study. In the Ex-4 pretreated group, the dopamine response to cocaine was less pronounced, with a maximum increase of 300% compared with basal levels, and had returned to basal levels at the end of the experimental time (significant difference from the saline pretreated group at time points 160 and 180 min, Bonferroni post hoc test after significant treatment effect in two-way ANOVA, (F(13,136)=7.15, p=0.001)) (Fig. 5).
Figure 5.
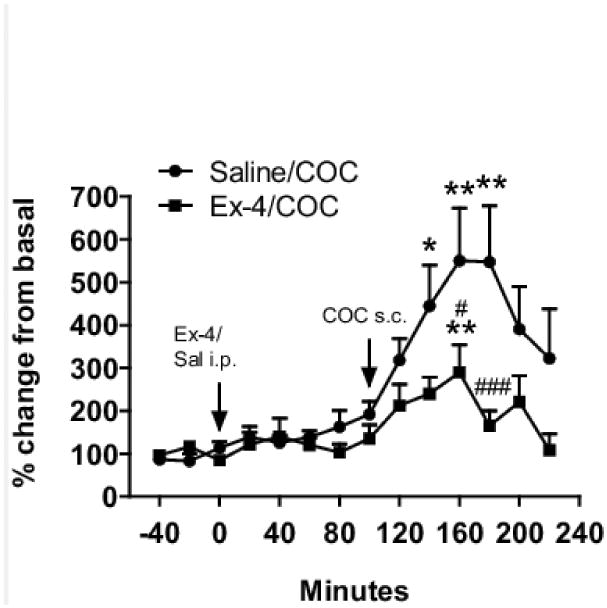
Extracellular striatal dopamine levels in response to cocaine and Ex-4. Cocaine (30 mg/kg, s.c., black circles) significantly increased dopamine levels in mice pretreated with saline. The cocaine-induced dopamine increase was attenuated when mice were pretreated with Ex-4 (30 μg/kg, i.p., black squares). ** p<0.01, * p<0.05 vs. own basal level, Bonferroni post-hoc t-test after significant one-way ANOVA ### p<0.001, # p<0.05 vs. veh-COC, Bonferroni post-hoc t-test after significant two-way ANOVA.
4. Discussion
In the present study, we report for the first time that stimulation of the GLP-1 receptor causes a marked reduction in acute and chronic cocaine self-administration. The GLP-1 analogue Ex-4 used in this study crosses the blood-brain barrier [12,13]. Furthermore, our striatal microdialysis and c-fos data strongly indicate that the observed effects of Ex-4 on cocaine reward are centrally mediated. In accordance with this, it is well known that GLP-1 receptors are expressed in the brain [5], including the midbrain and striatum [6], i.e., nuclei that play a pivotal role in drug reward and that receive direct input from GLP-1 producing neurons in NTS [7]. The anorectic effect of GLP-1 is well-established [9,10], and this effect was observed following both peripheral and intracerebroventricular injections of GLP-1 or GLP-1 analogues [9,10]. Ex-4 inhibits food-rewarded behavior as well as alcohol consumption in rats by mechanisms involving mesolimbic brain structures [14,23]. These data point to a possible role of GLP-1 in behaviors driven by drugs of abuse. Consistent with this, Ex-4 has very recently been shown to attenuate the locomotor response to the psychostimulant d-amphetamine [16] and cocaine-induced place preference [17], as well as alcohol-mediated behavior [18] in rodents. The rodent data on alcohol-mediated behavior is in accordance with a recent clinical report suggesting that the GLP-1 analogue liraglutide reduces alcohol intake in type 2 diabetes patients (Kalra et al., American Diabetes Association Annual Meeting 2011, Poster 1029).
Notably, our data suggest that Ex-4 reduced the efficacy of cocaine as a reinforcer, rather than reducing the potency of cocaine, in which case a rightward shift of the dose-effect curve would be observed [24]. Indeed, the dose-response curve for acute cocaine self-administration was shifted down, without a leftward or rightward shift, in animals pretreated with Ex-4, 30μg/kg. This profile suggests that the effect of GLP-1 receptor stimulation cannot be overcome by administration of higher doses of cocaine, supporting the potential utility of GLP-1 receptor stimulation in the management of the reinforcing properties of cocaine. The reduction in cocaine self-administration in our experiments cannot be explained by non-specific rate-decreasing effects. This is because Ex-4 did not significantly affect nose-poking for vehicle. Importantly, we also investigated the effects of GLP-1 receptor stimulation on the chronic reinforcing properties of cocaine at an even lower dose of Ex-4 (10μg/kg).
Parallel to the acute setting, in the chronic experiment Ex-4 attenuated cocaine self-administration. Since chronic cocaine self-administration represents a valuable animal model for addiction, these data strongly point to GLP-1 receptor signaling as a potential target for treating psychostimulant abuse. In this experiment, Ex-4 affected nose pokes emitted at the active hole when saline was available. However, Ex-4 did not affect nose pokes emitted at the inactive hole or during the time-out for any dose of cocaine available. Therefore these data indicate that the effect of Ex-4 on chronic cocaine self-administration cannot be solely due to non-specific rate lowering effects.
Using the lowest effective dose used in the acute self-administration experiment, we analyzed c-fos expression and striatal dopamine levels after cocaine injections with or without pre-treatment of Ex-4. An increase in extracellular dopamine levels in striatal areas is central to the reinforcing effects of cocaine and other drugs of abuse [2,25]. Using microdialysis, we found that the cocaine-induced striatal dopamine increase was significantly reduced by pretreatment with Ex-4, indicating that the inhibitory effects of GLP-1 receptor stimulation on cocaine self-administration could stem from the suppression of the cocaine effects on striatal dopamine levels. These results are in line with previous studies, demonstrating Ex-4 mediated attenuation of amphetamine-, cocaine-, and alcohol-induced dopamine increase in dialysate samples from accumbens in mice [18,26].
It has previously been shown that an intra-accumbal injection of GLP-1 increases the amount of c-fos-positive nuclei in rats [27]. In this study, we demonstrate the effect of a systemically administered GLP-1 receptor agonist on cocaine-induced c-fos labeling using in situ hybridization. Our data show that Ex-4 fully blocks the dorsal striatal c-fos response to cocaine. In accordance with our present self-administration data, microdialysis data and c-fos data, Ex-4 also inhibits cocaine-induced hyperlocomotion. The GLP-1 receptor agonist itself modestly inhibits basal locomotor activity. This is different from our acute cocaine self-administration data, where Ex-4 did not influence basal nose poking. The reason for this result is not clear, but may be because nose poking and locomotion are per se different types of behavior even though both are exploratory by nature.
The effects of GLP-1 receptor stimulation on cocaine-induced behaviors could also be mediated by more downstream neuronal structures, e.g., the medium spiny GABAergic output neurons, which receive direct input from midbrain dopaminergic neurons and project to the globus pallidus/ventral pallium and substantia nigra/VTA [28]. Most of the medium spiny GABAergic output neurons, which project directly to the VTA/substantia nigra in the so-called “direct pathway”, contain dopamine D1 receptors, and dopamine D1 receptors play a key role in mediating the hyperlocomotor effects of psychostimulants [29]. Earlier studies have also demonstrated that D1 receptor-mediated activation of the Gs/cAMP/PKA signaling cascade and subsequent downstream signaling events are critically involved in the manifestation of these psychostimulant-induced behavioral effects [30]. Here, we show that Ex-4 inhibits dopamine D1 receptor-mediated hyperlocomotion. This suggests that Ex-4 potentially exerts its effect both via regulating D1 receptor signaling pathways and by affecting cocaine-induced striatal dopamine release.
In conclusion, we have shown that the GLP-1 receptor agonist Ex-4 can attenuate cocaine self-administration, cocaine-induced dopamine release and c-fos expression and cocaine-induced hyperlocomotion. Our study indicates that the GLP-1 receptor is a potential target for the future treatment of psychostimulant abuse.
Highlights.
GLP-1 receptor stimulation reduces acute and chronic cocaine self-administration.
Exendin-b4 reduces cocaine-induced elevation of striatal DA and c-fos expression.
GLP-1 receptor stimulation decreases DA D1 receptor agonist induced hyperactivity.
Acknowledgments
The Ivan Nielsen Foundation and the Lundbeck Foundation supported the present work. We thank Birgit Hansen for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF. Drugs of Abuse - Anatomy, Pharmacology and Function of Reward Pathways. Trends Pharmacol Sci. 1992;13:177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 3.Novak U, Wilks A, Buell G, McEwen S. Indetical mRNA for preproglucagon in pancreas and gut. Eur J Biochem. 1987;164:553–8. doi: 10.1111/j.1432-1033.1987.tb11162.x. [DOI] [PubMed] [Google Scholar]
- 4.Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665–70. doi: 10.3109/00365529609009147. [DOI] [PubMed] [Google Scholar]
- 5.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: Evidence that Exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 6.Merchenthaler I, Lane M, Shrughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–58. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holst JJ, Deacon CF, Vilsbøl T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161–8. doi: 10.1016/j.molmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Tang-Christensen M, Larsen PL, Göke R, Fink-Jensen A, Jessup DS, Møller M, et al. Central administration of GLP-1 (7-36) amide inhibits food and water intake in rats. J Am Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 10.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 11.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes. 2003;27:313–8. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 13.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–12. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, Exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–20. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy IA, Stanwood GD, Galli A. Moving beyond energy homeostasis: New roles for glucagon-like peptide-1 in food and drug reward. Neurochem Int. 2013;73:49–55. doi: 10.1016/j.neuint.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A. Exendin-4 decreases amphetamine-induced locomotor activity. Physiol Behav. 2012;106:574–8. doi: 10.1016/j.physbeh.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry. 2012;18(9):961–2. doi: 10.1038/mp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egecioglu E, Steensland P, Freriksson I, Feltmann K, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013;38(8):1259–70. doi: 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen G, Jensen M, Weikop P, Dencker D, Christiansen SH, Løland CJ, et al. Neuropeptide Y Y5 receptor antagonism attenuates addiction-related effects of cocaine in mice. Psychopharmacology. 2012;222:565–77. doi: 10.1007/s00213-012-2651-y. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DPD, et al. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology. 2011;216:367–78. doi: 10.1007/s00213-011-2225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paxinos G, Franklin KJB. The mouse brain in stereotaxic coordinates. Academic; New York: 2001. [Google Scholar]
- 22.Weikop P, Egestad C, Kehr J. Application of triple-probe microdialysis for fast pharmacokinetic/pharmacodynamic evaluation of dopaminergic activity of drug candidates in the rat brain. J Neurosci Meth. 2004;140:59–65. doi: 10.1016/j.jneumeth.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Shirazi RS, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. Plos One. 2013;8:e61965. doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mello NS, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacol. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 25.Wise RA. Roles for nigrostriatal – not just mesocorticolimbic – dopamine in reward and addiction. Trends Neurosci. 2009;32:517–24. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, Exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. Plos One. 2013;8:e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dossat AM, Lilly N, Kay K, Williams DJ. Glucagon-Like Peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 29.Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 30.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–17. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]


