Abstract
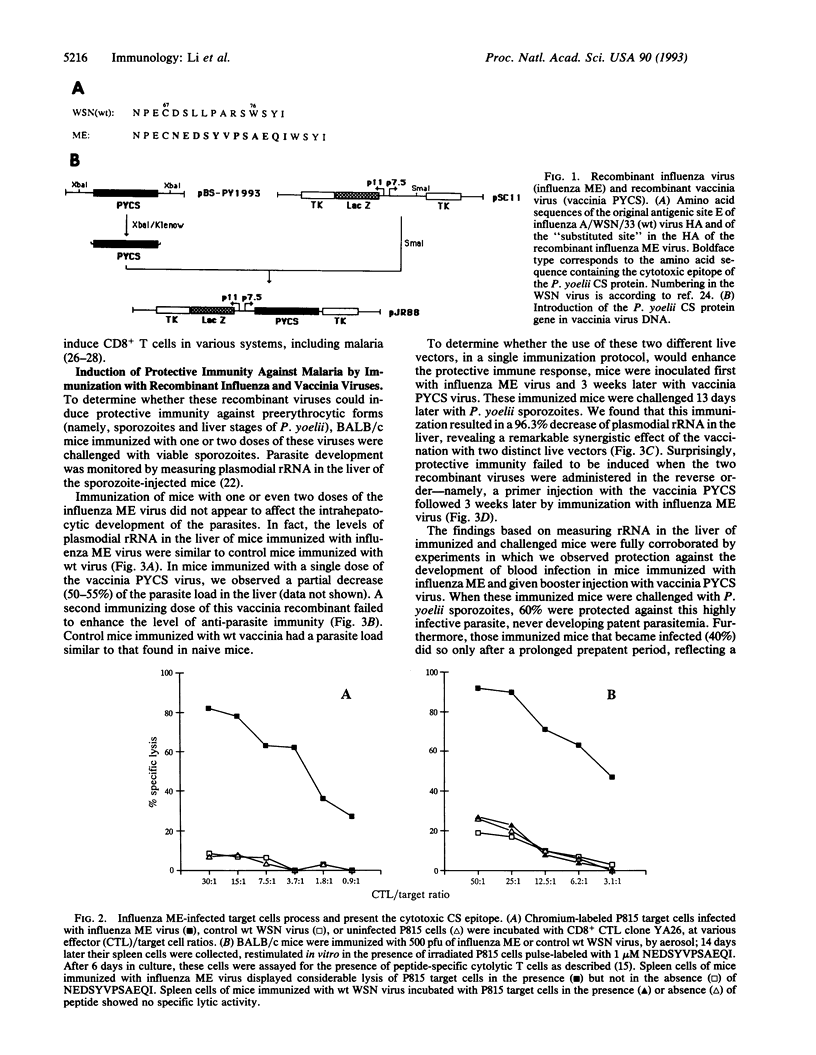
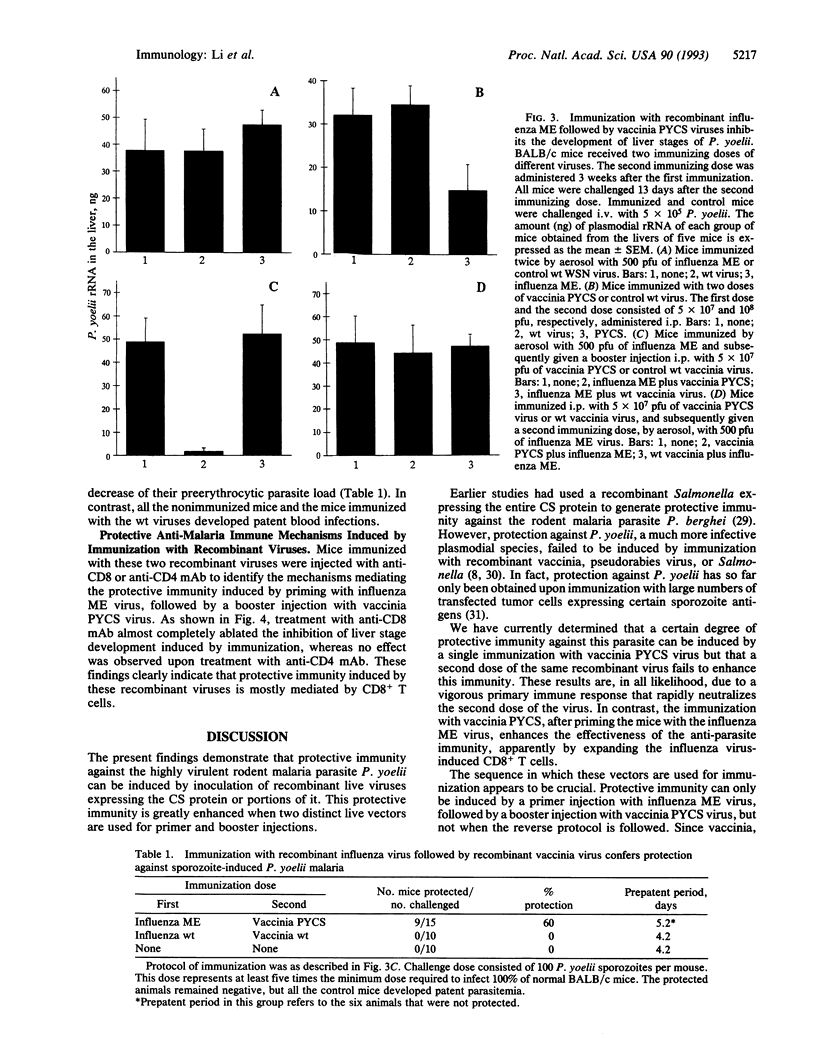
Live vectors expressing foreign antigens have been used to induce immunity against several pathogens. However, for the virulent rodent malaria parasite Plasmodium yoelii, the use of recombinant vaccinia virus, pseudorabies virus, or Salmonella, expressing the circumsporozoite protein of this parasite, failed to induce protection. We generated a recombinant influenza virus expressing an epitope from the circumsporozoite protein of P. yoelii known to be recognized by CD8+ T cells and demonstrated that this vector induced class I major histocompatibility complex-restricted cytotoxic T cells against this foreign epitope. Immunization of mice with this recombinant influenza virus, followed by a recombinant vaccinia virus expressing the entire circumsporozoite protein, induced protective immunity against sporozoite-induced malaria. The sequence of immunization appears to be crucial, since a primer injection with recombinant vaccinia virus, followed by a booster injection with recombinant influenza virus, failed to induce protection. The protection induced by immunization with these recombinant viruses is mostly mediated by CD8+ T cells, as treatment of mice with anti-CD8 monoclonal antibody abolishes the anti-malarial immunity. The use of different live vectors for primer and booster injections has a synergistic effect on the immune response and might represent an effective general strategy for eliciting protective immune responses to key antigens of microbial pathogens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arreaza G., Corredor V., Zavala F. Plasmodium yoelii: quantification of the exoerythrocytic stages based on the use of ribosomal RNA probes. Exp Parasitol. 1991 Jan;72(1):103–105. doi: 10.1016/0014-4894(91)90127-i. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moller C., Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984 Oct 11;311(5986):578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenvit Y., Mellouk S., Cole C., Bechara R., Leef M. F., Sedegah M., Yuan L. F., Robey F. A., Beaudoin R. L., Hoffman S. L. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J Immunol. 1991 Feb 1;146(3):1020–1025. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Grillot D., Rénia L., Müller I., Corradin G., Louis J. A., Mazier D., Lambert P. H. Peptide-primed CD4+ cells and malaria sporozoites. Immunol Lett. 1990 Aug;25(1-3):59–63. doi: 10.1016/0165-2478(90)90092-5. [DOI] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Hodges W. M., Hruby D. E. Protection against streptococcal pharyngeal colonization with a vaccinia: M protein recombinant. Science. 1989 Jun 23;244(4911):1487–1490. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Davis A. R., Nayak D. P. Complete sequence analysis shows that the hemagglutinins of the H0 and H2 subtypes of human influenza virus are closely related. Virology. 1981 May;111(1):113–124. doi: 10.1016/0042-6822(81)90658-9. [DOI] [PubMed] [Google Scholar]
- Jonjić S., del Val M., Keil G. M., Reddehase M. J., Koszinowski U. H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988 May;62(5):1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Charoenvit Y., Kumar S., Sedegah M., Beaudoin R. L., Hoffman S. L. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991 May 3;252(5006):715–718. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Drillien R., Spehner D., Skory S., Schmitt D., Wiktor T., Koprowski H., Lecocq J. P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Li S. Q., Schulman J. L., Moran T., Bona C., Palese P. Influenza A virus transfectants with chimeric hemagglutinins containing epitopes from different subtypes. J Virol. 1992 Jan;66(1):399–404. doi: 10.1128/jvi.66.1.399-404.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Pye D., Edwards S. J., Anders R. F., O'Brien C. M., Franchina P., Corcoran L. N., Monger C., Peterson M. G., Vandenberg K. L., Smythe J. A. Failure of recombinant vaccinia viruses expressing Plasmodium falciparum antigens to protect Saimiri monkeys against malaria. Infect Immun. 1991 Jul;59(7):2403–2411. doi: 10.1128/iai.59.7.2403-2411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. M., Cordey A. S., Arreaza G., Corradin G., Romero P., Maryanski J. L., Nussenzweig R. S., Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991 Jun;3(6):579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Nussenzweig R. S., Romero P., Zavala F. The in vivo cytotoxic activity of CD8+ T cell clones correlates with their levels of expression of adhesion molecules. J Exp Med. 1992 Apr 1;175(4):895–905. doi: 10.1084/jem.175.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D., Rodriguez J. R., Rodriguez J. F., Trauber D., Esteban M. Highly attenuated vaccinia virus mutants for the generation of safe recombinant viruses. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1287–1291. doi: 10.1073/pnas.86.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D., Zhou Y. W., Rodriguez J. R., Durbin R. K., Jimenez V., McAllister W. T., Esteban M. Regulated expression of nuclear genes by T3 RNA polymerase and lac repressor, using recombinant vaccinia virus vectors. J Virol. 1990 Oct;64(10):4851–4857. doi: 10.1128/jvi.64.10.4851-4857.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P., Maryanski J. L., Cordey A. S., Corradin G., Nussenzweig R. S., Zavala F. Isolation and characterization of protective cytolytic T cells in a rodent malaria model system. Immunol Lett. 1990 Aug;25(1-3):27–31. doi: 10.1016/0165-2478(90)90086-6. [DOI] [PubMed] [Google Scholar]
- Romero P., Maryanski J. L., Corradin G., Nussenzweig R. S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989 Sep 28;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- Sadoff J. C., Ballou W. R., Baron L. S., Majarian W. R., Brey R. N., Hockmeyer W. T., Young J. F., Cryz S. J., Ou J., Lowell G. H. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988 Apr 15;240(4850):336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Sedegah M., Beaudoin R. L., Majarian W. R., Cochran M. D., Chiang C. H., Sadoff J., Aggarwal A., Charoenvit Y., Hoffman S. L. Evaluation of vaccines designed to induce protective cellular immunity against the Plasmodium yoelii circumsporozoite protein: vaccinia, pseudorabies, and Salmonella transformed with circumsporozoite gene. Bull World Health Organ. 1990;68 (Suppl):109–114. [PMC free article] [PubMed] [Google Scholar]
- Sedegah M., Chiang C. H., Weiss W. R., Mellouk S., Cochran M. D., Houghten R. A., Beaudoin R. L., Smith D., Hoffman S. L. Recombinant pseudorabies virus carrying a plasmodium gene: herpesvirus as a new live viral vector for inducing T- and B-cell immunity. Vaccine. 1992;10(9):578–584. doi: 10.1016/0264-410x(92)90436-n. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. R., Mellouk S., Houghten R. A., Sedegah M., Kumar S., Good M. F., Berzofsky J. A., Miller L. H., Hoffman S. L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990 Mar 1;171(3):763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]



