Abstract
Early equine pregnancy shares many features with that of more intensively assessed domestic animals species, but there are also characteristic differences. Some of those are poorly understood. Descent of the equine conceptus into the uterine lumen occurs at day 5 to 6 after ovulation but is only possible when the embryo secretes prostaglandin E2. Although maintenance of equine pregnancy probably involves secretion of a conceptus derived anti-luteolytic factor, this agent has not been identified. Rapid growth, conceptus mobility and presence of an acellular capsule at the time of maternal recognition of pregnancy, i.e. between days 12 and 14, are prerequisites to avoid pregnancy loss. Progesterone together with 5α-pregnanes is secreted by the corpus luteum and induces the production of endometrial histotroph which is responsible for conceptus nutrition until placention. A stable contact between the outer trophoblast layer of the allantochorion and the luminal epithelium of the endometrium is not established before days 40 to 42 of pregnancy.
Keywords: Conceptus, Horse, Maternal recognition, Pregnancy
Introduction
In mammals, maintenance of pregnancy depends on the continuous production of progesterone. The rule is that extension of corpus luteum lifespan beyond the length of one physiological estrous cycle requires either a luteotrophic (e.g. as in humans) or an anti-luteolytic (e.g. as in ruminants or pigs) factor which is produced by the conceptus. Although presumed by many authors [1–5] that maintenance of pregnancy in the horse will involve secretion of an anti-luteolytic factor by the conceptus, this agent has so far not been identified. Knowledge on early pregnancy in the horse thus lacks an important component. In other species the anti-luteolytic factor does not only inhibit luteolysis but is also involved in modulation of endometrial functions in preparation to pregnancy [6]. In contrast to other domestic animals, some horse-specific limitations challenge the research on early pregnancy: access to experimental animals or genital organs is mostly limited in a species where meat consumption is not common in many countries and thus slaughterhouse material is almost not readily available. Superovulatory treatment with the aim to produce multiple conceptuses is difficult in the horse, i.e. mares cannot be superovulated to a meaningful extent [7]. At present no efficient superovulatory drug for the horse is available. Furthermore, late entry of the conceptus into the uterus and limited success of in vitro produced embryos makes research on early stages of pregnancy difficult in this species. The knowledge on early equine pregnancy in some aspects is therefore quite rudimentary in comparison to other domestic animal species. Nevertheless, many mechanisms and features – some of them quite unique among domestic animals - have been well characterized. Ongoing research may eventually lead to the solution to the riddle of maternal recognition of pregnancy in the horse. In this review, the present knowledge is critically summarized.
Current knowledge on maternal recognition of pregnancy in the horse
The horse is a seasonal breeding species with reproductive activity being associated with long days, i.e. occurring in spring and early summer. During the breeding season, cycle length is about 22 days with 5 to 7 days of oestrus. Functional luteolysis occurs on day 15 after ovulation [8]. Initiation of the luteolytic cascade in the horse was for a long time suggested to happen on day 10 after ovulation or even earlier [9, 10]. More recent research demonstrated successful transfer of day 10 embryos to mares that were either on day 10 or day 12 after ovulation. This proves that the luteolytic cascade in the non-pregnant mare is not initiated before day 12 after ovulation [11]. It can thus be concluded that the anti-luteolytic mechanism of the equine conceptus has to be active between days 12 and 14 after ovulation.
In the non-pregnant mare, luteolysis is initiated by endometrial secretion of prostaglandin (PGF)2α. On day 15 of the oestrous cycle, expression of cyclooxygenase 2 (COX2) by uterine epithelial cells of non-pregnant mares is markedly increased while it is inhibited in pregnant mares. Regulation of endometrial expression of COX2 is therefore considered a key event in either induction of luteolysis or maternal recognition of pregnancy in the horse [12, 13]. In agreement with the situation in other species, endometrial PGF2α release is stimulated by oxytocin [13]. In the mare, there is no significant synthesis of luteal oxytocin, but oxytocin has been localized in the endometrium [14]. However, administration of exogenous oxytocin was unable to induce endometrial PGF2α release during early pregnancy despite increased expression of endometrial oxytocin receptors. Therefore, a paracrine-autocrine system involving endometrial oxytocin and PGF2α most likely accelerates luteolysis in the non-pregnant mare [8].
For the horse conceptus, the signal or mechanism that inhibits luteolysis has not been identified. Unlike the ruminant conceptus, the equine conceptus does not produce interferons that inhibit endometrial PGF2α release [15]. The fact that equine conceptuses produce estrogens in high amounts from day 10 of pregnancy onwards [16] has stimulated research on estrogens as a potential anti-luteolytic agent in this species. This hypothesis could not be supported because estrogens – when provided at physiological concentrations - did not extend corpus luteum lifespan in horse mares [1, 4, 5]. The nature and origin of the antiluteolytic signal in the horse conceptus thus differs from domestic ruminants and pigs. In 1989, Sharp et al. [2] published evidence that the antiluteolytic agent secreted by the equine conceptus has a molecular weight between 1,000 and 6,000. However, molecules fitting into this molecular mass like PGE2 or insulin failed to prolong lifespan of the corpus luteum in cyclic mares when infused into the uterine lumen [5, 17]. Development of an endometrial explant in vitro culture system appeared promising to support further research for identification and characterization of the equine conceptus factor responsible for maternal recognition of pregnancy [13]. Unfortunately further relevant results have not been published.
Before and on day 14 of pregnancy, the equine yolk sac produces a characteristic pattern of proteins that completely changes thereafter. It was suggested that one or more of these proteins might be involved in the anti-luteolytic mechanism of the horse conceptus [3], but this has never been proven. The switch in protein expression by the yolk sac around day 14 is most likely associated with development of the mesoderm with its blood-forming islets [3, 18]. Uterocalin which has mainly received consideration as an endometrial protein (see below) is also expressed in conceptus tissue with decreasing expression between days 8 and 14 of pregnancy [19].
Persistence of the corpus luteum is also seen in a certain percentage of non-pregnant mares after introduction of a glass marble [20] or fluid-filled rubber ball [21] into the uterine lumen during the first days after ovulation. The presence of a spherical intrauterine device has thus been suggested to resemble the presence of a conceptus by exerting contact or pressure directly on the uterine wall [21]. This may induce changes in the endometrial epithelia similar to those induced by presence of a conceptus. Interestingly, the effect seems to depend on adequate perfusion and drainage of the endometrium and is less effective in aged mares [22]. These results suggest that the embryonic signal for maternal recognition of pregnancy in the horse might be at least in part mechanical rather than secretory in origin. This assumption was further supported by modulation of prostaglandin production and prolonged corpus luteum lifespan reported after intrauterine administration of different plant oils into the uterine lumen of luteal phase mares [23]. The authors could not exclude the possibility that physical interference with the endometrium was involved in this phenomenon. However, in contradiction to this hypothesis, intrauterine administration of mineral oil did not prevent luteolysis.
Sources of progestin during equine pregnancy
In domestic animal species, pregnancy is maintained by secretion of progesterone from the corpus luteum, the placenta or a combination of both. The situation is more complicated in pregnant mares where not only different sources for progestin secretion exist, but also a variety of progestins as well as estrogens are secreted [24, 25]. From ovulation until approximately day 40 of pregnancy, progestins and estrogens are solely secreted from the primary corpus luteum [26–29]. Besides progesterone the progestins 5α-pregnane-3,20-dione and 3β-hydroxy-5α-pregnan-20-one are detectable in the circulation [30]. Progestin concentrations in blood of mares increase rapidly after ovulation and peak around day 5 of pregnancy. From then onwards concentrations in maternal plasma gradually decline suggesting only a weak luteotrophic signal in early pregnant mares [31]. A second increase in concentration of progestin in maternal plasma around day 40 of pregnancy is based on the formation of secondary corpora lutea. Their formation is initiated by secretion of equine chorionic gonadotropin (eCG) from the endometrial cups from day 37 after ovulation [32]. A further support of pregnancy arises with the start of placental steroid synthesis around day 60 of gestation. Placental steroids again consist of different progestins, mainly 5α-pregnanes. From this time, circulating concentrations of progestin in the pregnant mare are considered a mixture of luteal and placental progestins until the feto-placental unit becomes the only source of progestins from day 160 of gestation onwards [30], when the function of the primary corpus luteum and secondary corpora lutea ceases [33].
Development of the early equine conceptus
In the horse, fertilization rate following natural service is greater than 90 % [34]. First cleavage of the fertilized equine oocyte occurs approximately 24 h after fertilization, subsequent divisions of the blastomeres follow at 12- to 24-h intervals [35]. Morphological reorganization of the nucleolus coinciding with activation of embryonic transcription takes place at the 6- to 8-cell stage, i.e. at the fourth embryonic cell cycle [36]. The early equine zygote is characterized by marked asymmetry in the distribution of cellular organelles and inclusions. This is suggested to contribute to the more ellipsoidal shape of the early equine embryo [37]. At the 8- to 16-cell stage, tight junctions between individual blastomeres are formed, causing aggregation and subsequent compaction of the cells. Thereafter, individual blastomeres cannot longer be identified, continuous cell division and tight junction formation leads to formation of a compact morula which consists of at least 32 blastomeres [38]. In the horse, the compact morula is the latest developmental stage found in the oviduct [39, 40]. It will develop into a blastocyst (Fig. 1) after entering the uterine lumen at approximately 6 days after ovulation. In the horse, transport of the embryo from the oviduct into the uterine lumen is selective and depends on the release of prostaglandin E2 by the conceptus shortly before the time of entering the uterus, i.e. on days 5 and 6 after ovulation [41, 42]. While segregation of the inner cell mass from the trophoblast at the time of blastocyst formation in ruminant and pig conceptuses is rapid and distinct, the cells of the inner cell mass in horse blastocysts remain much more dispersed. Differentiation between morulae and early blastocysts may therefore be difficult [37]. Already at the time of blastocyst formation, conceptus size is highly variable [43, 44]. It is influenced not only by day of pregnancy, but also by factors such as age of mare, the method of processing the semen used for breeding and number of ovulations per estrus [44–46]. Despite the fact that horses are seasonal breeders, conception rate as well as conceptus quality and growth is not impaired in mares that are spontaneously cyclic during the non-breeding season [46].
Fig. 1.
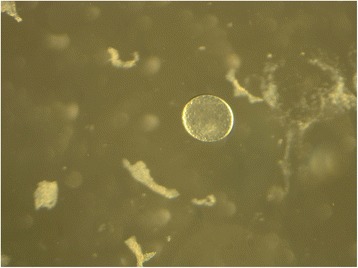
Blastocyst collected from the uterus of a mare on day 7 after ovulation. The zona pellucida is clearly visible
In contrast to ruminants and pigs, the increase in size of the equine conceptus is initially caused mainly by water influx and only to a minor degree by multiplication of cells [38]. During blastocyst expansion the formation of an osmotic gradient by α1/β1 Na+/K + −ATPase is the driving force of water influx into the blastocoel of equine embryos [47, 48]. After completion of endoderm formation around day 8, the blastocoel is termed yolk sac. Starting around day 10, osmolarity of the yolk sac fluid decreases. The yolk sac fluid is markedly hypotonic until about day 18 when osmolarity gradually increases [37]. Hypoosmolarity within the yolk sac seems to contradict the hypothesis of a Na+/K+ trans-trophoblast gradient responsible for blastocoel expansion before day 8 [49]. The control of equine yolk sac expansion is most probably mediated by changes in the permeability of the apical ectodermal membrane to water through differences in the abundance of aquaporin (AQP) 5. Vasopressin in the yolk sac could participate in the regulation of AQP5 function in a similar manner as in kidney collecting ducts [50, 51]. Subtrophoblastic compartments described in equine blastocysts seem to undergo a sharp increase in tonicity relative to the interior of the yolk sac, forming a third compartment which might be responsible for maintenance of the ion gradient in the equine conceptus larger than 6 mm in diameter [52].
The horse conceptus remains spherical much longer than the ruminant or pig conceptus which loses the spherical shape soon after hatching from the zona pellucida. From day 6 until approximately day 23 of pregnancy, the horse conceptus is surrounded by an acellular mucin-like glycoprotein capsule (Fig. 2) [38, 53–55]. Expansion of the capsule facilitates shedding of the zona pellucida. The capsule continues the protective function of the zona pellucida and is thus considered essential to the continuation of pregnancy [56]. Transfer of embryos into synchronous recipient mares after removal of the capsule dramatically impairs pregnancy rates [55]. The progestin-dependent endometrial protein uterocalin functionally correlates with capsule formation and persistence [57] which is in agreement with the finding that in vitro produced horse embryos fail to form a normal acellular capsule [58]. Nevertheless, addition of uterocalin to the culture media did not result in physiologic formation of a capsule in in vitro produced equine embryos [59]. Therefore, contact with the complex uterine environment seems to be essential for capsule formation.
Fig. 2.
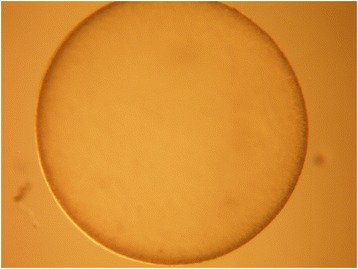
Horse conceptus collected from the uterus of a mare on day 7 after ovulation with the acellular capsule being clearly visible
Between days 10 and 15, i.e. at the time of maternal recognition of pregnancy, the equine embryo moves constantly through the uterine cavity (Figs. 3 and 4). This feature is suggested to compensate for the relatively small trophoblast surface area in this species [60, 61]. Restriction of conceptus mobility to only part of the uterine lumen results in failure of pregnancy in the horse [62]. Embryonic mobility depends on local peristaltic contractions of the myometrium that are most likely induced by prostaglandins synthesized and secreted from the conceptus itself [63–65]. Besides mobility, adequate size of the conceptus is a prerequisite for maternal recognition of pregnancy [60, 61] while retarded growth and inappropriate development are considered major reasons for early pregnancy loss in mares [66, 67, 68, 69, 76]. However, it has to be considered that active migration of the spherical blastocyst also occurs in ruminant and pig embryos after hatching and before development into tubular and then filamentous forms [70] and is therefore not completely unique to the equine species.
Fig. 3.
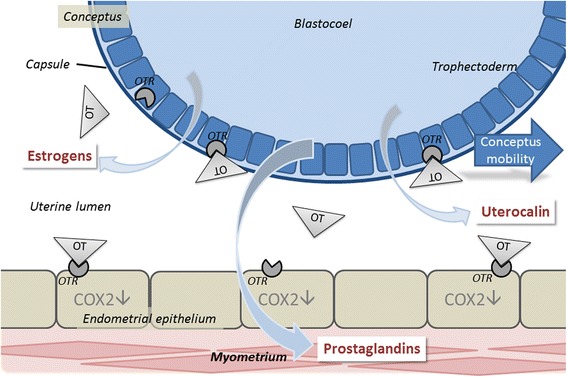
Schematic representation of interactions between the conceptus and the uterus as currently proposed at the time of maternal recognition of pregnancy on days 12/13 after ovulation: The conceptus propels through the uterine lumen dependent on the action of conceptus-derived prostaglandins on the myometrium. In addition, the conceptus secretes estrogens and arginine into the uterine lumen. Endometrial oxytocin (OT) stimulates conceptus growth by action on OT receptors (OTR) in the trophectoderm. Due to down-regulation of cyclooxygenase 2 (COX2) in the endometrial epithelium, endometrial oxytocin cannot stimulate endometrial synthesis of prostaglandin F2α, therefore corpus luteum function is maintained
Fig. 4.
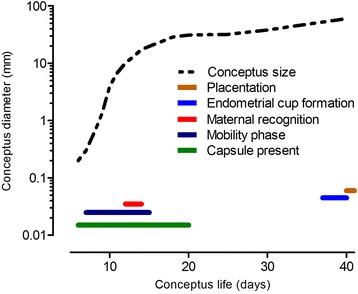
Mean conceptus diameter (mm) of the horse conceptus between days 7 and 40 after ovulation and time of some significant events that are involved in the establishment of equine pregnancy
In horses, the yolk sac is suggested to be an important source of nutrition for the conceptus during the first 3 to 4 weeks of pregnancy [71]. It thus persists beyond developmental stages when it becomes nonfunctional in conceptuses of most domestic animals. As a morphological structure, the yolk sac is often recognized at parturition of the foal. Architecture of the conceptus at the time of fixation, i.e. around day 16 of pregnancy, is believed to play an important role in its orientation within the uterine lumen [71]. Blister-like structures formed between ectoderm and mesoderm in the trilaminar part of day 14 and day 16 conceptuses may be involved [18].
Endometrial function in the mare during early conceptus development
In all mammals, establishment and maintenance of pregnancy depends on the presence of progesterone. In the mare, the presence of progesterone is a prerequisite for conceptus mobility, fixation at the basis of one uterine horn and orientation within in the uterus [72]. Expression of progesterone receptors in the trophoblast may allow for direct effects of progesterone on the conceptus [73, 74]. However, the main task of progesterone is the preparation of the endometrium for pregnancy. Paradoxically, this requires down-regulation of progesterone receptors in endometrial epithelia as a prerequisite for the expression of pregnancy-associated proteins [75]. Mares have a similar pattern of endometrial progesterone receptors during early pregnancy as other mammals. Progesterone receptors are absent in the endometrial epithelia from day 20 of pregnancy, but remain abundant in stromal cells [76]. Treatment of mares with a synthetic progestin from day 5 after ovulation resulted in enhanced downregulation of epithelial endometrial progesterone receptors already on day 11 after ovulation [74]. In cows, a positive relationship between concentrations of progesterone in maternal plasma and development of the embryo has been demonstrated. High concentrations of progesterone in the early postovulatory phases of the estrous cycle stimulate a stronger antiluteolytic signal [77, 78].
In many domestic animals, rodents and primates, the trophectoderm of the conceptus produces interferons (IFN) during the peri-implantation period. IFNτ (IFNT) is unique to ruminants and has been identified as their conceptus’ signal for maternal recognition of pregnancy. Besides, IFNs are involved in the regulation of uterine receptivity, decidualization, as well as placental growth and development. They induce the expression of IFN-stimulated genes in the uterus in a temporal and cell-specific manner [70]. IFNδ (IFND) has been demonstrated not only in pigs [79], but also in horses [80]. In this species, two IFND genes have been identified and are expressed between days 16 and 22 of pregnancy. This suggests involvement of IFND in conceptus-maternal interactions in the horse, but the expression occurs beyond the time of maternal recognition of pregnancy.
Duration of the pre-implantation period varies considerably among species, but is prolonged in the horse. The outer trophoblast layer of the allantochorion finally establishes a stable, microvillous contact with the luminal epithelium of the endometrium around days 40 to 42 and placentation commences thereafter [81]. Before placentation, the equine conceptus is completely dependent on nutritional support by histotroph secreted from the luminal epithelium and the endometrial glands [82]. Histotroph is produced in all mammalian uteri and consists of a complex mixture of proteins and molecules. Its production depends on progesterone action and – in sheep has been demonstrated to be IFNT-stimulated [6, 70]. At the blastocyst stage, the energy substrate for mammalian conceptuses switches from pyruvate to glucose. In sheep, concentrations of glucose and the amino acids arginine, leucine and glutamine increase in the uterine lumen between days 10 and 15 of pregnancy. This is paralleled by increased expression of specific transporters of those nutrients in the uterine epithelia. These changes are indispensable for the survival and development of the conceptus [6]. The same level of knowledge does not exist for the horse so far. However, changes at the mRNA level of the maternal endometrium during equine early pregnancy have been investigated utilizing microarray techniques. Pronounced changes occurred around the time of recognition of pregnancy. A high proportion of genes with altered transcription is regulated by estrogens, progesterone or PGE2. It is thus feasible that in the mare changes in mRNA abundance are also directly related to maternal progesterone secretion and/or conceptus-derived factors such as estrogens or PGE2. Because several of the affected genes are also involved in regulation of early gestation in species other than the horse it is suggested that a subset of genes crucial to endometrial receptivity is highly conserved among species [83, 84]. The importance of progesterone for histotroph production and maintenance of pregnancy in the horse has been long emphasized (reviewed by Sharp 2000). Similar to ruminants, a pronounced rise in progestins during the early post-ovulatory phase in pregnant mares contributes to improved development of the conceptus [45, 85] while deprivation of progesterone due to luteolysis leads to immediate changes in endometrial protein secretion [86]. Among others retinol binding protein [87], uteroferrin [88], uterocalin [82] and SLC36A2 (solute carrier family 36 (proton/amino acid symporter), member 2) [83] have been suggested to be of significance for maintenance of early pregnancy in the horse. Uterocalin has received specific interest because it has been suggested to facilitate lipid transport across the acellular embryonic capsule [82]. Histological evaluation of conceptuses collected on days 14 and 16 of pregnancy supports the hypothesis of a highly absorptive trophoblast during this time of development [18]. Furthermore, changes in the expression of a total of 42 members of the solute carrier group of membrane transport proteins were determined, 30 of them being upregulated and 12 being down-regulated. This suggests that these transporters contribute to nutrient exchange between the histotroph and the developing conceptus with unique subsets which are characteristic for different stages of conceptus development [19].
Conclusion
The majority of information available with respect to early equine pregnancy and conceptus development supports the idea of an anti-luteolytic mechanism responsible for maintenance of corpus luteum function beyond the physiological events of the estrous cycle. Despite intensive research, the nature of the embryonic signal for luteostasis in mares remains a mystery. It may be suggested that in the horse, luteolysis is prevented by a more complex conceptus-related mechanism and not only by a single substance. The reason why such a mechanism remains undetected until now is unclear. However, it appears feasible that the rapid development of molecular biological methods will eventually allow scientists to solve the riddle.
Abbreviations
- AQP
Aquaporin
- cAMP
Cycloadenosinmonophosphat
- COX2
Cyclooxygenase 2
- eCG
Equine chorionic gonadotropin
- IFN
Interferon
- mRNA
Messenger ribonucleic acid
- PG
Prostaglandin
Footnotes
Competing interests
The authors declared that they have not competing interests.
Authors’ contributions
CA wrote this review, and internal research reported was jointly designed and interpreted by CA and SB. All authors have read, discussed and approved this manuscript.
Authors’ information
Christine Aurich, DVM, Dipl. ECAR, is a veterinarian working as associate professor at the University for Veterinary Sciences in Vienna. She is head of the Centre for Artificial Insemination and Embryo Transfer and thus not only involved in research on early equine pregnancy, but has also ample experience in clinical work on equine fertility and assisted reproduction.
Sven Budik is a biologist at the Centre for Artificial Insemination and Embryo Transfer, Vetmeduni Vienna, with expertise in early conceptus development and molecular biology. He is responsible for the majority of laboratory work behind the research on early equine pregnancy at the unit.
Contributor Information
Christine Aurich, Phone: +43-1-250776400, Email: christine.aurich@vetmeduni.ac.at.
Sven Budik, Email: sven.budik@vetmeduni.ac.at.
References
- 1.Berg SL, Ginther OJ. Effect of estrogens on uterine tone and life span of the corpus luteum in mares. J Anim Sci. 1978;47:203–8. doi: 10.2527/jas1978.471203x. [DOI] [PubMed] [Google Scholar]
- 2.Sharp DC, McDowell KJ, Weithenauer J, Thatcher WW. The continuum of events leading to maternal recognition of pregnancy in mares. J Reprod Fertil Suppl. 1989;37:101–7. [PubMed] [Google Scholar]
- 3.McDowell KJ, Sharp DC, Fazleabas AT, Roberts RM. Synthesis and release of proteins by endometrium from pregnant and non-pregnant mares, and by conceptus membranes: characterization by two-dimensioal gel electrophoresis. J Reprod Fertil. 1990;89:107–115. doi: 10.1530/jrf.0.0890107. [DOI] [PubMed] [Google Scholar]
- 4.Goff AK, Sirois J, Pontbrian D. Effect of oestradiol on oxytocin-stimulated prostaglandin F2alpha release in mares. J Reprod Fertil. 1993;98:107–12. doi: 10.1530/jrf.0.0980107. [DOI] [PubMed] [Google Scholar]
- 5.Vanderwall DK, Woods GL, Weber JA, Lichtenwalner AB. Corpus luteum function in nonpregnant mares following intrauterine administration of prostaglandin E(2) or estradiol-17beta. Theriogenology. 1994;42:1069–83. doi: 10.1016/0093-691X(94)90855-9. [DOI] [PubMed] [Google Scholar]
- 6.Bazer FW, Jingyoung K, Song G, Ka H, Tekwe CD, Wu G. Select nutrients, progesterone and interferone tau affect conceptus metabolism and development. Ann N Y Acad Sci. 2012;1271:88–96. doi: 10.1111/j.1749-6632.2012.06741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCue PM, LeBlanc MM, Squires EL. eFSH in clinical equine practice. Theriogenology. 2007;68:429–33. doi: 10.1016/j.theriogenology.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Aurich C. Reproductive cycles of horses. Anim Reprod Sci. 2011;124:220–8. doi: 10.1016/j.anireprosci.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Goff AK, Pontbriand D, Sirois J. Oxytocin stimulation of plasma 15-keto-13,14-dihydro prostaglandin F-2 alpha during the oestrous cycle and early pregnancy in the mare. J Reprod Fertil Suppl. 1987;53:253–60. [PubMed] [Google Scholar]
- 10.Stout TAE, Lamming GE, Allen WR. Oxytocin administration prolongs luteal function in cyclic mares. J Reprod Fertil. 1999;116:315–20. [DOI] [PubMed]
- 11.Wilsher S, Clutton-Brock A, Allen WR. Successful transfer of day 10 horse embryos: influence of donor-recipient asynchrony on embryo development. Reproduction. 2010;139:575–85. doi: 10.1530/REP-09-0306. [DOI] [PubMed] [Google Scholar]
- 12.Boerboom D, Brown KA, Vaillancourt D, Poitras P, Goff AK, Watanabe K, et al. Expression of key prostaglandin synthases in equine endometrium during late diestrus and early pregnancy. Biol Reprod. 2004;70:391–99. doi: 10.1095/biolreprod.103.020800. [DOI] [PubMed] [Google Scholar]
- 13.Ealy AD, Eroh ML, Sharp DC. Prostaglandin H synthase type is differentially expressed in endometrium based on pregnancy status in pony mares and response to oxytocin and conceptus secretions in explant culture. Anim Reprod Sci. 2010;117:99–105. doi: 10.1016/j.anireprosci.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Bae SE, Watson ED. A light microscopic and ultrastructural study on the presence and location of oxytocin in the equine endometrium. Theriogenology. 2003;60:909–21. doi: 10.1016/S0093-691X(02)01362-6. [DOI] [PubMed] [Google Scholar]
- 15.Baker CB, Adams MH, McDowell KJ. Lack of expression of alpha or omega interferons by the horse conceptus. J Reprod Fertil Suppl. 1991;44:439–43. [PubMed] [Google Scholar]
- 16.Zavy MT, Mayer R, Vernon MW, Bazer FW, Sharp DC. An investigation of the uterine-luminal environment of non-pregnant and pregnant Pony mares. J Reprod Fertil Suppl. 1979;27:403–11. [PubMed] [Google Scholar]
- 17.Rambags BP, van Rossem AW, Blok EE, de Graaf-Roelfsema E, Kindahl H, van der Kolk JH, et al. Effects of exogenous insulin on luteolysis and reproductive cyclicity in the mare. Reprod Domest Anim. 2008;43:422–8. doi: 10.1111/j.1439-0531.2007.00929.x. [DOI] [PubMed] [Google Scholar]
- 18.Walter I, Budik S, Aurich C. Transmission electron microscopy (TEM) of equine conceptuses at 14 and 16 days of gestation. Reprod Fertil Dev. 2010;22:405–15. doi: 10.1071/RD08280. [DOI] [PubMed] [Google Scholar]
- 19.Klein C, Troedsson MHT. Transcriptional profiling of equine conceptuses reveals new aspects of embryo-maternal communication in the horse. Biol Reprod. 2011;84:872–85. doi: 10.1095/biolreprod.110.088732. [DOI] [PubMed] [Google Scholar]
- 20.Nie GJ, Johnson KE, Braden TD, Wenzel JGW. Use of an intra-uterine glass ball protocol to extend luteal function in mares. J Equine Vet Sci. 2003;23:266–73. doi: 10.1053/jevs.2003.75. [DOI] [Google Scholar]
- 21.del Alamo MM R, Reilas T, Kindahl H, Katila T. Mechanisms behind intrauterine device-induced luteal persistence in mares. Anim Reprod Sci. 2008;107:94–106. doi: 10.1016/j.anireprosci.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Katila T. Clinical commentary: techniques to suppress oestrus in mares. Equine Vet Educ. 2015;27:344–5. doi: 10.1111/eve.12324. [DOI] [Google Scholar]
- 23.Wilsher S, Allen WR. Intrauterine administration of plant oils inhibits luteolysis in the mare. Equine Vet J. 2011;43:99–105. doi: 10.1111/j.2042-3306.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 24.Thorburn GD. A speculative review of parturition in the mare. Equine Vet J Suppl. 1993;14:41–9. doi: 10.1111/j.2042-3306.1993.tb04808.x. [DOI] [PubMed] [Google Scholar]
- 25.Ousey JC. Peripartal endocrinology in the mare and foetus. Reprod Domest Anim. 2004;39:222–31. doi: 10.1111/j.1439-0531.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 26.Holtan DW, Nett TM, Estergreen VL. Plasma progestins in pregnant, postpartum and cycling mares. J Anim Sci. 1975;40:251–60. doi: 10.2527/jas1975.402251x. [DOI] [PubMed] [Google Scholar]
- 27.Atkins DT, Harms PG, Sorensen AM, Jr, Fleeger JL. Isolation, identification and quantitation of serum 5α-pregnane-3,20-dione and its relationship to progesterone in the pregnant mare. Steroids. 1976;28:867–80. doi: 10.1016/0039-128X(76)90036-2. [DOI] [PubMed] [Google Scholar]
- 28.Moss GE, Estergreen VL, Becker SR, Grant BD. The source of the 5α-pregnanes that occur during gestation in mares. J Reprod Fertil Suppl. 1979;27:511–19. [PubMed] [Google Scholar]
- 29.Daels PF, DeMoraes ML, Stabenfeldt GH, Hughes JP, Lasley BI. The corpus luteum: source of estrogen during early pregnancy in the mare. J Reprod Fertil Suppl. 1990;44:501–8. [PubMed] [Google Scholar]
- 30.Holtan DW, Houghton E, Silver M, Fowden AL, Ousey J, Rossdale PD. Plasma progestogens in the mare, fetus and newborn foal. J Reprod Fertil Suppl. 1991;44:517–28. [PubMed] [Google Scholar]
- 31.Allen WR. Fetomaternal interactions and influences during early pregnancy. Reproduction. 2001;121:513–27. doi: 10.1530/rep.0.1210513. [DOI] [PubMed] [Google Scholar]
- 32.Urwin VE, Allen WR. Pituitary and chorionic gonadotrophic control of ovarian function during early pregnancy in equids. J Reprod Fertil Suppl. 1982;32:371–81. [PubMed] [Google Scholar]
- 33.Squires EL, Ginther OJ. Collection technique and progesterone concentration of ovarian and uterine venous blood in mares. J Anim Sci. 1975;40:275–281. doi: 10.2527/jas1975.402275x. [DOI] [PubMed] [Google Scholar]
- 34.Ball BA, Little TV, Weber JA, Woods GL. Survival of Day-4 embryos from young, normal mares and aged, subfertile mares after transfer to normal recipient mares. J Reprod Fertil. 1989;89:187–194. doi: 10.1530/jrf.0.0850187. [DOI] [PubMed] [Google Scholar]
- 35.Bezard J, Magistrini M, Duchamp G, Palmer E. Chronology of equine fertilisation and embryonic development in vivo and in vitro. Equine Vet J Suppl. 1989;8:105–10. [Google Scholar]
- 36.Grøndahl C, Hyttel P. Nucleogenesis and ribonucleid acid synthesis in preimplantation equine embryos. Biol Reprod. 1996;55:769–74. doi: 10.1095/biolreprod55.4.769. [DOI] [PubMed] [Google Scholar]
- 37.Betteridge KJ. Equine embryology: an inventory of unanswered questions. Theriogenology. 2007;68S:S9–S21. doi: 10.1016/j.theriogenology.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Betteridge KJ, Eaglesome MD, Mitchell D, Flood PF, Beriault R. Development of horse embryos up to twenty-two days after ovulation: observations on fresh specimens. J Anat. 1982;135:191–209. [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman DA, Weber JA, Geary RT, Woods GL. Time of embryo transport through the mare oviduct. Theriogenology. 1991;36:823–30. doi: 10.1016/0093-691X(91)90348-H. [DOI] [PubMed] [Google Scholar]
- 40.Battut I, Colchen S, Fieni F, Tainturier D, Bruyas JF. Success rates when attempting to nonsurgically collect equine embryos at 144, 156 or 168 hours after ovulation. Equine Vet J Suppl. 1997;25:60–62. doi: 10.1111/j.2042-3306.1997.tb05102.x. [DOI] [PubMed] [Google Scholar]
- 41.Weber JA, Freeman DA, Vanderwall DK, Woods GL. Prostaglandin E2-secretion by oviductal transport-stage equine embryos. Biol Reprod. 1991;45:540–3. doi: 10.1095/biolreprod45.4.540. [DOI] [PubMed] [Google Scholar]
- 42.Weber JA, Freeman DA, Vanderwall DK, Woods GL. Prostaglandin E2 hastens oviductal transport of equine embryos. Biol Reprod. 1991;45:544–6. doi: 10.1095/biolreprod45.4.544. [DOI] [PubMed] [Google Scholar]
- 43.Colchen S, Battut I, Fieni F, Tainturier D, Siliart S, Bruyas JF. Quantitative histological analysis of equine embryos at exactly 156 and 168 h after ovulation. J Reprod Fertil Suppl. 2000;56:527–37. [PubMed] [Google Scholar]
- 44.Panzani D, Rota A, Marmorini P, Vannozzi I, Camillo F. Retrospective study of factors affecting multiple ovulations, embryo recovery, quality, and diameter in a commercial equine embryo transfer program. Theriogenology. 2014;82:807–14. doi: 10.1016/j.theriogenology.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Willmann C, Koblischke P, Hoffmann B, Schuler G, Aurich C. Effects of altrenogest treatment of early pregnant mares on pregnancy rate, conceptus development and plasma progesterone concentration. Theriogenology. 2011;55:421–8. doi: 10.1016/j.theriogenology.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Aurich C, Budik S. Season does not influence embryo recovery rate and conceptus size until day 14 after ovulation in the horse. Reprod Domest Anim. 2015. doi: 10.1111/rda.12490. [DOI] [PubMed]
- 47.Watson AJ, Natale DR, Barcroft LC. Molecular regulation of blastocyst formation. Anim Reprod Sci. 2004;82–83:583–92. doi: 10.1016/j.anireprosci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Waelchli RO, Mac Phee DJ, Kidder GM, Betteridge KJ. Evidence for the presence of sodium - and potassium - dependent adenosine triphosphatase α1 and β1 subunit isoforms and their probable role in blastocyst expansion in the preattachment horse conceptus. Biol Reprod. 1997;57:630–40. doi: 10.1095/biolreprod57.3.630. [DOI] [PubMed] [Google Scholar]
- 49.Waelchli RO, Betteridge KJ. Osmolality of equine blastocyst fluid from day 11 to day 25 of pregnancy. Reprod Fertil Dev. 1996;8:981–8. doi: 10.1071/RD9960981. [DOI] [PubMed] [Google Scholar]
- 50.Budik S, Walter I, Tschulenk W, Helmreich M, Deichsel K, Pittner F, Aurich C. Significance of aquaporins and sodium potassium ATPase subunits for expansion of the early equine conceptus. Reproduction. 2008;135:497–508. doi: 10.1530/REP-07-0298. [DOI] [PubMed] [Google Scholar]
- 51.Budik S, Palm S, Walter I, Helmreich M, Aurich C. Increasing expression of oxytocin and vasopressin receptors in the equine conceptus between days 10 and 16 of pregnancy. Reprod Fertil Dev. 2012;24:641–48. doi: 10.1071/RD11167. [DOI] [PubMed] [Google Scholar]
- 52.Crews LJ, Waelchli RO, Huang CX, Canny MJ, McCully ME, Betteridge KJ. Electrolyte distribution and yolk sac morphology in frozen hydrated equine conceptuses during the second week of pregnancy. Reprod Fertil Dev. 2007;19:804–814. doi: 10.1071/RD07050. [DOI] [PubMed] [Google Scholar]
- 53.Flood PF, Betteridge KJ, Diocee MS. Transmission electron microscopy of horse embryos 3–16 days after ovulation. J Reprod Fertil Suppl. 1982;32:319–27. [PubMed] [Google Scholar]
- 54.Oriol JG, Sharom FJ, Betteridge KJ. Developmentally regulated changes in the glycoproteins of the equine embryonic capsule. J Reprod Fertil. 1993;99:653–64. doi: 10.1530/jrf.0.0990653. [DOI] [PubMed] [Google Scholar]
- 55.Stout TAE, Meadows S, Allen WR. Stage-specific formation of the equine blastocyst capsule is instrumental to hatching and to embryonic survival in vitro. Anim Reprod Sci. 2005;87:269–81. doi: 10.1016/j.anireprosci.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Betteridge KJ. The structure and function of the equine capsule in relation to embryo manipulation and transfer. Equine Vet J Suppl. 1989;8:92–100. [Google Scholar]
- 57.Crossett B, Allen WR, Stewart F. A 19 kDa protein secreted by the endometrium of the mare is a novel member of the lipocalin family. Biochem J. 1996;320:137–43. doi: 10.1042/bj3200137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremoleda JL, Stout TAE, Lagutina I, Lazzari G, Bevers MM, Colenbrander BB, Galli C. ffects of in vitro production on horse embryo morphology, cytoskeletal characteristics and blastocyst capsule formation. Biol Reprod. 2003;69:1895–906. doi: 10.1095/biolreprod.103.018515. [DOI] [PubMed] [Google Scholar]
- 59.Smits K, Govaere J, Peelman LJ, Goossens K, de Graaf DC, Vercauteren D, Vandaela L, Hoogewijs M, Wydooghe E, Stout T, Van Soom A. Influence of the uterine environment on the development of in vitro-produced equine embryos. Reproduction. 2012;143:173–181. doi: 10.1530/REP-11-0217. [DOI] [PubMed] [Google Scholar]
- 60.Ginther OJ. Mobility of the equine conceptus. Theriogenology. 1983;19:603–11. doi: 10.1016/0093-691X(83)90180-2. [DOI] [PubMed] [Google Scholar]
- 61.Ginther OJ. Dynamical physical interactions between the equine embryo and uterus. Equine Vet J Suppl. 1985;3:41–7. [Google Scholar]
- 62.McDowell KJ, Sharp DC, Grubaugh W, Thatcher WW, Wilcox CJ. Restricted conceptus mobility results in failure of pregnancy maintenance in mares. Biol Reprod. 1988;39:340–8. doi: 10.1095/biolreprod39.2.340. [DOI] [PubMed] [Google Scholar]
- 63.Watson ED, Sertich PL. Prostaglandin production by horse embryos and the effect of co-culture of embryos with endometrium from pregnant mares. J Reprod Fertil. 1989;87:331–6. doi: 10.1530/jrf.0.0870331. [DOI] [PubMed] [Google Scholar]
- 64.Stout TAE, Allen WR. Role of prostaglandins in intrauterine migration of the equine conceptus. Reproduction. 2001;121:771–5. doi: 10.1530/rep.0.1210771. [DOI] [PubMed] [Google Scholar]
- 65.Stout TAE, Allen WR. Prostaglandin E(2) and F(2 alpha) production by equine conceptuses and concentrations in conceptus fluids and uterine flushings recovered from early pregnant and dioestrous mares. Reproduction. 2002;123:261–8. doi: 10.1530/rep.0.1230261. [DOI] [PubMed] [Google Scholar]
- 66.Ginther OJ, Bergfelt DR, Leith GS, Scraba ST. Embryonic loss in mares: incidence and ultrasonic morphology. Theriogenology. 1985;24:73–86. doi: 10.1016/0093-691X(85)90213-4. [DOI] [PubMed] [Google Scholar]
- 67.Woods GL, Baker CB, Hillman RB, Schlafer DH. Recent studies relating to early embryonic death in the mare. Equine Vet J Suppl. 1985;3:104–7. [Google Scholar]
- 68.Adams GP, Kastelic JP, Bergfelt DR, Ginther OJ. Effect of uterine inflammation and ultrasononically detected uterine pathology on fertility in the mare. J Reprod Fertil Suppl. 1987;35:445–54. [PubMed] [Google Scholar]
- 69.Bergfelt DR, Ginther OJ. Embryonic loss in mares: role of the embryonic vesicle, corpus luteum and progesterone. J Reprod Fertil. 1991;95:339–347. doi: 10.1530/jrf.0.0950339. [DOI] [PubMed] [Google Scholar]
- 70.Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138:195–209. doi: 10.1530/REP-09-0158. [DOI] [PubMed] [Google Scholar]
- 71.Sharp DC. The early fetal life of the equine conceptus. Anim Reprod Sci. 2000;60–61:679–89. doi: 10.1016/S0378-4320(00)00138-X. [DOI] [PubMed] [Google Scholar]
- 72.Kastelic JP, Adams GP, Ginther OJ. Role of progesterone in mobility, fixation, orientation, and survival of the equine embryonic vesicle. Theriogenology. 1987;27:655–63. doi: 10.1016/0093-691X(87)90059-8. [DOI] [PubMed] [Google Scholar]
- 73.Rambags BPB, van Tol HTA, van den Eng MM, Colenbrander B, Stout TAE. Expression of progesterone and oestrogen receptors by early intrauterine equine conceptuses. Theriogenology. 2008;69:366–75. doi: 10.1016/j.theriogenology.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Willmann C, Budik S, Walter I, Aurich C. Influences of treatment of early pregnant mares with the progestin altrenogest on embryonic development and gene expression in the endometrium and conceptus. Theriogenology. 2011;76:61–73. doi: 10.1016/j.theriogenology.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 75.Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:1879–98. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- 76.Wilsher S, Gower S, Allen WR. Immunohistolocalisation of progesterone and oestrogen receptors at the placental interface in mares during early pregnancy. Anim Reprod Sci. 2011;129:200–8. doi: 10.1016/j.anireprosci.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Mann GE, Lamming GE. The influence of progesterone during early pregnancy in cattle. Reprod Domest Anim. 1999;34:269–74. doi: 10.1111/j.1439-0531.1999.tb01250.x. [DOI] [Google Scholar]
- 78.Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryonic development and inhibition of the luteolytic mechanism in cows. Reproduction. 2001;121:175–80. doi: 10.1530/rep.0.1210175. [DOI] [PubMed] [Google Scholar]
- 79.Cencic A, Guillomot M, Koren S, LaBonnariére C. Trophoblastic interferons: do they modulate uterine cellular markers at the time of conceptus attachment in the pig? Placenta. 2003;24:862–9. doi: 10.1016/S0143-4004(03)00135-8. [DOI] [PubMed] [Google Scholar]
- 80.Cochet M, Vaiman D, Lefèvre F. Novel interferon delta genes in mammals: cloning of one gene from the sheep, two genes expressed by the horse conceptus and discovery of related sequences in several taxa by genomic database screening. Gene. 2009;433:88–99. doi: 10.1016/j.gene.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 81.Allen WR, Wilsher S. A review of implantation and early placentation in the mare. Placenta. 2009;30:1005–15. doi: 10.1016/j.placenta.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 82.Stewart F, Kennedy MW, Suire S. A novel uterine lipocalin supporting pregnancy in equids. Cell Mol Life Sci. 2000;57:1373–8. doi: 10.1007/PL00000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klein C, Scoggin KE, Ealy AD, Troedsson MHT. Transcriptional profiling of equine endometrium during the time of maternal recognition of pregnancy. Biol Reprod. 2010;83:102–13. doi: 10.1095/biolreprod.109.081612. [DOI] [PubMed] [Google Scholar]
- 84.Merkl M, Ulbrich SE, Otzdorff C, Herbach N, Wanke R, Wolf E, Handler J, Bauersachs S. Microarray analysis of equine endometrium at days 8 and 12 of pregnancy. Biol Reprod. 2010;83:874–86. doi: 10.1095/biolreprod.110.085233. [DOI] [PubMed] [Google Scholar]
- 85.Köhne M, Kuhl J, Ille N, Erber R, Aurich C. Treatment with human chorionic gonadotrophin before ovulation increases progestin concentration in early equine pregnancies. Anim Reprod Sci. 2014;149:187–193. doi: 10.1016/j.anireprosci.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Hayes MA, Quinn BA, Keirstaead ND, Katavolos P, Waelchli RO, Betteridge KJ. Proteins associated with the early intrauterine equine conceptus. Reprod Domest Anim. 2008;43(Suppl. 2):232–7. doi: 10.1111/j.1439-0531.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- 87.McDowell KJ, Adams MH, Franklin KM, Baker CM. Changes in equine endometrial retinol-binding protein RNA during the estrous cycle and early pregnancy and with exogenous steroids. Biol Reprod. 1995;52:438–43. doi: 10.1095/biolreprod52.2.438. [DOI] [PubMed] [Google Scholar]
- 88.McDowell KJ, Sharp DC, Fazleabas A, Robers RM, Bazer FW. Partial characterisation of the equine uteroferrin-like protein. J Reprod Fertil. 1982;32:329–34. [PubMed] [Google Scholar]


