Abstract
Complicated Grief, marked by a persistent and intrusive grief lasting beyond the expected period of adaptation, is associated with a relative inability to disengage from idiographic loss-relevant stimuli (O’Connor & Arizmendi, 2014). In other populations, functional magnetic resonance imaging (fMRI) studies investigating the neural networks associated with this bias consistently implicate the anterior cingulate cortex (ACC) during emotion regulation. In the present study, twenty-eight older adults were categorized into three groups based on grief severity: Complicated Grief (n=8), Non-Complicated Grief (n=9), and Nonbereaved, married controls (n=11). Using a block design, all participants completed 8 blocks (20 stimuli per block) of the ecStroop task during fMRI data acquisition. Differences in neural activity during grief-related (as opposed to neutral) stimuli across groups were examined. Those with Complicated Grief showed an absence of increased rostral ACC (rACC) and fronto-cortical recruitment relative to Nonbereaved controls. Activity in the orbitofrontal cortex (x=6, y=54, z=−10) was significantly elevated in the Non-Complicated Grief group when compared to Nonbereaved controls. Post hoc analysis evidenced activity in the dorsal ACC in the Complicated Grief and Nonbereaved groups late in the task. These findings, supported by behavioral data, suggest a relative inability to recruit the regions necessary for successful completion of this emotional task in those with Complicated Grief. This deficit was not observed in recruitment of the orbitofrontal cortex and the rACC during processing of idiographic semantic stimuli in Non-Complicated Grief.
Keywords: Complicated Grief, Grief, Magnetic Resonance Imaging, Psychopathology, Attention, ecStroop
Introduction
Complicated Grief (CG), termed Persistent Complex Bereavement Disorder by the DSM-5 (American Psychiatric Association, 2013), affects approximately 6.7% of all individuals who have experienced bereavement, a number that increases to 20.3% and 23.6 % in spousal and child bereavement respectively (Kersting, Brähler, Glawsmer, & Wagner, 2011). Features such as intense sorrow, and persistent yearning and longing for the deceased characterize CG. People with this syndrome experience a wide array of symptoms including intense anger, emotional numbness and even the desire to die in order to be with the deceased loved one (American Psychiatric Association, 2013). In contrast with the natural grief process, CG involves an unremitting pattern of symptoms enduring for at least 12 months, and can last for many years after the loss event. It is hypothesized that the individual suffering from CG has failed to integrate the loss event into their life, which can precipitate increased morbidity and debilitating psychological symptoms (Schultze-Florey et al., 2012; Shear, 2012).
CG is distinct from other known pathologies in many ways; yet affected individuals do exhibit some cognitive response patterns common to other affective disorders (Prigerson et al., 2009; Robinaugh, LeBlanc, Vuletich, & McNally, 2014). One such pattern is an attentional bias toward, or relative inability to disengage from psychopathology-related stimuli. Experimentally, attentional bias has been measured using the emotional Stroop (eStroop) task. Within this paradigm, bias manifests in CG as a delayed reaction time in trials with idiographic loss-related words when compared to those with Non-Complicated Grief (Non-CG) and Nonbereaved controls (Maccallum & Bryant, 2010; Mancini & Bonanno, 2012; O’Connor & Arizmendi, 2014). These studies lend support to a conflict resolution hypothesis; those with CG show a relatively inhibited ability to disengage from an emotionally relevant stimulus (emotional interference) toward a goal-directed behavior (a correct response). Clinically, a diminished capacity to attend away from the content of the semantic stimuli maintains psychopathological behavior (Koster, Lissnyder, Derakshan, & De Raedt, 2011; Williams, Mathews, & Macleod, 1996).
1.1 Neural correlates of emotional response in grief
A recent review indicates relatively few neuroimaging studies have involved grief (O’Connor, 2012). In one study, data from individuals recorded while viewing pictures of either a stranger or their lost loved one indicate significant recruitment of the dorsal anterior cingulate cortex (dACC) and the insula, as well as the posterior cingulate cortex (PCC; Gündel, O’Connor, Littrell, Fort, & Lane, 2003). Pronounced dACC activation has also been evidenced in acutely grieving mothers who have recently lost an unborn child when viewing images of smiling unfamiliar infants (Kersting et al., 2009). In both instances Nonbereaved controls showed no increased activation in these regions, thereby implicating greater recruitment of the anterior cingulate cortex (ACC) in grief-related emotional response only. However, neither study utilized a task specifically requiring the participants to ignore task-irrelevant content in favor of a goal-directed response.
Extensive literature exists on the role of the ACC in task-related conflict resolution and emotion regulation writ large. Evidence from the eStroop paradigm indicates the existence of two separate but related types of conflict resolution (Williams et al., 1996). One involves conflict due to stimulus incongruence and the other involves task-irrelevant emotional interference (Mohanty et al., 2007; Algom, Chajut, & Lev, 2004). One longstanding model asserts a functional differentiation of the ACC. However, recent evidence in non-clinical populations has blurred the distinction between the dACC and rACC systems. A more integrated hypothesis has been proposed which asserts that the dACC is involved in appraisal and monitoring of emotion, while the rACC demonstrates a regulatory function by inhibiting negative emotional processing in the amygdala (Etkin, Egner, & Kalisch, 2011). In this integrative model, the dACC is involved in reappraisal and resolution of both emotional and non-emotional conflict, while increased rACC activity is associated primarily with resolution of negative emotional conflict and may be recruited by other regions when there is a need for inhibition of limbic response (Kanske & Kotz, 2011).
In clinical samples, studies of individuals with various types of psychopathology have repeatedly demonstrated pronounced ventral/rostral ACC and medial prefrontal dysfunction during the experience of psychopathology-related stimuli. Studies of emotional conflict resolution indicate deficient rACC response in social and trait anxiety (Klumpp, Post, Angstadt, Fitzgerald, & Phan, 2011; Klumpp, Ho, Taylor, Phan, Abelson, & Liberzon, 2013), anxious youth (Price et al., 2014; Swartz et al., 2014), and Generalized Anxiety Disorder (GAD; for review see Mochcovitch, da Rocha Freire, Garcia, & Nardi, 2014). Additionally, frontal hypo-activation and cortico-limbic decoupling has been shown in Major Depressive Disorder (MDD), Panic Disorder, and Post Traumatic Stress Disorder (PTSD; Ball, Ramsawh, Campbell-Sills, Paulus, & Stein, 2013; Etkin, & Schatzberg, 2011; Milad et al., 2009; van Tol, 2013). Collectively, these studies indicate decreased functioning in the ostensible cognitive control network, which may contribute to deficits in emotion regulation and even maintenance of disorder. Importantly, this dysregulation does not occur in healthy controls viewing emotionally salient stimuli; instead conflict resolution may actually be enhanced (Kanske & Kotz, 2010).
Acutely bereaved individuals experiencing the loss of a pet also display similar neural patterning (Freed, Yanagihara, Hirsch, & Mann, 2009). Evidence from this study indicates that those individuals experiencing intrusive thoughts and avoidance behaviors showed reduced amygdalar-rACC/DLPFC connectivity when exposed to grief related words in an eStroop task. Importantly, participants in this study exhibited grief acuity similar to that in CG, but were not assessed for grief-related maladaptation, a defining feature of CG. No known neuroimaging study exists examining resolution of emotional conflict in a CG sample.
1.2 The emotional-counting Stroop and ACC activation
The emotional-counting Stroop (ecStroop) task is an imaging-friendly variant of the eStroop task (Whalen, Bush, Shin, & Rauch, 2006). Similar to the traditional eStroop, the ecStroop is designed to measure response latency to emotionally salient as compared to neutral stimuli in order to assess for emotional interference, evidenced by increased response latency (Whalen et al., 2006). The participant is required to count the number of individual words presented on the screen (count varies from 1 to 4 across trials). The participant indicates this count via button-press response, thus utilizing a motor response rather than vocal response, which can lead to errant motion artifacts in neuroimaging data (Hajnal et al., 1994). While the ecStroop does not utilize directly incongruent stimuli, as does the eStroop (e.g., the word “happy” presented in color), the emotional words induce automatic vigilance “to threat in the environment” that temporarily delays ongoing activity – word counting (Algom et al., 2004;). Therefore, this paradigm has demonstrated utility in targeting emotional interference.
The ecStroop during fMRI data acquisition in familial major depression, specific phobia, and PTSD (Mannie et al., 2008; Shin et al., 2001) yields group- and condition-specific effects of emotional interference. However, one study in phobics indicates increased recruitment of the rACC in orthogonal emotion-neutral stimuli contrasts, when compared to controls (Britton et al., 2009). Thus it appears that conflicting fMRI evidence exists with regard to the ecStroop and clinical psychopathology.
Studies utilizing the ecStroop primarily involve individuals with fear and anxiety-related clinical psychopathology. Therefore, the emotional stimuli are negatively valenced, such as threat, fear, and pain. However, reminders of loss do not elicit only negative emotions. Instead, memories of the deceased often elicit pleasurable feelings commonly associated with yearning and attachment (Shear, Frank, Houck, & Reynolds, 2005). The nucleus accumbens, a brain locus associated with reward, was engaged when adults with CG viewed images of their deceased loved one (O’Connor et al., 2008). Understanding the interplay of approach and avoidance behaviors in bereavement and CG is currently under investigation in the field. The current study examines individuals with CG as they experience both neutral and personalized grief-related words in an ecStroop paradigm, and compares to those who are experiencing expected bereavement adjustment (Non-CG), and married, healthy Nonbereaved controls. Based upon the relevant extant literature, we hypothesized that:
Significantly greater activity in the rostral ACC (rACC), associated with emotional stimulus processing, will be seen during the Grief vs. Neutral stimuli in Non-CG compared to Nonbereaved groups.
Given the opposing results from the ecStroop literature and psychopathology literature, the CG group will show either increased or decreased recruitment of the rACC compared to the Nonbereaved controls, instantiating either a need for greater recruitment of this area to perform the emotional conflict task or an inability to successfully recruit this area.
Method
2.1 Participants
Twenty-eight older adults were recruited as a part of a larger study by advertisement at metropolitan senior centers and direct mailing lists. Exclusion criteria were: (1) presence of current major psychiatric disorder (e.g. Major Depressive Disorder, alcohol or substance dependence) as assessed with a structured clinical interview for, (2) use of psychotropic medications initiated since the death event, (3) immunosuppressive medication, (4) current major medical illness and (5) Mini-Mental State Examination (MMSE) score of less than or equal to 18. Additionally, participants were screened for ferromagnetic material and fear of small, enclosed spaces.
Participants were between 62 and 82 years of age (M = 71.9), were predominantly female (81%), and predominantly Caucasian (82%). Of this sample, 8 met criteria for CG, 9 met criteria for Non-CG, and 11 were Nonbereaved, married control participants. The Nonbereaved controls were excluded if they experienced the death of a first-degree family member or close friend in the past three years. The UCLA Institutional Review Board approved the study.
2.2 Procedure
All bereaved participants were given the 19-item Inventory of Complicated Grief (ICG; Prigerson et al., 1995). This inventory is a psychometrically sound measure of grief severity, as well as a valid tool for differentiating CG from Non-CG (Prigerson et al., 1995). A score of 30 or higher (out of 76) indicates a clinically significant likelihood that CG is present (Shear et al., 2005). This clinical cutoff was used as the criterion to differentiate the CG and Non-CG groups for the current study. The Beck Depression Inventory II was used to further assess the presence of depressive symptoms.
An ecStroop (Whalen et al., 2006) was employed using a list of 73 grief-related words developed from interviews with bereaved participants in prior research studies (Gündel et al., 2003; O’Connor et al., 2008). Grief-related words were matched with neutral words based on number of letters and syllables, part of speech, and frequency in the English language. The participants rated words that were most relevant to their own grief experience; those deemed most relevant were presented during the task (Williams et al., 1996). Each Nonbereaved participant was matched to a bereaved participant and given the bereaved participant’s idiographic list, ensuring consistency in the stimuli presented across all three groups. Behavioral data presented here were taken from participant performance on three blocks of the ecStroop completed outside the scanner, as such data from the scanner task was lost due to technical issues.
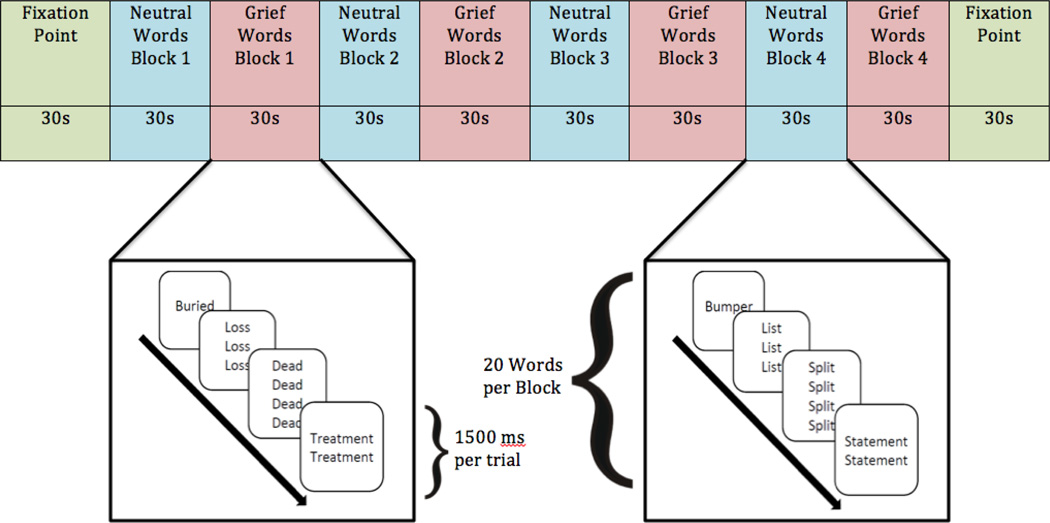
The ecStroop was administered in eight alternating blocks; four blocks of 20 grief-related words and four blocks of 20 matched neutral words. Each trial was presented for 1.45 seconds after a .05 second orienting stimulus (i.e., a centered “+”). Trials were administered in a “jittered” fashion per Whalen and colleagues (2006). The eight Stroop blocks were bookended by 30-second fixation point blocks (Figure 1). Participants viewed stimuli through stereoscopic 3D goggles (Resonance Technologies, Inc.)
Figure 1.
Emotional Counting Stroop Task Design. Participants viewed four blocks of neutral words alternating with four block of grief-related words. Each block consisted of the same words presented in different order. Participants were told to ignore the semantic content of the word and respond with the number of words presented, as quickly and as accurately as possible The task began and ended with a fixation-point block, which consisted of a “+” sign presented in the center of the screen for the duration of the block.
Scanning took place in a Siemens Trio 3T scanner at the UCLA Ahmonson-Lovelace Brain Mapping Center. A high-resolution (1 × 1 × 1 mm voxel size) structural T1 weighted echo-planar image (spin-echo, TR = 2200 ms; TE = 3.4 ms; matrix size: 256×256;176 axial slices; FOV = 256 mm; 1-mm thick) was acquired for each participant using a magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence. Additionally, T2*-weighted functional scans were acquired (36 interleaved slices, 3-mm thick, FOV = 200 mm cubic voxels; 3.1 × 3.1 × 3.0 mm slice; matrix size: 64×64; repetition time (TR) = 3000 msec; echo time (TE) = 25 msec; flip angle = 90°) for each participant.
2.3 Data analysis
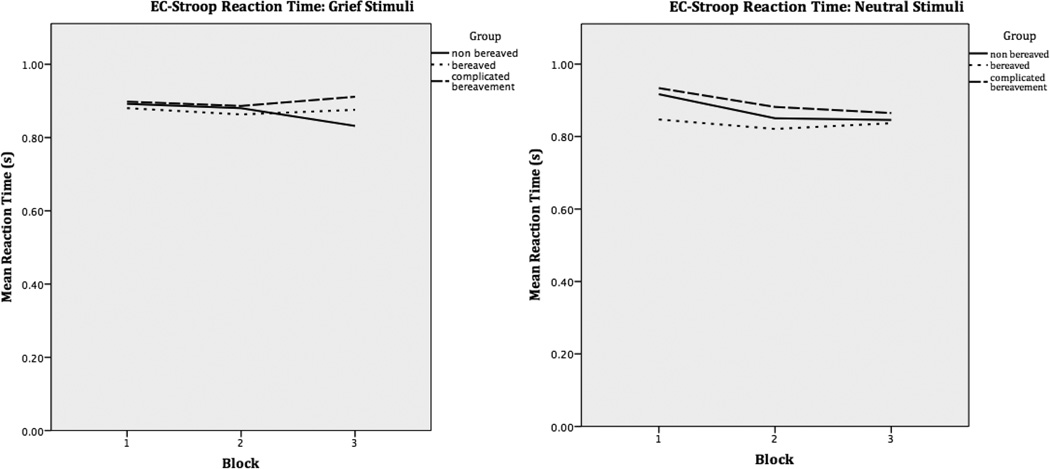
Behavioral analyses were conducted using SPSS (version 22). A one-way analysis of variance was conducted to determine group differences in average reaction time during the third block of grief words. This specific time point was chosen as the third block in the grief trials exhibited the most separation in average reaction times (see fig 2). Importantly, the data from this analysis are from the fMRI-completed subgroup of a larger participant pool described in our previous work, and not all fMRI-completing individuals analyzed here participated in the task outside the scanner (O’Connor & Arizmendi, 2014). Time interval between task performance outside the scanner and in the scanner was two or more weeks, thus no practice effect is suspected.
Figure 2.
Averaged participant reaction times during three blocks of the computerized ecStroop task performed outside the scanner. Only those participants that completed the computerized task both outside and in the scanner environment are represented here.
Image processing and statistical analyses were carried out using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm). Acquired DICOM images were realigned, normalized to the MNI template, and smoothed with an 8mm Gaussian kernel, full width half maximum. Data were corrected for rigid body motion artifacts using the Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect/) toolbox. Analysis used the general linear model with a block design. In the two-level random effects approach implemented in SPM 8, contrasts for individuals were aggregated for within-group analysis and for between-group analysis. All group level analyses were thresholded using an uncorrected p-value of .005, with a cluster size threshold of 5. All coordinates are reported in MNI format.
First level fMRI analyses consisted of contrasts at the individual level. T-contrasts were conducted for activation in grief-related blocks compared to neutral blocks, and vice versa. Contrast models for each participant included an outlier regressor produced by the ART program. Task-correlated movement outliers were not included as a regressor, since task-related movement is common in block designs, and if removed, can be detrimental to task-related variance (Johnstone et al., 2006).
Second level analyses were conducted at the group level. Contrasts created during first level analyses were grouped into the predetermined CG, Non-CG, and Nonbereaved groups described previously. Contrasts between each group were conducted to determine group-level differences in neural activity related to the Grief > Neutral condition. Finally, a three-way comparison was conducted to investigate regional activation found commonly across all three groups.
To further query our data regarding the rACC, a region of interest (ROI) analysis was performed to acquire parameter estimates pertaining to the hemodynamic response in this region. The ROI seed region (seed region x=−10, y=36, z=2) was drawn from Stroop and ecStroop studies that found activity in this region during task performance (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Weissman-Fogel et al., 2011). ROI analysis was carried out using the MarsBaR region of interest toolbox for SPM 8 (marsbar.sourceforge.net).
Results
The three groups (CG, Non-CG, and Nonbereaved) did not differ on any demographic variables (Table 1). Additionally, groups did not significantly differ on scores of depression, or basic cognitive function level.
Table 1.
Participant characteristics. Continuous variables: mean (±SD), F value (ANOVA); categorical variables: n (%), X2 value (Chi Square Test).
| Non-bereaved (N=11) |
Non-Complicated Grief (N=9) |
Complicated Grief (N=8) |
F-value/X2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | |||
| Age | 70.73 | 4.61 | 74.22 | 4.87 | 70.75 | 3.24 | 1.95 | 0.16 |
| Gender (Female) | 8 | 73% | 8 | 89% | 7 | 88% | 1.01 | 0.58 |
| Ethnicity (non-Caucasian) | 4 | 36% | 0 | 0% | 1 | 13% | 4.98 | 0.29 |
| Employment (Retired) | 5 | 45% | 7 | 78% | 6 | 75% | 9.31 | 0.16 |
| Education (Post graduate) | 5 | 45% | 3 | 33% | 4 | 50% | 4.07 | 0.67 |
| Years married/partnered | 39.73 | 13.48 | 30.44 | 18.34 | 33.5 | 17.47 | 0.85 | 0.44 |
| Body mass index | 25.09 | 3.79 | 25.76 | 7.18 | 25.69 | 4.24 | 0.04 | 0.96 |
| Alcohol (Drinks per week) | 0.36 | 0.64 | 1 | 1.6 | 3.25 | 5.08 | 2.47 | 0.11 |
| BDI-II | 1.6 | 2.07 | 5.71 | 7.57 | 11.29 | 14.42 | 2.48 | 0.11 |
| ICG | - | - | 17.44 | 7.35 | 31.88 | 4.42 | 23.25 | <0.001 |
| MMSE | 28.09 | 2.07 | 28.89 | 1.54 | 28.63 | 2.33 | 0.42 | 0.66 |
Results from behavioral analysis show this subgroup of participants was underpowered to detect the ecStroop effect as predicted, but results are in a direction consistent with those found in previous studies. There was no significant difference in reaction time between groups during block 3, F(2,19) = 0.87, p = 0.44. However, the grief reaction times show a trend such that those with CG to have a slower overall reaction time, and perform worse on the task as it progresses across blocks. This differs from Nonbereaved controls, who improved over the course of the task. With regard to the neutral blocks, all three groups performed equally and showed some improvement in task performance across the blocks, as hypothesized (Figure 2).
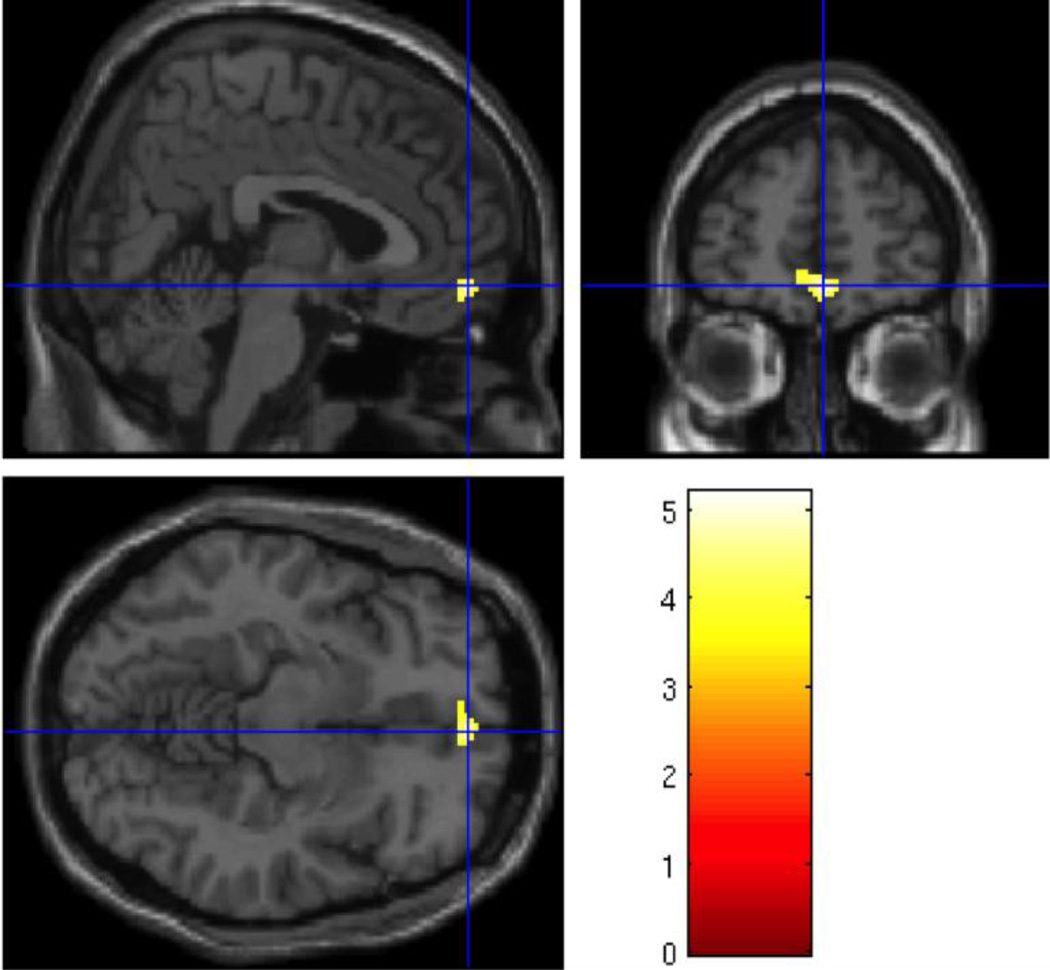
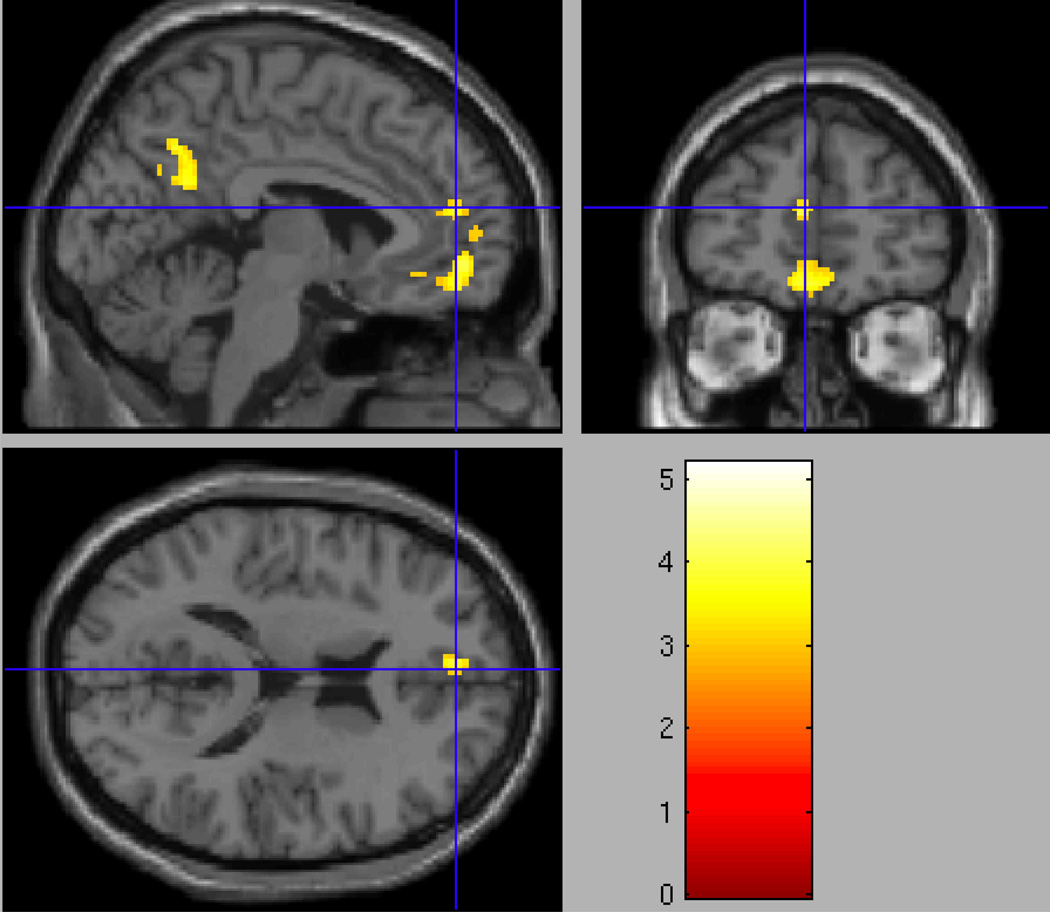
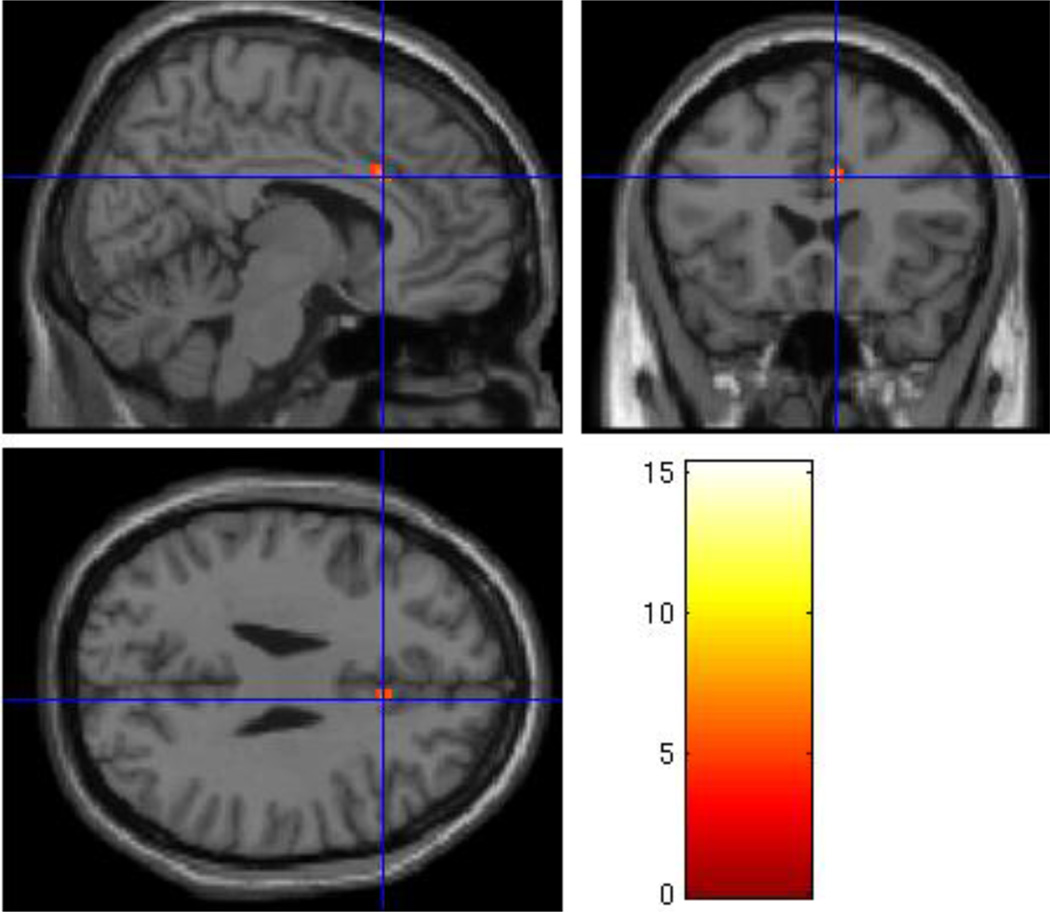
As expected, analysis of the Nonbereaved group alone showed no differential activation in the Grief > Neutral contrast with respect to the rACC. When compared to the Nonbereaved control group, the Non-CG group showed significantly greater activity in grief trials compared to neutral trials in the orbitofrontal cortex (OFC; Table 2), with peak activation in the right medial OFC (x=4, y=54, z=−10; t=5.13; p = .001, uncorrected; see Figure 3). Similarly significant activity was found in a neighboring cluster posterior to the OFC, which included the rACC (x=−6, y=48, z=14; t=4.25; p < .005, uncorrected; Figure 4).
Table 2.
Group comparisons of Grief-related > Neutral (words). Group contrasts not listed did not show significant activations. Peak activation clusters at p = .005, uncorrected.
| Region | MNI Coordinates | Brodmann’s Area |
Cluster | T | ||
|---|---|---|---|---|---|---|
| Non-Complicated Grief > Nonbereaved | x | y | z | |||
| Right Orbitofrontal Cortex | 4 | 54 | −10 | 10 | 452 | 5.13 |
| Left Caudate | −6 | 14 | −12 | 25 | 20 | 3.67 |
| Left Anterior Cingulate | −6 | 48 | 14 | 10 | 41 | 4.25 |
| Superior Temporal Gyrus | −58 | −64 | 28 | 39 | 121 | 5.19 |
| Left Precuneus | −2 | −60 | 40 | 7 | 241 | 4.01 |
| Non-Complicated > Complicated Grief | ||||||
| Superior Temporal Gyrus | −54 | −2 | −6 | 10 | 48 | 5.10 |
| Right Insula | 42 | −12 | 0 | 13 | 38 | 4.76 |
| Left Insula | −38 | −24 | 6 | 13 | 28 | 4.84 |
| Right Angular Gyrus | 52 | −62 | 28 | 39 | 26 | 4.34 |
Figure 3.
Regional Grief > Neutral activation in the orbitofrontal cortex (x=4, y=54, z=−10, p=.001, uncorrected) in the Non-Complicated Grief > Nonbereaved group analysis.
Figure 4.
Regional Grief > Neutral activation in the rostral/pregenual anterior cingulate cortex (x=−6, y=48, z=14; t=4.25; p < .005, uncorrected) in the Non-Complicated Grief > Nonbereaved group analysis.
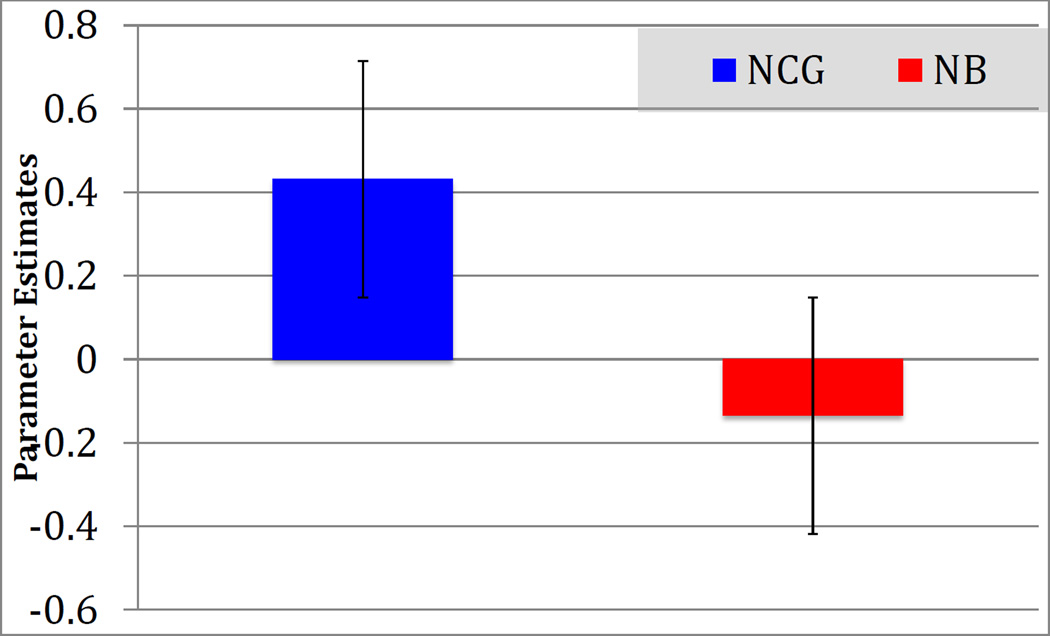
To determine the direction of the differential activation in right orbitofrontal cortex and rACC in the Non-CG > Nonbereaved group in the Grief > Neutral condition, a voxel of interest (VOI) analysis was completed using the peak activation coordinates derived from the whole-brain contrast (x=4, y=54, z=−10). Parameter estimates for the Non-CG group indicated significant activation (M = .43, SD = .31) while those for the Nonbereaved group indicated no activation and even slight deactivation (M = −.14, SD = .20; See Figure 5).
Figure 5.
Parameter estimates in the orbitofrontal VOI (x=4, y=54, z=−10) contrast; Grief > Neutral stimuli in the Non-Complicated Grief group compared to Nonbereaved controls. Estimates indicate that NCG participants showed significant recruitment of this region, while NB showed little or no recruitment differences across types of stimulus.
The Non-CG > CG contrast showed greater activation in the left superior temporal gyrus and bilaterally in the insula CG did not show significantly greater activations than Non-CG in any regions (Table 2). No regions were significantly activated when contrasting the CG from the Nonbereaved group. Similarly, comparing CG and Nonbereaved groups in an ROI analysis of the rACC, based on an a priori seed region from the literature, yielded null results.
3.1 Post-hoc analysis
Corresponding behavioral data gathered outside the scanner in a larger group that included the present participants potentially indicate a time-delayed regulatory response in the CG group, wherein successful regulation occurred only in latter blocks of the task (O’Connor & Arizmendi, 2014). In order to determine whether those with CG recruited frontocortical regions in a time-delayed manner, a post-hoc analysis examining the activation in block four of grief stimuli compared to block one of grief stimuli was conducted (Figure 1). Grief Block 4 > Grief Block 1 analysis yielded no significant activity in the rACC region in separate ROI analyses in any of the three groups, implying that this region was not being activated late in the task.
However, grief-related words produced significantly greater dACC activation in Grief Block 4 > Grief Block 1 in the CG group (x=8, y=22, z=28, t=7.66; p < .001, uncorrected; Figure 6) and to a lesser extent, in the Nonbereaved group (x=6, y=22, z=28, t=4.62; p < .005, uncorrected). The same contrast analysis yielded no significant activation in the Non-CG group in the Grief Block 4 > Grief Block 1 contrast. Between group analyses comparing parameter estimates of grief-related activity in the dACC did not differ significantly between CG and Nonbereaved groups, t(17) = .67, p = .51. These groups were not compared to the Non-CG group, as that group showed no increased dACC activation.
Figure 6.
Regional Grief Block 4 > Grief Block 1 activation in the dorsal anterior cingulate cortex (x=8, y=22, z=28) in the Complicated Grief group.
Discussion
The current study investigated neural activity associated with grief severity in older spousally bereaved adults. Informed by both the psychopathology and ecStroop literature, we hypothesized that those suffering from CG would show differential rACC activation when compared to other groups, due to its association with regulation of response to emotionally salient stimuli. We further hypothesized that those with Non-CG would show significantly higher rACC recruitment, and Nonbereaved individuals would show little or no increase in activity in the rACC. Results indicate that those with Non-CG showed significantly higher recruitment of the brain regions of a priori interest (rACC), with peak activation in this cluster found anteriorly, in the right OFC. However, this pattern of activation was not observed in participants with CG, nor those in the Nonbereaved group.
To summarize, Non-CG showed greater rACC activation than the Nonbereaved group, and those with CG did not show differential activation relative to the Nonbereaved group. One interpretation of this data suggests that when confronted with the distressing, universal experience of bereavement, the CG and Non-CG groups responded with different neural strategies (corroborated by neuropsychological performance). Given the theoretical importance of investigating the rACC activation between CG and Non-CG, we investigated the regional activation at a lower level of significance, even though there was no statistically significant difference. Indeed, in this contrast, only 25% of those with CG showed rACC activation and 40% of those with Non-CG showed rACC activation, and at much higher levels. With higher numbers of participants, this may have shown a statistically significant difference.
Although no prior tasks requiring task-related response to emotional stimuli have been used in imaging studies of CG, reduced activity in the rACC observed in the present study falls in line with imaging studies examining emotional responses in other pathologies. Deficient activation in rACC is associated with GAD, bereavement due to pet loss (but not CG per se) and PTSD. Two of these studies also indicated a deficit in functional connectivity between the rACC and the amygdala, implying a deficit not only in recruiting relevant control regions, but also in the communication necessary for this region to influence the emotion-relevant limbic network. Further evidence comes from studies in ecStroop literature; PTSD and familial history of depression also show recruitment deficits in rACC regions during task performance. Although psychopathology symptoms are sometimes found in those with CG, the three groups in the present study did not have co-occurring clinically significant psychopathology.
Although results here mirror those seen in tasks of emotional regulation, the rACC has been implicated in various other emotion-related psychological processes. The personal relevance of stimuli and the evaluation of one’s own current emotional state have both been associated with rACC activity (Smith, Allen, Thayer & Lane, 2015; Lee & Siegle, 2009). Both of these mental processes may co-occur with the conflict resolution in the ec-Stroop or directly contribute to performance of the ecStroop task and as such, it is not possible to discriminate which of these mental processes (i.e., emotion regulation, personal relevance or evaluation of current emotional state) is specifically associated with the rACC activation in the present study.
It may seem unexpected that we found no difference between CG and Nonbereaved groups in the rACC activation, where previous studies have found a deficit in functional recruitment in psychopathology when compared to controls. However, we do not believe that the reduced functional ACC activity seen in both CG and NB control groups in the present study means that these two groups are functionally similar. We know that those with CG showed significantly longer response latencies than controls, indicating a behavioral disruption in the CG group but not in the controls (O’Connor & Arizmendi, 2014).
4.1 CG and avoidance
Theorists studying CG suggest that the interplay of approach and avoidance behavior is implicated in the maintenance of grief symptoms. Those with CG regularly engage in avoidance behavior, such as refraining from engaging in activities previously enjoyed with the deceased (Shear et al., 2007). In this context, avoidance serves to reduce fear of intense sadness and yearning for the deceased. In line with this, fMRI evidence indicates that when presented with photos of the deceased, those with CG show significantly greater activity in the nucleus accumbens, a region associated with desire and wanting (O’Connor et al., 2008). This evidence suggests that individuals with CG have an intrusive longing for the deceased, and actively manage this longing by engaging in avoidance behavior.
Although avoidance behavior may be exercised successfully in daily living, evidence from emotion conflict tasks indicates successful emotion regulation may be disrupted when those with CG are directly confronted with reminders of the deceased. This disruption is likely one aspect of avoidance that comes to bear during an emotion regulation task.
Behaviorally, this is manifested as a delayed reaction time that endures for the entirety of a performance regulation task (O’Connor and Arizmendi, 2014; Maccallum & Bryant, 2010; Mancini & Bonanno, 2012). From a neuroanatomical standpoint, the current findings may be interpreted as a poorer performance associated with significant deficit in the ability to recruit brain regions required to resolve a conflict and generate a behavioral response (including the rACC and the insula). Instead, those with CG, when forced to confront semantic reminders of their loss event, experience the emotion with little or no ability to engage regulatory processes. Thus, avoidance may look like an emotional/ cognitive “overload” that requires increased cognitive effort in order to be resolved (Warren et al., 2010). Only individuals in the CG group appear to be unable to compensate, resulting in considerably slower reaction time and a recruitment deficit in the cortical regions implicated in emotion regulation. However, this interpretation must be made cautiously, as the task may have conflated the conflict resolution and the personal relevance of the stimuli or the self-evaluation of the elicited emotional state.
4.2 Delayed conflict resolution and the dACC
We considered that the task design would mask any temporal effects in MR signal as the task progresses. Furthermore, reaction time data from a previous study by the current authors demonstrate notable improvement in the Non-CG group across grief blocks, indicating an effect of time, or learning, in the task (O’Connor & Arizmendi, 2014). This reaction time improvement was not observed in the CG group in the prior study. Significantly greater activation in the dACC in Grief Block 4 > Grief Block 1 was seen for the CG and Nonbereaved groups. For those with no symptomatology (e.g., the Nonbereaved healthy controls) these data, coupled with previous research, indicate that the dACC may be involved in the processing of idiographic stimuli in asymptomatic individuals (Etkin, 2011).
The lack of published research directly related to the grief population precludes making any evidence-based inferences about this finding. However, a few possibilities are plausible. The first posits that some small regulatory effect may be coming “online” nearer to the end of the task. This hypothesis is supported by evidence indicating the co-dependent role of the dACC and rACC and, in this context, suggests that those with CG may be able to engage in emotion regulation but only after a severe delay (Etkin et al., 2011). However, this hypothesis would also suggest a late-onset regulatory effect in the Nonbereaved group as well – a delay that is not corroborated by their behavioral data (O’Connor & Arizmendi, 2014). An alternative explanation is that both the CG and Nonbereaved groups are engaging in error/task performance monitoring later in the task. There is considerable evidence implicating the dACC success of task performance (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). Furthermore, Ridderinkhof and colleagues (2004) also suggest that while the dACC is required for performance evaluation, the rACC is recruited for modulation of behavior. As evidenced by reaction time data, neither group modulated behavior over the course of the task, as those with CG consistently performed poorly, and those in the Nonbereaved group consistently performed well (O’Connor & Arizmendi, 2014). Finally, these results may not suggest error-monitoring activity, but instead simply arise as a function of time spent performing the task, or time on task (Grinband et al., 2011).
4.3 Non-CG and Emotion Processing
Although the CG group failed to recruit the rACC, those in the Non-CG group showed significant grief-related recruitment of the midline cortical right OFC and rACC when compared to Nonbereaved controls. Furthermore, parameter estimates of this region indicate directional differences in BOLD signal between the Non-CG group and the Nonbereaved controls. In fact, the recruitment of the rACC in the control group was slightly higher in neutral-word trials compared to grief-related stimuli (Figure 5) suggesting that grief-related stimuli had no differential effect on Nonbereaved individuals. Similar to results observed in the Non-CG group, these regions are hypothesized to come “on line” in order reappraise negative stimuli and evaluate reward (Golkar et al., 2012). Taken together with grief-related behavioral data previously published on this sample, it is likely that those with grief, but not CG, were successful in recruiting the relevant regulatory regions (O’Connor & Arizmendi, 2014). Those in the Nonbereaved group required no such increase in regulatory input.
Although not an area of interest in this study, data here also indicate significantly increased posterior cingulate and precuneus activity in the NCG group, consistent with findings in other imaging studies of grief (Figure 4; Table 2; Gündel, O’Connor, Littrell, Fort, & Lane, 2003). Not surprisingly, this area is often associated with recall of autobiographical memory and memory retrieval especially when exposed to semantic stimuli (Cavanna & Trimble, 2006).
4.4 Limitations
Study limitations include the fact that no behavioral data were recorded during task completion in the scanner, thus all discussion regarding reaction time patterns in these groups is based on data collected from a larger inclusive group outside the fMRI environment. Future studies should collect concurrent behavioral and fMRI data during task completion to clarify the association between conflict resolution and regional activation specifically. In addition, main effects seen in the NCG, although significant, do not survive family-wise error correction, although this is of lesser concern when considering the large cluster size activated, and the emphasis on avoidance of Type-II (missed true effects) in affect-related fMRI literature (Lieberman & Cunningham, 2009).
4.5 Summary
The present study is the first to examine the neural correlates of behavior regulation during an emotion elicitation task in those suffering from CG. Similar to previous imaging studies investigating emotion regulation in psychopathology, those with CG did showed significant dysfunction in the rACC in response to grief-related stimuli. However, this group did show activation in the dACC during the last grief-related block of the task. It is possible that this pattern of activation indicates that the dACC may be “coming online” later in the task during performance and error monitoring when the rACC is not successfully recruited for regulatory purposes. Those with Non-CG exhibited recruitment in prefrontal regions (medial OFC and rACC) during grief-related trials, indicating successful engagement in emotion regulation. As expected, Nonbereaved individuals showed no significant change in grief vs. neutral stimuli, likely because these grief-specific words were not salient. Overall, these results support a model of CG that implicates avoidance and disruption of emotion regulation as a meaningful component of psychopathology onset and maintenance.
Highlights.
We examined functional activity during emotion processing in bereaved adults.
We compared three groups: Complicated Grief (CG), Non-Complicated Grief (NCG), and Nonbereaved.
CG individuals showed an absence of increased fronto-cortical recruitment relative to controls.
Activity in orbitofrontal cortex was elevated in the NCG group compared to Nonbereaved.
CG individuals show relative inability to recruit regions for grief-related emotion processing.
Acknowledgments
The Complicated Grief in Older Adults study was supported by a grant from the National Institutes of Aging (K01-AG028404) awarded to Dr. Mary-Frances O’Connor, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algom D, Chajut E, Lev S. A rational look at the emotional Stroop phenomenon: A generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133(3):323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychological medicine. 2013;43(07):1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Gold AL, Deckersbach T, Rauch SL. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depression and Anxiety. 2009;26(9):796–805. doi: 10.1002/da.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive an emotional influences in anterior cingulate cortex. Trend in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. American Journal of Psychiatry. 2011;168(9):968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Freed PJ, Yanagihara TK, Hirsch J, Mann JJ. Neural Mechanisms of Grief Regulation. Biological Psychiatry. 2009;66(1):33–40. doi: 10.1016/j.biopsych.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, Schalling M, Ingvar M, Öhman A. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS ONE. 2012;7(11):e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57(2):303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündel H, O’Connor M-F, Littrell L, Fort C, Lane RD. Functional neuroanatomy of grief: An fMRI study. The American Journal of Psychiatry. 2003;160(11):1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magnetic Resonance in Medicine. 1994;31(3):283–291. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Human Brain Mapping. 2006;27(10):779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Emotion speeds up conflict resolution: a new role for the ventral anterior cingulate cortex? Cerebral Cortex. 2010:bhq157. doi: 10.1093/cercor/bhq157. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Emotion triggers executive attention: anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Human brain mapping. 2011;32(2):198–208. doi: 10.1002/hbm.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kersting A, Brähler E, Glaesmer H, Wagner B. Prevalence of complicated grief in a representative population-based sample. Journal of Affective Disorders. 2011;131(1–3):339–343. doi: 10.1016/j.jad.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Kersting A, Ohrmann P, Pederson A, Kroker K, Samberg D, Bauer J, Kugel H, Koelkebeck K, Steinhard J, Heindel W, Arolt V, Suslow T. Neural activation underlying acute grief in women after the loss of an unborn child. American Journal of Psychiatry. 2009;166(12):1402–1410. doi: 10.1176/appi.ajp.2009.08121875. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depression and anxiety. 2011;28(3):194–201. doi: 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2013;3(1):7. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical psychology review. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kredenster MS. Speech given at the Annual Convention of the Anxiety and Depression Association of America. Chicago, IL: 2014. Reliability and Validity of the Inventory of Complicated Grief in a Manitoba first nation population bereaved by suicide. [Google Scholar]
- Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Social and Cognitive and Affective Neuroscience. 2012;7:521–34. doi: 10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccallum F, Bryant RA. Attentional bias in complicated grief. Journal of Affective Disorders. 2010;125(1–3):316–322. doi: 10.1016/j.jad.2010.01.070. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter C. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mancini AD, Bonanno GA. The persistence of attachment: Complicated grief, threat, and reaction times to the deceased’s name. Journal of Affective Disorders. 2012;139(2):256–263. doi: 10.1016/j.jad.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie ZN, Norbury R, Murphy SE, Inkster B, Harmer CJ, Cowen PJ. Affective modulation of anterior cingulate cortex in young people at increased familial risk for depression. British Journal of Psychiatry. 2008;192:356–361. doi: 10.1192/bjp.bp.107.043398. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318(5852):987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE. A systematic review of fMRI studies in generalized anxiety disorder: Evaluating its neural and cognitive basis. Journal of Affective Disorders. 2014;167:336–342. doi: 10.1016/j.jad.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho M-HR, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- O’Connor M-F. Immunological and neuroimaging biomarkers of complicated grief. Dialogues in Clinical Neuroscience. 2012;14(2):141–148. doi: 10.31887/DCNS.2012.14.2/mfoconnor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Arizmendi BJ. Neuropsychological correlates of Complicated Grief in older spousally bereaved adults. The Journals of Gerontology, series B: Psychological Sciences and Social Sciences. 2014;69:12–18. doi: 10.1093/geronb/gbt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Schultze-Florey CR, Irwin MR, Arevalo JM, Cole SW. Divergent gene expression responses to Complicated Grief and Non-complicated Grief. Brain, Behavior, and Immunity. 2014;37:78–83. doi: 10.1016/j.bbi.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates the brain’s reward center. NeuroImage. 2008;42(2):969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, Ryan ND. Looking under the hood of the dot–probe task: an fmri study in anxious youth. Depression and Anxiety. 2014;31(3):178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Horowitz MJ, Jacobs SC, Parkes CM, Aslan M, Goodkin K, Raphael B, Marwit SJ, Wortman C, Meimeyer RA, Bonnano G, Block SD, Kissane D, Boelen P, Maercker Litz BT, Johnson JG, First MB, Maciejewski PK. Prolonged Grief Disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Medicine. 2009:e1000121. doi: 10.1371/journal.pmed.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Reynolds CF, III, Bierhals AJ, Newsom JT, Fasiczka A, Frank E, Doman J, Miller M. Inventory of complicated grief: A scale to measure maladaptive symptoms of loss. Psychiatry Research. 1995;59(1–2):65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robinaugh DJ, LeBlanc NJ, Vuletich HA, McNally RJ. Network analysis of Persistent Complex Bereavement Disorder in conjugally bereaved adults. Journal of Abnormal Psychology. 2014;123(3):510–522. doi: 10.1037/abn0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Florey CR, Martinez-Maza O, Magpantay L, Breen EC, Irwin MR, Gündel H, et al. When grief makes you sick: Bereavement induced systemic inflammation is a question of genotype. Brain, Behavior, and Immunity. 2012;26(7):1066–1071. doi: 10.1016/j.bbi.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK. Grief and mourning gone awry: pathway and course of complicated grief. Dialogues in Clinical Neuroscience. 2012;14:119–128. doi: 10.31887/DCNS.2012.14.2/mshear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear K, Frank E, Houck PR, Reynolds CF., III Treatment of Complicated Grief: A randomized controlled trial. Journal of the American Medical Association. 2005;293(21):2601–2608. doi: 10.1001/jama.293.21.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear K, Monk T, Houck P, Melhem N, Frank E, Reynolds C, Sillowash R. An attachment-based model of complicated grief including the role of avoidance. European Archives of Psychiatry and Clinical Neuroscience. 2007;257(8):453–461. doi: 10.1007/s00406-007-0745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Smith R, Allen JJ, Thayer JF, Lane RD. Altered functional connectivity between medial prefrontal cortex and the inferior brainstem in major depression during appraisal of subjective emotional responses: A preliminary study. Biological psychology. 2015;108:13–24. doi: 10.1016/j.biopsycho.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Phan KL, Angstadt M, Klumpp H, Fitzgerald KD, Monk CS. altered activation of the rostral anterior cingulate cortex in the context of emotional face distractors in children and adolescents with anxiety disorders. Depression and Anxiety. 2014 doi: 10.1002/da.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol MJ, Veer IM, van der Wee NJ, Aleman A, van Buchem MA, Rombouts SA, Johnstone T. Whole-brain functional connectivity during emotional word classification in medication-free Major Depressive Disorder: Abnormal salience circuitry and relations to positive emotionality. NeuroImage: clinical. 2013;2:790–796. doi: 10.1016/j.nicl.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SL, Bost KK, Roisman GI, Silton RL, Spielberg JM, Engels AS, et al. Effects of adult attachment and emotional distractors on brain mechanisms of cognitive control. Psychological Science. 2010;21(12):1818–1826. doi: 10.1177/0956797610388809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Tenenbaum H, Goldberg M, Freeman B, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. PAIN. 2011;152(2):384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally R, Willhelm S, McInerney Jenike MA, Rauch SL. The emotional counting stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;12:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, Shin LM, Rauch SL. The emotional counting Stroop: A task for assessing emotional interference during brain imaging. Nature Protocols. 2006;1:293–296. doi: 10.1038/nprot.2006.45. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]