Abstract
Primary oral melanoma is known to be an extremely rare and aggressive neoplasm arising from the mucosal epithelium of the oral cavity especially upper jaw (palate or alveolar gingivae). Malignant melanoma that does not originate in the skin is a very rare disease and is considered one of the most deadly of all human neoplasms. Oral malignant melanoma (OMM) represents about 1% of all melanomas and approximately 0.5% of all oral malignancies. OMM has been reported in patients aged 20 to 80 years and has a male predilection. Because most mucosal melanotic lesions are painless in their early stages, so delayed recognition and subsequent treatment result in worst prognosis. Here, we report three cases with significant heterogeneity in morphological features and biologic behavior.
Keywords: Malignant, melanoma, neoplasm
INTRODUCTION
Malignant melanoma (MM) is a potentially aggressive tumor of melanocytic origin.[1] Primary oral MM represents about 0.2-8% of all melanomas.[2] Only about 1% of all melanomas arise in the oral mucosa and these account for 0.5% of all oral malignancies.[1] The most frequently affected oral sites are palate and maxillary gingival.[1] Less frequently they can be found in mucosal epithelium, stria vascularis of inner ears, retina, and uveal tract.[3] The clinical presentation of this condition can vary widely, from a typically pigmented macular or proliferative lesion to a nonpigmented, soft vascular tumor, single or multiple, primary or metastatic. Nonpigmented forms of MM often cannot be distinguished clinically from other benign or malignant oral tumors and only biopsy can establish the diagnosis.[4]
The initial symptom and sign of oral malignant melanoma (OMM) is often swelling, which is usually pigmented. OMM may be uniformly brown or black, or may show variation of colour, with black, brown, grey, purple and red shades, or depigmentations.[1] Satellite foci around the primary also may be present, giving some support to the theory of “field cancerization” already proposed for other histological types of malignancy.[5]
In amelanotic melanomas, pigmentation is absent. The tumor surface may be smooth, with an intact overlying mucosa, or may be ulcerated. Other presenting signs and symptoms include bleeding, ill-fitting dentures, pain, increased mobility of teeth, and delayed healing of extraction sockets. Regional lymphadenopathy may be present and connotes a poor prognosis.[1]
Here, we are reporting three cases of MM with different presentations and treatment modalities. The frequently delayed diagnosis of OMM may be primarily due to insufficient knowledge and experience with this rare cancer, resulting in lack of awareness among professionals. As all aggressive cancers, it requires early diagnosis and treatment and it is necessary to draw the attention of health care professionals into performing a more detailed oral mucosa examination and biopsies of all nodular or macular suspect lesions on palate and gum, regardless of color, and pigmentation.[3]
CASE REPORTS
Case 1
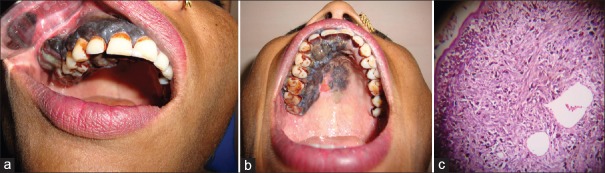
A 32-year-old female patient reported the department of oral and maxillofacial pathology with chief complaint of pain on palate since 1 year. The lesion was rapidly progressive and pain was continuous and intense in nature. On intraoral examination, lesion extended from maxillary left central incisor to maxillary right third molar as well as involving whole of the buccal gingiva. The lesion on gingiva presented as band-like thickening extending from marginal gingiva to mucobuccal fold also involving interdental papilla and buccal frenum [Figure 1a]. It was associated with surface nodularity, erythematous areas, and spontaneous bleeding. Lesion on the palatal side was extending from left central incisor to right third molar from cemento-enamel junction CEJ to 3 mm away from midpalatine raphae as well as patchy pigmentation was also seen on the midline of palate with erythematous zone [Figure 1b]. The right submandibular lymph node was palpable about 1.5 cm in dimensions, firm, and tender. Infrastructural maxillectomy was performed with safe margins of 1 cm.
Figure 1.
(a) Intraorally, the lesion on gingiva presented as band-like thickening extending from marginal gingiva to mucobuccal fold also involving interdental papilla and buccal frenum, (b) Lesion on the palatal side extended from CEJ to 3 mm away from midpalatine raphae as well as patchy pigmentation was also seen on the midline of palate with erythematous zone, (c) Photomicrograph (H and E, ×100)
Case 2
A 30-year-old female patient reported with chief complaint of a black colored lesion on the floor of mouth since 7 months. The lesion extended from mandibular right retromolar area to left retromolar area. The lesion was predominantly present on anterior region of the floor of mouth also involving lingual frenum and openings of the salivary glands [Figure 2a]. Along with this, diffuse ulceration was present on posterior region of left side of mandible. The lymph nodes were not palpable. Orthopantomograph and chest radiograph was done, which revealed no significant findings.
Figure 2.
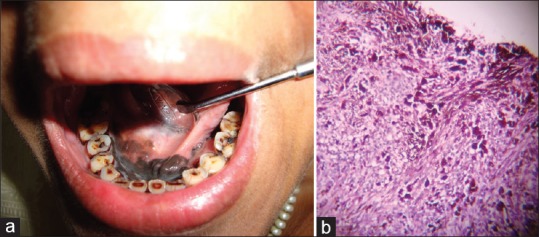
(a) The lesion was predominantly present on anterior region of the floor of mouth also involving lingual frenum and openings of the salivary glands, (b) Photomicrograph (H and E, ×100)
Case 3
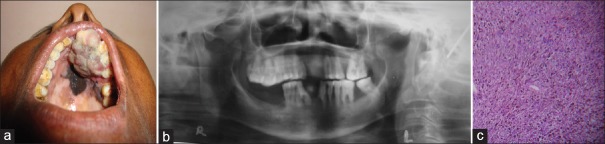
A 55-year-old female patient presented with complaint of a painful swelling in the left maxillary region for last 8 months. The medical and dental histories were noncontributory. History of tobacco consumption in the chewing form was positive. The oral examination revealed an expansile mass of 3 × 4 cm in the left maxillary region involving anterior and premolar teeth. The lesion was focally erythematous, with well-defined borders and an intact, irregular and lobulated palatal mucosal surface [Figure 3a]. Radiographic examination using orthopantomograph [Figure 3b] and computed tomography (CT) revealed a well-defined lesion with areas of bony destruction. The left submandibular lymph nodes were palpable and measured roughly 2 cm in greatest dimensions, were mobile and nontender.
Figure 3.
(a) An expansile mass of 3 × 4 cm in the left maxillary region involving anterior and premolar teeth. The lesion was focally erythematous, with well-defined borders and an intact, irregular, and lobulated palatal mucosal surface, (b) Orthopantomograph revealed a well-defined lesion with areas of bony destruction, (c) Photomicrograph (H and E, ×100)
The histopathological examination [Figures 1c, 2b, 3c] collectively revealed malignant cells in nests, clusters, and in organoid arrangements. The cells appeared to have large nuclei with prominent nucleoli and abundant cytoplasm. At few places, the cells also appeared to be spindled and melanin component predominated in all the cases. The cases were then confirmed as OMM.
DISCUSSION
MM was first described by Weber in 1859. It was recognized as a distinct clinical entity as “melanotic sarcoma” by Lucke in 1869 as also highlighted by Rimal et al.[6] A melanoma is a malignant tumor comprising melanocytes, cells derived from the neural crest that constitute the melanin pigment in the basal layer of epithelium.[7] This pigmentation is observed diffusely as racial pigmentation, which can be patchy and focally distributed as melanotic macules and nevi. It is considered primary OMM when the following criteria described by Greene (1953) are fulfilled:
Demonstration of melanoma in the oral mucosa
Presence of junctional activity
Inability to demonstrate extraoral primary melanoma.[8]
As neuroectodermal derivatives, melanocytes are known to migrate to the skin, retina, uveal tract, and other ectodermally derived mucosae. Melanocytes migrate much less frequently to endodermally derived mucosae, such as the nasopharynx, larynx, tracheobronchial tree, and esophagus. This explains the lower frequency of melanoma in these locations. Melanocytes have also been documented in the deep stroma of oral mucosa. Because mucosal melanomas are frequently found at mucocutaneous junctions, they were once believed to arise only as extensions of melanocytic hyperplasia from adjacent skin.[7]
It is interesting that the most prevalent site for head and neck melanomas (the nasal cavity) is in close anatomic proximity to the most common site in the oral cavity (the palate). It has been speculated that mucosal melanomas may arise from both pigment-producing cells and Schwann cells found in the mucous membrane.[9]
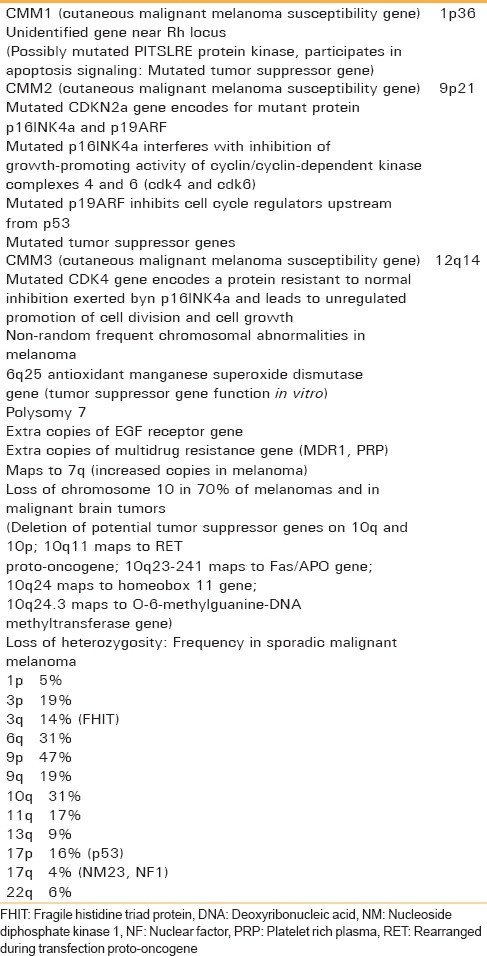
A study demonstrates the loss of heterozygosity at 12p13 and loss of p27K1P1 protein expression contribute to melanoma progression. Cytogenetic analysis and evaluation of melanocyte-specific gene-1 appears to be very helpful for understanding the pathogenesis of OMM [Table 1].[10]
Table 1.
Cytogenetic abnormalities in melanoma[10]
On the contrary, the specific cause of occurrence of amelanotic melanoma is the lack of melanotic pigmentation in these lesions. It is proposed that there is a deficiency in tyrosine and an enzyme required for melanin production. Others believe that this enzyme system is intact and can produce melanin, but the quantity is insufficient to be seen with histologic methods. The latter theory is favored because electron microscopy has revealed the presence of melanosomes in all amelanotic melanomas examined to date.[11]
Tobacco use, chronic irritation from ill-fitting dentures,[1] and alcohol[7] has been mentioned as possible risk factors.[1] Ingested and inhaled environmental carcinogens at high internal body temperature may play some role.[1] Although, ultraviolet-B light is believed to be the most important factor during sun exposure.[7] According to epidemiologic data, those with blond hair, blue eyes, and those who tend to burn or freckle easily after sun exposure are at the highest risk.[7] Some melanoma associated antigens become expressed during the transformation process from a benign melanocytic nevus to melanoma; most of these are related to the melanin production process and most are human leukocyte antigen-restricted.[1] Studies have postulated that DNA repair capacity may modify the risk of melanoma in the presence of other strong risk factors.[3]
MM of the skin has been divided into four types: (i) Lentigo maligna melanoma, (ii) superficial spreading melanoma, (iii) nodular melanoma, and (iv) acral-lentiginous melanoma.[7] Oral melanotic macules (increased melanotic pigmentation in the basal and occasionally in the immediately suprabasal keratinocytes), melanoacanthoma or melanoacanthosis (probably reactive proliferation of both keratinocytes and melanocytes), and oral melanocytic nevi can represent oral lesions preceding OMM.[7] Tanaka et al.,[12] identified five types of OMM on the basis of the clinical appearance: Pigmented nodular type, nonpigmented nodular type, pigmented macular type, pigmented mixed type, and nonpigmented mixed type.
In 1995 WESTOP Banff Workshop recommended that oral mucosa should be classified separately from cutaneous lesions and terminology should include descriptive terms such as melanoma in situ and invasive melanoma. In association with these categories, two further types were considered. One type is described as invasive melanoma with an in situ component (mixed in situ and invasive oral mucosal melanomas) and the other type is defined as an atypical melanocytic proliferation (borderline lesion) to identify lesions that may have originated from an in situ melanoma.[7]
The histological microstaging system of Clark [Table 2][13] used in cutaneous melanoma cannot be applied to oral mucosa because of the lack of histologic landmarks analogous to papillary and reticular dermis. The 2002 (revised) TNM Melanoma Staging system of the American Joint Committee on Cancer applying the T-symbol for the thickness and the ulceration status of the tumor, the symbol N for the regional lymph nodes. The symbol M for distant metastases and the serum Lactic Dehydrogenase level does not provide specific guidelines for OMM. A recent histopathological microstaging for stage 1 subclassifies three levels [Table 3].[1]
Table 2.
Classification of tumor based on clark's level[7]

Table 3.
Clinical staging system for OMM with histopathological microstaging for stage I[1]

The highest age standardized rates (ASRW) per 100,000 were observed among both men (55.8) and women (41.1) in Queensland, Australia. The lowest ASR (W) were found for men (0.1) in Harbin, China and for women (0.1) in Nagpur, India.[2] The age of reported patient's ranges from 20 to 80 years, some authors have reported a male preponderance.[1] The palatal mucosa is the most commonly affected area in the oral cavity, followed by maxillary gingival (77% from these sites).[6] Reported cases of melanoma were also seen on the mucosa of mandible, maxilla, and tongue.[14,15] The occurrence of developing amelanotic lesions in melanomas of the oral mucosa varies but even in amelanotic melanomas, small amounts of melanin are often produced and a few flecks may be detected on close inspection.[16] Melanocytic activity, clinically corresponding to a light-brown to black pigmentation gradually fading in the surrounding mucosa, is seen in most patients.[5] OMM typically presents an aggressive vertical growth phase, with invasion of the underlying submucosa.[17] Clinically, OMM lesions may be macular or nodular[3] as also seen in our cases. Colors can range from brown, grey, black, white, purple, and red shades. Satellite lesions are frequently present surrounding the initial tumor.[3]
Melanomas may be difficult to distinguish on a clinical basis from solitary oral melanotic macules, labial lentigines, foreign bodies, or racial pigmentation. Amelanotic lesions may simulate pyogenic granulomas.[7] MM must also be differentiated from other forms of pigmented oral disease, including drug, disease or smoking-associated melanosis, oral melanotic macule, Kaposi's sarcoma, physiologic or racial pigmentation, melanocytic nevus, melanoacanthoma, amalgam tattoo, vascular blood-related pigments, oral melanotic macule, physiologic pigmentation, Peutz-Jeghers syndrome, Addison disease, and drug-induced pigmentation.[7]
On histologic evaluation, the tumor is composed of cells that range from compact spindled through ovoid to large epithelioid and clear cells. The clear cells often have grossly enlarged nuclei and prominent amphophilic or slightly eosinophilic nucleoli that are central when single. Mitoses are frequent and often abnormal. Large cytoplasmic pigment granules are prominent in areas, and a lighter more finely dispersed granular pigment is usually present in many cells. Cells are arranged in loosely cohesive sheets, which in places can form alveolar clefts; in clear cell zones, a tendency to form nests or islands is present.[18] Malignant cells of OMM show a wide range of shapes, including spindle, plasmocytoid, clear cell, and epithelioid ones. Atypical melanocytes have been defined as melanocytes with hyperchromatic and angular nuclei, but with infrequent mitotic figures.[1] Many of these features were also evident in our cases. The histologic spectrum of melanoma also includes rare instances of an additional osseous and/or chondroid component within the substance of the neoplasm.[19] In 1971, Conley et al.,[20] reported a rare variant of spindle cell melanoma, generally located in the head and neck and coined the term, “desmoplastic malignant melanoma (DMM)”. The histogenesis of DMM is unclear. Currently, it is believed that the desmoplastic component arises from the intraepidermal melanocytic component and undergoes reactive fibroplasia into the spindle neoplasm.
Immunohistochemistry such as positive staining for vimentin, S-100 protein, HMB-45, MART-1/Melan A, tyrosinase, NKI/C-3, and microphthalmia transcription factor (MiTF) aid the diagnosis. Vimentin is the most consistent but the least useful diagnostically.[17] Immunostaining with HMB-45, cytokeratin, and neuron-specific enolase are also required for establishing the differential diagnosis.[21]
During the transformation process from a benign melanocytic nevus to the premalignant stage (atypical melanocytic hyperplasia/dysplastic nevus) to melanoma, several melanoma-associated antigens, cell-to-cell and cell matrix interacting antigens, signaling and transport function receptors and antigens, mutated tumor suppressor gene products, and proliferating antigens are expressed [Table 4].[10]
Table 4.
Tumor-associated antigens in melanoma[10]

A high degree of p53 immunonegativity may indicate an advanced stage of disease in HNMM, and recent studies have demonstrated a correlation between p53 immunonegativity and decreased responsiveness to chemotherapy in some types of tumors. Also, MDM2 immunonegativity may suggest the chemosensitivity of HNMM.[3] Melan A is positive in approximately 80% of melanomas. The positivity of MiTF is in the range of over 90%.[17]
The diagnostic modalities clinically include the so-called ABCD checklist (“Asymmetry”, “Border” irregularities, “Color” variegation and “Diameter” > 6 mm); “rubbing a gauze” method (Delgado et al.)[22]; cytological examination (Garzino-Demo et al.)[23] and radiographically by computed tomography, magnetic resonance (MR) imaging or positron emission tomography (Goerres et al.).[24] CT scan depicts MM as an expansile, homogeneously enhancing mass. Melanin has paramagnetic properties that can affect signal on MR images, on which melanotic melanomas have a characteristic intensity pattern: They appear hyperintense on T1-weighted sequences and hypotense on T2-weighted sequences.[25]
Histopathologic examination of an incisional or excisional biopsy remains the most accurate diagnostic tool.[5] Though it has been mentioned that incisional biopsy may negatively affect the prognosis, it has also been stated that biopsy of melanoma does not increase the risk of metastasis and does not affect the prognosis.[9] However, hematic and lymphatic dissemination is possible that may be followed by metastatic adenopathy especially in the tumors with vertical growth. The profuse vascularization could influence in the elevated incidence of metastasis.[8] Two separate measures of tumor invasion have been employed. Clark's levels of melanoma invasion evaluate the depth of invasion based upon anatomical compartments of the skin. A more reproducible method of assessing invasion is based upon measuring tumor thickness (Breslow tumor thickness) from the superficial aspect of the granular cell layer of the epidermis to the greatest depth of tumor cell penetration into the deep aspect of the skin.[10]
Previously, surgery was the primary treatment for malignant mucosal melanoma of the head and neck. However, at the time of diagnosis, most patients with Head and Neck Malignant Melanoma (HNMM) have already progressed to the vertical growth phase, with the cancer having already invaded the underlying submucosal tissues, causing significant destruction. With this degree of invasion, complete resection is difficult; this is compounded by the tendency of the tumors to spread rapidly, subsequently involving large areas of mucosa, making the margins difficult to delineate.[3] Melanoma in situ should have a 0.5 to 1.0 cm margin; thin melanomas (0.76 mm) should have a 1 to 2 cm margin; and intermediate and thick lesions (0.76 mm) should have a 2 to 3 cm margin.[11] According to Tanaka et al.,[12] neck dissection should be reserved for cases with preoperatively confirmed lymph node metastases and the choice of the neck dissection modality should be guided by the extent and level of lymph nodes.
A protocol adopted by Umeda and Shimada refers to the extent of the margins:
Excision of the primary lesion, preferably using an intraoral approach and involving at least 1.5 cm of healthy tissue
Excision of any lymph node metastases (stage II)
Consider chemotherapy.
Adjuvant chemotherapy with decarbazine, platinum analogs, nitrosureas, and microtubular toxins has been used for palliative purposes or for therapy of metastatic melanoma but does not seem to influence survival. Umeda and Shimada suggested dimethyl triazeno imidazole carboxamide, nimustine hydrochloride, or vincristine as drugs of choice for post operative chemotherapy.[1,26]
Postoperative radiotherapy is generally recommended if poor prognostic pathologic features are present, such as multiple positive nodes, or extranodal spread of metastastic melanoma, even though OMMs are regarded as poorly radiosensitive. Apparently, postoperative radiotherapy does not seem to improve the survival rate, even though on this point, no agreement exists in the literature. Current postoperative radiotherapy for cutaneous melanoma delivers 30 Gy or 24 Gy, for example, in five fractions of 6 Gy spread over 2.5 weeks or 3 weeks. Other irradiation modalities such as intraoral mould (60Co, 192Ir, or 198Au), intraoral electron beam or interstitial brachytherapy have also been used.[1] The additional use of the microbial immunostimulant, OK-432, injected around the tumor has been advocated for the treatment of melanoma and has been reported to be useful for prolonging survival periods in oral lesions.[16] Gene therapy is still in an experimental phase.[1]
Tumor thickness greater than 5 mm, presence of vascular invasion, necrosis, polymorphous tumor cell morphology, and the inability to properly resect the lesion with negative margins have been associated with poor survival in OMM.[1] As far as biological aggressiveness is concerned, it has been hypothesized that amelanotic melanomas are more anaplastic and have sacrificed the differentiated function to produce and store pigment.[16] The 5-year overall survival rate for HNMM ranges from 21% to 40% and for OMM it is 15% with a median survival of 25 months. Gingival melanoma has a better 5-year survival rate than palatal melanoma, with a longer median survival period (46 months versus 22 months).[1]
In a study on 35 patients with OMM, Tanaka et al.,[12] observed a 5-years cumulative survival rate of 35.5% in the group of patients treated with irradiation alone, while it was 15.4% for patients treated with surgery. On review of the approximately 1000 cases reported, it was found that the 5-year survival rate was 17% and the 10-year survival rate was 5%.[9]
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Meleti M, Leemans CR, Mooi WJ, Vescovi P, van der Waal I. Oral malignant melanoma: A review of the literature. Oral Oncol. 2007;43:116–21. doi: 10.1016/j.oraloncology.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Umeda M, Komatsubara H, Shibuya Y, Yokoo S, Komori T. Premalignant melanocytic dysplasia and malignant melanoma of the oral mucosa. Oral Oncol. 2002;38:714–22. doi: 10.1016/s1368-8375(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 3.Sortino-Rachou AM, Cancela Mde C, Voti L, Curado MP. Primary oral melanoma: Population-based incidence. Oral Oncol. 2009;45:254–8. doi: 10.1016/j.oraloncology.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrakopoulos I, Lazaridis N, Skordalaki A. Primary malignant melanoma of the oral cavity. Report of an unusual case. Aust Dent J. 1998;43:379–81. [PubMed] [Google Scholar]
- 5.Meleti M, Leemans CR, Mooi WJ, van der Waal I. Oral malignant melanoma: The amsterdam experience. J Oral Maxillofac Surg. 2007;65:2181–6. doi: 10.1016/j.joms.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Rimal J, Kasturi DP, Sumanth KN, Ongole R, Shrestha A. Intra-oral amelanotic malignant melanoma: Report of a case and review of literature. J Nepal Dent Assoc. 2009;10:49–52. [Google Scholar]
- 7.Femiano F, Lanza A, Buonaiuto C, Gombos F, Di Spirito F, Cirillo N. Oral malignant melanoma: A review of the literature. J Oral Pathol Med. 2008;37:383–8. doi: 10.1111/j.1600-0714.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 8.Aquas SC, Quarracino MC, Lence AN, Lanfranchi-Tizeira HE. Primary melanoma of the oral cavity: Ten cases and review of 177 cases from literature. Med Oral Patol Oral Cir Bucal. 2009;14:E265–71. [PubMed] [Google Scholar]
- 9.Gorsky M, Epstein JB. Melanoma arising from the mucosal surfaces of the head and Neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:715–9. doi: 10.1016/s1079-2104(98)90209-8. [DOI] [PubMed] [Google Scholar]
- 10.Hicks MJ, Flaitz CM. Oral mucosal melanoma: Epidemiology and pathobiology. Oral Oncol. 2000;36:152–69. doi: 10.1016/s1368-8375(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 11.Ducic Y, Pulsipher DA. Amelanotic melanoma of the palate: Report of case. J Oral Maxillofac Surg. 2001;59:580–3. doi: 10.1053/joms.2001.22695. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka N, Amagasa T, Iwaki H, Shioda S, Takeda M, Ohashi K, et al. Oral malignant melanoma in Japan. Oral Surg Oral Med Oral Pathol. 1994;78:81. doi: 10.1016/0030-4220(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 13.Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–26. [PubMed] [Google Scholar]
- 14.Tong Su, Liu B, Chen XM, Zhang WF, Zhao YF. Melanoma involving the mucosa on mandible and both maxilla simultaneously. Oral Oncol Extra. 2006;42:112–4. [Google Scholar]
- 15.Rowland HN, Schnetler JF. Primary malignant melanoma arising in the dorsum of the tongue. Br J Oral Maxillofac Surg. 2003;41:197–8. doi: 10.1016/s0266-4356(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 16.Notani K, Shindoh M, Yamazaki Y, Nakamura H, Watanabe M, Kogoh T, et al. Amelanotic malignant melanomas of the oral mucosa. Br J Oral Maxillofac Surg. 2002;40:195–200. doi: 10.1054/bjom.2001.0713. [DOI] [PubMed] [Google Scholar]
- 17.Andreadis D, Poulopoulos A, Nomikos A, Epivatianos A, Barbatis C. Diagnosis of metastatic malignant melanoma in parotid gland. Oral Oncol Extra. 2006;42:137–9. [Google Scholar]
- 18.Lombardi T, Haskell R, Morgan PR, Odell EW. An unusual intraosseous melanoma in the maxillary alveolus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:677–82. doi: 10.1016/s1079-2104(05)80251-3. [DOI] [PubMed] [Google Scholar]
- 19.Rinaggio J, Hameed M, Baredes S. Melanoma with cartilaginous differentiation originating within the mucosa of the nasal cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:861–5. doi: 10.1016/j.tripleo.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma) Cancer. 1971;28:914–36. doi: 10.1002/1097-0142(1971)28:4<914::aid-cncr2820280415>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Kao SY, Yang JC, Li WY, Chang RC. Maxillary amelanotic melanoma: A case report. J Oral Maxillofac Surg. 2001;59:700–3. doi: 10.1053/joms.2001.23409. [DOI] [PubMed] [Google Scholar]
- 22.Delgado Azañero WA, Mosqueda Taylor A. A practical method for clinical diagnosis of oral mucosal melanomas. Med Oral. 2003;8:348–52. [PubMed] [Google Scholar]
- 23.Garzino-Demo P, Fasolis M, Maggiore GM, Pagano M, Berrone S. Oral mucosal melanoma: A series of case reports. J Craniomaxillofac Surg. 2004;32:251–7. doi: 10.1016/j.jcms.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Goerres GW, Stoeckli SJ, von Schulthess GK, Steinert HC. FDG PET for mucosal malignant melanoma of the head and neck. Laryngoscope. 2002;112:381–5. doi: 10.1097/00005537-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama Y, Murakami S, Kawai T, Ishida T, Fuchihata H. Primary malignant melanoma in the oral mucosal membrane with metastasis in the cervical lymph node: MR appearance. AJNR Am J Neuroradiol. 1998;19:954–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Manganaro AM, Hammond HL, Dalton MJ, William TP. Oral melanoma: Case reports and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:670–6. doi: 10.1016/s1079-2104(05)80250-1. [DOI] [PubMed] [Google Scholar]