Abstract
Lasers have been introduced in dentistry as an alternative to conventional knife surgery. The advantage to the operator includes a clean dry field that enhances visibility and reduces the procedure time. The patient benefits by minimal postoperative pain and swelling. The paper discusses use of carbon dioxide laser in five conditions commonly encountered in oral cavity.
Keywords: Dental lasers, laser depigmentation, laser frenectomy
INTRODUCTION
The research on use of lasers in dentistry dates back as early as 1960s, but it was only in 1985 that the first documented use of a laser in periodontal surgery was published.[1]
Wigdor et al.,[2] described the advantages of lasers over cold steel surgical procedures as follows:
Dry and bloodless surgery
Minimal postoperative swelling and scarring
Reduces bacteremia
Instant sterilization of the surgical site
Reduced mechanical trauma
Minimal postoperative pain.
Studies on healing period of laser wounds compared to scalpel wounds have been rather inconclusive: Some suggest a faster healing rate,[3,4,5] some suggest slower[6,7,8,9,10] while some others report no significant difference.[11,12]
The mechanism behind laser hemostasis has been suggested to be increased platelet activation at the point of wound causing sealing of blood vessels.[13] Because lasers provide excellent hemostasis, the need for suturing is significantly reduced. Some lasers can even be used to weld the edges of tissue together and this in itself provides less need for suturing.[14,15,16] The reduction in postoperative swelling appears to be related to sealing of lymphatic vessels.[1] The interaction of laser energy with the pigmented portion of bacteria found in oral cavity contributes to reduction of bacterial count at the surgical site.[17,18,19,20,21,22] Another advantage of laser use in practise is the psychological effect on patient. It instills a greater confidence in the mind of the patient for the doctor who is seen as using the most latest and sophisticated equipment for treatment.[23]
CARBONDIOXIDE LASER (CO2)
The CO2 laser is a gas-active medium laser that incorporates a sealed tube containing a gaseous mixture with CO2 molecules pumped via electrical discharge current. The light energy, whose wavelength is 10,600 nm, is placed at the end of the mid-infra-red invisible non-ionizing portion of the spectrum, and it is delivered through a hollow tube-like waveguide. The CO2 laser's ability to provide the required power in continuous and gated modes using focused or nonfocused hand-pieces gives this instrument the versatility and precision required for soft tissue surgical procedures.
The wavelength is well absorbed by water, second only to erbium family. It can easily cut and coagulate soft tissue and in addition has a shallow depth of penetration into soft tissue, which gives it an edge in treating mucosal lesions.
This wavelength has the highest absorption in hydroxyapatite of any dental laser, about 1000 times greater than erbium. Therefore, tooth structure adjacent to soft tissue surgical site must be shielded from the incident laser beam. The continuous wave emission and the technology of delivery system limit hard-tissue applications because carbonization and crazing of tooth structure can occur due to the long pulse duration and low-peak powers.[24]
SOFT TISSUE SURGERY USING CO2 LASER
Frenectomy
Figure 1 shows the preoperative view of a frenum that was placing excessive tension on the marginal gingival between the two maxillary central incisors. The treatment was planned to remove the frenum pull. The area was anesthetized with 2% lignocaine and adrenaline in the ratio of 1:80,000 The frenum was excised with CO2 laser set at 5W-focused mode [Figure 2]. Figure 3 shows the immediate postoperative view. Note the char layer (biologic bandage) over the surgical site. Figure 4 shows the healing at 4 weeks.
Figure 1.
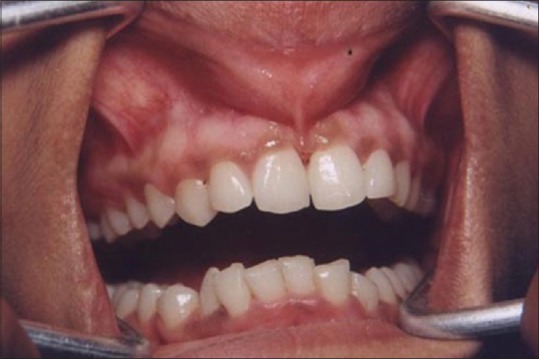
Preoperative view: High maxillary frenum
Figure 2.
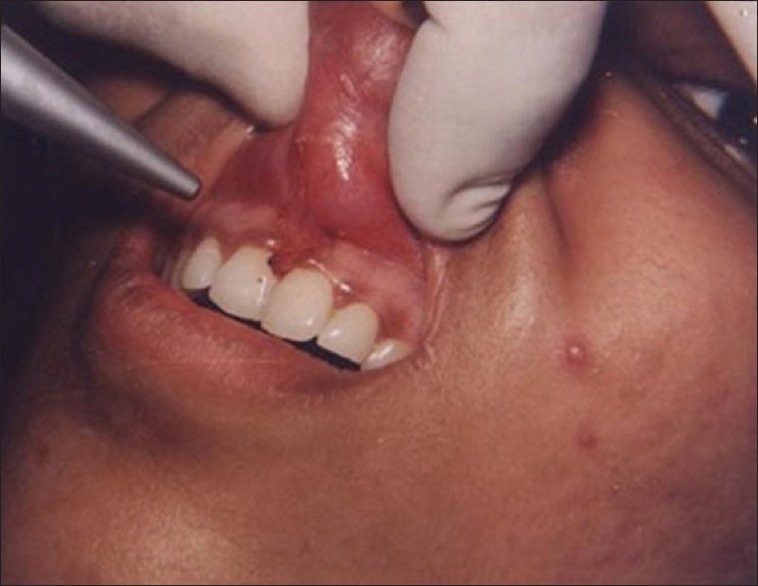
Frenum excised with laser
Figure 3.
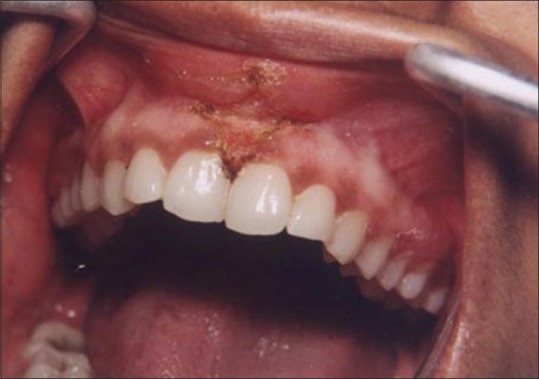
Immediate postoperative view
Figure 4.
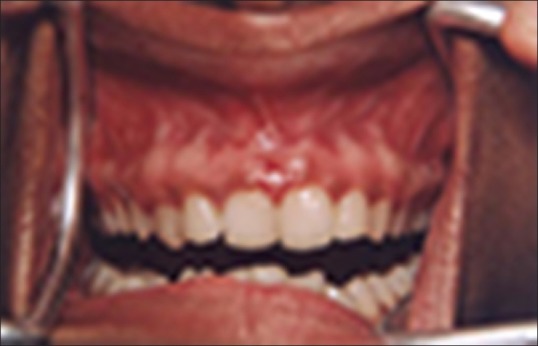
Healing at 4 weeks
Excisional biopsy
A laser may be used as a light scalpel to make relatively deep, thin cuts similar to that made with a scalpel. The technique requires the use of laser in a focused mode. Figures 5 and 6 show the buccal and occlusal views of a lesion that was planned for excisional biopsy using the laser. The lesion is first outlined using an intermittent, pulsed mode with a rate of 10 to 20 pulses per se cond to allow for superficial mark on the surface of the target. Once this is done, the laser is set to 5 w continuous mode power and the dots are joined to create the desired incision [Figure 7]. When the appropriate depth has been reached, excision is performed by grasping the tissue with a forceps, slightly pulling it and horizontally undermining the tissue similar to that done by the blade. At this stage, care should be taken to prevent inadvertent damage to adjacent and posterior tissues by the laser when it passes through the specimen. Because bleeding and scarring are minimal, sutures are generally not required. The wound is left to granulate [Figure 8].
Figure 5.
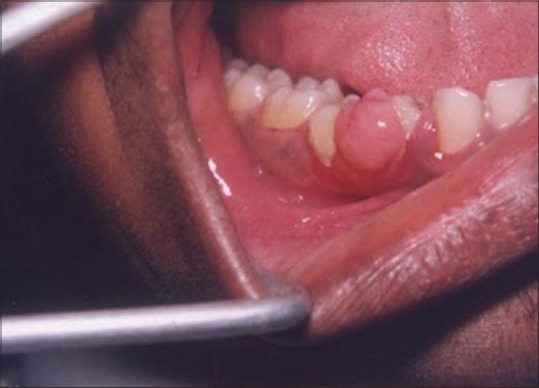
Buccal view of the lesion
Figure 6.
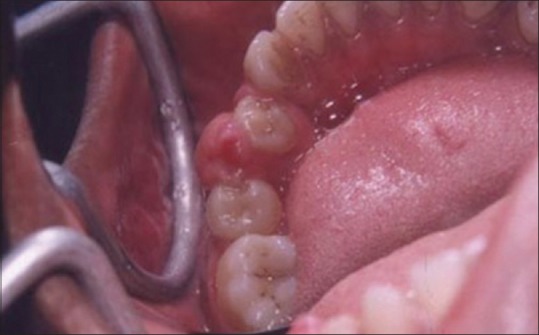
Occlusal view of the lesion
Figure 7.
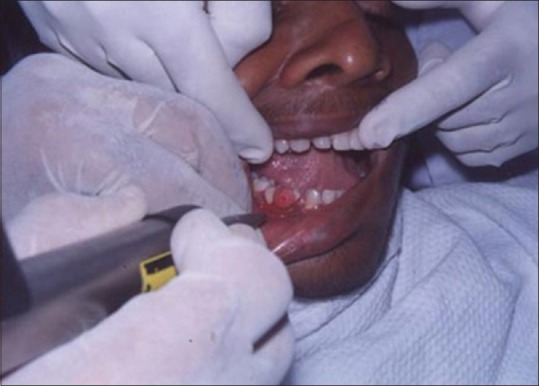
Laserization of the site
Figure 8.
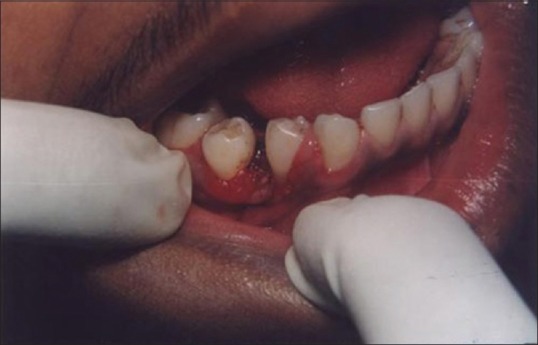
Immediate postoperative view
Figures 9 to 12 show a similar case of epulis excision using the CO2 laser.
Figure 9.
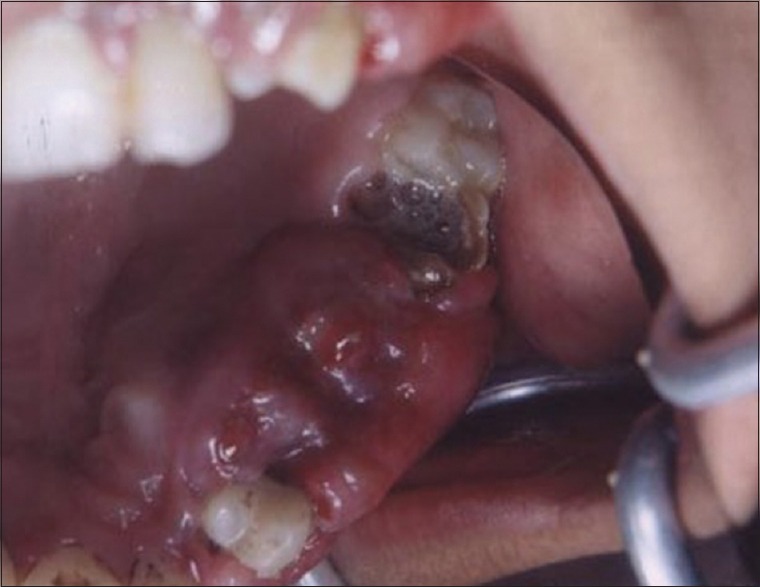
Pre-operative view of epulis
Figure 12.
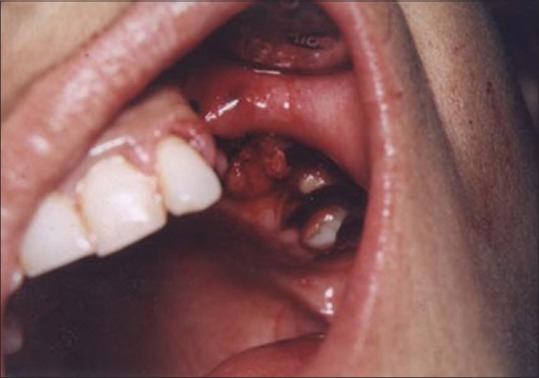
Healing site at 2 weeks
Figure 10.
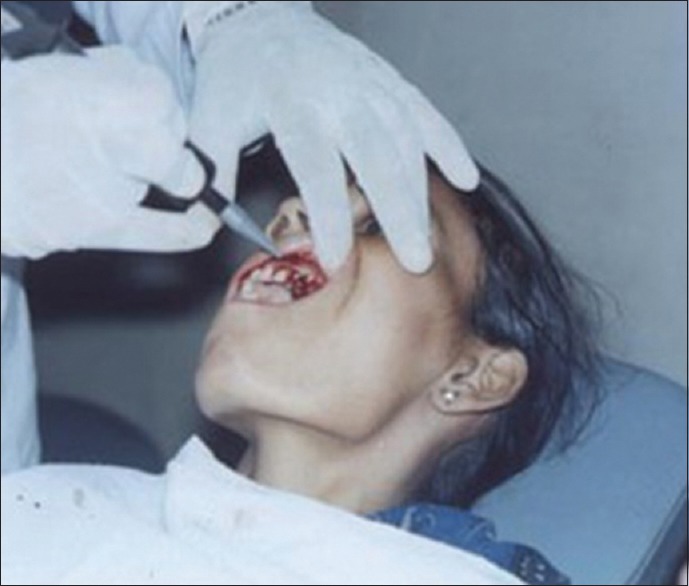
Laser treatment in progress
Figure 11.
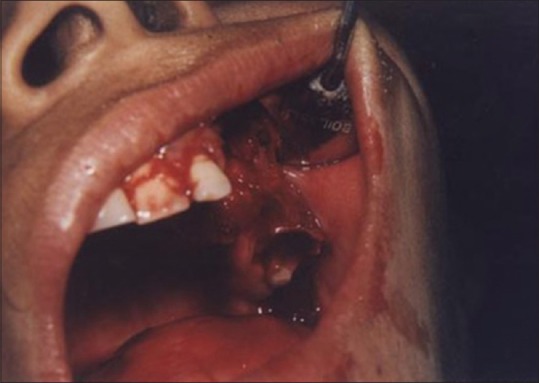
Immediate postoperative view
Laser depigmentation
Figure 13 shows the intraoral view of the mouth of a male patient who was unhappy with the appearance of “dark gums.” On examination, it was found that patient had melanin pigmentation in buccal gingival of lower anterior teeth and between the upper central incisors. The area planned for depigmentation was anesthetized with 2% lignocaine with adrenaline in the ratio of 1:100,000. The CO2 laser set at 4 W continuous in a defocused mode was used. In this manner, it is possible to separate the epithelium from underlying connective tissue by creating a blister. Because the melanocytes are found in the basement membrane of epithelium, they will be permanently eliminated with the tissue that is removed. Figure 14 shows the view immediately after laserization. Note the char layer. After 2 weeks, the site has healed completely, and shows the normal gingival color [Figure 15].
Figure 13.
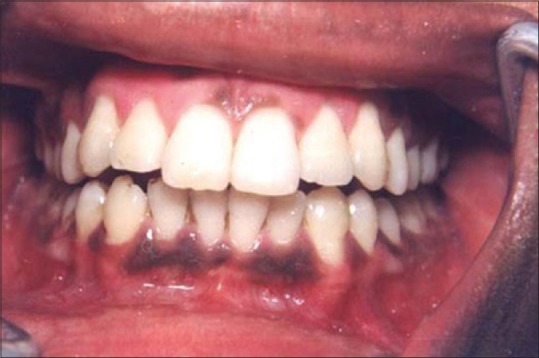
Preoperative melanin pigmentation of gums
Figure 14.
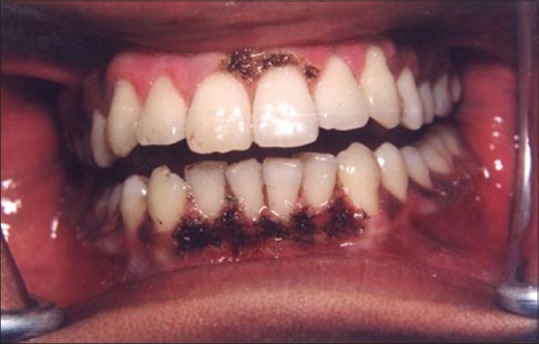
Appearance immediately after laserization
Figure 15.
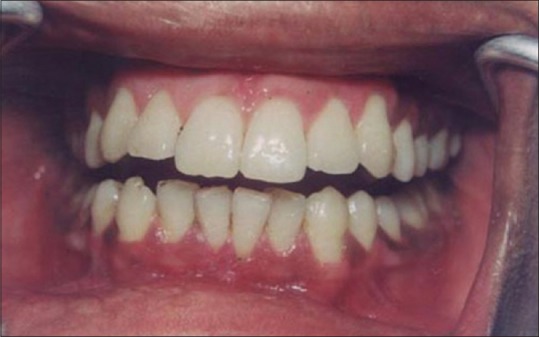
Healing at 2 weeks
Use of lasers in treating white lesions
The successful treatment of white lesions in the oral cavity purely depends on the etiology and histopathology of the lesion. While the treatment of certain white lesions as lichen planus and hyperkeratosis can be easily accomplished, other lesions such as candidiasis and erythema multiforme may be totally unresponsive. Figure 16 shows a hyperkeratotic lesion on the lateral margin of tongue. After anesthetizing the area, the laser energy is directed at the lesion in defocused mode and slowly moved in a circular mode. The power used was 2 W [Figure 17]. The process caused blister formation that separates epithelium from the underlying connective tissue. The re-evaluation appointment at 7 days shows the complete healing of the site [Figure 18].
Figure 16.
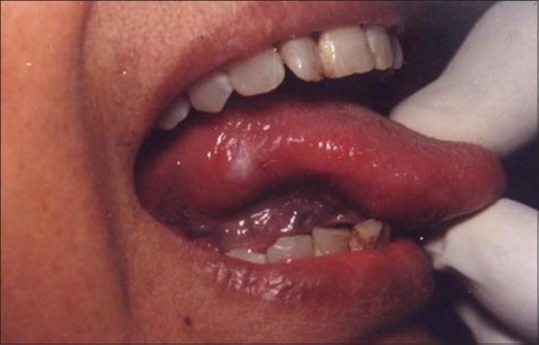
White lesion on the lateral border of tongue
Figure 17.
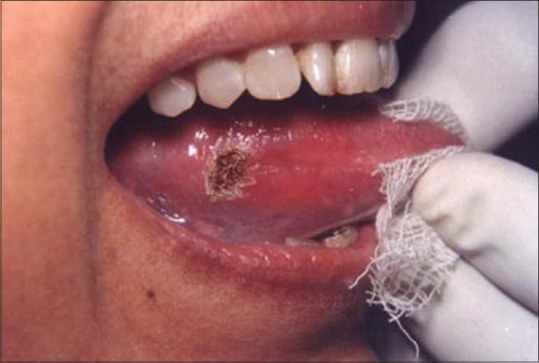
Site immediately after laser treatment
Figure 18.
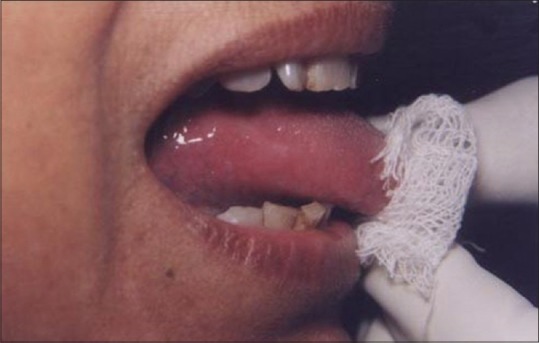
Healing at 1 week
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Pick RM, Pecaro BC, Silberman CJ. The laser gingivectomy. The use of CO2 laser for the removal of phenytoin hyperplasia. J Periodontol. 1985;56:492–4. doi: 10.1902/jop.1985.56.8.492. [DOI] [PubMed] [Google Scholar]
- 2.Wigdor HA, Walsh JT, Jr, Featherstone JD, Visuri SR, Fried D, Waldvogel JL. Lasers in dentistry. Lasers Surg Med. 1995;16:103–33. doi: 10.1002/lsm.1900160202. [DOI] [PubMed] [Google Scholar]
- 3.Carruth JA. Resection of the tongue with carbon dioxide laser. J Laryngol Otol. 1982;96:529–43. doi: 10.1017/s002221510009280x. [DOI] [PubMed] [Google Scholar]
- 4.Hall RR, Hill DW, Beach AD. A carbon dioxide surgical laser. Ann R Coll Surg Engl. 1971;48:181–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Howell RM, Cohen DM, Powell GL, Green JG. The use of low level laser therapy to treat apthous ulcers. Ann Dent. 1988;47:16–8. [PubMed] [Google Scholar]
- 6.Pogrel MA, Yen CK, Hansen LS. A comparison of carbon dioxide laser, liquid nitrogen cryosurgery, and scalpel wounds in healing. Oral Surg Oral Med Oral Pathol. 1990;69:269–73. doi: 10.1016/0030-4220(90)90285-z. [DOI] [PubMed] [Google Scholar]
- 7.Loumanen M. A comparative study of healing of laser and scalpel incision wounds in rat oral mucosa. Scand J Dent Res. 1987;95:65–73. doi: 10.1111/j.1600-0722.1987.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 8.Pick RM, McCullum Y, Kaminsky EJ. Comparative wound healing of the scalpel, Nd: YAG laser and electrosurgery in oral mucosa. Innov Technologie Biologie Med. 1990;11:116–21. [Google Scholar]
- 9.Hukki J, Krogerus L, Castren M, Schroder T. Effect of different contact laser scalpels on skin and subcutaneous fat. Lasers Surg Med. 1988;8:276–82. doi: 10.1002/lsm.1900080309. [DOI] [PubMed] [Google Scholar]
- 10.Visser H, Mausberg R. Free gingival grafts using A CO2 laser: Results of a clinical study. J Clin Laser Med Surg. 1996;14:85–8. doi: 10.1089/clm.1996.14.85. [DOI] [PubMed] [Google Scholar]
- 11.Mihashi S, Jako GJ, Incze J, Strong MS, Vaughon CW. Laser surgery in otolaryngology: Interaction of CO2 laser and soft tissue. Ann N Y Acad Sci. 1976;267:263–94. doi: 10.1111/j.1749-6632.1976.tb41614.x. [DOI] [PubMed] [Google Scholar]
- 12.Research, Science and Therapy Committee of the American Academy of Periodontology. Lasers in Periodontics. J Periodontol. 2002;73:1231–9. doi: 10.1902/jop.2002.73.10.1231. [DOI] [PubMed] [Google Scholar]
- 13.Mordon S, Begu S, Buys B, Tourne-Peteilh C, Devoisselle JM. Study of platelet behaviour in vivo after endothelial stimulation with laser irradiation using fluorescence in trivital videomicsocopy and PEG-ylated liposome staining. Microvasc Res. 2002;64:316–25. doi: 10.1006/mvre.2002.2435. [DOI] [PubMed] [Google Scholar]
- 14.Phillips AB, Ginsberg BY, Shin SJ, Soslow R, Ko W, Poppas DP. Laser welding for vascular anastomosis using albumin solder: An approach for MID-CAB. Lasers Surg Med. 1999;24:264–8. doi: 10.1002/(sici)1096-9101(1999)24:4<264::aid-lsm3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Bass LS, Treat MR. Laser tissue welding: A comprehensive review of current and future clinical applications. Laser Surg Med. 1995;17:315–49. doi: 10.1002/lsm.1900170402. [DOI] [PubMed] [Google Scholar]
- 16.Martinot VL, Mordon SR, Mitchell VA, Pellerin PN, Brunetand JM. Determination of efficient parameters for argon laser-assisted anastomosis in rats: Macroscopic, thermal, and histological evaluation. Lasers Surg Med. 1994;14:168–73. doi: 10.1002/lsm.1900150204. [DOI] [PubMed] [Google Scholar]
- 17.Radvar M, Creanor SL, Gilmour WH, Payne AP, McGadey J, Foye RH, et al. An evaluation of the effects of an Nd: YAG laser on subgingival calculus, dentin and cementum: An in vitro study. J Clin Periodontol. 1995;22:71–7. doi: 10.1111/j.1600-051x.1995.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 18.Midda M. Lasers in periodontics. Periodontol Clin Invest. 1992;14:14–7. [PubMed] [Google Scholar]
- 19.Rooney M, Midda M, Leeming J. A laboratory investigation of the bactericidal effect of an NdYAG laser. Br Dent J. 1994;176:61–4. doi: 10.1038/sj.bdj.4808364. [DOI] [PubMed] [Google Scholar]
- 20.Whitters CJ, MacGarlane TW, McKenzie D, Strang R. The bacterial activity of pulsed Nd: YAG laser radiation in vitro . Lasers Med Sci. 1994;9:297–303. [Google Scholar]
- 21.Pinero J. Nd: YAG assisted periodontal curettage to prevent bacteremia before cardio-vascular surgery. Lasers Surg Med. 1997;9:13. [Google Scholar]
- 22.Wilson M. Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J. 1994;44:181–9. [PubMed] [Google Scholar]
- 23.Weiser MT. Practise management. Dent Clin North Am. 2000;44:907–21. [PubMed] [Google Scholar]
- 24.Pogrel MA, Muff DF, Marshall GW. Structural changes in dental enamel induced by high energy continuous wave carbon dioxide laser. Lasers Surg Med. 1993;13:89–96. doi: 10.1002/lsm.1900130115. [DOI] [PubMed] [Google Scholar]


