Abstract
Winter ecology of putative vectors of eastern equine encephalomyelitis virus (EEEV) in northern Florida was investigated at field locations with evidence of historic EEEV winter transmission. Light traps and resting shelters were used to sample the mosquito community in the vicinity of eight sentinel flocks throughout the winter period (November–April) of 2013 and 2014 in Walton County, FL. Overall mosquito activity was relatively low, although mosquitoes were captured during each week of the study period. Mosquito activity was linked to morning temperature, and females were captured when ambient morning temperatures were quite low (1–5°C). Anopheles crucians Wiedemann, Culex erraticus (Dyar and Knab), Culex territans Walker, and Culiseta melanura (Coquillett) were the most commonly collected mosquito species (of 20 total species). Analysis of blood-engorged mosquitoes revealed a number of mosquito species feeding upon chickens, other birds, amphibians, and domestic and wild mammals. Cs. melanura fed primarily upon chickens and songbirds (Passeriformes), suggesting that this mosquito species is the likely winter vector of EEEV to sentinel chickens in northern Florida. Both resident and nonresident songbird species were fed upon, constituting 63.9 and 36.1% of total songbird meals, respectively. Our results suggest important roles for Cs. melanura and songbird hosts for the winter transmission of EEEV in northern Florida.
Keywords: Culiseta melanura, eastern equine encephalitis, winter, arbovirus, avian hosts
Eastern equine encephalomyelitis virus (EEEV) is a pathogenic zoonotic arbovirus, endemic to eastern North America, Central America, and northern South America (Mullen and Durden 2005). In North America, seasonality of epizootic transmission of EEEV varies with latitude. In northern portion of the virus’ range (Connecticut, Maine, Massachusetts, and New Hampshire), epizootic transmission is confined to a very limited portion of the year (July–October) and transmission of EEEV outside of late summer is exceedingly rare (Andreadis et al. 1998, MMWR 2006, Lubelczyk et al. 2013). In the southern part of the virus’ range, particularly Florida, epizootic transmission peaks in June and July, although equine and human cases of EEE can occur throughout the year (Bigler et al. 1976). This year-round transmission of EEEV in Florida could have implications for northern parts of the virus’ range. Birds that become infected with EEEV during the winter or early spring in Florida and then return to their breeding ranges could initiate local amplification of EEEV in those northern locales.
Throughout the range of NA-EEEV, the mosquito Culiseta melanura (Coquillett) is considered the primary enzootic vector of the virus (Howard et al. 1988, Komar and Spielman 1994, Armstrong and Andreadis 2010). This mosquito species feeds mainly upon birds (Molaei et al. 2006a, Burkett-Cadena et al. 2008, Bingham et al. 2012), although reptiles (Burkett-Cadena et al. 2008) and mammals (Molaei et al. 2006b, Bingham et al. 2014) may be fed upon in some cases. A number of other mosquito species have been implicated as vectors of EEEV in the southern United States, although mostly as bridge vectors, transmitting the virus from amplification hosts (birds) to dead-end hosts (mammals). These include Aedes canadensis (Theobald), Aedes sollicitans (Walker), Anopheles crucians, Coquillettidia perturbans (Walker), and Culex erraticus, among others. Given the potential importance of wintertime transmission of EEEV in Florida to the year-round local transmission of the virus and its potential importance to northern transmission foci, elucidating the role of potential vectors and reservoir hosts of EEEV during the winter period becomes important.
In the current study, mosquito sampling, bloodmeal analysis and EEEV pool-screening by RT-PCR were conducted at historic sites of EEEV transmission in Walton County, FL, in order to investigate winter-period ecology of EEEV. The major objectives were to determine which mosquito species are active during the winter and which host animals were being fed upon by the putative vector species. To this end, mosquitoes were sampled November–April of 2013 and 2014, with EEEV pool-screening and bloodmeal analysis conducted on field-collected female mosquitoes.
Materials and Methods
Sampling Sites
Study sites were located in Walton County, on the northern coast of the Gulf of Mexico (Fig. 1). Walton County is relatively rural, with population density of 22.3 persons per square kilometer (U.S. Census Bureau data, 2013), compared with Florida overall population density (140.8 persons per square kilometer). Major land classifications include upland forests, tree plantations, wetlands, cropland, and residential (VanderKelen et al. 2012). Walton County is served by two independent mosquito abatement districts, North Walton Mosquito Control (DeFuniak Springs) and South Walton County Mosquito Control District (Santa Rosa Beach). Both districts participate year-round in the statewide arbovirus surveillance program that monitors sentinel chickens for arbovirus exposure. Seroconversion of sentinel chickens to EEEV in Walton County is not uncommon during the winter period (November–April). Over the past decade, the number of winter period chicken seroconversions to EEEV has been highest in November and December, and lowest in March and April (Florida Department of Health). However, yearly and monthly variation in EEEV seroconversion in sentinel chickens is quite high. For example, 24 chickens were seropositive for EEEV in the winter period of 2010–2011, while no EEEV seroconversions were detected in chickens the following winter (2011–2012).
Fig. 1.
Location of Walton County, FL (shaded black), and the range of EEEV in North America (shaded gray). Map redrawn after Mullen and Durden (2009).
Because historical data demonstrated winter period transmission of EEEV to sentinel chickens, mosquito sampling sites were localized in the vicinities of eight sentinel chicken flocks maintained by North Walton Mosquito Control District. Seven of the eight sentinel flocks have been maintained for more than a decade, while one flock was recently relocated (2013), owing to a request from the landowner of property where the flock was located. Habitats surrounding the eight sentinel flocks are primarily rural residential property, intermixed with forest and agricultural plots. Dominant forest types include upland hardwood, wetland mixed forest, pine tree plantations, and upland coniferous forest, habitats that have been previously associated with EEEV activity in the state (VanderKelen et al. 2012). Wetlands typically associated with EEEV transmission (marshes and hardwood swamps) were minor constituent habitats in lands surrounding the sentinel flocks. A wide variety of domestic animals were found at most sites, and included poultry (chicken, emu, turkey, and waterfowl), mammalian livestock (alpaca, cow, goat, horse, llama, and pig), as well as pets (dog and cat).
Mosquito Sampling
Two methods of mosquito sampling were used to gain an account of the species active during the study period. The two sampling methods included resting shelter aspiration (Burkett-Cadena 2011) and automated carbon dioxide-baited updraft light traps (Fig. 2). Resting shelters were wire-frame models (Burkett-Cadena et al. 2011), consisting of a cylinder of galvanized steel field fencing placed inside a heavy-duty black plastic garbage bag. A handheld rechargeable vacuum (Black and Decker Dustbuster), modified to utilize mesh-bottom collection canisters (BioQuip Products, Rancho Dominguez, CA), was used to aspirate resting females from the resting shelters twice weekly, throughout the sampling season, with limited exceptions due to inclement weather. Automated carbon dioxide-baited light traps were modifications of a system currently used by Sarasota County (FL) Mosquito Control. In general, the trapping system consists of electronic timers automating the timing and duration of light, suction, and carbon dioxide dispensation functions to operate the trap within designated time periods, resulting in fewer trips to field locations to set and retrieve traps. The flow of compressed carbon dioxide (from standard gas cylinders) is dispensed through a gas regulator (200 ml/min) connected to an irrigation hose-end timer (Galcon, Inc., San Rafael, CA) that controls the timing and duration of gas dispensations. From the output of the hose-end timer, gas is fed to the intake area of the trap using 4 mm i.d. drip irrigation tubing, which terminated in a gas orifice restrictor (0.178 mm). The light used was 12 V DC LED 9-mm miniature bayonet base bulb (three lumens), color “warm white” (SuperbrightLEDs.com, model BA9s). The light was located next to the intake of the trap, at the point of carbon dioxide emission. Suction for the trap was created by a 12 V DC computer processor cooling fan, mounted inside a PVC coupler (90 mm i.d.) with clear acrylic tubes (84 cm i.d.) extending 65 mm beyond the pipe coupler in both directions. The intake (lower) arm of the tube was inserted through a hole cut into the upper surface of the trap chamber, which was a 9.4-liter Slimline beverage container (Arrow Plastic Manufacturing Co., Elkgrove, IL). A pocket of PVC window screen prevented mosquitoes in the trap chamber from being sucked through the fan. An aluminum baking tray served as a rain shield. The light and fan were powered by a 12 V, 18 Ah rechargeable sealed lead-acid battery. Operating hours of the light and fan were automatically controlled by programmable digital 12 V DC timers, model CN101A (OKtimer, China). Timers regulated the cycles of trap operation such that carbon dioxide, light, and suction were produced during crepuscular periods (0600–0900 hours and 1630–1930 hours) daily. Trap collection chambers were retrieved from the field twice weekly. During nontrapping hours, an air-actuated gate, made of transparency film, prevented escape of captured insects from the collection chamber. A notable feature of the trapping system includes the use of honey-coated nucleic acid preservation cards (FTA cards, Whatman plc) that sample arbovirus infection in trapped mosquitoes after sugar feeding (Hall-Mendelin et al. 2010).
Fig. 2.
Automated updraft carbon-dioxide baited light trap. Panel A: Diagram of trap. Panel B: Trap in field setting. See Materials and Methods for complete description.
Collection chambers and aspirator canisters were returned to the laboratory, where mosquitoes were freeze-killed then sorted by species. Blood-engorged females were individually preserved for bloodmeal identification. FTA cards and nonengorged females (up to 50) were pool screened for EEEV by RT-PCR, as described below.
Screening of Samples for EEEV by RT-PCR
Mosquito pools were homogenized using a high-speed mechanical homogenizer (TissueLyser; Qiagen, Valencia, CA) after the addition of a copper BB and 1 ml of biological field diluent (90% minimum essential medium with Hanks’ salts, 10% fetal bovine serum, 200 U/ml penicillin, 200 µg/ml streptomycin, 2.5 µg/ml amphotericin B, and 50 µg/ml kanamycin). Homogenates were centrifuged at 10,500 × g for 7 min at 4°C.
FTA cards were ripped into smaller fragments, changing gloves between cards. One milliliter of biological field diluent was added to the card fragments and tubes were vortexed every 5 min for 20 min, keeping the tubes on ice between vortexes. RNA was prepared from 140 µl of the resulting supernatant for both mosquito pools and FTA cards using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations. The Qiacube platform (Qiagen, Valencia, CA) was used to automate RNA extraction and the isolated RNA was stored at −80°C. A real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) assay was then conducted using the iTaq Universal Probes One-Step Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Primers, probe, and reaction conditions for EEEV RNA detection were those recommended by Lambert and others (Lambert et al. 2003) with the exception that reactions were performed in a final volume of 20 µl, and used 4 µl of the RNA extract.
Bloodmeal Analysis
Individual blood-engorged female mosquitoes were homogenized in 225 µl of phosphate buffered saline (pH 7.4) using a disposable plastic pestle. DNA was prepared using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The extracted DNA served as a template for two PCR-based assays. The initial nested PCR used a set of universal vertebrate primers targeting the vertebrate cytochrome B gene as described previously (Hassan et al. 2003). Samples that did not amplify in the first assay were then tested by a second PCR assay targeting the 16 S rRNA gene. Primers used in the second PCR assay were those of Kitano et al. (2007) using the reaction conditions described previously (Burkett-Cadena et al. 2008). Samples producing a detectable amplicon were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA) and were then sent to the Eurofins MWG Operon sequencing facility (Huntsville, AL) for sequence determination. Sequences with a match percentage of ≥95% in the NCBI BLAST database were accepted as belonging to the identified bloodmeal source.
Statistics
Statistical comparisons of sampling methods (light trap versus resting shelter aspiration) were determined using Poisson regression (Coxe et al. 2009, McElduff et al. 2010), with individual tests constructed for each mosquito species (PROC GENMOD, SAS Institute 2013, Cary, NC). Significant differences among treatments determined using contrast statements (α = 0.05). Linear regression (PROC REG, SAS Institute 2013) was used to examine the relationship between mosquito activity and morning temperature (0700 hours). For this analysis, only aggregated species mean from resting shelters were used, as light traps combined collections over a 3- or 4-d period into a single trap chamber, complicating comparisons of day-to-day variation in activity for light traps. Temperature data were obtained from the National Climatic Data Center weather station at DeFuniak Springs, Walton County, FL (county seat; (http://www.ncdc.noaa.gov, accessed 7 April 2015)).
Results
Mosquitoes were captured each week of the winter period in Walton County, FL (Fig. 3A), and activity of females demonstrated a statistically significant (df = 45; P < 0.001) positive linear relationship with morning temperature (Fig. 3B). The best-fit line of the scatterplot of morning temperature (0700 hours) and average mosquito activity suggests that mosquitoes are active in Walton County at morning temperatures of −0.17°C and above. Morning temperature fell below zero (−1.1°C) on only a single sampling day (6 January), with no mosquitoes being collected on that day.
Fig. 3.
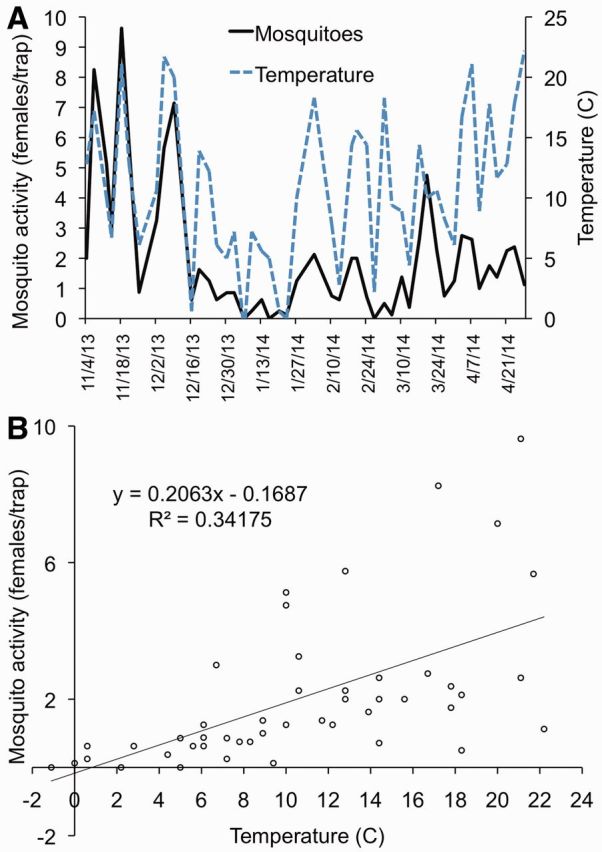
Mosquito and EEEV activity and winter temperature in Walton County, FL (2013-2014). Panel A: Average of total females (20 species) collected from resting shelters (solid line) and morning (0700 hours) temperature (dashed line). Subzero temperatures are not displayed. Panel B: Scatter plot and best-fit line of relationship between mosquito activity (average of total females from Panel A) and morning temperature (0700 hours) at reference site. Temperature data from National Climatic Data Center.
Twenty species of mosquitoes were collected during the study period, about a third of the species known to occur in Walton County (Darsie and Ward 2005). Total numbers of mosquitoes were relatively low, despite extensive sampling utilizing two methods. An. crucians, Cx. erraticus, Cx. territans, and Cs. melanura were the most commonly encountered species (Table 1). Of these, Ae. canadensis, Aedes sticticus (Meigen), and An. crucians were more effectively sampled by carbon dioxide baited light traps (Table 1). Anopheles punctipennis (Say), Anopheles quadrimaculatus Say, Culex erraticus, Culex nigripalpus Theobald, Culex restuans Theobald, Culex territans, Culiseta melanura, and Uranotaenia sapphirina (Osten Sacken) were more effectively sampled by resting shelters (Table 1). Several species were collected in very low numbers, including one to three total specimens each of Aedes albopictus (Skuse), Aedes japonicus (Theobald), Aedes infirmatus Dyar and Knab, Coquillettidia perturbans, Culex coronator Dyar and Knab, Culex peccator Dyar and Knab, and Psorophora ferox (Humboldt).
Table 1.
Relative abundance and sampling method comparison of mosquito species collected during winter period (November–April) at sites in Walton County, FL (2013–2014)
| Relative abundance (percent of total collection) |
CO2-baited light trap |
Resting shelter |
Poisson regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nov. | Dec. | Jan. | Feb. | Mar. | April | Mean | Mean | Wald’s chi-square | P | |
| Mosquito | (N = 285) | (N = 177) | (N = 53) | (N = 99) | (N = 200) | (N = 212) | ||||
| Ae. canadensis | 0 | 0 | 0 | 0 | 0.5 | 10.4 | 0.08 | 0.01 | 10.1 | 0.002 |
| Ae. sticticus | 0 | 0 | 0 | 0 | 2.0 | 11.3 | 0.10 | 0.01 | 12.2 | 0.001 |
| An. crucians | 7.0 | 14.1 | 28.3 | 15.2 | 29.5 | 14.6 | 0.43 | 0.17 | 15.2 | <0.001 |
| An. punctipennis | 2.5 | 6.2 | 15.1 | 8.1 | 0 | 1.9 | 0.02 | 0.09 | 14.1 | <0.001 |
| Cs. melanura | 22.4 | 14.1 | 13.2 | 3.0 | 23.0 | 29.7 | 0.14 | 0.51 | 74.3 | <0.001 |
| Cx. erraticus | 31.8 | 18.1 | 18.9 | 48.5 | 35.5 | 9.9 | 0.20 | 0.66 | 91.3 | <0.001 |
| Cx. nigripalpus | 9.4 | 16.9 | 5.7 | 0 | 0 | 0 | 0.04 | 0.14 | 20.1 | <0.001 |
| Cx. restuans | 1.1 | 0 | 1.9 | 0 | 2.0 | 7.5 | 0.03 | 0.05 | 3.9 | 0.048 |
| Cx. territans | 18.2 | 23.7 | 7.6 | 21.2 | 5.0 | 10.9 | <0.01 | 0.45 | 25.0 | <0.001 |
| Ur. sapphirina | 3.9 | 2.8 | 1.9 | 0 | 0 | 0 | <0.01 | 0.05 | 7.2 | 0.007 |
| Minor species (N = 14) | 3.8 | 4.1 | 7.4 | 4 | 2.5 | 3.8 | – | – | – | – |
Mean values for sampling methods are females captured per sampling occasion at a site. Species representing less than 1% of the total samples, individually, are combined as “Minor species”.
Only four mosquito species (An. crucians, Cs. melanura, Cx. erraticus and Cx. territans) were collected in each month of sampling (Table 1). Aedes canadensis and Ae. sticticus were collected in March and April only (Table 1). Culex nigripalpus and Ur. sapphirina were collected in November, December, and January only (Table 1). An. punctipennis was collected in all months with the exception of March. Cx. restuans was collected in all months except for December and February.
No samples (FTA cards or mosquito pools) tested positive for EEEV by RT-PCR. A single seroconversion to EEEV in sentinel chickens maintained at the study sites was detected from a blood sample drawn on 16 December 2013.
In total, 145 blood-engorged mosquitoes were collected during the sampling period, of which 117 bloodmeal hosts were successfully identified (80.7%). Twenty-eight species of host animals were identified, belonging to three classes of vertebrates (Amphibia, Aves, and Mammalia). The majority of identified bloodmeals (69.2%) were from two mosquito species (Table 2), Cs. melanura (n = 59), and Cx. erraticus (n = 24). These two species fed predominantly upon birds, taking 91.3 and 87.5% of bloodmeals from avian hosts, respectively. Chickens constituted 24.6 and 58.3% of total bloodmeals of Cs. melanura and Cx. erraticus, respectively (Table 2). Nonavian meals of Cs. melanura and Cx. erraticus originated from mammals (cow, human, and domestic cat). Single meals identified for Ae. sticticus and An. quadrimaculatus were from white-tailed deer. An. crucians meals were from chicken (n = 4), cow (n = 2), human (n = 1), llama (n = 1), and white-tailed deer (n = 1). An. punctipennis bloodmeals were from chicken (n = 1), human (n = 1), eastern cottontail rabbit (n = 2), and llama (n = 1). Coquillettidia perturbans bloodmeals (n = 2) were from summer tanager and white-tailed deer. Cx. nigripalpus bloodmeals were from chicken (n = 6), ruby-crowned kinglet (n = 1), and llama (n = 1). A single bloodmeal of Cx. restuans was from white-eyed vireo. Cx. territans bloodmeals were primarily from frogs, including river frog (n = 1), southern leopard frog (n = 1), bullfrog (n = 1), and pine woods tree frog (n = 2), with nonamphibian bloodmeals from chicken (n = 1) and human (n = 3).
Table 2.
Winter period (November–April) host associations of mosquitoes from Walton County, FL (2013–2014)
| Mosquito | Total | Amphibian |
Avian (nonchicken) |
Chicken |
Mammal |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | SE | N | % | SE | N | % | SE | N | % | SE | ||
| Ae. sticticus | 1 | 0 | 0 | – | 0 | 0 | – | 0 | 0.0 | – | 1 | 100.0 | 0.0 |
| An. crucians | 9 | 0 | 0 | – | 0 | 0 | – | 4 | 44.4 | 6.2 | 5 | 55.6 | 4.9 |
| An. punctipennis | 5 | 0 | 0 | – | 0 | 0 | – | 1 | 20.0 | 16.0 | 4 | 80.0 | 4.0 |
| An. quadrimaculatus | 1 | 0 | 0 | – | 0 | 0 | – | 0 | 0.0 | – | 1 | 100.0 | 0.0 |
| Cq. perturbans | 2 | 0 | 0 | – | 1 | 50 | 25.0 | 0 | 0.0 | – | 1 | 50.0 | 25.0 |
| Cs. melanura | 57 | 0 | 0 | – | 38 | 66.7 | 0.6 | 14 | 24.6 | 1.3 | 5 | 8.8 | 1.6 |
| Cx. erraticus | 24 | 0 | 0 | – | 7 | 29.2 | 2.9 | 14 | 58.3 | 1.7 | 3 | 12.5 | 3.7 |
| Cx. nigripalpus | 8 | 0 | 0 | – | 1 | 12.5 | 10.9 | 6 | 75.0 | 3.1 | 1 | 12.5 | 10.9 |
| Cx. restuans | 1 | 0 | 0 | – | 1 | 100.0 | 0.0 | 0 | 0.0 | – | 0 | 0.0 | – |
| Cx. territans | 9 | 5 | 55.6 | 4.9 | 0 | 0 | – | 1 | 11.1 | 9.9 | 3 | 33.3 | 7.4 |
Avian bloodmeals were relatively diverse, originating from 17 different bird species. Bloodmeals from poultry (chicken, emu, greater white-fronted goose, and turkey) constituted 57.9% of total avian meals. Nonpoultry avian bloodmeals were overwhelmingly from songbirds (order Passeriformes; 97.3%), belonging to 13 species (Table 3). Resident (summer resident and year-round) songbird species accounted for 63.9% of total songbird meals, and nonresident (winter resident and transient) species accounted for 36.1% of songbird meals (Table 3). Most songbird bloodmeals were from Cs. melanura, which constituted 91.3 and 92.3% of resident and nonresident songbird bloodmeals, respectively.
Table 3.
Songbird (Passeriformes) hosts of mosquitoes during winter period (November-April) 2013–2014 in Walton County, FL
| Status | Common name | Scientific name | Week of | Mosquito species (N) |
|---|---|---|---|---|
| Nonresident | Black-and-white warbler | Mniotilta varia | 7 Nov. 2013 | Cs. melanura (1) |
| Nonresident | Grasshopper sparrow | Ammodramus savannarum | 9 Dec. 2013 | Cs. melanura (1) |
| Nonresident | Hermit thrush | Catharus guttatus | 10 Mar. 2014 | Cs. melanura (1) |
| Nonresident | Hermit thrush | Catharus guttatus | 18 Nov. 2013 | Cs. melanura (2) |
| Nonresident | House wren | Troglodytes aedon | 7 Nov. 2013 | Cs. melanura (1) |
| Nonresident | House wren | Troglodytes aedon | 18 Nov. 2013 | Cs. melanura (1) |
| Nonresident | Ruby-crowned kinglet | Regulus calendula | 9 Dec. 2013 | Cx. nigripalpus (1) |
| Nonresident | Tennessee warbler | Vermivora peregrina | 2 Dec. 2013 | Cs. melanura (1) |
| Nonresident | Tennessee warbler | Vermivora peregrina | 18 Nov. 2013 | Cs. melanura (3) |
| Nonresident | Yellow-rumped warbler | Setophaga coronata | 25 Nov. 2013 | Cs. melanura (1) |
| Resident | Carolina wren | Thryothorus ludovicianus | 7 Nov. 2013 | Cs. melanura (1) |
| Resident | Northern cardinal | Cardinalis cardinalis | 7 Nov. 2013 | Cs. melanura (3) |
| Resident | Northern cardinal | Cardinalis cardinalis | 9 Dec. 2013 | Cs. melanura (1) |
| Resident | Northern cardinal | Cardinalis cardinalis | 14 April 2014 | Cs. melanura (2) |
| Resident | Northern cardinal | Cardinalis cardinalis | 17 April 2014 | Cs. melanura (1) |
| Resident | Northern cardinal | Cardinalis cardinalis | 18 Nov. 2013 | Cs. melanura (3) |
| Resident | Northern cardinal | Cardinalis cardinalis | 18 Feb. 2014 | Cs. melanura (1) |
| Resident | Northern cardinal | Cardinalis cardinalis | 21 April 2014 | Cs. melanura (1) |
| Resident | Northern cardinal | Cardinalis cardinalis | 24 April 2014 | Cs. melanura (2) |
| Resident | Red-winged Blackbird | Agelaius phoeniceus | 24 April 2014 | Cs. melanura (1) |
| Resident | Summer tanager | Piranga rubra | 17 April 2014 | Cq. perturbans (1) |
| Resident | Tufted titmouse | Baeolophus bicolor | 17 April 2014 | Cs. melanura (3) |
| Resident | Tufted titmouse | Baeolophus bicolor | 28 April 2014 | Cs. melanura (1) |
| Resident | White-eyed vireo | Vireo griseus | 7 April 2014 | Cs. melanura (1) |
| Resident | White-eyed vireo | Vireo griseus | 21 April 2014 | Cx. restuans (1) |
Discussion
Mosquitoes were active throughout the winter period (November–April), although day-to-day variation in mosquito activity was quite high (Fig. 3A). Mosquitoes were active even on days with very low morning temperatures (<5°C), but activity diminished on days with morning temperatures near 0°C (Fig. 3B). Day-to-day variation in mosquito activity was linked to morning temperature, particularly in the period from November through February. Mosquito activity was lowest between mid-December and mid-March, despite multiple days with relatively high morning temperatures during this same period (Fig. 3A). It is particularly notable that Cs. melanura was active throughout the winter, constituting 3 to 30% of total samples, depending on the month (Table 1). In addition, Cx. erraticus, a species that has been implicated as a potential vector of EEEV in Tennessee (Cohen et al. 2009), Florida (Bingham et al. 2014), and Alabama (Cupp et al. 2003), and often considered a warm-season species, was also found to be relatively abundant and active through the winter, constituting 10 to 49% of total monthly samples.
In previous fieldwork in Walton County, carbon dioxide-baited light-traps operated from May–August (VanderKelen et al. 2012) yielded a quite similar mosquito community to that observed in the cold-season sampling (Table 1). The four mosquito species commonly encountered from light traps in the warm season (An. crucians, Cs. melanura, Cx. erraticus, and Cx. nigripalpus) were likewise the most common species (from light traps) in the cold season (Table 1). Other species were far more seasonal, with their numbers decreasing as the warm season waned (Cx. nigripalpus and Ur. sapphirina) or increasing with spring warming (Ae. canadensis and Ae. sticticus), in general agreement with seasonal patterns observed in other studies (O’Meara and Evans 1983, Irby and Apperson 1992, Burkett-Cadena et al. 2008).
Sentinel chicken arbovirus surveillance during the study period in North Walton County yielded a single seroconversion to EEEV, from a blood sample collected from a sentinel chicken on 16 December 2013 at a site named “Punchbowl.” This seroconversion followed a noticeable peak in mosquito activity (Fig. 3A). During the month-long period preceding the seroconversion, three mosquito bloodmeals were identified, all from Cs. melanura at the Punchbowl site. The hosts were northern cardinal (18 November), yellow-rumped warbler (25 November), and Tennessee warbler (2 December). No samples (FTA cards or mosquito pools) tested positive for EEEV by RT-PCR, possibly because of low sample sizes or limited virus transmission during the study period.
Overall patterns of host use by mosquitoes reflected broad host associations that have been reported from previous work. Avian-biased biting by Cs. melanura, Cx. erraticus, Cx. nigripalpus, and Cx restuans was not unexpected. The total lack of reptilian bloodmeals was somewhat surprising, although a recent study from Hillsborough County, FL (roughly 2° south latitude of Walton County), found that the portion of bloodmeals from reptiles decreases dramatically in the winter (Bingham et al. 2014). This reduction in reptile biting is likely owing to brumation and hibernation of various reptiles, making them less accessible to host-seeking mosquitoes (Bingham et al. 2014). Mixed mammal and bird biting by An. crucians, An. punctipennis, and Cq. perturbans was in agreement with past work in Florida (Bingham et al. 2014). Cx. territans took a majority of bloodmeals from frogs, as expected (Burkett-Cadena et al. 2008), but also took a substantial number of meals from mammals (33.3%) and birds (11.1%). As reported previously for reptiles (Bingham et al. 2014), the lower reliance upon amphibian hosts is likely because of reduced availability of these ectotherms in winter.
That chickens were relatively important hosts for the most commonly collected mosquito species is not particularly surprising, given that resting shelters were placed 50–100 m from sentinel flocks. However, this information does provide a useful indication of mosquito species that potentially transmit EEEV to sentinel chickens during the winter, when examined in light of the other host groups fed upon by each mosquito species. Mosquitoes found to feed upon chickens as well as suspected reservoir hosts are likely more important as EEEV vectors than mosquitoes that feed upon chickens, but not upon reservoir hosts. An. crucians, for example, took 36.4% of bloodmeals from chickens. However, all other hosts of An. crucians were large mammals (ungulates and humans), which are generally not considered amplification hosts of EEEV (Tate et al. 2005). Cs. melanura, on the other hand, fed upon chickens (Table 2), but also fed upon known amplification hosts (songbirds). Poultry (including chickens, geese, and turkeys) were major avian hosts of An. crucians, An. punctipennis, Cx. erraticus, and Cx. territans, constituting 100% of avian meals for these species.
Songbird (Aves: Passeriformes) bloodmeals are of particular interest in this study, as this host groups constitutes the most likely source of EEEV for local winter transmission. Cq. perturbans, Cx. nigripalpus, and Cx. restuans each took a single bloodmeal from songbirds (Table 3). Only Cs. melanura fed heavily upon songbirds, however, taking 57.9% of avian meals from songbirds. The high relative abundance of Cs. melanura during the winter and its propensity for feeding upon both reservoir hosts (songbirds) and sentinel animals (chickens) suggests that this mosquito is the likely winter vector of EEEV in Walton County, and perhaps elsewhere in the temperate south.
Bloodmeals from wading birds (Ciconiiformes) were not encountered from mosquitoes collected during the winter period. Previous field investigations on host utilization during winter in the Tampa Bay region (Hillsborough County) in Florida found that wading birds, particularly yellow-crowned night heron (Nyctanassa violacea) and black-crowned night heron (Nycticorax nycticorax), were important hosts for wetland mosquitoes, including Cs. melanura and Cx. erraticus (Bingham et al. 2014). The discrepancy between results of the current study and previous work (Bingham et al. 2014), with regards to mosquitoes feeding upon wading birds during the winter period, demonstrates the geographic variation in host use by EEEV vectors, and highlights the need for field studies from multiple locations, conducted synchronously.
The majority of resident songbird bloodmeals (60.9%) were from northern cardinal (Table 3), a species repeatedly implicated as an enzootic host of EEEV. Northern cardinal has been found with high EEEV antibody seroprevalence in Michigan (McLean et al. 1985) and New Jersey (Crans et al. 1994). Northern cardinal was also found to be a preferred host species of Cs. melanura and Cx. erraticus in Alabama (Estep et al. 2011) and constituted 21.7% of Cs. melanura bloodmeals from Hillsborough County, FL (Bingham et al. 2014). In Walton County, EEEV risk (sentinel chicken exposure) was found to increase with northern cardinal density (Estep et al. 2013). The preponderance of northern cardinal feeds by Cs. melanura during the winter and early spring in the current study supports the idea that this host is an important enzootic host for EEEV throughout the year.
Six nonresident songbird species were fed upon by Cs. melanura in the current study. Five of these species (grasshopper sparrow, house wren, Tennessee warbler and yellow-rumped warbler, hermit thrush, and black-and-white warbler) are reported as hosts of Cs. melanura from New York (Molaei et al. 2006), Massachusetts, or both (Molaei et al. 2013). Hermit thrush and black-and-white warbler are of broader potential interest in the ecology of EEEV, based on the current findings and those from previous investigations at other sites. Hermit thrush was found to be EEEV viremic in Louisiana in March (Stamm 1958) and antibody positive to EEEV in Maryland (1 of 8 birds; Dalrymple et al. 1972), but not in New Jersey (0 of 18; Crans et al. 1994). Hermit thrush was also a minor host of Cs. melanura at an EEEV focus in New York (Molaei et al. 2006), but not Massachusetts (Molaei et al. 2013). Hatch-year black-and-white warbler was found EEEV viremic in New Jersey in late September and antibody positive (14.7%) at the same location (Crans et al. 1994). In Maryland, 9% of 66 black-and-white warbler were EEEV antibody-positive (Dalrymple et al. 1972). Black-and-white warbler was also fed upon by Cs. melanura in New York (Molaei et al. 2006) and Massachusetts (Molaei et al. 2013). An ecological assessment of bird characteristics that contribute to higher antibody prevalence among bird species in New Jersey found that summer resident species and species with lower nesting heights had significantly higher antibody prevalence than other species (Crans et al. 1994). Hermit thrush and black-and-white warblers are summer residents (in New Jersey) and are ground nesters (Crans et al. 1994). Based on their summer and winter ranges, their importance as a host for Cs. melanura in both seasons and disparate habitats, and their exposure to EEEV, these two species fit the profile of transporter hosts, importing EEEV from southern U.S. winter transmission sites to northern foci where EEEV transmission is limited to nonwinter months. Other nonresident species fed upon by Cs. melanura in the current work (grasshopper sparrow, house wren, Tennessee warbler, and yellow-rumped warbler) had low antibody prevalence to EEEV (Dalrymple et al. 1972, Crans et al. 1994) or were not found to be Cs. melanura hosts (Molaei et al. 2006, 2013) in northern sites.
Winter transmission of EEEV in Florida is an important, yet poorly documented aspect of EEEV ecology. Future studies should incorporate measures of bird relative abundance to better elucidate the seasonal relationship between resident and nonresident songbirds and vectors of EEEV.
Acknowledgments
We would like to thank Brett Bishop for assistance with mosquito collection and the private landowners who allowed access to their property for mosquito collections. Wade Brennan and Matthew Smith, Sarasota County Mosquito Control, generously shared their time and experience with their automated light trap system, which was the basis for the trapping system used in the current work. Jim Newman (UF) produced the digital illustration and photograph of the updraft trap. This research was supported by grants from the National Institute of Allergy and Infectious Diseases, Projects R01AI49724 and R56AI01372 to T.R.U.
References Cited
- Andreadis T. G., Anderson J. F., Tirrell-Peck S. J. 1998. Multiple isolations of eastern equine encephalitis and highlands J viruses from mosquitoes (Diptera: Culicidae) during a 1996 epizootic in southeastern Connecticut. J. Med. Entomol. 35: 296–302. [DOI] [PubMed] [Google Scholar]
- Armstrong P. M., Andreadis T. G. 2010. Eastern equine encephalitis virus in mosquitoes and their role as bridge vectors. Emerg. Infect. Dis. 16: 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler W. J., Lassing E. B., Buff E. E., Prather E. C., Beck E. C., Hoff G. L. 1976. Endemic eastern equine encephalomyelitis in Florida: A twenty-year analysis, 1955-1974. Am. J. Trop. Med. Hyg. 25: 884–890. [DOI] [PubMed] [Google Scholar]
- Burkett-Cadena N. D., Graham S. P., Hassan H. K., Guyer C., Eubanks M. D., Katholi C. R., Unnasch T. R. 2008. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am. J. Trop. Med. Hyg. 79: 809–815. [PMC free article] [PubMed] [Google Scholar]
- Bingham A. M., Graham S. P., Burkett-Cadena N. D., White G. S., Hassan H. K., Unnasch T. R. 2012. Detection of eastern equine encephalomyelitis virus RNA in North American snakes. Am. J. Trop. Med. Hyg. 87: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham A. M., Burkett-Cadena N. D., Hassan H. K., McClure C.J.W., Unnasch T. R. 2014. Field investigations of winter transmission of eastern equine encephalitis virus in Florida. Am. J. Trop. Med. Hyg. 91: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett-Cadena N. D. 2011. A wire-frame shelter for collecting resting mosquitoes. J. Am. Mosq. Control Assoc. 27: 152–155. [DOI] [PubMed] [Google Scholar]
- Cohen S. B., Lewoczko K., Huddleston D. B., Moody E., Mukherjee S., Dunn J. R., Jones T. F., Wilson R., Moncayo A. C. 2009. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am. J. Trop. Med. Hyg. 81: 452–456. [PubMed] [Google Scholar]
- Coxe S., West S. G., Aiken L. S. 2009. The analysis of count data: A gentle introduction to Poisson regression and its alternatives. J. Personality Assess. 91: 121–136. [DOI] [PubMed] [Google Scholar]
- Crans W. J., Caccamise D. F., McNelly J. R. 1994. Eastern equine encephalomyelitis virus in relation to the avian community of a coastal cedar swamp. J. Med. Entomol. 31: 711–728. [DOI] [PubMed] [Google Scholar]
- Cupp E. W., Klingler K., Hassan H. K., Viguers L. M., Unnasch T. R. 2003. Transmission of eastern equine encephalomyelitis virus in central Alabama. Am. J. Trop. Med. Hyg, 68: 495–500. [PMC free article] [PubMed] [Google Scholar]
- Dalrymple J. M., Young O. P., Eldridge B. F., Russell P. K. 1972. Ecology of arboviruses in a Maryland freshwater swamp. III. Vertebrate hosts. Am. J. Epidemiol. 96: 129–140. [DOI] [PubMed] [Google Scholar]
- Darsie R. F., Ward R. A. 2005. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. University Press of Florida, Gainesville, FL, USA. [Google Scholar]
- Estep L. K., McClure C.J.W., Burkett-Cadena N. D., Hassan H. K., Hicks T. L., Unnasch T. R., Hill G.E. 2011. A multi-year study of mosquito feeding patterns on avian hosts in a southeastern focus of eastern equine encephalitis virus. Am. J. Trop. Med. Hyg. 84: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep L. K., McClure C.J.W., VanderKelen P. T., Burkett-Cadena N. D., Sickerman S., Hernandez J., Jinright J., Hunt B., Lusk J., Hoover V., et al. 2013. Risk of exposure to eastern equine encephalomyelitis virus increases with the density of northern cardinals. PLoS ONE 8: e57879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Mendelin S., Ritchie S. A., Johansen C.A., Zborowski P., Cortis G., Dandridge S., Hall R. A., van den Hurk A. F. 2010. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc. Natl. Acad. Sci. USA 107: 11255–11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. K., Cupp E. W., Hill G. E., Katholi C. R., Klingler K., Unnasch T. R. 2003. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 69: 641–647. [PubMed] [Google Scholar]
- Howard J. J., Morris C. D., Emord D. E., Grayson M. A. 1988. Epizootiology of eastern equine encephalitis virus in upstate New York, USA. VII. Virus surveillance 1978-85, description of 1983 outbreak, and series conclusions. J. Med. Entomol. 25: 501–514. [DOI] [PubMed] [Google Scholar]
- Irby W. S., Apperson C. S. 1992. Spatial and temporal distribution of resting female mosquitoes (Diptera: Culicidae) in the coastal plain of North Carolina. J. Med. Entomol. 29: 150–159. [DOI] [PubMed] [Google Scholar]
- Kitano T., Umetsu K., Tian W., Osawa M. 2007. Two universal primer sets for species identification among vertebrates. Int. J. Legal Med. 121: 423–427. [DOI] [PubMed] [Google Scholar]
- Komar N., Spielman A. 1994. Emergence of eastern encephalitis in Massachusetts. Ann. N. Y. Acad. Sci. 740: 157–168. [DOI] [PubMed] [Google Scholar]
- Lambert A. J., Martin D. A., Lanciotti R. S. 2003. Detection of North American eastern and western equine encephalitis viruses by nucleic acid amplification assays. J. Clin. Microbiol, 41: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelczyk C., Mutebi J.-P., Robinson S., Elias S. P., Smith L. B., Juris S. A., Foss K., Lichtenwalner A., Shively K. J., Hoenig D. E., et al. 2013. An epizootic of eastern equine encephalitis virus, Maine, USA in 2009: Outbreak description and entomological studies. Am. J. Trop. Med. Hyg. 88: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElduff F., Cortina-Borja M., Chan S., Wade A. 2010. When t-tests or Wilcoxon-Mann-Whitney tests won't do. Adv. Physiol. Ed. 34: 128–133. [DOI] [PubMed] [Google Scholar]
- McLean R. G., Frier G., Parham G. L., Francy D. B., Monath T. P., Campos E. G., Therrien A., Kerschner J., Calisher C. H. 1985. Investigations of the vertebrate hosts of eastern equine encephalitis during an epizootic in Michigan, 1980. Am. J. Trop. Med. Hyg. 34: 1190–1202. [DOI] [PubMed] [Google Scholar]
- (MMWR) Morbidity and Mortality Weekly Report. 2006. Eastern equine encephalitis–New Hampshire and Massachusetts, August-September 2005.30: 697–700. [Google Scholar]
- Molaei G., Oliver J. A., Andreadis T. G., Armstrong P. M., Howard J. J. 2006a. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am. J. Trop. Med. Hyg. 75: 1140–1147. [PubMed] [Google Scholar]
- Molaei G., Andreadis T. G. 2006. Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, U.S.A. J. Med. Entomol. 43: 1088–1093. [DOI] [PubMed] [Google Scholar]
- Molaei G., Andreadis T. G., Armstrong P. M., Thomas M.i.C., Deschamps T., Cuebas-Incle E., Montgomery W., Osborne M., Smole S., Matton P., et al. 2013. Vector-host interactions and epizootiology of eastern equine encephalitis virus in Massachusetts. Vector Borne Zoonotic Dis. 13: 312–323. [DOI] [PubMed] [Google Scholar]
- Mullen G. R., Durden L. A. 2009. Medical and veterinary entomology, 2nd ed Academic Press, Amsterdam. [Google Scholar]
- O’Meara G. F., Evans D. S. 1983. Seasonal patterns of abundance among three species of Culex mosquitoes in a south Florida wastewater lagoon. Ann. Entomol. Soc. Am. 76: 130–133. [Google Scholar]
- SAS Institute. 2012. SAS computer program, version 9.3. SAS Institute, Cary NC. [Google Scholar]
- Stamm D. D. 1958. Studies on the ecology of equine encephalomyelitis. Am. J. Public Health 48: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate C. M., Howerth E. W., Stallknecht D. E., Allison A. B., Fischer J. R., Mead D.G. 2005. Eastern equine encephalitis in a free-ranging white-tailed deer (Odocoileus virginianus). J. Wildl. Dis. 41: 241–245. [DOI] [PubMed] [Google Scholar]
- VanderKelen P. T., Downs J. A., Burkett-Cadena N. D., Ottendorfer C. L., Hill K., Sickerman S., Hernandez J., Jinright J., Hunt B., Lusk J., et al. 2012. Habitat associations of eastern equine encephalitis transmission in Walton County Florida. J. Med. Entomol. 49: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]