Abstract
Background:
Low levels of adiponectin (ADIPOQ; HGNC ID; HGNC:13633), an adipokine, are associated with obesity, adiposity, excess energy balance, and increased risk of colorectal neoplasia. Given the reported association of increased body mass index (BMI) and low-level physical activity with KRAS-mutated colorectal tumor, we hypothesized that low-level plasma adiponectin might be associated with increased risk of KRAS-mutant colorectal carcinoma but not with risk of KRAS wild-type carcinoma.
Methods:
We conducted molecular pathological epidemiology research using a nested case-control study design (307 incident rectal and colon cancer case patients and 593 matched control individuals) within prospective cohort studies, the Nurses’ Health Study (152 case patients and 297 control individuals, with blood collection in 1989–1990) and the Health Professionals Follow-up Study (155 case patients and 296 control individuals, with blood collection in 1993–1995). Multivariable conditional logistic regression models and two-sided likelihood ratio tests were used to assess etiologic heterogeneity of the associations.
Results:
The association of low-level plasma adiponectin with colorectal cancer risk statistically significantly differed by KRAS mutation status (P heterogeneity = .004). Low levels of plasma adiponectin were associated with KRAS-mutant colorectal cancer (for the lowest vs highest tertile: multivariable odds ratio [OR] = 2.83, 95% confidence interval [CI] = 1.50 to 5.34, P trend = .002) but not with KRAS wild-type cancer (for the lowest vs highest tertile: multivariable OR = 0.83, 95% CI = 0.49 to 1.43, P trend = .48). In secondary analyses, the association between plasma adiponectin and colorectal cancer did not appreciably differ by BRAF or PIK3CA oncogene mutation status.
Conclusions:
Low-level plasma adiponectin is associated with KRAS-mutant colorectal cancer risk but not with KRAS wild-type cancer risk.
Adiponectin (ADIPOQ; HGNC ID; HGNC:13633), an adipocyte-derived protein or adipokine, modulates a number of metabolic processes and plays an important role in energy homeostasis (1–3). Low levels of circulating plasma adiponectin are considered a marker of excess energy balance status (3) and have been associated with obesity, type 2 diabetes mellitus, and insulin resistance (3). Low-level plasma adiponectin is associated with increased risk of colorectal cancer (CRC) (4–7), colorectal adenoma (7–9), and various other tumors (10). Few previous studies could measure adiponectin level in plasma from individuals years before CRC diagnosis, and could assess plasma adiponectin as a potential biomarker for future CRC risk (4–6). It remains uncertain whether low-level plasma adiponectin in an individual without cancer is associated with higher incidence of CRC with specific molecular features, such as KRAS mutation status.
CRCs develop through a multistep carcinogenic process with an accumulation of epigenetic and genetic changes, which differ from tumor to tumor (11). The KRAS gene has been established as one of important drivers in colorectal carcinogenesis, and its activating mutations (in codons 12, 13, 61, and 146) are present in approximately 40% of CRCs (12–15). Additionally, KRAS mutation in CRC has been shown to be a predictive biomarker for resistance to anti–epidermal growth factor receptor (EGFR) therapy (16–18). Previous studies showed that high body mass index (BMI) and physical inactivity are associated with KRAS-mutated colorectal neoplasia (19–21). Therefore, we hypothesized that the association between low-level plasma adiponectin and risk of developing CRC might be stronger for KRAS-mutated CRC than for KRAS wild-type CRC.
To test this hypothesis, we conducted a nested case-control study within two prospective cohort studies. We examined the association of plasma adiponectin levels with risk of developing CRC classified by tumor KRAS mutation status using participants in prospective cohorts with blood specimens (collected [7.8 years on average] before the diagnosis of CRC [307 case patients]) and 593 control individuals, matched on sex, age, and month/year of blood draw. As secondary analyses, we examined BRAF and PIK3CA mutations, in addition to KRAS mutation. These three genes encode proteins that mediate signaling cascades triggered by EGFR, and KRAS mutation is associated positively with PIK3CA mutation, and inversely with BRAF mutation in CRC (16–18,22–25).
Methods
Study Population
We conducted this nested case-control study within two prospective cohort studies, the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) (26,27). The NHS enrolled 121 700 female nurses, age 30 to 55 years, in 1976, and the HPFS enrolled 51 529 male health professionals, age 40 to 75 years, in 1986. From enrollment, cohort participants were sent self-administered questionnaires biennially regarding medical conditions, family history, diet (diet was assessed every four years), and lifestyle.
Blood specimens were collected from 32 826 women from the NHS (in 1989–1990) and 18 225 men from the HPFS (in 1993–1995). The procedures and protocols of blood collection and processing were similar in the NHS and HPFS (4,5); within 26 hours of blood draw, specimens were shipped on ice to the laboratory, where, upon arrival, they were separated into plasma, red cell, white cell components. All specimens were stored in continuously monitored liquid nitrogen freezers (<-130°C) (the median storage time of blood samples: 18.8 years for NHS and 16.3 years for HPFS). The Human Research Committee at the Brigham and Women’s Hospital and the Harvard T. H. Chan School of Public Health approved this study. All participants provided informed consent.
Ascertainment of Case Patients and Control Individuals
Figure 1 shows selection of each triad (one case patient and two matched control individuals) in our nested case-control design. Figures 2 and 3 show summary flow diagrams of the selection of CRC case patients and matched control individuals. CRC case patients were ascertained through self-report and investigation of the National Death Index and medical record review. We included both colon and rectal carcinoma case patients, considering the colorectal continuum model (28,29). Among 51 051 participants who had provided blood specimens, 360 women and 287 men subsequently developed CRC during follow-up (through October 1, 2008 in the NHS; and through January 1, 2008 in the HPFS). The median time from blood draw to diagnosis of CRC is 9.7 years in women, 6.4 years in men, and 7.8 years in the combined cohort. Collection of paraffin-embedded archival tissue blocks was attempted from hospitals where participants with CRC had undergone tumor resection.
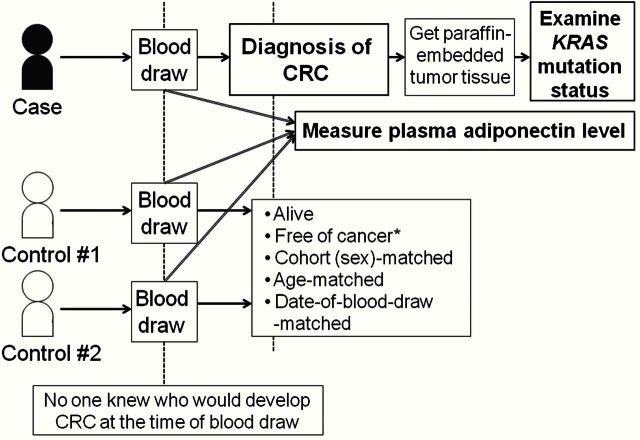
Figure 1.
Illustration of a nested case-control design within two prospective cohort studies. A selection of each case patient and matched control individuals is shown. Plasma adiponectin level was measured after the diagnosis of colorectal cancer for case patients and matching control individuals. *Except nonmelanoma skin cancer. CRC = colorectal cancer.
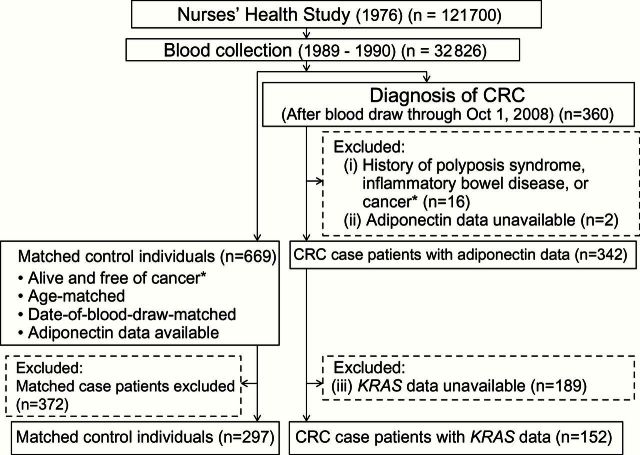
Figure 2.
Flow diagram of this nested case-control study in the Nurses’ Health Study. *Except nonmelanoma skin cancer. CRC = colorectal cancer.
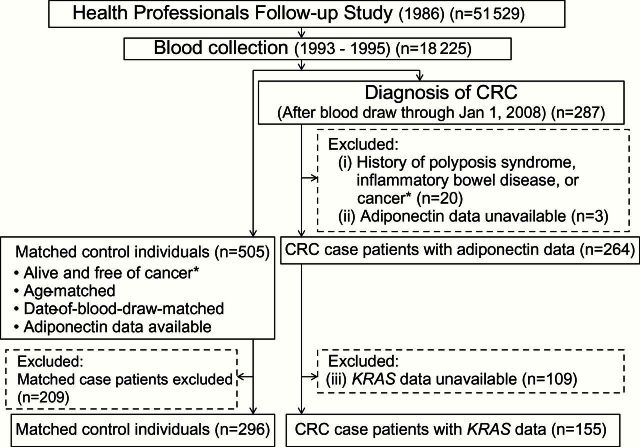
For the current study, we included CRC case patients if: 1) at the time of blood draw, there had been no personal history of polyposis syndrome, inflammatory bowel disease, or cancer (except for nonmelanoma skin cancer); 2) adiponectin was successfully measured in prediagnosis blood specimen; and 3) tumor KRAS mutation status was successfully determined. As a result, the numbers of included (excluded) CRC case patients were 152 (208) in the NHS (Figure 2) and 155 (132) in the HPFS (Figure 3). For each CRC case patient, we used risk set sampling to randomly select up to two control individuals matched on sex, age, and month/year of blood draw from participants who were alive and free of cancer at the time of the CRC case patient diagnosis (Figure 1). A total of 297 control individuals from the NHS and 296 control individuals from the HPFS could be included in this study.
Figure 3.
Flow diagram of this nested case-control study in the Health Professionals Follow-up Study. *Except nonmelanoma skin cancer. CRC = colorectal cancer.
Plasma Adiponectin Assay
Adiponectin levels were measured from blood samples using enzyme-linked immunosorbent assay (ELISA) (ALPCO Diagnostics, Salem, NH) with a sensitivity of 0.04ng/mL (4). Paired quality control samples were randomly interspersed among the case-control samples. All assays were conducted by laboratory personnel blinded to quality control, case-control status, or any other participant information. Samples from case patients and their matched control individuals were analyzed in the same batch. The intra-assay coefficient of variation from quality control samples was 8.6%. The inter-assay coefficient of variation was 12.4%.
Sequencing of KRAS, BRAF, and PIK3CA
DNA was extracted from paraffin-embedded tumor tissue, and polymerase chain reaction and pyrosequencing targeted for KRAS (codons 12, 13, 61, and 146) (13,30), BRAF (codon 600) (31), and PIK3CA (exons 9 and 20) were performed as previously described (32,33).
Covariates
We categorized plasma adiponectin level into tertiles using sex-specific cutoff levels based on the distribution among the control individuals of each cohort. The presence of mutation in any of KRAS codons 12, 13, 61, and 146 was classified as KRAS-mutant, while the absence of mutation in all KRAS codons 12, 13, 61, and 146 was classified as KRAS wild-type. The presence of mutation in BRAF codon 600 was classified as BRAF-mutant, while the absence of mutation in BRAF codon 600 was classified as BRAF wild-type. The presence of mutation in any of PIK3CA exons 9 and 20 was classified as PIK3CA-mutant, while the absence of mutation in PIK3CA exons 9 and 20 was classified as PIK3CA wild-type. In the final multivariable models, the following variables were included: fasting status at blood draw, BMI, physical activity, CRC family history, multivitamin use, aspirin use, hormone replacement therapy, lower endoscopy history, smoking history, total caloric intake, intakes of red meat, processed meat, calcium, folate, alcohol, and plasma 25-hydroxyvitamin D (4,5).
Statistical Analysis
Details of the statistical analysis are described in the Supplementary Methods (available online). All statistical analyses were carried out using SAS (version 9.3, SAS Institute, Cary, NC). All P values were two-sided. Our primary hypothesis testing was heterogeneity testing between “the association of low-level plasma adiponectin with KRAS-mutant CRC” and “that with KRAS wild-type CRC” in the combined cohort. Nonetheless, we considered the risk of false-positive results because of multiple hypothesis testing. In this study, we examined three tumor markers (KRAS, BRAF, and PIK3CA). Therefore, for our primary hypothesis testing, the alpha level for statistical significance was adjusted to .017 (= .05/3). All the other analyses (including evaluations of individual odds ratio [OR] and sex-specific analyses) were secondary and exploratory.
We estimated cohort-specific odds ratios and 95% confidence intervals (CIs) for each CRC subtype (KRAS-mutant or KRAS wild-type), using conditional logistic regression models that accounted for the matching variables, ie, age at blood draw and date of blood collection.
We examined and confirmed consistency between the two cohorts using Q statistics in the relationship of adiponectin with KRAS-mutated CRC and that with KRAS wild-type CRC. Then, we merged the data from the two cohorts and tested etiologic heterogeneity in the combined cohort, including sex (cohort) as a stratification variable. To test heterogeneity, we used the likelihood ratio test by comparing the model in which the association with exposures was allowed to vary by tumor subtypes with a model in which all the associations were held constant (34,35).
Results
Baseline Characteristics of Study Subjects
Table 1 shows baseline characteristics of the study subjects. Compared with control individuals, the median of plasma adiponectin level of CRC case patients was lower in both men and women. In men, compared with control individuals, CRC case patients tended to have higher BMI, CRC family history, and a lower rate of experiencing lower endoscopy.
Table 1.
Baseline characteristics of study subjects within the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS)
| Baseline characteristics | Women (NHS) | Men (HPFS) | ||||
|---|---|---|---|---|---|---|
| Case patients (n = 152) |
Control individuals (n = 297) |
P | Case patients (n = 155) |
Control individuals (n = 296) |
P | |
| Mean age at blood draw (SD)*, y | 58.5 (6.7) | 58.5 (6.6) | − | 66.2 (8.4) | 66.2 (8.4) | − |
| Fasting at time of blood draw†, % | 69 | 70 | .84 | 52 | 53 | .93 |
| Mean body mass index (SD), kg/m2 | 25.2 (4.7) | 24.7 (4.4) | .32 | 26.1 (2.9) | 25.3 (2.8) | .02 |
| Mean physical activity, MET-hours per week (SD) | 15.2 (17.7) | 14.8 (16.6) | .60 | 32.1 (27.2) | 30.4 (26.8) | .70 |
| Mean pack-years of smoking before age 30 y among ever-smoker (SD) | 7.3 (7.0) | 6.1 (4.7) | .41 | 10.1 (6.3) | 9.1 (6.4) | .23 |
| Never smoker, % | 44 | 49 | .31 | 39 | 45 | .22 |
| Colorectal cancer in a parent or sibling, % | 16 | 15 | .93 | 21 | 14 | .04 |
| Current multivitamin use, % | 35 | 38 | .55 | 48 | 51 | .55 |
| Regular aspirin use (≥2 tablets/wk), % | 22 | 29 | .12 | 35 | 41 | .26 |
| Current hormone replacement therapy, % | 22 | 16 | .12 | − | − | − |
| History of previous lower endoscopy, % | 14 | 17 | .52 | 54 | 64 | .03 |
| Mean daily intake (SD) | ||||||
| Total calorie, kcal/d | 1.7 (0.46) | 1.7 (0.44) | .52 | 2.1 (0.58) | 2.0 (0.49) | .10 |
| Red meat, serving/d | 0.68 (0.34) | 0.63 (0.31) | .75 | 0.62 (0.40) | 0.56 (0.33) | .13 |
| Processed meat, serving/d | 0.29 (0.27) | 0.27 (0.26) | .56 | 0.38 (0.49) | 0.33 (0.32) | .93 |
| Calcium, mg/d | 922 (350) | 972 (362) | .20 | 917 (331) | 952 (366) | .52 |
| Folate, µg/d | 398 (172) | 415 (185) | .48 | 495 (204) | 529 (238) | .45 |
| Alcohol, g/d | 5.0 (7.0) | 6.0 (9.2) | .67 | 13.4 (16.3) | 10.8 (12.4) | .08 |
| Plasma 25-hydroxyvitamin D, median (IQR), ng/mL | 24.2 (17.5–29.5) | 25.9 (19.2–30.8) | .17 | 27.2 (22.2–33.1) | 27.8 (21.8–33.7) | .96 |
| Plasma adiponectin, median (IQR), µg/mL | 7.9 (5.0–10.8) | 8.2 (5.7–10.6) | .45 | 5.0 (3.0–7.0) | 5.6 (3.7–7.9) | .003 |
| Plasma adiponectin, tertile cutpoints based on controls, µg/mL | 6.7 and 10.0 (batch 1); 6.6 and 9.3 (batch 2) |
4.2 and 6.7 | ||||
| Plasma adiponectin, tertiles | .14 | .06 | ||||
| Tertile 1, No. | 63 | 102 | 60 | 98 | ||
| Tertile 2, No. | 46 | 101 | 53 | 94 | ||
| Tertile 3, No. | 43 | 94 | 42 | 104 | ||
* Age is one of the matching variables. HPFS = Health Professionals Follow-up Study; IQR = interquartile range; MET = metabolic equivalent task; NHS = Nurses’ Health Study; SD = standard deviation.
† Hours since last meal ≥ 8.
Supplementary Table 1 (available online) shows baseline characteristics of CRC patients with KRAS mutation data and those without KRAS data (the latter of which were excluded from this study). Baseline characteristics did not differ substantially according to the availability of KRAS data.
We examined the association of plasma adiponectin level with risk of KRAS-data-available CRC and risk of KRAS-data-unavailable CRC, in our overall nested case-control set in men, women, and the combined cohort (605 case patients and 1174 matched control individuals) (Supplementary Table 2, available online). The association of low-level adiponectin with CRC risk did not differ substantially according to the availability of KRAS data (P heterogeneity ≥ .29).
The median (interquartile range) levels of plasma adiponectin in CRC case patients according to KRAS, BRAF, and PIK3CA status are provided in Supplementary Table 3 (available online).
Plasma Adiponectin and Incident CRC Classified by KRAS Mutation Status
We tested our primary hypothesis that the association of low-level prediagnosis plasma adiponectin with CRC risk might differ by KRAS status in the combined cohort (Table 2). We detected KRAS mutations in 136 (44%) of 307 CRC case patients; the number of mutated case patients in each codon was 99 in codon 12, 28 in codon 13, six in codon 61, and five in codon 146. One case patient had mutations in codons 12 and 13 concomitantly, and another had mutations in codons 13 and 146 concomitantly. In the combined cohort, the association of low-level plasma adiponectin with CRC risk statistically significantly differed by KRAS status (P heterogeneity = .004). Low-level plasma adiponectin was associated with risk of KRAS-mutant CRC (for the lowest [T1] vs highest [T3] tertile; multivariable OR = 2.83, 95% CI = 1.50 to 5.34, P trend = .002 across tertiles of adiponectin level) but not with risk of KRAS wild-type CRC (for T1 vs T3; multivariable OR = 0.83, 95% CI = 0.49 to 1.43, P trend = .48 across tertiles).
Table 2.
Odds ratio for colorectal cancer by KRAS mutation status according to plasma adiponectin level in the Nurses’ Health Study and the Health Professionals Follow-up Study
| Colorectal cancer subtype | Tertile 3 | Tertile 2 | Tertile 1 | P trend* | P heterogeneity† |
|---|---|---|---|---|---|
| Women (NHS) | |||||
| KRAS wild-type | |||||
| No. of case patients/control individuals (82/161) | 26/50 | 26/58 | 30/53 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 0.89 (0.48 to 1.67) | 1.14 (0.57 to 2.24) | .79 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 0.79 (0.39 to 1.60) | 0.98 (0.44 to 2.14) | .86 | |
| KRAS-mutant | |||||
| No. of case patients/control individuals (70/136) | 17/44 | 20/43 | 33/49 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.21 (0.55 to 2.66) | 1.92 (0.86 to 4.26) | .12 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.34 (0.56 to 3.19) | 1.97 (0.82 to 4.75) | .14 | .21 |
| Men (HPFS) | |||||
| KRAS wild-type | |||||
| No. of case patients/control individuals (89/171) | 29/57 | 27/51 | 33/63 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.04 (0.53 to 2.03) | 1.10 (0.55 to 2.18) | .80 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 0.86 (0.40 to 1.89) | 0.92 (0.40 to 2.09) | .81 | |
| KRAS-mutant | |||||
| No. of case patients/control individuals (66/125) | 13/47 | 26/43 | 27/35 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 2.28 (1.00 to 5.21) | 3.17 (1.30 to 7.75) | .01 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 2.69 (1.04 to 6.93) | 4.21 (1.52 to 11.6) | .005 | .02 |
| Combined (women [NHS] and men [HPFS]) | |||||
| KRAS wild-type | |||||
| No. of case patients/control individuals (171/332) | 55/107 | 53/109 | 63/116 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 0.96 (0.60 to 1.51) | 1.11 (0.68 to 1.80) | .72 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 0.89 (0.54 to 1.45) | 0.83 (0.49 to 1.43) | .48 | |
| KRAS-mutant | |||||
| No. of case patients/control individuals (136/261) | 30/91 | 46/86 | 60/84 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.65 (0.94 to 2.89) | 2.42 (1.34 to 4.37) | .005 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.88 (1.03 to 3.42) | 2.83 (1.50 to 5.34) | .002 | .004 |
* Tests for trend (the Wald statistics, two-sided) were conducted using the median values for each tertile of plasma adiponectin. CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; OR = odds ratio.
† Likelihood ratio tests (two-sided) were used to test for heterogeneity (comparing the associations across tumor subtypes).
‡ Results were based on conditional logistic regression analysis with adjustment for age at blood draw and date of blood collection.
§ Results were based on conditional logistic regression analysis (which accounted for age at blood draw and date of blood collection) with additional adjustment for fasting status (<8 hours, ≥8 hours since last meal), body mass index (continuous, kg/m2), physical activity level (continuous, metabolic equivalent hour per week), family history of colorectal cancer (yes, no), multivitamin use (yes, no), regular aspirin use (yes, no), hormone replacement therapy (yes, no; NHS only), history of previous lower endoscopy (yes, no), pack-years of smoking before age 30 years (continuous), intake of total calorie (continuous, kcal/day), red meat intake (continuous, serving/day), processed meat intake (continuous, serving/day), calcium intake (continuous, mg/day), folate intake (continuous, μg/day), alcohol consumption (continuous, g/day), and plasma 25-hydroxyvitamin D (tertile, ng/mL).
The median (range) levels of plasma adiponectin across tertiles among controls are as follows:
Women (NHS) (Batch 1): 12.1 (10.0 to 26.0) μg/mL (Tertile 3), 8.2 (6.7 to 10.0) μg/mL (Tertile 2), 5.0 (1.8 to 6.7) μg/mL (Tertile 1)
Women (NHS) (Batch 2): 11.5 (9.3 to 27.0) μg/mL (Tertile 3), 8.1 (6.6 to 9.3) μg/mL (Tertile 2), 4.6 (1.4 to 6.6) μg/mL (Tertile 1)
Men (HPFS): 9.2 (6.7 to 28.0) μg/mL (Tertile 3), 5.4 (4.2 to 6.7) μg/mL (Tertile 2), 3.3 (1.3 to 4.2) μg/mL (Tertile 1)
Although sample size was limited, we conducted secondary cohort (sex)-specific analyses (Table 2). In men, low-level plasma adiponectin was associated with KRAS-mutant CRC (for T1 vs T3; multivariable OR = 4.21, 95% CI = 1.52 to 11.6, P trend = .005 across tertiles), but not with KRAS wild-type CRC (P trend = .81 across tertiles) (P heterogeneity = .02, between KRAS subtypes). Although no statistically significant association was observed in women, data were generally consistent between women and men. We confirmed consistencies between women and men by Q statistics (P heterogeneity ≥ .41).
In secondary analysis, we used log-transformed adiponectin value as a continuous variable and showed statistically significant heterogeneity in the association of low-level plasma adiponectin with CRC risk according to KRAS status (P heterogeneity = .004) (Supplementary Table 4, available online).
Plasma Adiponectin and Incident CRC Classified by BRAF or PIK3CA Mutation Status
As secondary analyses, we examined whether the association between low-level plasma adiponectin and CRC risk differed by mutation status of BRAF or PIK3CA (Table 3). In the combined cohort, low-level plasma adiponectin was associated with BRAF wild-type CRC (for T1 vs T3; multivariable OR = 1.81, 95% CI = 1.15 to 2.83, P trend = .01 across tertiles) but not with BRAF-mutant CRC (P trend = .15) (P heterogeneity = .01, between BRAF subtypes) (Table 3).
Table 3.
Odds ratio for colorectal cancer by mutation status of BRAF or PIK3CA according to plasma adiponectin level in the Nurses’ Health Study and the Health Professionals Follow-up Study
| Colorectal cancer subtype | Tertile 3 | Tertile 2 | Tertile 1 | P trend* | P heterogeneity† |
|---|---|---|---|---|---|
| Women (NHS) | |||||
| BRAF wild-type | |||||
| No. of case patients/control individuals (121/236) | 29/73 | 40/84 | 52/79 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.23 (0.69 to 2.17) | 1.84 (0.99 to 3.43) | .06 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.19 (0.63 to 2.25) | 1.76 (0.89 to 3.49) | .12 | |
| BRAF-mutant | |||||
| No. of case patients/control individuals (30/59) | 14/20 | 6/17 | 10/22 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 0.55 (0.19 to 1.63) | 0.69 (0.26 to 1.87) | .40 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 0.52 (0.15 to 1.74) | 0.54 (0.17 to 1.74) | .26 | .07 |
| PIK3CA wild-type | |||||
| No. of case patients/control individuals (123/239) | 31/73 | 38/75 | 54/91 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.16 (0.67 to 2.03) | 1.47 (0.83 to 2.61) | .20 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.16 (0.62 to 2.16) | 1.37 (0.71 to 2.63) | .37 | |
| PIK3CA-mutant | |||||
| No. of case patients/control individuals (20/40) | 9/14 | 5/18 | 6/8 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 0.43 (0.10 to 1.81) | 0.90 (0.20 to 4.08) | .94 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 0.35 (0.07 to 1.71) | 0.74 (0.15 to 3.67) | .78 | .54 |
| Men (HPFS) | |||||
| BRAF wild-type | |||||
| No. of case patients/control individuals (142/270) | 38/95 | 50/89 | 54/86 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.44 (0.85 to 2.43) | 1.76 (1.00 to 3.08) | .05 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.43 (0.79 to 2.61) | 1.90 (0.98 to 3.67) | .06 | |
| BRAF-mutant | |||||
| No. of case patients/control individuals (13/26) | 4/9 | 3/5 | 6/12 | ||
| Age-adjusted OR (95% CI) ‡ | 1 (referent) | 1.46 (0.18 to 11.8) | 1.19 (0.23 to 6.13) | .87 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.06 (0.12 to 9.58) | 0.91 (0.15 to 5.52) | .91 | .44 |
| PIK3CA wild-type | |||||
| No. of case patients/control individuals (117/224) | 33/82 | 40/68 | 44/74 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.52 (0.83 to 2.76) | 1.65 (0.89 to 3.05) | .10 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.55 (0.80 to 3.03) | 1.70 (0.84 to 3.43) | .13 | |
| PIK3CA-mutant | |||||
| No. of case patients/control individuals (28/53) | 6/19 | 12/19 | 10/15 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.96 (0.62 to 6.23) | 2.17 (0.60 to 7.78) | .20 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.62 (0.40 to 6.66) | 3.11 (0.64 to 15.1) | .17 | .52 |
| Combined (women [NHS] and men [HPFS]) | |||||
| BRAF wild-type | |||||
| No. of case patients/control individuals (263/506) | 67/168 | 90/173 | 106/165 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.33 (0.91 to 1.97) | 1.79 (1.18 to 2.72) | .007 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.41 (0.93 to 2.13) | 1.81 (1.15 to 2.83) | .01 | |
| BRAF-mutant | |||||
| No. of case patients/control individuals (43/85) | 18/29 | 9/22 | 16/34 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 0.67 (0.26 to 1.73) | 0.77 (0.34 to 1.79) | .49 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 0.60 (0.22 to 1.63) | 0.53 (0.21 to 1.33) | .15 | .01 |
| PIK3CA wild-type | |||||
| No. of case patients/control individuals (240/463) | 64/155 | 78/143 | 98/165 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.32 (0.88 to 1.98) | 1.55 (1.02 to 2.35) | .04 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.40 (0.91 to 2.17) | 1.46 (0.93 to 2.30) | .11 | |
| PIK3CA-mutant | |||||
| No. of case patients/control individuals (48/93) | 15/33 | 17/37 | 16/23 | ||
| Age-adjusted OR (95% CI)‡ | 1 (referent) | 1.06 (0.46 to 2.48) | 1.58 (0.62 to 4.06) | .38 | |
| Multivariable-adjusted OR (95% CI)§ | 1 (referent) | 1.07 (0.42 to 2.72) | 1.89 (0.67 to 5.29) | .28 | .72 |
* Tests for trend (the Wald statistics, two-sided) were conducted using the median values for each tertile of plasma adiponectin. CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; OR = odds ratio.
† Likelihood ratio tests (two-sided) were used to test for heterogeneity (comparing the associations across tumor subtypes).
‡ Results were based on conditional logistic regression analysis with adjustment for age at blood draw and date of blood collection.
§ Results were based on conditional logistic regression analysis (which accounted for age at blood draw and date of blood collection) with additional adjustment for fasting status (<8 hours, ≥8 hours since last meal), body mass index (continuous, kg/m2), physical activity level (continuous, metabolic equivalent hour per week), family history of colorectal cancer (yes, no), multivitamin use (yes, no), regular aspirin use (yes, no), hormone replacement therapy (yes, no; NHS only), history of previous lower endoscopy (yes, no), pack-years of smoking before 30 years of age (continuous), intake of total calorie (continuous, kcal/day), red meat intake (continuous, serving/day), processed meat intake (continuous, serving/day), calcium intake (continuous, mg/day), folate intake (continuous, μg/day), alcohol consumption (continuous, g/day), and plasma 25-hydroxyvitamin D (tertile, ng/mL). The median (range) levels of plasma adiponectin across tertiles among controls are the same as shown in Table 2.
We examined whether the association between plasma adiponectin level and CRC risk differed by combined KRAS/BRAF mutation status, excluding two KRAS-mutant BRAF-mutant case patients (Table 4). In the combined cohort, low-level plasma adiponectin appeared to be associated with KRAS-mutant BRAF wild-type CRC (for T1 vs T3; multivariable OR = 2.93, 95% CI = 1.54 to 5.56, P trend = .002 across tertiles) but not with the other subtypes (KRAS wild-type BRAF wild-type CRC and KRAS wild-type BRAF-mutant CRC) (P trend ≥ .15 across tertiles).
Table 4.
Odds ratio for colorectal cancer by combined mutation status of KRAS and BRAF according to plasma adiponectin level in the combined cohort of the Nurses’ Health Study and the Health Professionals Follow-up Study
| Colorectal cancer subtype | Tertile 3 | Tertile 2 | Tertile 1 | P trend* |
|---|---|---|---|---|
| Combined (women [NHS] and men [HPFS]) | ||||
| KRAS wild-type & BRAF wild-type | ||||
| No. of case patients/control individuals (130/251) | 37/79 | 45/87 | 48/85 | |
| Age-adjusted OR (95% CI)† | 1 (referent) | 1.13 (0.66 to 1.94) | 1.32 (0.73 to 2.38) | .32 |
| Multivariable-adjusted OR (95% CI)‡ | 1 (referent) | 1.06 (0.60 to 1.90) | 1.07 (0.56 to 2.04) | .79 |
| KRAS-mutant & BRAF wild-type | ||||
| No. of case patients/control individuals (133/255) | 30/89 | 45/86 | 58/80 | |
| Age-adjusted OR (95% CI)† | 1 (referent) | 1.58 (0.90 to 2.78) | 2.41 (1.33 to 4.39) | .006 |
| Multivariable-adjusted OR (95% CI)‡ | 1 (referent) | 1.87 (1.02 to 3.40) | 2.93 (1.54 to 5.56) | .002 |
| KRAS wild-type & BRAF-mutant | ||||
| No. of case patients/control individuals (41/81) | 18/28 | 8/22 | 15/31 | |
| Age-adjusted OR (95% CI)† | 1 (referent) | 0.59 (0.22 to 1.55) | 0.77 (0.33 to 1.81) | .44 |
| Multivariable-adjusted OR (95% CI)‡ | 1 (referent) | 0.54 (0.19 to 1.52) | 0.53 (0.21 to 1.36) | .15 |
*Tests for trend (the Wald statistics, two-sided) were conducted using the median values for each tertile of plasma adiponectin. CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; OR = odds ratio.
† Results were based on conditional logistic regression analysis with adjustment for age at blood draw and date of blood collection.
‡ Results were based on conditional logistic regression analysis (which accounted for age at blood draw and date of blood collection) with additional adjustment for fasting status (<8 hours, ≥8 hours since last meal), body mass index (continuous, kg/m2), physical activity level (continuous, metabolic equivalent hour per week), family history of colorectal cancer (yes, no), multivitamin use (yes, no), regular aspirin use (yes, no), hormone replacement therapy (yes, no; NHS only), history of previous lower endoscopy (yes, no), pack-years of smoking before age 30 years (continuous), intake of total calorie (continuous, kcal/day), red meat intake (continuous, serving/day), processed meat intake (continuous, serving/day), calcium intake (continuous, mg/day), folate intake (continuous, μg/day), alcohol consumption (continuous, g/day), and plasma 25-hydroxyvitamin D (tertile, ng/mL). The median (range) levels of plasma adiponectin across tertiles among controls are the same as shown in Table 2.
The association of plasma adiponectin level with CRC risk did not appreciably differ by PIK3CA mutation status (P heterogeneity = .72) (Table 3).
Sensitivity Analyses
To assess for potential bias arising from an influence of subclinical CRC on plasma adiponectin level at the time of blood collection, we conducted an analysis excluding 33 CRC case patients diagnosed within two years after blood draw (Supplementary Table 5, available online). This analysis yielded similar results to our main findings.
To assess the potential bias because of diabetic history, we also conducted an analysis excluding 12 CRC case patients with a history of diabetes at baseline (Supplementary Table 6, available online). This analysis also yielded similar results to our main findings.
Discussion
We conducted this study to test the hypothesis that the association of low-level plasma adiponectin with subsequent risk of developing CRC might be stronger for KRAS-mutant CRC than for KRAS wild-type CRC. This hypothesis is based on the combination of two distinct lines of evidence. First, low-level plasma adiponectin has been associated with CRC risk (4–7). Second, high BMI and physical inactivity have been associated with lower level of plasma adiponectin (3) and with higher risk of KRAS-mutant CRC (19–21). We found that the association of prediagnosis plasma adiponectin level with CRC risk statistically significantly differed by tumor KRAS mutation status and that low-level plasma adiponectin was associated with KRAS-mutant CRC but not with KRAS wild-type CRC.
Observational studies have suggested an inverse association between plasma adiponectin level and CRC risk (4–7). In our previous study, we showed that plasma adiponectin level was inversely associated with overall CRC incidence in men but not in women (4). Experimental studies suggest that adiponectin may also exert anticancer effects through direct mechanisms, such as inhibiting cancer cell proliferation (36,37) and inducing apoptosis (38,39), as well as indirectly through pathways associated with insulin resistance and inflammation (10,40). These potential mechanisms need to be examined in future research.
Analyses of tumor molecular characteristics are increasingly important in clinical and epidemiologic cancer research (41–47). Our current research, which examined an epidemiologic exposure (such as plasma adiponectin) and risk of cancer subtypes classified by molecular pathological features (such as KRAS status), represents molecular pathological epidemiology (MPE) research (48–50). Our current study has substantial strengths as well as specific caveats. Strengths and caveats of MPE research have been discussed in detail elsewhere (48–52). Our current study is unusual in that it identified an association between a plasma marker and a specific molecular subtype of CRC. None of the previous studies on plasma adiponectin and colorectal tumor risk (4–9) examined the molecular features of tumors that had arisen in the context of low-level adiponectin. Utilizing our database, we could assess etiologic heterogeneity according to tumor molecular features, accounting for matching factors and additionally adjusting for lifestyle factors, dietary intake, and medication use.
The possible association between low-level plasma adiponectin and KRAS-mutant CRC is intriguing. Epidemiological evidence suggests that alterations in energy balance associated with physical inactivity, high BMI, or excess caloric intake may be involved in specific mutational pathways in colorectal carcinogenesis, including KRAS mutation (19–21). Taking into account the potential link between excess energy balance changes and KRAS-mutant CRC (19–21), low-level plasma adiponectin, as an indicator of excess energy balance status, might play a role in the development of KRAS-mutant CRC. Low-level adiponectin may favor the evolution of KRAS-mutant premalignant cells in the colorectal tissue microenvironments. Further studies are required to clarify the role of adiponectin in the KRAS-mutated pathway of colorectal carcinogenesis.
As secondary analyses, considering the relationships of KRAS mutation with BRAF and PIK3CA mutations in CRC (13,22–25), we examined whether the association between plasma adiponectin level and CRC risk also differed by mutation status of BRAF or PIK3CA. The association of low-level plasma adiponectin with CRC risk did not appreciably differ by BRAF or PIK3CA status, independent of KRAS status.
Several limitations of our study deserve comment. First, we obtained only one baseline measure of plasma adiponectin. However, plasma adiponectin level has been shown to be generally stable over time, and a one-time measurement can be a surrogate of long-term plasma adiponectin status (53). In addition, the correlation between plasma and serum adiponectin levels is high (54). Second, we measured total adiponectin level but did not identify specific forms of adiponectin (55,56), and one study suggests that ALPCO ELISA assay used in this study might not be as optimal as other commercially available assays in identifying total adiponectin associated with metabolic traits (57). Third, some of control individuals with occult disease might have affected the results. Fourth, we excluded CRC case patients without KRAS data, which might have caused selection bias. However, the association of plasma adiponectin level with incident CRC did not differ substantially by the availability of KRAS data. Furthermore, baseline characteristics of CRC did not differ materially according to the availability of KRAS data. Fifth, most covariates in multivariable models were self-reported and error-prone. Nonetheless, our study population consisted of health professionals known for relatively high accuracy in their reporting data, as shown in previous validation studies (58–60). We still recognize measurement error and possible residual confounding as limitations of this study. Sixth, multiple comparisons were performed in our exploratory analyses, and therefore our results should be interpreted with caution. Finally, the sample size is limited because of the necessity of both prediagnosis plasma adiponectin and CRC tissue with KRAS data. Nevertheless, we have interpreted the results cautiously, considering the biological plausibility. Future studies with larger sample size are warranted to replicate our results.
Strengths of our study include our prospective collection of blood specimens prior to development of CRC, which represents a precious resource to evaluate plasma biomarkers as a potential. Second, our nested case-control design enabled us to match each case patient with control individuals in the same background population. Third, we had access to detailed information on lifestyle, dietary intake, and medication use, which allowed us to control for multiple potential confounders. Fourth, our tumor molecular data enabled us to conduct MPE research (47,61,62), in which we gained unique evidence for the link between low-level plasma adiponectin and KRAS-mutant CRC.
In conclusion, lower prediagnosis level of plasma adiponectin is associated with higher risk of KRAS-mutant CRC but not with KRAS wild-type CRC. Our data suggest that low-level adiponectin may play a role in the development of KRAS-mutant CRC. Additional studies should confirm our results and examine the potential for interventions targeting adiponectin and its signaling pathways in the prevention of CRC, especially KRAS-mutant CRC.
Funding
This work was supported by National Institutes of Health (NIH) grants (P01 CA87969 to SEH; P01 CA55075 to WCW; UM1 CA167552 to WCW; UM1 CA186107 to MJS; P50 CA127003 to CSF; R01 CA136950 to EC; R01 CA137178 and K24 DK098311 to ATC; R01 CA151993 and R35 CA197735 to SO; and K07 CA190673 to RN) and by grants from the Paula and Russell Agrusa Fund for Colorectal Cancer Research (to CSF), the Friends of the Dana-Farber Cancer Institute (to SO), and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. KI was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad and by Takashi Tsuruo Memorial Fund. MS is a trainee of the Harvard Transdisciplinary Research Center on Energetics and Cancer (TREC). PL is a Scottish Government Clinical Academic Fellow and was supported by a Harvard University Frank Knox Memorial Fellowship. SAK was supported by an early exchange postdoctoral fellowship grant from Asan Medical Center. KM is supported by a fellowship grant from the Uehara Memorial Foundation. ATC is a Damon Runyon Clinical Investigator.
Supplementary Material
ATC previously served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, Pozen Inc., and Pfizer Inc. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, Pozen Inc., or Pfizer Inc. No other conflict of interest exists.
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or other research foundations. The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
We deeply thank hospitals and pathology departments throughout the United States for generously providing us with tissue specimens. In addition, we would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
References
- 1. Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270 (45):26746–26749. [DOI] [PubMed] [Google Scholar]
- 2. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271 (18):10697–10703. [DOI] [PubMed] [Google Scholar]
- 3. Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116 (7):1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song M, Zhang X, Wu K, et al. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila). 2013;6 (9):875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei EK, Giovannucci E, Fuchs CS, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97 (22):1688–1694. [DOI] [PubMed] [Google Scholar]
- 6. Aleksandrova K, Boeing H, Jenab M, et al. Total and high-molecular weight adiponectin and risk of colorectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Carcinogenesis. 2012;33 (6):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu XT, Xu Q, Tong JL, et al. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis. 2011;12 (4):234–244. [DOI] [PubMed] [Google Scholar]
- 8. Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11 (10):3642–3646. [DOI] [PubMed] [Google Scholar]
- 9. Yamaji T, Iwasaki M, Sasazuki S, et al. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res. 2010;70 (13):5430–5437. [DOI] [PubMed] [Google Scholar]
- 10. Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86 (3):s858–s866. [DOI] [PubMed] [Google Scholar]
- 11. Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev Mol Diagn. 2012;12 (6):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosty C, Young JP, Walsh MD, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013;26 (6):825–834. [DOI] [PubMed] [Google Scholar]
- 13. Imamura Y, Lochhead P, Yamauchi M, et al. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature review. Mol Cancer. 2014;13 (1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eklof V, Wikberg ML, Edin S, et al. The prognostic role of KRAS, BRAF, PIK3CA and PTEN in colorectal cancer. Br J Cancer. 2013;108 (10):2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359 (17):1757–1765. [DOI] [PubMed] [Google Scholar]
- 17. De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304 (16):1812–1820. [DOI] [PubMed] [Google Scholar]
- 18. Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slattery ML, Curtin K, Anderson K, et al. Associations between dietary intake and Ki-ras mutations in colon tumors: a population-based study. Cancer Res. 2000;60 (24):6935–6941. [PubMed] [Google Scholar]
- 20. Slattery ML, Anderson K, Curtin K, et al. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutat Res. 2001;483(1–2):73–81. [DOI] [PubMed] [Google Scholar]
- 21. Slattery ML, Curtin K, Wolff RK, et al. Diet, physical activity, and body size associations with rectal tumor mutations and epigenetic changes. Cancer Causes Control. 2010;21 (8):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colussi D, Brandi G, Bazzoli F, et al. Molecular Pathways Involved in Colorectal Cancer: Implications for Disease Behavior and Prevention. Int J Mol Sci. 2013;14 (8):16365–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosty C, Young JP, Walsh MD, et al. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS One. 2013;8 (6):e65479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phipps AI, Buchanan DD, Makar KW, et al. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21 (10):1792–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reimers MS, Zeestraten EC, Kuppen PJ, et al. Biomarkers in precision therapy in colorectal cancer. Gastroenterol Rep (Oxf). 2013;1 (3):166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367 (17):1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369 (12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61 (6):794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61 (6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7 (3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogino S, Kawasaki T, Kirkner GJ, et al. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8 (5):582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nosho K, Kawasaki T, Ohnishi M, et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10 (6):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao X, Morikawa T, Lochhead P, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18 (8):2257–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51 (2):524–532. [PubMed] [Google Scholar]
- 35. Rosner B, Glynn RJ, Tamimi RM, et al. Breast cancer risk prediction with heterogeneous risk profiles according to breast cancer tumor markers. Am J Epidemiol. 2013;178 (2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sugiyama M, Takahashi H, Hosono K, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol. 2009;34 (2):339–344. [PubMed] [Google Scholar]
- 37. Kim AY, Lee YS, Kim KH, et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol. 2010;24 (7):1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brakenhielm E, Veitonmaki N, Cao R, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101 (8):2476–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Byeon JS, Jeong JY, Kim MJ, et al. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int J Cancer. 2010;127 (12):2758–2767. [DOI] [PubMed] [Google Scholar]
- 40. Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94 (9):1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonsalves WI, Mahoney MR, Sargent DJ, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106 (7):pii: dju106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noreen F, Roosli M, Gaj P, et al. Modulation of age- and cancer-associated DNA methylation change in the healthy colon by aspirin and lifestyle. J Natl Cancer Inst. 2014;106 (7):pii: dju161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochem Biophys Res Commun. 2014;455(1–2):70–83. [DOI] [PubMed] [Google Scholar]
- 44. Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148 (1):77–87 e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buchanan DD, Win AK, Walsh MD, et al. Family history of colorectal cancer in BRAF p.V600E-mutated colorectal cancer cases. Cancer Epidemiol Biomarkers Prev. 2013;22 (5):917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta RS, Song M, Bezawada N, et al. A prospective study of macrophage inhibitory cytokine-1 (MIC-1/GDF15) and risk of colorectal cancer. J Natl Cancer Inst. 2014;106 (4):dju016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ng JM, Yu J. Promoter Hypermethylation of Tumour Suppressor Genes as Potential Biomarkers in Colorectal Cancer. Int J Mol Sci. 2015;16 (2):2472–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102 (6):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60 (3):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bishehsari F, Mahdavinia M, Vacca M, et al. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20 (20):6055–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Epplein M, Bostick RM, Mu L, et al. Challenges and opportunities in international molecular cancer prevention research: An ASPO Molecular Epidemiology and the Environment and International Cancer Prevention Interest Groups Report. Cancer Epidemiol Biomarkers Prev. 2014;23 (11):2613–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campbell PT, Deka A, Briggs P, et al. Establishment of the cancer prevention study II nutrition cohort colorectal tissue repository. Cancer Epidemiol Biomarkers Prev. 2014;23 (12):2694–2702. [DOI] [PubMed] [Google Scholar]
- 53. Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49 (4):650–652. [DOI] [PubMed] [Google Scholar]
- 54. Huebinger RM, Xiao G, Wilhelmsen KC, et al. Comparison of protein concentrations in serum versus plasma from Alzheimer’s patients. Adv Alzheimer Dis. 2012;1 (3):51–58. [Google Scholar]
- 55. Neumeier M, Weigert J, Schaffler A, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79 (4):803–808. [DOI] [PubMed] [Google Scholar]
- 56. Joshi RK, Lee SA. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15 (1):397–405. [DOI] [PubMed] [Google Scholar]
- 57. Bluher M, Brennan AM, Kelesidis T, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007;30 (2):280–285. [DOI] [PubMed] [Google Scholar]
- 58. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23 (5):991–999. [DOI] [PubMed] [Google Scholar]
- 59. Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135 (10):1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 60. Willett WC. Nutritional Epidemiology. Third ed New York: Oxford University Press; 2012. [Google Scholar]
- 61. Tillmans LS, Vierkant RA, Wang AH, et al. Associations between cigarette smoking, hormone therapy, and folate intake with incident colorectal cancer by TP53 protein expression level in a population-based cohort of older women. Cancer Epidemiol Biomarkers Prev. 2014;23 (2):350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Campbell PT, Newton CC, Newcomb PA, et al. Association between Body Mass Index and Mortality for Colorectal Cancer Survivors: Overall and by Tumor Molecular Phenotype. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.