Abstract
Background
Treatment of Staphylococcus aureus colonization prior to surgery reduces risk of surgical site infection (SSI). The regimen of nasal mupirocin ointment and topical chlorhexidine gluconate is effective, but cost and patient compliance may be a barrier. Nasal povidone iodine solution may provide an alternative to mupirocin.
Methods
We conducted an investigator initiated, open label, randomized trial comparing SSI after arthroplasty or spine fusion in patients receiving topical 2% chlorhexidine gluconate (CHG) wipes with either twice daily application of mupirocin 2% ointment for the 5 days prior to surgery or two 30 second applications of povidone iodine 5% solution into each nostril within 2 hours of surgical incision. The primary study end point was deep SSI within the 3 months after surgery caused by any pathogen or S. aureus.
Results
In the intent-to-treat analysis, a deep SSI developed after 14 of 855 surgeries in the mupirocin group and 6 of 842 surgeries in the povidone iodine group; S. aureus deep SSI developed after 5 surgeries in the mupirocin group and 1 surgery in the povidone iodine group. In the per protocol analysis, S. aureus deep SSI developed in 5 of 763 surgeries in the mupirocin group and 0 of 776 surgeries in the povidone iodine group. Patients found to be S. aureus colonized before surgery were more likely to have a S. aureus deep SSI (OR 6.79; 95% CI 1.1–41.2; p=0.02).
Conclusions
Nasal povidone iodine may be considered as an alternative to mupirocin in a multifaceted approach to reduce SSI.
An estimated 290,000 surgical site infections occur after a procedure in the United States annually, accounting for 22% of all healthcare associated infections [1]. Deep surgical site infections (SSI) after arthroplasty or spine fusion surgery complicate up to 2% of cases, and result in revision surgery and prolonged antibiotic use [2, 3]. The patient morbidity and healthcare system cost is tremendous, with an estimated $566 million spent annually in hospital treatment costs for arthroplasty SSI alone [4]. Staphylococcus aureus is a frequent and feared cause of these infections, given its unique pathogenicity and ability to adhere to prosthetic material [5, 6]. Studies indicate S. aureus colonization prior to surgery is a risk of subsequent infection, with the nasal mucosa serving as a reservoir for S. aureus colonization and a source of secondary transmission to other body sites [7, 8].
Prevention of SSI by treatment of S. aureus colonization with intranasal topical mupirocin has been studied. A short-term suppression rate of 83% after multiple doses of nasal mupirocin was achieved in one randomized, placebo-controlled trial of 891 S. aureus colonized patients, resulting in a statistically significant reduction of invasive S. aureus infection [9]. Several controlled trials suggest a reduction in SSI with the use of pre-operative topical antiseptics [10, 11]. When nasal mupirocin was combined with use of chlorhexidine soap in a randomized, double-blind, placebo-controlled trial including 808 S. aureus colonized surgical patients, a significant reduction in deep S. aureus SSI was realized [12].
To reduce the risk of SSI after arthroplasty and spine fusion surgery at our institution, we historically provided a prescription for brand mupirocin ointment specifically formulated for application on intranasal mucosal surfaces twice a day for the five days prior to surgery, and instructions for the use of chlorhexidine soap the evening before surgery. After implementation of this protocol, we conducted an anonymous patient survey to measure compliance. Although 94% of patients used the chlorhexidine soap, only 86% applied the mupirocin ointment and 8% of patients stated they found it hard or very hard to purchase the mupirocin due to cost [13]. The brand nasal mupirocin ointment specifically produced for application on intranasal mucosal surfaces is only formulation currently available; although generic mupirocin ointment for topical use on skin is available at less cost, application of this formulation on mucosal surfaces may cause irritation. Our survey results, plus reports of emerging mupirocin resistance, led us to search for alternatives [14–19]. Povidone-iodine solution is a broad-spectrum antiseptic suitable for suppression of S. aureus in nasal secretions [20]. In contrast to the application of nasal mupirocin antibiotic ointment to eradicate S. aureus in the nares before surgery, the application of povidone iodine is intended to transiently suppress S. aureus in the nares during surgery. Our hypothesis was a one-time application of nasal povidone iodine just prior to surgery would be as effective as twice daily applications of nasal mupirocin during the five days before surgery in preventing SSI, and provide a more convenient option for patients at lower cost.
Methods
Study treatment
We conducted an investigator initiated, prospective, open-label, randomized trial of twice daily application of mupirocin 2% ointment specifically formulated for use on intranasal mucosal surfaces into each nostril for the 5 days prior to surgery compared with a two 30 second applications of povidone iodine 5% solution formulated as a nasal antiseptic into each nostril (4 applications total) within 2 hours of surgical incision. Both treatments were combined with the application of six 2% chlorhexidine wipes on specific body surfaces from chin to toes the evening prior and morning of surgery. Patients received verbal and written instructions and had access to 24/7 telephone number in case of study treatment related questions.
Subjects
From March 2011 through March 2012, we recruited subjects at least 18 years old who presented to the pre-surgical assessment clinic prior to primary or revision arthroplasty and spine fusion surgery. Exclusion criteria included pregnancy, breastfeeding, allergy to mupirocin or povidone iodine, interval from pre-surgical assessment clinic visit to surgery of less than 7 days and an infectious indication for surgery. The need for nasal intubation (typically for cervical spine surgery) was added as an exclusion criterion shortly after study initiation. All subjects underwent the routine pre-operative evaluation appropriate for their planned surgery, including pregnancy testing, tobacco cessation education, nasal culture for S. aureus, and blood samples for hematology and serum chemistry testing.
Randomization, perioperative surgical prophylaxis and evaluation of S. aureus isolates
Subjects were stratified by arthroplasty or spine fusion surgery, and then randomized 50:50 to either mupirocin or povidone iodine treatment groups in blocks of 100. Research personnel evaluated subjects in the pre-operative holding area to determine chlorhexidine compliance and to either apply povidone iodine or assess compliance with mupirocin.
Subjects received routine antimicrobial prophylaxis, surgical site preparation and surgical draping. Primary antimicrobial prophylaxis was cefazolin 1 gm; subjects with reported β-lactam allergy received clindamycin 600 mg and those colonized with methicillin-resistant S. aureus (MRSA) received vancomycin 1 gm. Antibiotic infusion was started within one hour of incision (two hours for vancomycin) and was re-dosed per accepted guidelines. Weight based dosing was employed at the discretion of the anesthesiologist. Standard pre-operative surgical site skin preparation consisted of a 2% chlorhexidine gluconate/70% isopropyl alcohol solution. If needed, electrical clippers were used for hair removal at the surgical site and patients were actively warmed in the intraoperative and postoperative period.
Subjects were re-assessed within 1 to 3 days after surgery to record patient satisfaction and adverse events related to study treatment. If the pre-operative nasal culture grew S. aureus, a repeat nasal culture was ordered. The S. aureus isolates from those subjects who developed a S. aureus SSI were retrieved from the Clinical Microbiology laboratory for additional testing. Identification of MRSA was based on routine criteria, including the coagulase tube test and the automated Vitek 2 system [BioMérieux, Marci l'Etoile, France] and mupirocin susceptibility was performed by E-test. Isolates with a mupirocin MIC of ≥8μg/mL considered mupirocin-resistant [19, 21]. Further characterization by spa typing was performed if the pre-operative and post-operative S. aureus isolates from the same subject were available [22–24].
End points
The primary study end point was onset of a deep SSI within the 3 months after surgery caused by any pathogen or S. aureus. Potential SSI were identified by review of microbiology reports, hospital readmissions, if a report was received from another healthcare facility (as mandated by New York State Department of Health regulations) and during Infection Prevention and Control (IPC) rounds on inpatient units. Patient records were reviewed and the SSI classified using the Center for Disease Control and Prevention’s National Healthcare Safety Network case definitions. IPC practitioners reviewing the records were blinded to study participation and receipt of study treatment; potential cases were discussed at a group meeting to ensure consistent application of the SSI case definition. Infections in subjects were retrieved from the IPC database maintained for routine SSI surveillance.
Statistical analysis
We expected no difference in SSI between treatment groups. Our baseline combined arthroplasty and spine fusion deep SSI rate was 1.5/100 procedures, with S. aureus as the infecting pathogen in 37% of cases. During the baseline period, all patients received a prescription for mupirocin ointment with instructions to apply to the nares twice a day for the five days prior to surgery and were provided 2% chlorhexidine wipes for use the evening prior and morning of surgery. We assumed a doubling of SSI rate in the povidone iodine group would be clinically relevant, and calculated a sample size of 3000 subjects would provide a power of 80% to detect a doubling of SSI rate to 3.0/100 procedures with an alpha level of 0.05 and a two sided Fisher’s exact test. Analysis was conducted using SAS version 9.1, Cary, NC. Categorical variables were analyzed using Fisher’s exact test.
The intent to treat group included those who were enrolled and met eligibility requirements for the study and the per protocol group included all eligible enrolled subjects who completed the assigned study regimen. Completion of the study regimen was defined as 2 applications of 6 chlorhexidine wipes to specific areas of skin from chin to toe, receiving appropriate perioperative antimicrobial prophylaxis and receiving either 7 to 10 applications of mupirocin to the nares over the 5 days before surgery or 2 applications of povidone-iodine each nostril within 2 hours of surgical incision.
Study oversight
The study was approved by the institutional review board at our institution and informed consent was obtained from all study participants. The authors designed the study, and were solely responsible for the collection, analysis, interpretation and presentation of the data. 3M Corporation, the manufacturer of the nasal povidone-iodine solution, provided financial support but had no role in the study design, collection of the data or preparation of this manuscript.
Results
Subjects
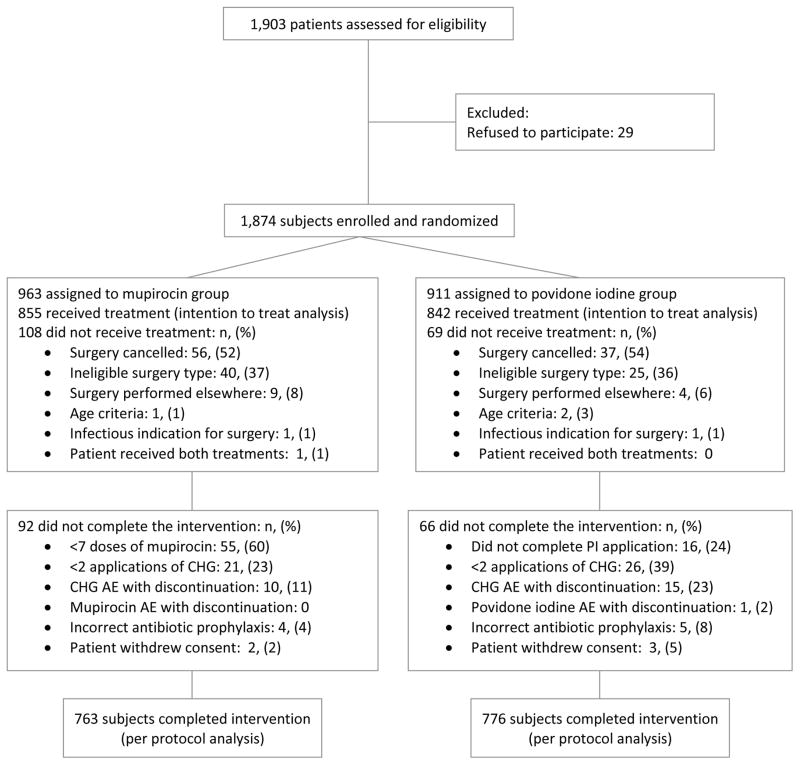
During the 12 month enrollment period, 1,874 of the 1,903 patients assessed were enrolled and randomized; 177 of the enrolled patients did not receive the study intervention, the surgery for most of these individuals was cancelled or the actual surgical procedure performed was not eligible for inclusion in the study. The demographic and clinical characteristics and surgery types of the remaining 1,697 subjects in the intent to treat analysis are provided in Tables 1 and 2. The 1,539 subjects who completed the intervention are included in the per protocol analysis (Figure 1).
Table 1.
Demographic Characteristics of Subjects in the Intention-to-Treat Analysis
| Characteristic | Mupirocin (n=855) | Povidone-iodine (n=842) | ||
|---|---|---|---|---|
| Age (years) | ||||
|
|
||||
| Median | 62.4 | 61.8 | ||
|
|
||||
| Range | 19.2–93.2 | 19.1–92.4 | ||
|
|
||||
| Female sex–no. (%) | 523 | (61) | 499 | (59) |
|
|
||||
| Race–no. (%) | ||||
|
|
||||
| White | 677 | (79) | 670 | (80) |
|
|
||||
| Black | 138 | (16) | 145 | (17) |
|
|
||||
| Asian | 23 | (2.7) | 21 | (2.5) |
|
|
||||
| Native Hawaiian/Pacific Islander | 0 | 1 | (0.1) | |
|
|
||||
| American Indian/Alaska native | 2 | (0.2) | 0 | |
|
|
||||
| Other | 20 | (1.9) | 6 | (0.7) |
|
|
||||
| Ethnic group–no. (%) | ||||
|
|
||||
| Hispanic | 97 | (11) | 88 | (10) |
|
|
||||
| Non-Hispanic | 746 | (87) | 749 | (89) |
Table 2.
Clinical and Surgical Characteristics of Subjects in the Intention-to-Treat Analysis
| Characteristic | Mupirocin (n=855) | Povidone-iodine (n=842) | ||
|---|---|---|---|---|
| BMI (kg/m2) | ||||
|
|
||||
| Median | 29.5 | 29.5 | ||
|
|
||||
| Range | 14.9–58.9 | 12.0–57.3 | ||
|
|
||||
| Current smoking – no. (%) | 104 | (12) | 114 | (13) |
|
|
||||
| Medical comorbidities – no. (%) | ||||
|
|
||||
| Diabetes mellitus | 110 | (13) | 104 | (12) |
|
|
||||
| Rheumatoid arthritis | 36 | (4.2) | 36 | (4.3) |
|
|
||||
| Pre-op S. aureus colonization - no. (%) | ||||
|
|
||||
| MSSA | 137 | (16) | 130 | (15) |
|
|
||||
| MRSA | 25 | (2.9) | 21 | (2.5) |
|
|
||||
| Any S. aureus | 162 | (19) | 151 | (18) |
|
|
||||
| Pre-op serum albumin (g/dL) | ||||
|
|
||||
| Median | 4.2 | 4.2 | ||
|
|
||||
| Range | 2.9–6.9 | 2.8–5.2 | ||
|
|
||||
| ASA score–no. (%) | ||||
|
|
||||
| 1 | 35 | (4.5) | 39 | (5.0) |
|
|
||||
| 2 | 486 | (62) | 524 | (68) |
|
|
||||
| 3 | 254 | (32) | 206 | (27)* |
|
|
||||
| 4 | 9 | (1.1) | 4 | (0.5) |
|
|
||||
| Receipt of blood products–no. (%) | 179 | (21) | 158 | (19) |
|
|
||||
| Post-op glucose ≥ 180 mg/dL - no. (%) | 40 | (4.7) | 46 | (5.5) |
| Procedure type – no. (%) | ||||
|
|
||||
| Spine fusion | 148 | (17) | 145 | (17) |
|
|
||||
| Spine fusion, revision | 12 | (1.4) | 10 | (1.2) |
|
|
||||
| Arthroplasty surgery | ||||
|
|
||||
| Knee | 299 | (35) | 297 | (35) |
|
|
||||
| Knee, revision | 24 | (2.8) | 24 | (2.8) |
|
|
||||
| Hip | 298 | (35) | 293 | (35) |
|
|
||||
| Hip, revision | 35 | (4.1) | 29 | (3.4) |
|
|
||||
| Shoulder | 33 | (3.9) | 42 | (5.0) |
|
|
||||
| Shoulder, revision | 7 | (0.8) | 1 | (0.1) |
|
|
||||
| Median operative time (minutes) | ||||
|
|
||||
| Spine fusion | 202 | 205 | ||
|
|
||||
| Spine fusion, revision | 256 | 299 | ||
|
|
||||
| Arthroplasty surgery, unilateral | ||||
|
|
||||
| Knee | 93 | 87 | ||
|
|
||||
| Knee, revision | 137 | 128 | ||
|
|
||||
| Hip | 94 | 93 | ||
|
|
||||
| Hip, revision | 138 | 123 | ||
|
|
||||
| Shoulder | 106 | 109 | ||
|
|
||||
| Shoulder, revision | 122 | 119 | ||
|
|
||||
| Bilateral arthroplasty – no. (%) | 49 | (6.2) | 73 | (9.3)* |
p<0.05 by chi-square
Figure 1.
Flow Diagram of Study Participants
End points
In the intent-to-treat analysis, S. aureus deep SSI developed after 5 of 855 surgeries in the mupirocin group and 1 of 842 surgeries in the povidone iodine group (p=0.2). A deep SSI caused by any pathogen developed after 14 surgeries in the mupirocin group and 6 surgeries in the povidone iodine group (p=0.1). In the per protocol analysis, S. aureus deep SSI developed in 5 of 763 surgeries in the mupirocin group and 0 of 776 surgeries in the povidone iodine group (p=0.03). The overall deep SSI rate was 1.6/100 procedures in the mupirocin group and 0.7/100 procedures in the povidone-iodine group in the intent to treat analysis. The infecting pathogens are provided in Table 3. The S. aureus deep SSI rate was 0.6/100 procedures in the mupirocin group and 0.1/100 procedures in the povidone-iodine group in the intent to treat analysis. In the per protocol analysis, the S. aureus deep SSI was 0.7/100 procedures in the mupirocin group and there were no infections in the povidone-iodine group (Tables 4 and 5).
Table 3.
Deep SSI Pathogens, Intent to Treat Analysis
| Mupirocin | Povidone-Iodine | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Methicillin-sensitive S. aureus | 4 | (24) | ||
|
|
||||
| Methicillin-resistant S. aureus | 1 | (6) | 1 | (17) |
|
|
||||
| Coagulase-negative Staphylococci | 4 | (24) | 1 | (17) |
|
|
||||
| S. agalactiae | 1 | (17) | ||
|
|
||||
| E. faecalis | 1 | (6) | 1 | (17) |
|
|
||||
| P. acnes | 2 | (12) | ||
|
|
||||
| E. coli | 1 | (6 | 1 | (17) |
|
|
||||
| P. mirabilis | 2 | (12) | ||
|
|
||||
| P. aeruginosa | 1 | (17) | ||
|
|
||||
| B. fragilis | 2 | (12) | ||
|
|
||||
| Total | 17 | 100 | 6 | 100 |
Table 4.
Any Deep Surgical Site Infection, by Analysis and Treatment
| # Subjects | # Deep SSI | p-value | |
|---|---|---|---|
| Intent to treat | |||
| Mupirocin | 855 | 14 | 0.1 |
| Povidone iodine | 842 | 6 | |
| Per protocol | |||
| Mupirocin | 763 | 13 | 0.06 |
| Povidone iodine | 776 | 5 | |
Table 5.
S. aureus Deep Surgical Site Infection, by Analysis and Treatment
| # Subjects | # S. aureus Deep SSI | p-value | |
|---|---|---|---|
| Intent to treat | |||
| Mupirocin | 855 | 5 | 0.2 |
| Povidone iodine | 842 | 1 | |
| Per protocol | |||
| Mupirocin | 763 | 5 | 0.03 |
| Povidone iodine | 776 | 0 | |
Adverse events and patient perception of study treatment
An adverse event resulted in study discontinuation in 10 of 855 (1.2%) subjects in the mupirocin group and 16 of 842 (1.9%) subjects in the povidone iodine group (P=0.24); most due to skin reactions to topical chlorhexidine (Figure 1). One patient in the povidone-iodine group discontinued the study after a vasovagal reaction during the application of the study medication. In the intent to treat analysis, those in the mupirocin group were more likely to report headache, rhinorrhea, congestion, sore throat or any treatment related symptom (Table 6). Patient perceptions of the study treatment were recorded for 555 mupirocin and 536 povidone iodine subjects. Although an equivalent proportion of subjects felt use of the study medication was very important to reduce risk of infection (57% of mupirocin and 60% of povidone iodine subjects), a significantly higher proportion of mupirocin subjects (213 of 555, 38%) reported application of the nasal treatment to be unpleasant compared to povidone iodine subjects (19 of 536, 3.6%), (p<0.0001).
Table 6.
Treatment related symptoms, Intent to Treat Analysis
| Mupirocin (n=855) | Povidone-iodine (n=842) | ||||
|---|---|---|---|---|---|
| Number (%) | Number (%) | P value | |||
| Study treatment | |||||
|
|
|||||
| Headache | 15 | (1.8) | 2 | (0.2) | 0.002 |
|
|
|||||
| Rhinorrhea | 47 | (5.5) | 1 | (0.1) | <0.0001 |
|
|
|||||
| Nasal irritation | 13 | (1.5) | 8 | (1.0) | 0.38 |
|
|
|||||
| Congestion | 15 | (1.8) | 3 | (0.4) | 0.007 |
|
|
|||||
| Cough | 6 | (0.7) | 3 | (0.4) | 0.5 |
|
|
|||||
| Pharyngeal pain | 10 | (1.2) | 0 | 0 | 0.002 |
|
|
|||||
| Any | 76 | (8.9) | 15 | (1.8) | <0.0001 |
|
|
|||||
| CHG wipe | |||||
|
|
|||||
| Pruritis | 7 | (0.8) | 12 | (1.4) | 0.26 |
|
|
|||||
| Rash | 4 | (0.5) | 3 | (0.4) | 1.0 |
|
|
|||||
| Any | 10 | (1.2) | 13 | (1.5) | 0.54 |
Risk factors
Receipt of mupirocin and pre-operative S. aureus colonization were significant risk factors for S. aureus deep SSI by univariate analysis (Table 7). There was an insufficient number of outcomes to perform a meaningful multivariate analysis, therefore we stratified outcome by pre-operative S. aureus colonization status for more information. In the 274 S. aureus colonized subjects, S. aureus deep SSI occurred in 3 of 141 mupirocin subjects and none of 136 povidone iodine subjects (p=0.08). In the 1,252 subjects characterized as not S. aureus colonized by pre-operative nasal culture, 2 S. aureus deep SSI occurred in the 617 mupirocin subjects and none of the 637 povidone iodine subjects (p=0.15).
Table 7.
Univariate Analysis of Risk Factors for deep S. aureus Surgical Site Infection
| Risk | RR (95% CI) | P value |
|---|---|---|
| Mupirocin | 1.01 (1.001–1.012) | 0.04 |
|
|
||
| Female sex | 1.00 (0.17–6.03) | 0.99 |
|
|
||
| Current smoking | 1.76 (0.19–15.6) | 0.61 |
|
|
||
| Pre-op culture = S. aureus | 6.79 (1.1–41.2) | 0.02 |
|
|
||
| Diabetes | 1.60 (0.19–14.9) | 0.65 |
|
|
||
| Post-op glucose ≥ 180mg/dL (day 1,2) | 1.60 (0.09–29.9) | 0.6 |
|
|
||
| Rheumatoid arthritis | 5.60 (0.6–50) | 0.08 |
|
|
||
| Immunosuppressive medication | 5.10 (0.29–89.9) | 0.77 |
|
|
||
| ASA score ≥3 | 1.69 (0.28–10.1) | 0.55 |
|
|
||
| Receipt of blood products | 0.98 (0.11–8.8) | 0.99 |
|
|
||
| BMI ≥ 30 kg/mm2 | 0.28 (0.03–2.5) | 0.22 |
|
|
||
| Pre-op albumin ≥ 3.5 g/dL | 0.33 (0.02–5.8) | 0.7 |
|
|
||
| Bilateral arthroplasty surgery | 0.94 (0.05–16.8) | 0.52 |
|
|
||
| Revision surgery | 2.70 (0.31–24.1) | 0.35 |
S. aureus antibiotic susceptibility testing and strain typing
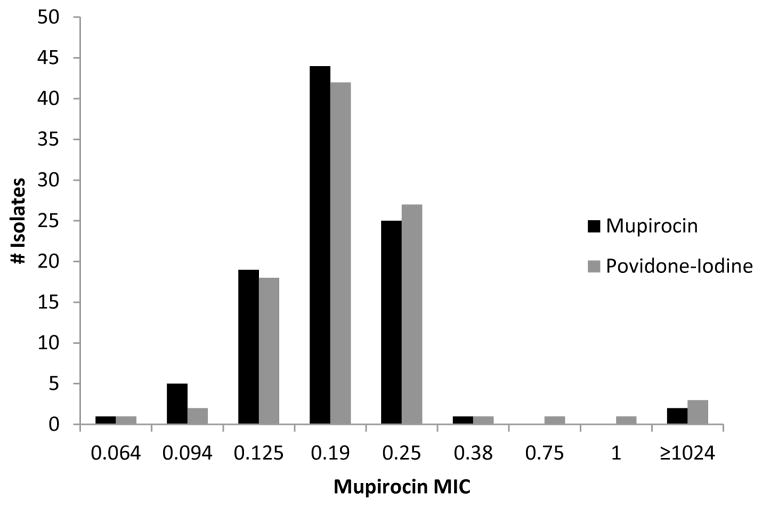
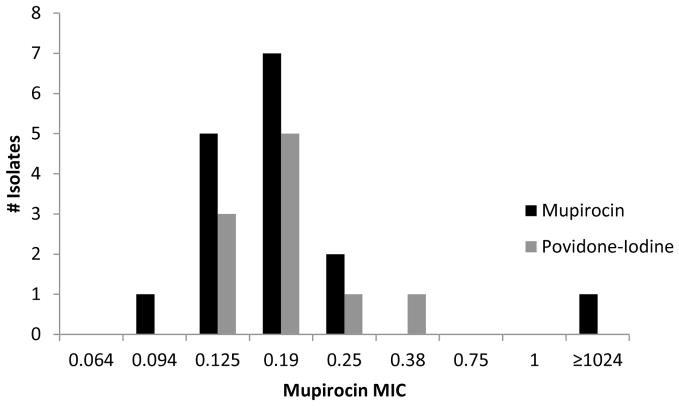
Available S. aureus isolates from pre-operative nasal culture, post-operative nasal cultures and surgical site infections were tested for methicillin and mupirocin susceptibility. The proportion of subjects colonized with MRSA and methicillin-sensitive S. aureus (MSSA) before surgery was equivalent in both treatment groups (Table 8). The methicillin susceptibility of the S. aureus isolate obtained from a deep SSI matched the pre-operative nasal culture in 4 of the 6 S. aureus deep SSI in the intent to treat analysis. In the remaining 2 S. aureus deep SSI (both in the mupirocin group), the pre-operative nasal culture was no growth (data not shown). Mupirocin resistance was detected in 2 of 97 (2%) MSSA isolates and 1 of 16 (6%) of MRSA isolates; distribution of mupirocin MIC was similar in both treatment groups (Figures 2 and 3). In the intent to treat analysis, subjects with a pre-operative nasal culture yielding S. aureus, the proportion of post-operative nasal culture with no growth was 78 of 85 (92%) mupirocin subjects and 45 of 84 (54%) povidone-iodine subjects, (p=0.03). No deep S. aureus SSI occurred in patients colonized with mupirocin resistant S. aureus. The S. aureus strain isolated from pre-operative culture was different by spa typing from the post-operative strain in 2 of 33 (6%) subjects (Table 9).
Table 8.
Methicillin Sensitivity of Pre-operative Nasal Culture S. aureus Isolates by Study Drug, Intent to Treat Analysis
| Mupirocin | Povidone-Iodine | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
|
|
||||
| MSSA | 135 | (16) | 130 | (15) |
|
|
||||
| MRSA | 24 | (3) | 21 | (3) |
|
|
||||
| Culture no growth | 692 | (81) | 683 | (81) |
|
|
||||
| Sample not obtained | 4 | (0) | 8 | (1) |
|
|
||||
| Total | 855 | 842 | ||
Figure 2.
Mupirocin Minimum Inhibitory Concentration of Pre-operative MSSA Isolates by Study Drug, Intent to Treat Analysis
Figure 3.
Mupirocin Minimum Inhibitory Concentration of Pre-operative MRSA Isolates by Study Drug, Intent to Treat Analysis
Table 9.
Mupirocin Sensitivity of Pre-operative Nasal, Post-operative Nasal and Deep Surgical Site Infection S. aureus Isolates by Study Treatment, Per Protocol Analysis
| Pre-operative | Post-operative | Deep SSI | Mupirocin | Povidone-iodine | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
|
|
||||||
| Sensitive | Sensitive | no SSI | 6* | (4) | 34^ | (25) |
|
|
||||||
| Sensitive | Resistant | no SSI | 0 | (0) | 1# | (1) |
|
|
||||||
| Sensitive | Isolate not tested | no SSI | 0 | (0) | 4 | (3) |
|
|
||||||
| Sensitive | Culture no growth | no SSI | 74 | (53) | 43 | (32) |
|
|
||||||
| Sensitive | Sample not obtained | no SSI | 24 | (17) | 19 | (14) |
|
|
||||||
| Resistant | Isolate not tested | no SSI | 1 | (1) | 0 | (0) |
|
|
||||||
| Resistant | Culture no growth | no SSI | 1 | (1) | 2 | (1) |
|
|
||||||
| Resistant | Sample not obtained | no SSI | 1 | (1) | 1 | (1) |
|
|
||||||
| Isolate not tested | no SSI | 28 | (20) | 31 | (23) | |
|
|
||||||
| Culture no growth | Sample not obtained | Sensitive | 2 | (1) | 0 | (0) |
|
|
||||||
| Sensitive | Culture no growth | Isolate not tested | 1 | (1) | 0 | (0) |
|
|
||||||
| Isolate not tested | Culture no growth | Sensitive | 2 | (1) | 0 | (0) |
Analysis of the 6 pre-post pairs by spa typing revealed 5 pairs were identical and one pair was unable to be typed
Analysis of the 34 pre-post pairs by spa typing revealed 26 pairs were identical, one pair was different and 7 pairs were unable to be typed
Analysis of the pre-post pair revealed different spa types
Discussion
Healthcare systems and providers are challenged to improve patient safety and control cost by identifying important, modifiable SSI risk factors amenable to intervention. The use of nasal mupirocin to suppress S. aureus colonization and prevent subsequent invasive infection has proven effective in controlled studies, yet compliance in actual use may be problematic due to side effects and out of pocket patient expenses. Our study suggests pre-operative nasal povidone iodine with topical chlorhexidine is similar to pre-operative nasal mupirocin with topical chlorhexidine in preventing S. aureus deep SSI after arthroplasty and spine fusion surgery. Although target enrollment was not met, a statistically significant reduction in S. aureus deep SSI in the per protocol analysis was observed. Subjects in the povidone iodine group experienced lower rates of treatment related symptoms and were less likely to report application of the treatment as unpleasant. Application of nasal povidone iodine by the patient care team just prior to surgery may ensure greater compliance.
Similar to other investigators, we identified pre-operative S. aureus colonization as a significant risk factor for subsequent S. aureus SSI [25–28]. In our study, all deep S. aureus SSI occurred in subjects with either a pre-operative nasal culture of no growth or a pre-operative nasal culture yielding S. aureus coupled with a post-operative culture of no growth. We feel this likely represents either incomplete suppression of S. aureus colonization at sites other than the nares or possibly an intra-operative or post-operative exposure from exogenous source. Mupirocin was more effective than povidone-iodine at clearing nasal S. aureus colonization. This result is not unexpected given the different mechanisms of the study treatments – the antibiotic mupirocin is intended to eradicate colonization in the nares, while the antiseptic povidone iodine only suppresses S. aureus for the duration of surgery. In two cases, the pre-operative and post-operative S. aureus spa type differed, and in one of these cases was associated with acquisition of mupirocin resistance in a patient who received povidone iodine. This finding may be due to colonization with heterogeneous S. aureus strains, postoperative re-colonization or acquisition of mupirocin resistance (in one case) due to a transient hypermutable state. Although mupirocin resistance was not associated with infection in our study, the number of resistant isolates was low and for several subjects with deep S aureus SSI, either the pre-operative culture was no growth or the isolate was unavailable for mupirocin susceptibility testing.
The use of mupirocin to decolonize the nares of patients prior to orthopedic surgery has been demonstrated as a cost effective intervention [29–32]. Brand nasal mupirocin is currently the only formulation available for application to the nasal mucosa and costs approximately $130/course, while nasal povidone iodine costs approximately $20/application – given the equal efficacy of both treatments in our study, povidone iodine provides more value, as defined as quality of outcomes divided by cost [33]. Implementing cost effective interventions to reduce SSI is even more critical as the payors move to reimburse healthcare providers base on episode of care, which requires hospitals and physicians to control costs and assume financial risk for outcomes [34].
Our study has several limitations. First, we failed to achieve our target enrollment due to an overestimation of number of potential subjects during study period. Regardless of under enrollment, the study effect was large enough that a statistical difference was noted in number of deep S. aureus SSI infections in the per protocol analysis. Second, the small sample size precluded a multivariate analysis. Although this is true, the randomization provided well balanced treatment groups with respect to clinical, demographic and surgical variables. Third, nasal culture alone was used as a screen for S. aureus colonization, which has a sensitivity of only 48% to 66%, and we did not quantify the amount of S. aureus in the nares [35, 36]. Although nasal culture alone may miss colonized subjects, we feel study outcome was unaffected as all subjects received treatment. We agree that certain colonized patients shed more S. aureus from the nares than other colonized patients, and this potential effect on S. aureus SSI warrants further study. Fourth, a portion of post-operative nasal cultures were not performed or S. aureus isolates did not undergo mupirocin susceptibility testing. We feel this did not introduce a bias or nullify our conclusions as the number of missed cultures and isolates were equally balanced between groups. Finally, the study was performed at one institution and the results may not be applicable to other locations with different patient characteristics, or differing frequencies of S. aureus strain types or mupirocin resistance.
In conclusion, the use of nasal povidone iodine may be considered as an alternative to mupirocin and a component of a multifaceted approach to reduce SSI.
Acknowledgments
Funding/Support
This project was supported by a grant from 3M Corporation
Footnotes
Role of the Sponsor
3M had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit this manuscript for publication.
Additional Contributions
We thank Robert S. Holzman MD, Vasudha Reddy, MPH and Anna Stachel, MPH for their assistance in statistical analysis and careful review of this manuscript. They received no compensation related to this study or from the sponsor for their contributions.
Presentations
This work was presented at the annual IDWeek meeting in San Diego, CA on October 20, 2012 in the Late Breaker Symposium
This trial was registered on ClinicalTrials.gov; registration identification number NCT01313182; URL http://clinicaltrials.gov/show/NCT01313182
Conflict of Interest Disclosures
M. Phillips, 3M corporation (research grant which funded this study); had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
A. Rosenberg, 3M Corporation (research grant which funded this study)
B. Shopsin, 3M Corporation (research grant which funded this study) and grant funding is: R01 AI103268-01
G. Cuff, no potential conflicts of interest
F. Skeete, no potential conflicts of interest
A. Foti, no potential conflicts of interest
K. Kraemer, no potential conflicts of interest
K. Inglima, no potential conflicts of interest
B. Press, no potential conflicts of interest
J. Bosco, 3M Corporation (research grant which funded this study)
Contributor Information
A. Rosenberg, Email: Andrew.Rosenberg@nyumc.org.
B. Shopsin, Email: Bo.Shopsin@med.nyu.edu.
G. Cuff, Email: Germaine.Cuff@nyumc.org.
F. Skeete, Email: Faith.Skeete@nyumc.org.
A. Foti, Email: alyciafoti7@gmail.com.
K. Kraemer, Email: Candy.Kraemer@nyumc.org.
K. Inglima, Email: Kenneth.Inglima@nyumc.org.
B. Press, Email: Robert.Press@nyumc.org.
J. Bosco, Email: Joseph.Bosco@nyumc.org.
References
- 1.Klevens RM, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renaud A, Lavigne M, Vendittoli PA. Periprosthetic joint infections at a teaching hospital in 1990–2007. Can J Surg. 2012;55(6):394–400. doi: 10.1503/cjs.033610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz SM, et al. Infection risk for primary and revision instrumented lumbar spine fusion in the Medicare population. J Neurosurg Spine. 2012;17(4):342–7. doi: 10.3171/2012.7.SPINE12203. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz SM, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61–5 e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Felden B, et al. The Staphylococcus aureus RNome and its commitment to virulence. PLoS Pathog. 2011;7(3):e1002006. doi: 10.1371/journal.ppat.1002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez CJ, Jr, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluytmans JA, et al. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171(1):216–9. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 8.Wertheim HF, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–62. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 9.Perl TM, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346(24):1871–7. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 10.Hayek LJ, Emerson JM, Gardner AM. A placebo-controlled trial of the effect of two preoperative baths or showers with chlorhexidine detergent on postoperative wound infection rates. J Hosp Infect. 1987;10(2):165–72. doi: 10.1016/0195-6701(87)90143-5. [DOI] [PubMed] [Google Scholar]
- 11.Lynch W, et al. Cost-effectiveness analysis of the use of chlorhexidine detergent in preoperative whole-body disinfection in wound infection prophylaxis. J Hosp Infect. 1992;21(3):179–91. doi: 10.1016/0195-6701(92)90074-v. [DOI] [PubMed] [Google Scholar]
- 12.Bode LG, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 13.Ramos N, et al. Surgical site infection prevention initiative - patient attitude and compliance. Bull NYU Hosp Jt Dis. 2011;69(4):312–5. [PubMed] [Google Scholar]
- 14.Cookson B, et al. Mupirocin-resistant Staphylococcus aureus. Lancet. 1992;339(8793):625. doi: 10.1016/0140-6736(92)90920-x. [DOI] [PubMed] [Google Scholar]
- 15.Annigeri R, et al. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int. 2001;21(6):554–9. [PubMed] [Google Scholar]
- 16.McNeil JC, et al. Mupirocin resistance in Staphylococcus aureus causing recurrent skin and soft tissue infections in children. Antimicrob Agents Chemother. 2011;55(5):2431–3. doi: 10.1128/AAC.01587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bathoorn E, et al. Emergence of high-level mupirocin resistance in coagulase-negative staphylococci associated with increased short-term mupirocin use. J Clin Microbiol. 2012;50(9):2947–50. doi: 10.1128/JCM.00302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layton MC, et al. An outbreak of mupirocin-resistant Staphylococcus aureus on a dermatology ward associated with an environmental reservoir. Infect Control Hosp Epidemiol. 1993;14(7):369–75. doi: 10.1086/646764. [DOI] [PubMed] [Google Scholar]
- 19.Jayakumar S, et al. Prevalence of High and Low Level Mupirocin Resistance among Staphylococcal Isolates from Skin Infection in a Tertiary Care Hospital. Journal of Clinical and Diagnostic Research. 2013;7(2):238–242. doi: 10.7860/JCDR/2013/4694.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill RL, Casewell MW. The in-vitro activity of povidone-iodinecream against Staphylococcus aureus and its bioavailability in nasal secretions. J Hosp Infect. 2000;45(3):198–205. doi: 10.1053/jhin.2000.0733. [DOI] [PubMed] [Google Scholar]
- 21.Eltringham I. Mupirocin resistance and methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 1997;35(1):1–8. doi: 10.1016/s0195-6701(97)90162-6. [DOI] [PubMed] [Google Scholar]
- 22.Shopsin B, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37(11):3556–63. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallin M, et al. Validation of pulsed-field gel electrophoresis and spa typing for long-term, nationwide epidemiological surveillance studies of Staphylococcus aureus infections. J Clin Microbiol. 2007;45(1):127–33. doi: 10.1128/JCM.01866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallin M, Friedrich AW, Struelens MJ. spa typing for epidemiological surveillance of Staphylococcus aureus. Methods Mol Biol. 2009;551:189–202. doi: 10.1007/978-1-60327-999-4_15. [DOI] [PubMed] [Google Scholar]
- 25.Mangram AJ, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250–78. doi: 10.1086/501620. quiz 279–80. [DOI] [PubMed] [Google Scholar]
- 26.Yano K, et al. Positive nasal culture of methicillin-resistant Staphylococcus aureus (MRSA) is a risk factor for surgical site infection in orthopedics. Acta Orthop. 2009;80(4):486–90. doi: 10.3109/17453670903110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalmeijer MD, et al. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21(5):319–23. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 28.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slover J, et al. Cost-effectiveness of a Staphylococcus aureus screening and decolonization program for high-risk orthopedic patients. J Arthroplasty. 2011;26(3):360–5. doi: 10.1016/j.arth.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 30.VandenBergh MF, et al. Cost-effectiveness of perioperative mupirocin nasal ointment in cardiothoracic surgery. Infect Control Hosp Epidemiol. 1996;17(12):786–92. doi: 10.1086/647237. [DOI] [PubMed] [Google Scholar]
- 31.Young LS, Winston LG. Preoperative use of mupirocin for the prevention of healthcare-associated Staphylococcus aureus infections: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2006;27(12):1304–12. doi: 10.1086/509837. [DOI] [PubMed] [Google Scholar]
- 32.Courville XF, et al. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2012;33(2):152–9. doi: 10.1086/663704. [DOI] [PubMed] [Google Scholar]
- 33.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 34.Enquist M, et al. Managing episodes of care: strategies for orthopaedic surgeons in the era of reform. J Bone Joint Surg Am. 2011;93(10):e55. doi: 10.2106/JBJS.J.01703. [DOI] [PubMed] [Google Scholar]
- 35.Matheson A, et al. Nasal swab screening for methicillin-resistant Staphylococcus aureus--how well does it perform? A cross-sectional study. Infect Control Hosp Epidemiol. 2012;33(8):803–8. doi: 10.1086/666639. [DOI] [PubMed] [Google Scholar]
- 36.Senn L, et al. Which anatomical sites should be sampled for screening of methicillin-resistant Staphylococcus aureus carriage by culture or by rapid PCR test? Clin Microbiol Infect. 2012;18(2):E31–3. doi: 10.1111/j.1469-0691.2011.03724.x. [DOI] [PubMed] [Google Scholar]