Abstract
Objective
Human neuroimaging studies of reward processing typically involve tasks that engage decision-making processes in the dorsal striatum or focus upon the ventral striatum's response to feedback expectancy. These studies are often compared to the animal literature; however, some animal studies include both feedback and nonfeedback events that activate the dorsal striatum during feedback expectancy. Differences in task parameters, movement complexity, and motoric effort to attain rewards may partly explain ventral and dorsal striatal response differences across species. We therefore used a target capture task during functional neuroimaging that was inspired by a study of single cell modulation in the internal globus pallidus during reward-cued, rotational arm movements in nonhuman primates.
Methods
In this functional magnetic resonance imaging study, participants used a fiberoptic joystick to make a rotational response to an instruction stimulus that indicated both a target location for a capture movement and whether or not the trial would end with feedback indicating either a small financial gain or a neutral outcome.
Results
Portions of the dorsal striatum and pallidum demonstrated greater neural activation to visual cues predicting potential gains relative to cues with no associated outcome. Furthermore, both striatal and pallidal regions displayed a greater response to financial gains relative to neutral outcomes.
Conclusions
This reward-dependent modulation of dorsal striatal and pallidal activation in a target-capture task is consistent with findings from reward studies in animals, supporting the use of motorically complex, tasks as translational paradigms to investigate the neural substrates of reward expectancy and outcome in humans.
Keywords: striatum, pallidum, functional magnetic resonance imaging, reward, skeletomotor, translational
Introduction
Positive reinforcers serve a number of basic functions. They promote selected behaviors, speed actions, maintain stimulus-response associations and, in humans, induce subjective feelings of pleasure and other positive emotions (Thut et al., 1997). Given the importance of reinforcers in promoting adaptive behavior, identifying the brain substrate underlying reinforced movement has been a central task of behavioral neuroscience.
Advances in functional brain imaging have made possible the visualization of the distributed brain systems underlying reward processing in humans. Findings from functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies indicate that the key anatomical nodes in the reward system are midbrain dopaminergic neurons, the ventral striatum (which includes the nucleus accumbens), the ventral pallidum, the orbitofrontal cortex, and the anterior cingulate (Haber & Knutson, 2010; Volkow et al., 1996; Wallis & Kennerley, 2011). These brain areas appear to be involved in the appetitive or anticipatory aspects of reward (Rademacher et al., 2010). The ventral striatum has also been shown to respond to the delivery of rewards in both human (Daniel & Pollmann, 2014; Delgado, Nystrom, Fissell, Noll, & Fiez, 2000) and nonhuman primates (Apicella, Ljungberg, Scarnati, & Schultz, 1991). The anterior caudate, which receives projections from the prefrontal cortex and midbrain dopaminergic neurons, may also be engaged as part of its role in goal-directed behavior (Balleine & O'Doherty, 2010; Yin & Knowlton, 2006). Of the brain regions mediating reward, the ventral striatum has been theorized to contribute a central role to reward processing due to massive projections of midbrain dopaminergic neurons into this region (Daniel & Pollmann, 2014). An important current theory holds that dopaminergic neurons code differences between anticipated and actual rewards (Daniel & Pollmann, 2014; Schultz, 2007). This reward error prediction theory is supported by animal studies showing high bursts of neuronal activity when rewards are unpredictable or when stimuli predict the probabilistic occurrence of rewards, but not when reward occurs 100% of the time (Daniel & Pollmann, 2014; Schultz, 2002). In addition to contributing to the basic neuroscience of reward, imaging studies of reward have stimulated the development of neurobiological theories of psychological phenomena that engage reward associated brain systems – including accounts of addiction, depression, schizophrenia, eating disorders, and financial decision making (Chau, Roth, & Green, 2004; Neary & Batterham, 2010; Volkow, et al., 1996; Wu, Sacchet, & Knutson, 2012).
Functional MRI studies in humans have temporally separated the component processes involved in reward expectation and reward processing. A common finding is that the expectation of reward preferentially activates the ventral striatum, including the nucleus accumbens, whereas reward outcome preferentially activates the ventromedial prefrontal cortex (Knutson, Fong, Adams, Varner, & Hommer, 2001; O'Doherty, Deichmann, Critchley, & Dolan, 2002). Other human studies of reward focus upon its response to decision-making outcomes (Delgado, et al., 2000), reporting activation in more dorsal regions of the striatum. A recent meta-analysis of 142 neuroimaging studies of reward valence processing confirmed contributions of the orbitofrontal cortex and anterior cingulate in reward processing and identified a sub-region of the ventral striatum, the nucleus accumbens, as a brain area broadly involved in different stages of reward processing (Liu, Hairston, Schrier, & Fan, 2011).
Investigators using functional brain imaging to study the neural substrates of reward have often interpreted their findings by relating them to single cell recording studies in animals (Haber & Knutson, 2010; Rademacher, et al., 2010), but differences in task structure across species may limit interpretation. For example, nonhuman primate studies of reward outcome typically involve events that predict feedback, such as a tone indicating the animal should approach a target to receive a reward, but they may also include events that predict no feedback, such as a tone indicating the animal should return to the starting position in preparation for the next trial (Gdowski, Miller, Bastianen, Nenonene, & Houk, 2007).
Perhaps a more important difference is that in many human fMRI studies, the response is a brief, ballistic button press (Elliott, Friston, & Dolan, 2000; Ernst et al., 2004; Knutson, Westdorp, Kaiser, & Hommer, 2000; Tanaka et al., 2004), whereas in animal studies the response is often a capture movement that engages both proximal and distal musculature (Tremblay, Hollerman, & Schultz, 1998), requiring greater movement control and accuracy. These differences in response requirements between human and animal reward processing studies might explain why neurons in the dorsal striatum (comprised of the caudate nucleus and putamen) are often found to be responsive during movements elicited by reward cues in animal studies (Hollerman, Tremblay, & Schultz, 1998; Kawagoe, Takikawa, & Hikosaka, 1998), whereas the ventral striatum is often activated in human fMRI studies of reward cuing (Delgado, Miller, Inati, & Phelps, 2005; Galvan et al., 2005; Jensen et al., 2007; Knutson, et al., 2001). Differences in response requirements might also explain why some studies of reward-cued movement in non-human primates have found neurons in the internal globus pallidus, outside of the ventral pallidum, that discharge during reward-cued capture-movements (Gdowski, et al., 2007; Gdowski, Miller, Parrish, Nenonene, & Houk, 2001). Single cell recording data support the view that the basal ganglia are involved in setting movement parameters, thus contributing to movement control (DeLong et al., 1984; Evarts & Wise, 1984). When movements are complex and unfold over time, continual updating of amplitude, force, direction, and velocity parameters is required to control the movement (DeLong, 1973; Kornhuber, 1971). This provides a setting where reward-cue modulation of the basal ganglia activity may occur more broadly than the ventral striatum and pallidum's response to reward cues involving ballistic movements.
Some support does exist for the thesis that reward-cued movements may activate different components of the basal ganglia when paradigms require greater movement control than that needed for ballistic movements. For example, this hypothesis is supported by a human fMRI study involving action and movement control in the oculomotor system (Harsay et al., 2011). The investigators found that although both the dorsal and ventral striatum activated in anticipation of future rewards, the dorsal striatum was directly involved in the modulation of oculomotor behavior through motivational processes (Harsay, et al., 2011). What the profile of basal ganglia activation in the skeletomuscular system might be for reward-cued movements executed in a motor control paradigm remains an open question.
In this study, fMRI was used during a movement paradigm requiring action control to investigate the neural substrate of reward-cued movement. We adapted a paradigm, originally designed for nonhuman primates (Gdowski, et al., 2001), for human use during fMRI. Based on findings from primate studies, we hypothesize that reward related skeletomotor movements will modulate the BOLD signal compared with non-reward movements (Balleine, Delgado, & Hikosaka, 2007; Gdowski, et al., 2001; Turner & Anderson, 2005). Specifically, we predict that the caudate nucleus, pallidum, and putamen will exhibit greater context dependent modulation for visual cues predicting potential financial gains relative to cues associated with no outcome. Furthermore, striatal regions will also exhibit a greater response to financial gains relative to neutral outcomes. To determine whether reward expectancy and reward outcome selectively alter neural activity in the basal ganglia, the BOLD response in motor and sensory areas (supplementary motor area, precuneus, and the precentral gyrus) involved in visually cued movement will also be studied (Benton & Tranel, 1993; Brown et al., 2004).
Materials and Methods
Subjects
Twenty right-handed participants (8 male, 12 female) aged 27.7±8.53 years (range 18-42) with 16.08±3 years of education (range 12-23.5) gave written informed consent in accordance with the University of California at San Diego Institutional Review Board. Exclusion criteria included: past history of dependence on alcohol, stimulants, opiates, or hallucinogens; serious medical conditions; color blindness; psychiatric illness (e.g., anxiety, depression); and conditions contraindicative to magnetic resonance imaging. Participants received $50 for their participation and were told they may earn up to an additional $25 based upon their performance. All participants, regardless of performance, received the additional $25 in compensation.
Experimental Design
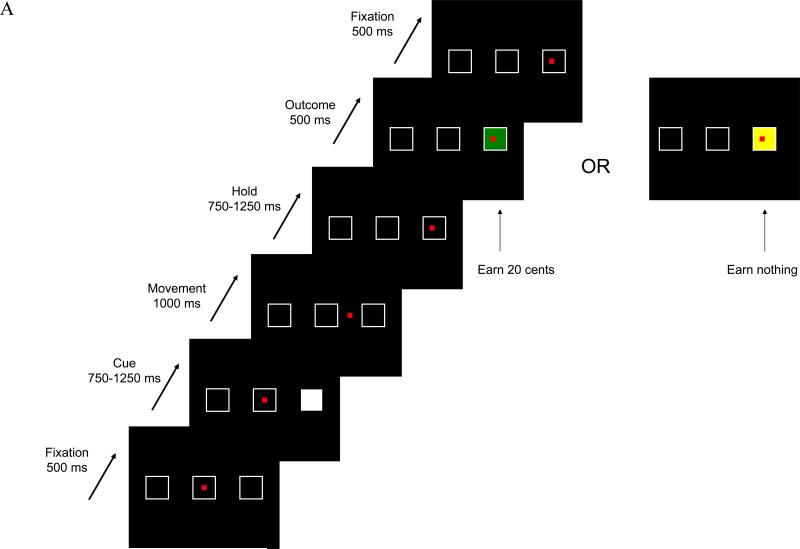
Participants performed a reward expectancy task with probabilistic reward. Targets were presented on a projection monitor controlled by a Dell Inspiron computer running Presentation (Neurobehavioral Systems, Inc., Albany, CA, www.neurobs.com), which also recorded choices and response times. Responses were made with a fiberoptic joystick (Current Designs, Philadelphia, PA, www.curdes.com) to manipulate a red square cursor. Three square targets with white sides on a black background were presented. Participants initially aligned the cursor to the center target. Visual cues were randomly ordered through the use of an m-sequence (Buracas & Boynton, 2002): Feedback Cues, occurring 50% of the time and in which one of the targets was illuminated in white, indicating that either a 1) positive or 2) neutral outcome would be presented following the movement (Figure 1A); 3) Nonfeedback Cues, occurring 25% of the time and in which one of the targets was illuminated in gray, indicating that no outcome stimulus would occur on this trial after a movement was performed (Figure 1B); and 4) Fixation Cues, occurring 25% of the time, where the participant remained fixated at the current location. For Feedback and Nonfeedback Cues, fixation occurred for 500 ms before one of the two remaining squares was randomly selected as the target and illuminated. After a variable length cue period (750 – 1250 ms), the illuminated target was extinguished, and the participant had 1000 ms to acquire the target with the cursor; the participant then held position inside the target frame for a variable period (750 – 1250 ms). For a Feedback Cue, the target was re-illuminated in either green (positive outcome) or yellow (neutral outcome) following the hold period; both positive and neutral outcomes were equally likely. Positive outcomes were worth 20 cents, and there was no financial gain for the neutral outcome. The current cursor location then served as the fixation position for the next trial. For a Nonfeedback Cue, no outcome stimulus was provided, and participants held position within the target frame until the next trial period. Each trial lasted approximately 4 sec, with jittered hold and outcome presentation periods (Figure 1C), both to ensure that activation patterns did not become phase-locked with the slice acquisition rate and to minimize anticipatory movements. Each functional run contained 63 trials and lasted 221 – 284 sec. The eight functional runs were separated by a 30 sec rest period.
Figure 1.
Examples of the two behavioral trial types. A) For the Feedback Cue, the white stimulus predicting future feedback is presented; upon extinguishing, the participant uses the fiberoptic joystick to move to the previously lit target's position and holds during the delay period. Feedback is provided with either a green filled target, indicating the participant earned 20 cents for the trial, or a yellow target, indicating the participant did not earn any money for this trial. B) For the Nonfeedback Cue, the gray stimulus indicating no feedback for this trial is presented; upon extinguishing, the participant uses the joystick to move to the target's location. C) The timeline for a typical trial.
MRI
Functional MRI was performed with gradient-recalled echoplanar imaging (TR=2000 ms; TE=30 ms; flip angle=90°; 64 × 64 matrix; 32 4 mm (no gap) contiguous oblique slices aligned to AC-PC with an in-plane resolution of 3.75 mm × 3.75 mm; 150 volumes) with a General Electric Signa HDx 3 T scanner (Kwong et al., 1992; Ogawa et al., 1992). The first four volumes of each run were discarded so as to discount T1 saturation. High resolution T1-weighted SPGR anatomical images (TR=8 ms; TE=min full; 256 × 192 matrix; 124 1.2 mm contiguous sagittal slices) were obtained for subsequent spatial normalization.
Behavioral Analysis
The joystick was sampled at 60 Hz and filtered offline with a Butterworth lowpass filter at 10 Hz to remove jitter. Response time was defined as the interval of time from when the target was extinguished to the onset of joystick movement. Movement duration time was defined as the interval of time from the onset of joystick movement to the point in time at which maximum displacement occurred. In order to calculate these parameters, movement onset and offset were determined with a three-step algorithm (Teasdale, Bard, Fleury, Young, & Proteau, 1993). To calculate movement onset, the algorithm traced backward from the event with the peak tangential velocity to the event that was 10% of its peak value, t2, and traced back further to event t1, which was 10% of the velocity value of t2. Movement onset was then defined as the average value (plus standard deviation) between events t1 and t2; averaging minimizes the contribution of noise and is more accurate than an absolute criterion (Teasdale, et al., 1993). Movement offset was calculated in a similar fashion, but with the average calculated from events t1 and t2 located on the downward slope of the tangential velocity profile (i.e., tracing forward from the peak tangential velocity event).
MRI Statistical Analysis
Imaging data were analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, London UK)(Friston et al., 1995) in Matlab 7.5.0.338 (R2007b). Data were motion corrected to the first functional scan, coregistered to the individual's high-resolution MRI using mutual information, spatially normalized to the Montreal Neurologic Institute (MNI) template with a 12-parameter affine transformation followed by nonlinear warping (Ashburner & Friston, 1999), resampled to 2 mm isotropic voxels, and smoothed with a 6 mm isotropic Gaussian kernel. Two participants failed to complete all eight runs due to technical problems; therefore only their completed runs were included in the analyses. Each participant's data were analyzed using the general linear model with an event-related design. The onset times of cue and outcome stimuli were convolved with the canonical hemodynamic response with time and dispersion derivatives. Contrasts were constructed at the subject level for comparing the cue stimuli (Feedback Cues vs. Nonfeedback Cues), and for comparing outcome stimuli (Positive Outcomes vs. Neutral Outcomes).
A region of interest (ROI) analysis was employed on structures associated with reward and goal-oriented movements. Anatomical representations of the precentral gyrus, supplementary motor area, precuneus, pallidum, putamen, and caudate nucleus were obtained from the AAL atlas (Tzourio-Mazoyer et al., 2002), a toolbox available for SPM. Due to issues with signal dropout in ventral regions, we did not pursue an ROI analysis within the ventral striatum (including the nucleus accumbens). Statistical maps for each ROI were thresholded at p<.05 using the False Discovery Rate (FDR, Benjamini & Hochberg, 1995) for multiple comparisons using a small volume correction (SVC). To account for testing multiple ROIs (6 per hemisphere), we applied a Bonferroni-corrected threshold of p<.004. In order to test for both condition and temporal (run) effects, the MarsBar toolbox (http://marsbar.sourceforge.net) (Brett, Anton, Valabregue, & Poline, 2002) was used to extract the average percent signal change within our reward-related ROIs for each of the four conditions (Feedback Cue, Nonfeedback Cue, Positive Outcome, Neutral Outcome), and the somatosensory regions for trial type (Feedback Cue, Nonfeedback Cue). These extractions were 5 mm spheres centered upon the peak activations found within our ROIs as determined by SVC. The radius was chosen so as to ensure the extraction was smaller than the smallest ROI, and so that there would be the same number of observations within each extraction. Hypotheses related to these ROIs were tested by a 2 (condition) by 8 (run) mixed effects ANOVA with subject as a random effect. The condition effect either contrasted Feedback Cues with Nonfeedback Cues or Positive Outcomes with Neutral Outcomes. We used Mauchly's W to test for violations of sphericity in the run effect and in the run by condition interaction. When Mauchly's test indicated a significant deviation from sphericity, we used the Huynh-Feldt adjustment to the degrees of freedom. Finally, a voxelwise group analysis was employed to look for other regions that showed significant changes in our contrasts of interest(pFDR<.05).
Results
Response Behavior
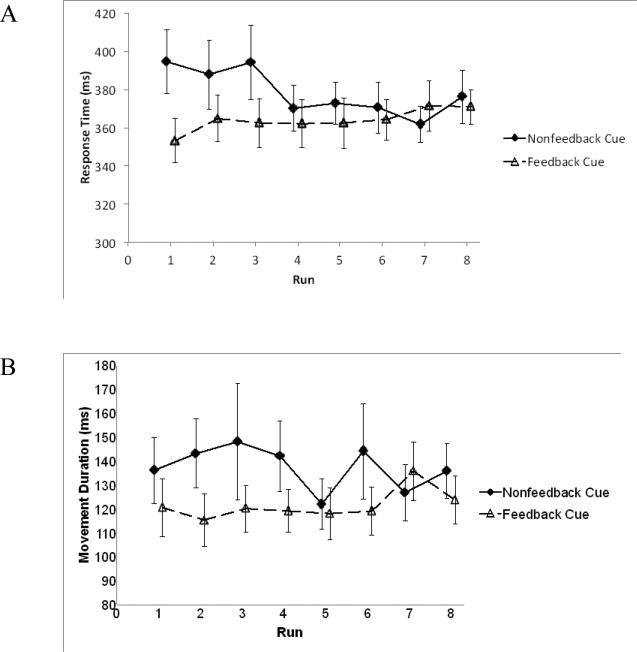
Motor response with the joystick was broken down into two components: 1) the time to initiate the response, and 2) the movement duration. Overall, participants tended to initiate and respond more slowly for the Nonfeedback Cue than for the Feedback Cue. A 2 (Cue Type: Feedback, Nonfeedback) by 8 (run) mixed-effects ANOVA with subject as a random effect reported an interaction between measures for movement response time, F(7,133)=2.56, p=.02, ηp2=.12. There was also a significance for trial type, F(1, 19)=6.15, p=.02, ηp2=.24 (Figure 2A). While movement duration failed to show an interaction between trial type and run, it was also significant for trial type, F(1, 19)=10.09, p=.005, ηp2=.35 (Figure 2B). Run effects were not found to be significant for either the movement response time or duration. All participants successfully captured all targets.
Figure 2.
A) Response time in relation to Cue stimuli (Feedback Cue, Nonfeedback Cue) across 8 runs. Overall, response initiation was slower for the Nonfeedback Cues relative to the Feedback Cues. B) Movement duration in relation to Cue stimuli (Feedback Cue, Nonfeedback Cue) across 8 runs. Participants exhibited longer movement durations for the Nonfeedback Cues relative to the Feedback Cues. Error bars indicate standard errors.
Hypothesized Effect of Cue Type
Subcortical regions of interest
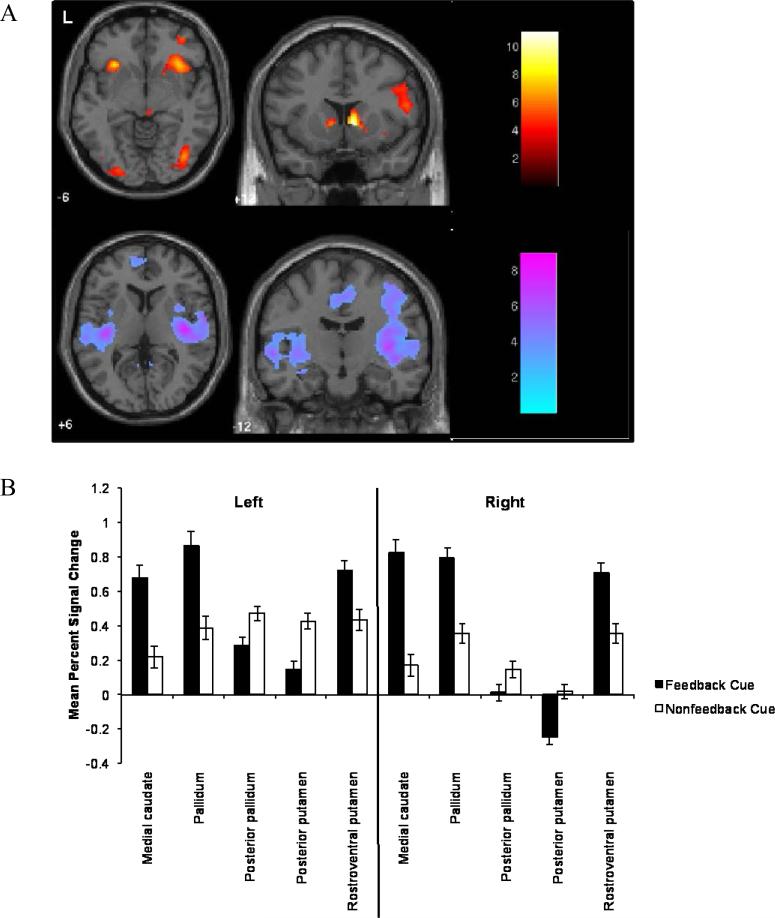
Using SVC, we compared activation differences between Feedback Cues and Nonfeedback Cues within the pallidum, putamen and caudate nucleus. Most ROIs reported significant clusters (FDR-corrected) for Feedback Cues relative to Nonfeedback Cues (Table 1, Figure 3). Several of these regions also reported significant clusters for Nonfeedback Cues relative to Feedback Cues; these latter results included the posterior zones of the pallidum and putamen. We also extracted and averaged the percent signal change within each ROI for the Feedback Cues and Nonfeedback Cues for each run. A 2 (Cue Type: Feedback, Nonfeedback) by 8 (run) ANOVA did not show an interaction within subcortical ROIs. However, a difference of cue type was found for the bilateral medial caudate nucleus [Left: F(1,19)=33.46, p<.001, ηp2=.64; Right: F(1,19)=105.50, p<.001, ηp2=.85], the bilateral rostroventral putamen [Left: F(1,19)=11.14, p=.003, ηp2=.38; Right: F(1,19)=37.83, p<.001, ηp2=.67], the bilateral posterior putamen [Left: F(1,19)=20.98, p<.001, ηp2=.52; Right: F(1,19)=26.44, p<.001, ηp2=.58], the bilateral pallidum [Left: F(1,19)=29.59, p<.001, ηp2=.61; Right: F(1,19)=56.97, p<.001, ηp2=.75], and the bilateral posterior pallidum [Left: F(1,19)=12.26, p=.002, ηp2=.39; Right: F(1,19)=8.51, p=.009, ηp2=.31].
Table 1.
Significant Clusters of BOLD Response within Regions of Interest in Relation to the Cue Stimuli
| Region | L/R | Cluster Size | X | Y | Z | Z-Score | pFDR |
|---|---|---|---|---|---|---|---|
| Feedback Cue > Nonfeedback Cue | |||||||
| Subcortical Regions | |||||||
| Medial Caudate Nucleus | L | 79 | −8 | 10 | 0 | 4.7 | < 0.001* |
| R | 242 | 10 | 12 | 0 | 6.1 | < 0.001* | |
| Pallidum | L | 15 | −10 | 4 | 2 | 4.3 | < 0.001* |
| R | 18 | 14 | 8 | 0 | 4.8 | < 0.001* | |
| Rostroventral Putamen | L | 4 | −14 | 10 | −2 | 3.3 | 0.01 |
| R | 116 | 16 | 12 | −2 | 4.3 | < 0.001* | |
| Sensorimotor Regions | |||||||
| Precentral Gyrus (4) | R | 587 | 42 | 2 | 36 | 4.7 | < 0.001* |
| Supplementary Motor Area | L | 246 | 2 | 22 | 52 | 4.1 | 0.01 |
| R | 430 | 6 | 24 | 46 | 4.6 | < 0.001* | |
| Nonfeedback Cue > Feedback Cue | |||||||
| Subcortical Regions | |||||||
| Posterior Pallidum | L | 23 | −26 | −12 | −4 | 3.5 | .01 |
| R | 19 | 28 | −10 | −2 | 2.7 | .002* | |
| Posterior Putamen | L | 236 | −32 | −16 | −2 | 4.2 | .003* |
| R | 348 | 36 | −10 | 2 | 4.1 | <.001* | |
| Sensorimotor Regions | |||||||
| Precentral Gyrus (4) | L | 527 | −26 | −28 | 62 | 3.8 | .01 |
| R | 377 | 38 | −18 | 60 | 4.0 | .004* | |
| Precuneus | L | 1711 | 2 | −62 | 38 | 5.0 | <.001* |
| R | 1273 | 2 | −60 | 36 | 4.9 | <.001* | |
| Supplementary Motor Area (6) | L | 400 | 0 | −12 | 48 | 3.8 | .03 |
| R | 701 | 6 | −4 | 46 | 4.1 | .01 | |
Note: Brodmann's areas for appropriate regions are in parentheses. Z-score refers to the activation peak within the cluster. Coordinates are given in MNI space. FDR: false discovery rate.
Significant at Bonferroni-corrected threshold of FWE p<.007 (with SVC).
Figure 3.
Differences in response to Feedback and Nonfeedback Cues. A) Upper panel: The contrast of Feedback Cues > Nonfeedback Cues revealed effects within several a priori predicted regions, including the medial caudate. Lower panel: The contrast of Nonfeedback Cues > Feedback Cues revealed differences within the posterior putamen and posterior pallidum. B) Average percent signal change extracted from a priori selected regions of interest for Feedback Cues and Nonfeedback Cues. Anterior subregions (medial caudate) tended to be more responsive to Feedback Cues, whereas posterior subregions (putamen) were more responsive to Nonfeedback Cues. Error bars indicate standard errors.
Several regions also demonstrated run effects. Although the main effect of run fell just short of being significant in the left medial caudate nucleus [F(7,133)=1.88, p=.08, η2=.09], several non-linear trends were significant. The data suggested that early runs showed larger effects than later runs. The right medial caudate nucleus, bilateral rostroventral putamen, and bilateral pallidum also showed a pattern of significant non-linear effects reflecting larger effects for early runs.
Cortical regions of interest
Using SVC, the right precentral gyrus and bilateral supplementary motor area both exhibited a greater response for Feedback Cues relative to Nonfeedback Cues (Table 1). Several cortical regions also demonstrated significant clusters for Nonfeedback Cues relative to Feedback Cues; these included the bilateral precuneus and more posterior zones of the bilateral supplementary motor area and precentral gyrus. A 2 (Cue Type: Feedback, Nonfeedback) by 8 (run) ANOVA detected a difference of cue type within the left precuneus [F(1,19)=15.28, p=.001, ηp2=.45] and the right precuneus [F(1,19)=6.09, p=.023, ηp2=.24]. The right precentral gyrus also indicated a cue type effect [F(1,19)=8.73, p=.008, ηp2=.32].
Hypothesized Effect of Feedback
Subcortical regions of interest
SVC was used to compare positive and neutral outcomes within the subcortical ROIs (Table 2, Figure 4). All ROIs exhibited a significant response to Positive Outcomes relative to Neutral Outcomes; no regions were more responsive to Neutral Outcomes relative to Positive Outcomes. The average percent signal change within each ROI was extracted and subjected to a 2 (Outcome: Positive, Neutral) by 8 (run) ANOVA. None of the ROIs demonstrated a run by outcome interaction. Several regions were significant for type of outcome, including the bilateral medial caudate nucleus [Left: F(1,19)=56.75, p<.001, ηp2=.75; Right: F(1,19)=77.62, p<.001, ηp2=.80], the bilateral rostroventral putamen [Left: F(1,19)=63.98, p<.001 ηp2=.77; Right: F(1,19)=59.19, p<.001, ηp2=.76], and the bilateral pallidum [Left: F(1,19)=40.81, p<.001, ηp2=.68; Right: F(1,19)=42.26 p<.001, ηp2=.69].
Table 2.
Significant Clusters of BOLD Response within Regions of Interest in Relation to the Outcome Stimuli
| Region | L/R | Cluster Size | X | Y | Z | Z-Score | p FDR |
|---|---|---|---|---|---|---|---|
| Positive Outcomes > Neutral Outcomes | |||||||
| Subcortical Regions | |||||||
| Medial Caudate Nucleus | L | 651 | −10 | 14 | −2 | 5.1 | <.001* |
| R | 770 | 12 | 16 | −2 | 5.6 | <.001* | |
| Pallidum | L | 119 | −18 | 6 | −4 | 5.0 | <.001* |
| R | 96 | 18 | 8 | −2 | 5.2 | <.001* | |
| Rostroventral Putamen | L | 552 | −22 | 6 | −8 | 6.0 | <.001* |
| R | 610 | 16 | 12 | −2 | 5.6 | <.001* | |
| Sensorimotor Regions | |||||||
| None | |||||||
| Neutral Outcomes > Positive Outcomes | |||||||
| None | |||||||
Note: Brodmann's areas for appropriate regions are in parentheses. Z-score refers to the activation peak within the cluster. Coordinates are given in MNI space. FDR: false discovery rate.
Significant at Bonferroni-corrected threshold of FWE p < 0.007 (with SVC).
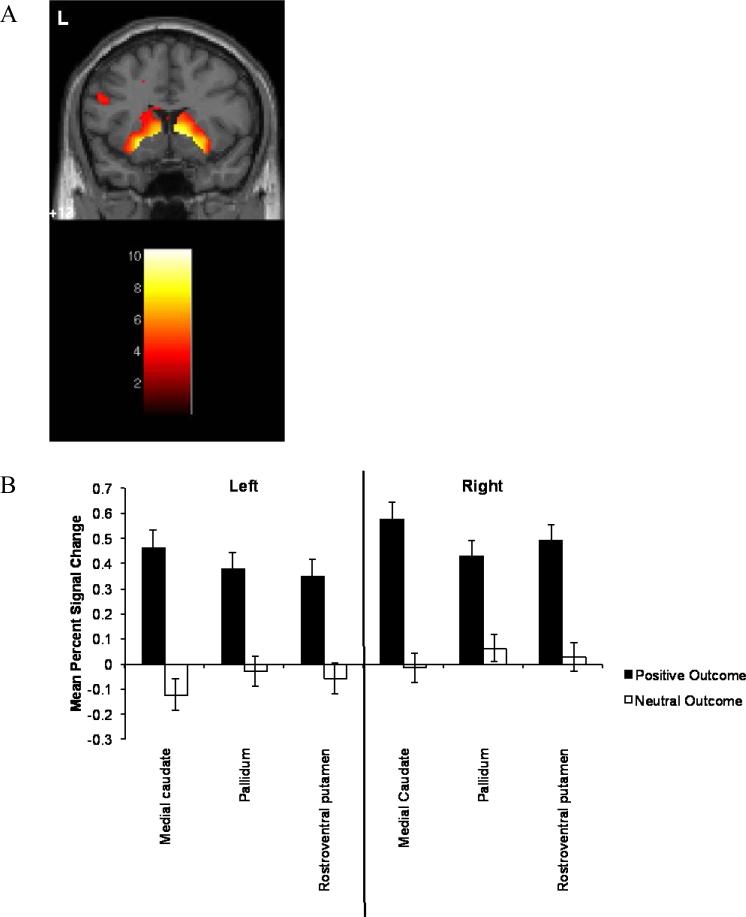
Figure 4.
Differences in response to Positive Outcomes and Neutral Outcomes. A) The contrast of Positive Outcomes > Neutral Outcomes revealed effects within several a priori predicted regions, including medial caudate. B) Average percent signal change extracted from a priori selected regions of interest for Positive Outcomes and Neutral Outcomes. Overall, regions of interest were more responsive for Positive Outcomes than for Neutral Outcomes. Error bars indicate standard errors.
Several regions demonstrated a significant linear increase in the BOLD response over runs: bilateral medial caudate nucleus [Left: Flin(1,19)=10.32, p=.005, ηp2=.35; Right: Flin(1,19)=14.84, p=.001, ηp2=.44]; bilateral rostroventral putamen [Left: Flin(1,19)=28.57, p<.001, ηp2=.60; Right: Flin(1,19)=19.69, p<.001, ηp2=.51], and the bilateral pallidum [Left:Flin(1,19)=24.62, p<.001, ηp2=.56; Right: Flin(1,19)=23.44, p<.001, ηp2=.55]. For these ROIs the advantage of later trials over the first trial leveled off, adding a quadratic trend to the linear effect.
Cortical regions of interest
None of the cortical ROIs demonstrated a differential response to Positive Outcomes relative to Neutral Outcomes.
Exploratory Voxelwise Analysis
Effects of cue type
A secondary voxelwise analysis at p<.01 (FDR-corrected) and a minimum of 10 contiguous voxels per cluster reported additional regions active for Feedback Cues relative to Nonfeedback Cues (Supplemental Table 1). These additional regions included the right inferior parietal lobule, the right middle frontal gyrus, the bilateral anterior insula, the right middle occipital gyrus, and a cluster that included the right inferior frontal gyri. In comparison, additional regions that were significantly activated for Nonfeedback Cues relative to Feedback Cues included the inferior parietal lobule bilaterally, the left middle frontal gyrus, and the right superior frontal gyrus (Supplemental Table 2).
Effects of outcome
A secondary voxelwise analysis at p<.01 (FDR-corrected) and a minimum of 10 contiguous voxels per cluster reported additional regions active for Positive Outcomes relative to Neutral Outcomes, including the bilateral inferior parietal lobule and bilateral superior frontal gyrus (Supplemental Table 3). No regions survived significance for the Neutral Outcomes relative to Positive Outcomes contrast.
Comparison of Trial Type with Behavioral Response
Finally, multiple regression was used to determine whether the contrast difference comparing Feedback Cues to Nonfeedback Cues was related to the individual's mean response duration between the two cue types. SVC within the ROIs failed to find a relationship between the difference in trial types and mean response duration (p<.05, FDR-corrected). Similarly, a voxelwise analysis revealed no regions surviving the p<.05 (FDR-corrected) threshold.
Discussion
Our study yielded two main results. First, as hypothesized, portions of the dorsal striatum and pallidum exhibited greater context dependent modulation for visual cues predicting potential financial gains compared to cues associated with no outcome. These results extend the finding of dorsal striatal activation during anticipation of future reward reported in the oculomotor system (Harsay, et al., 2011) to the skeletomotor system. Second, both striatal and pallidal regions displayed a greater response to financial gains compared to neutral outcomes. Our finding of context dependent modulation of basal ganglia response converges with the pallidal results reported by Gdowski and colleagues (2007; 2001) in monkey and with the role of the dorsal striatum in reward and decision-making described in the model by Balleine and colleagues (2007). Overall, these finding support the notion of a broader effect of context dependent modulation within subregions of the basal ganglia.
Interestingly, the direction of the reward expectancy modulation varied – some subregions within each basal ganglia nucleus displayed increases in the BOLD response, whereas other areas displayed decreases. The directional difference in modulation can be explained by variations in the neuronal response to cues predicting reward or by differences in metabolic-blood flow coupling (Buxton, 2009; T. L. Davis, Kwong, Weisskoff, & Rosen, 1998). Primate data indicate that both explanations are plausible. Single cell recordings of the activity of individual neurons in the external globus pallidus of monkeys during a probabilistic visuomotor task show that some neurons increase their activity to predicted reward, whereas others decrease their activity (Arkadir, Morris, Vaadia, & Bergman, 2004). This differential response to reward cues is compatible with the view that dopamine release has a brief excitatory effect on some neurons and a slow inhibitory effect on others (Shepherd, 1994, p. 472). Although the BOLD signal appears to be more tightly regulated by alterations of local field potentials rather than by neural spikes, sustained neural firing of a pool of neurons can alter nearby local field potentials (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001; Mishra et al., 2011).
Differential metabolic-blood flow coupling associated with neural activity in different neural pools within the basal ganglia is an alternative to the purely neural account of the differential BOLD response. A study of the WAG/Rij rat found regions within the caudateputamen where cerebral blood flow decreased even though multiunit neural activity increased during either whisker stimulation or spike-wave discharges (Mishra, et al., 2011). Cerebral blood volume (CBV) within the caudate-putamen is modulated in part by dopamine, with D1/D5 receptors mediating increases in CBV and D2/D3 receptors mediating decreases (Choi, Chen, Hamel, & Jenkins, 2006). Based on data showing reduced decreases of CBV to pain stimulation in the striatum of rodents following administration of a D2 antagonist (Shih et al., 2009), Mishra and colleagues (2011) hypothesize that dopamine release following neural activation causes vasoconstriction in the striatum that in turn reduces the BOLD signal. These differential vascular effects in basal ganglia subregions could confound the expression of the BOLD signal, perhaps leading to different directions of the effect, even if the neural effects were in the same direction. If true, a calibrated BOLD study would be necessary to identify the true directions of the effects (Ances et al., 2008).
All locations within the striatum and pallidum showing a significant BOLD response to outcome type revealed a significant response to positive reward outcome. While the animal literature has demonstrated striatal neurons that fire to either reward prediction or delivery (Hollerman, et al., 1998; Lau & Glimcher, 2007), few human neuroimaging studies have reported activation to both anticipatory cues and reward outcome within a single experiment in the absence of learning or decision-making. Human studies have generally either demonstrated responses to rewarding outcomes (Delgado, Locke, Stenger, & Fiez, 2003; Delgado, et al., 2000) or only to the anticipatory phase if one is present (Knutson & Greer, 2008). However, the anticipatory element in these kinds of tasks predicts a specific outcome (e.g., receipt of a reward), whereas our task's anticipatory cue predicted only that an outcome (either positive or neutral) would be presented. In that regard, our task is similar to that of Breiter and colleagues (2001); in their paradigm, participants were presented with one of three different anticipatory cues, each of which was associated with three different probabilistic outcomes. They reported that several of their regions of interest increased activation both to the cues associated with the greatest potential gain as well as the most rewarding outcome for each cue. Thus, our findings support the notion that the striatum can encode both anticipation and outcome during the same paradigm when sufficient uncertainty is present.
We also saw differences in regional specificity to cue type, with anterior striatal regions more responsive to feedback cues, and posterior regions more responsive to no feedback cues. While the anterior striatum has been associated with reward anticipation in humans (Tricomi, Delgado, & Fiez, 2004) as well as nonhuman primates (Lauwereyns, Watanabe, Coe, & Hikosaka, 2002), the role of the posterior striatum in outcome anticipation is less certain. Prediction error studies in humans have suggested that posterior regions are more linked to punishment prediction error signals (Mattfeld, Gluck, & Stark, 2011; Seymour, Daw, Dayan, Singer, & Dolan, 2007), although a meta-analysis suggests this relationship may follow a U-shaped profile (Bartra, McGuire, & Kable, 2013), with an emphasis on cues predicting non-neutral outcomes. The no feedback cue may have been interpreted as predicting a negative outcome, as it led to no potential for monetary gain. Such an interpretation is supported by studies demonstrating that the context of alternative options can influence the subjective value of a stimulus (Breiter, et al., 2001; Bunzeck, Dayan, Dolan, & Duzel, 2010; Nieuwenhuis et al., 2005).
There was no differential brain response within motor and visual areas when positive feedback was compared with no feedback. In comparison, supplementary motor area, precentral gyrus, and precuneus all exhibited a modulated BOLD response to cued outcome. These results indicate that corticostriatal pathways that have been postulated to be involved only in the acquisition of cued actions and habits might also be involved in probabilistic decision-making, at least for reward modulated motor control tasks (Balleine, et al., 2007). The reward cue modulation of sensorimotor areas indicates that the neural effects of reward expectancy extend well beyond the basal ganglia. Our exploratory voxelwise analyses suggested that the impact of reward expectancy on neural activity includes occipital and parietal cortex, as well as limbic cortex (insula) and multiple regions within the lateral frontal cortex (See supplementary material). Overall our results support theories about the brain-substrate of reward expectancy and reward outcome that emphasize the contributions of multiple brain systems, including emotional, attentional, motor and memory systems, in accounting for the neural effects of reward (e.g., Delgado & Dickerson, 2012).
These results must be viewed in light of the study's limitations. The participants we studied were generally young and college-educated, and our results should only be generalized to similar samples. Evidence suggests that older adults may have reduced reward expectancy, but not outcome, response in the striatum (Samanez-Larkin, Worthy, Mata, McClure, & Knutson, 2014). Education may also have had an influence; some studies suggest performance differences in decision making, such as with the Iowa Gambling Task, (C. Davis et al., 2008; Evans, Kemish, & Turnbull, 2004), and the influence of feedback during decision making processes may be modulated by IQ, which is in turn correlated with education (Brand, Laier, Pawlikowski, & Markowitsch, 2009). Thus, our reward expectancy findings in particular are limited to young, well-educated adults. A second limitation of the current approach is the use of secondary versus primary reinforcers. In humans, both primary (e.g., juice) and secondary (e.g., money) reinforcers activate similar neural substrates (Kim, Shimojo, & O'Doherty, 2011; McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007; McClure, Laibson, Loewenstein, & Cohen, 2004), thereby supporting the use of financial rewards. Third, due to signal dropout we did not acquire adequate data within the ventral striatum (including the nucleus accumbens) and orbitofrontal cortex. Future imaging studies using approaches to minimize susceptibility distortion (Deichmann, Gottfried, Hutton, & Turner, 2003) should help minimize this problem. Fourth, although we were interested in whether complex movements in humans would demonstrate a similar dorsal striatal response to future rewards as seen in the animal literature, we did not perform a direct comparison of simple vs. complex movements. Simple motor responses are common in the literature, thus we wanted to focus on more complex motor responses. Because there were 8 functional runs, there was insufficient time for also including a simple response task. Moreover, we were concerned about the potential for negative transference of the simple motor task if it were in the same session as the complex version. Finally, we did not model the movement or hold periods to avoid overfitting, but note that we did see response differences due to cue type, which might reflect preparatory or motor response activity. Future studies examining response complexity during reward processing are needed to better determine whether more complex responses are region-specific.
These findings demonstrate the importance of context in human reward processing neuroimaging paradigms. Furthermore, robust modulation of dorsal striatal and pallidal response to reward expectancy and reward outcome was seen in a probabilistic reward task involving larger scale arm movements as those seen in non-human primate studies of probabilistic reward (Gdowski, et al., 2007; Gdowski, et al., 2001), supporting the importance of response complexity in the interpretation of neuronal processing across species.
Supplementary Material
Acknowledgements
This research was supported by NIH grant K01DA15499.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Amanda Bischoff-Grethe, Veterans Affairs San Diego Healthcare System, San Diego, California and Department of Psychiatry, University of California, San Diego.
Richard B. Buxton, Department of Radiology, University of California, San Diego
Martin P. Paulus, Laureate Institute for Brain Research, Tulsa, OK
Adam S. Fleisher, Department of Neurosciences, University of California, San Diego and Banner Alzheimer's Institute, Phoenix, Arizona.
Tony T. Yang, Department of Psychiatry, Division of Child and Adolescent Psychiatry, University of California, San Francisco.
Gregory G. Brown, Veterans Affairs San Diego Healthcare System, San Diego, California and Department of Psychiatry, University of California, San Diego
References
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage. 2008;39:1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Experimental Brain Research. 1991;85:491–500. doi: 10.1007/BF00231732. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- Arkadir D, Morris G, Vaadia E, Bergman H. Independent coding of movement direction and reward prediction by single pallidal neurons. The Journal of Neuroscience. 2004;24:10047–10056. doi: 10.1523/JNEUROSCI.2583-04.2004. doi: 10.1523/JNEUROSCI.2583-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AIDHBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of Neuroscience. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. doi: npp2009131 [pii] 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. Retrieved from http://www.jstor.org/stable/2346101. [Google Scholar]
- Benton AL, Tranel D. Visuoperceptive, visuospatial, and visuoconstructive disorders. In: Heilman KE, Valenstein E, editors. Clinical neuropsychology. 3rd ed. Grune and Stratton; New York: 1993. pp. 165–213. [Google Scholar]
- Brand M, Laier C, Pawlikowski M, Markowitsch HJ. Decision making with and without feedback: the role of intelligence, strategies, executive functions, and cognitive styles. Journal of Clinical and Experimental Neuropsychology. 2009;31:984–998. doi: 10.1080/13803390902776860. doi: 10.1080/13803390902776860. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. doi: 10.1016/S0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox.. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. Jun 2-6, 2002. 2002. [Google Scholar]
- Brown GG, Caligiuri M, Meloy MJ, Eberson SC, Kindermann SS, Frank LR, Lohr JB. Functional brain asymmetries during visuomotor tracking. Journal of Clinical and Experimental Neuropsychology. 2004;26:356–368. doi: 10.1080/13803390490510086. doi: 10.1080/13803390490510086. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Dayan P, Dolan RJ, Duzel E. A common mechanism for adaptive scaling of reward and novelty. Human Brain Mapping. 2010;31:1380–1394. doi: 10.1002/hbm.20939. doi: 10.1002/hbm.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buracas GT, Boynton GM. Efficient design of event-related fMRI experiments using M-sequences. Neuroimage. 2002;16:801–813. doi: 10.1006/nimg.2002.1116. doi: 10.1006/nimg.2002.1116. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to functional magnetic resonance imaging: Principles and techniques. Cambridge University Press; New York: 2009. [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Current Psychiatry Reports. 2004;6:391–399. doi: 10.1007/s11920-004-0026-8. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Daniel R, Pollmann S. A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem. 2014;114:90–100. doi: 10.1016/j.nlm.2014.05.002. doi: 10.1016/j.nlm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Fox J, Patte K, Curtis C, Strimas R, Reid C, McCool C. Education level moderates learning on two versions of the Iowa Gambling Task. Journal of the International Neuropsychological Society. 2008;14:1063–1068. doi: 10.1017/S1355617708081204. doi: 10.1017/S1355617708081204. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Dickerson KC. Reward-related learning via multiple memory systems. Biological Psychiatry. 2012;72:134–141. doi: 10.1016/j.biopsych.2012.01.023. doi: 10.1016/j.biopsych.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. doi: 10.3758/CABN.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. Retrieved from http://jn.physiology.org. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Putamen: activity of single units during slow and rapid arm movements. Science. 1973;179:1240–1242. doi: 10.1126/science.179.4079.1240. doi: 10.1126/science.179.4079.1240. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE. Functional organization of the basal ganglia: contributions of single-cell recording studies. Ciba Foundation Symposium. 1984;107:64–82. doi: 10.1002/9780470720882.ch5. doi: 10.1002/9780470720882.ch5. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable Neural Responses in Human Reward Systems. The Journal of Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. Retrieved from http://www.jneurosci.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Evans CE, Kemish K, Turnbull OH. Paradoxical effects of education on the Iowa Gambling Task. Brain and Cognition. 2004;54:240–244. doi: 10.1016/j.bandc.2004.02.022. doi: 10.1016/j.bandc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Wise SP. Basal ganglia outputs and motor control. Ciba Foundation Symposium. 1984;107:83–102. doi: 10.1002/9780470720882.ch6. doi: 10.1002/9780470720882.ch6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. doi: 10.1002/hbm.460020402. [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. The Journal of Neuroscience. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdowski MJ, Miller LE, Bastianen CA, Nenonene EK, Houk JC. Signaling patterns of globus pallidus internal segment neurons during forearm rotation. Brain Research. 2007;1155:56–69. doi: 10.1016/j.brainres.2007.04.028. doi: 10.1016/j.brainres.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdowski MJ, Miller LE, Parrish T, Nenonene EK, Houk JC. Context dependency in the globus pallidus internal segment during targeted arm movements. Journal of Neurophysiology. 2001;85:998–1004. doi: 10.1152/jn.2001.85.2.998. Retrieved from http://jn.physiology.org. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Cohen MX, Oosterhof NN, Forstmann BU, Mars RB, Ridderinkhof KR. Functional Connectivity of the Striatum Links Motivation to Action Control in Humans. The Journal of Neuroscience. 2011;31:10701–10711. doi: 10.1523/JNEUROSCI.5415-10.2011. doi: 10.1523/JNEUROSCI.5415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. Journal of Neurophysiology. 1998;80:947–963. doi: 10.1152/jn.1998.80.2.947. Retrieved from http://jn.physiology.org/ [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Human Brain Mapping. 2007;28:294–302. doi: 10.1002/hbm.20274. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience. 1998;1:411–416. doi: 10.1038/1625. Retrieved from http://www.nature.com/neuro/index.html. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Overlapping Responses for the Expectation of Juice and Money Rewards in Human Ventromedial Prefrontal Cortex. Cerebral Cortex. 2011;21:769–776. doi: 10.1093/cercor/bhq145. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH. Motor functions of cerebellum and basal ganglia: the cerebellocortical saccadic (ballistic) clock, the cerebellonuclear hold regulator, and the basal ganglia ramp (voluntary speed smooth movement) generator. Kybernetik. 1971;8:157–162. doi: 10.1007/BF00290561. doi: 10.1007/BF00290561. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. Journal of Neuroscience. 2007;27:14502–14514. doi: 10.1523/JNEUROSCI.3060-07.2007. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. Retrieved from http://www.nature.com. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CE. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learning & Memory. 2011;18:703–711. doi: 10.1101/lm.022889.111. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. The Journal of Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, Blumenfeld H. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. The Journal of Neuroscience. 2011;31:15053–15064. doi: 10.1523/JNEUROSCI.0101-11.2011. doi: 10.1523/JNEUROSCI.0101-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary MT, Batterham RL. Gaining new insights into food reward with functional neuroimaging. Forum of Nutrition. 2010;63:152–163. doi: 10.1159/000264403. doi: 10.1159/000264403. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, Alting von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005;25:1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. doi: Doi 10.1016/J.Neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Worthy DA, Mata R, McClure SM, Knutson B. Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cogn Affect Behav Neurosci. 2014;14:672–682. doi: 10.3758/s13415-014-0297-4. doi: 10.3758/s13415-014-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. Journal of Neuroscience. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. Neurobiology. Third ed. Oxford University Press; New York: 1994. [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. The Journal of Neuroscience. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neuroscience. 2004;7:887–893. doi: 10.1038/nn1279. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Bard C, Fleury M, Young DE, Proteau L. Determining movement onsets from temporal series. Journal of Motor Behavior. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. Journal of Neurophysiology. 1998;80:964–977. doi: 10.1152/jn.1998.80.2.964. Retrieved from http://jn.physiology.org. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. The Journal of Neuroscience. 2005;25:2965–2976. doi: 10.1523/JNEUROSCI.4036-04.2005. doi: 10.1523/JNEUROSCI.4036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Logan J, Wang GJ, Ding YS, Dewey S. PET evaluation of the dopamine system of the human brain. Journal of Nuclear Medicine. 1996;37:1242–1256. Retrieved from http://jnm.snmjournals.org. [PubMed] [Google Scholar]
- Wallis JD, Kennerley SW. Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex. Annals of the New York Academy of Sciences. 2011;1239:33–42. doi: 10.1111/j.1749-6632.2011.06277.x. doi: 10.1111/j.1749-6632.2011.06277.x. [DOI] [PubMed] [Google Scholar]
- Wu CC, Sacchet MD, Knutson B. Toward an affective neuroscience account of financial risk taking. Frontiers in Neuroscience. 2012;6:159. doi: 10.3389/fnins.2012.00159. doi: 10.3389/fnins.2012.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.