Abstract
Dexamethasone was administered to healthy horses daily for 7 days. Blood samples were collected at 3 time points from both treatment and non-treatment groups, and analyzed via thromboelastography (TEG). There were no significant differences in TEG parameters between treated and untreated horses, or within treatment groups over time.
Résumé
Évaluation de la coagulation par une thrombo-élastographie chez des chevaux en santé ayant reçu de de la dexaméthasone. La dexaméthasone a été administrée à des chevaux en santé pendant 7 jours. Des échantillons de sang ont été prélevés à trois moments auprès des groupes de traitement et des groupes sans traitement et ensuite analysés par thrombo-élastographie (TEG). Il n’y avait aucune différence significative dans le temps pour les paramètres TEG entre les chevaux traités et non traités ou à l’intérieur des groupes de traitement.
(Traduit par Isabelle Vallières)
Glucocorticoid therapy increases clotting factors in humans (1) and is associated with increased clot strength in canine blood (2,3). Canine and human patients with naturally occurring hyperadrenocorticism are more likely to develop thromboembolic disease (4,5). Thromboelastography (TEG) is a whole-blood viscoelastic coagulation assay that has been used to demonstrate hypercoagulable states in dogs (2). Thromboelastography has been validated for use in dogs, cats, and horses (6–8).
Dexamethasone is used in equine practice to treat a variety of conditions, including non-infectious respiratory diseases such as recurrent airway obstruction (9). Despite their common use, there are no studies looking into the effects of corticosteroids on coagulation in horses.
The objective of this study was to investigate exogenous glucocorticoid (dexamethasone) administration on coagulation of equine blood. We hypothesized that healthy horses administered dexamethasone would have a hypercoagulable TEG tracing compared with untreated horses, and that dexamethasone treated horses would show evidence of hypercoagulability compared with baseline values.
Ten healthy adult horses were used. Animal Care Committee approval was obtained. Horses were assessed as healthy based on history, physical examination, complete blood (cell) count (CBC) and serum biochemistry. Horses were housed on pasture prior to the study and during the washout period, and in outdoor pens during the sampling period. A 3-day acclimation period to pens was provided prior to taking baseline samples. Diet consisted of mixed grass hay (alfalfa, brome, and fescue) q12h. Horses had ad libitum access to water.
This was a blinded, randomized, controlled crossover study. Each horse was randomly distributed into 1 of 2 treatment groups for Phase 1. Twenty-four hours following baseline sampling, the treatment group received dexamethasone (Dexamethasone 5; Dexamethasone Injectable Sterile Solution USP; Vétoquinol, Lavaltrie, Quebec), 0.05 mg/kg body weight (BW), IM, q24h for 7 d while the untreated group received an equivalent volume of sterile saline IM. Injections were given in the cervical region with a BD Precision Glide Needle (20G × 1 1/2″) (Becton-Dickenson, Franklin Lakes, New Jersey, USA). Injection site (right or left side) was alternated each day. After a 7-week washout period, Phase 2 was completed as a crossover study. Investigators who administered treatments, sampled blood, and performed TEG analysis were blinded. Blood samples were taken via atraumatic jugular venipuncture using 20 Ga needles [Jorvet Quick Draw Blood Collection Needle (20 G × 1 1/2″); Jorgenson Laboratories, Loveland, Colorado, USA, and BD Vacutainer Standard Yellow Holder (Becton-Dickinson)] and Vacutainer tubes (BD Vacutainer K2 EDTA Plus Blood Collection Tubes and BD Vacutainer Serum Plus Blood Collection Tubes; Becton-Dickinson) 2 h following treatment. A discard sample of at least 7 mL was drawn (BD Vacutainer Serum Plus Blood Collection Tubes; Becton-Dickinson) before collecting 2.7 mL of blood in 3.2% sodium citrate [BD Vacutainer Buffered Sodium Citrate (9NC) Blood Collection Tubes (0.105M ~3.2%); Becton-Dickinson] to achieve a 9:1 blood-to-citrate ratio. Thromboelastography analyses were performed on Thromboelastograph Hemostasis Analyzers (TEG 5000; Haemonetics Corporation, Haemoscope Division, Niles, Illinois, USA) by one operator (JW). Blood samples were held at room temperature until time of analysis. Thirty minutes after blood collection, the sample was gently inverted 5 times and 1 mL of citrated blood was added to a kaolin vial. This vial was gently inverted 5 times and 340 μL of the resulting mixture was added to a preheated reaction cup (37°C) containing 20 μL of 0.2 M CaCl2. Each sample was run in duplicate. Reaction time (R), clotting time (K), angle (α), and maximum amplitude (MA) were recorded.
Two-way repeated measures analysis of variance (ANOVA) with post-hoc Bonferroni were used to compare data between groups for each TEG parameter. One-way repeated measures ANOVAs were used for within-group comparisons and, where a statistically significant difference was found, a post-hoc Dunnett’s was performed using Day 0 as the comparison column. Normality was tested with a Kolmogorov-Smirnov test. A P value < 0.05 was considered significant. Graphpad Prism (version 5) was used for analyses.
All horses were considered healthy prior to beginning each phase. One horse was removed from the study in Phase 1 in accordance with our animal care protocol due to intolerance of venipuncture. A second horse was removed during Phase 2 when it suffered a limb laceration. Data from these 2 horses were removed from analysis. The horses included for analysis consisted of 2 geldings and 6 mares that were 16.9 y [±5.2 (SD)] old and weighed 479.4 [±73.7 (SD)] kg. Body condition scores ranged from 5 to 6 out of 9. Breeds included Quarter Horse, Thoroughbred, and Standardbred.
One horse had a mildly decreased red blood cell (RBC) count with hematocrit and hemoglobin concentration within reference intervals (RI) in both study phases (6.3 × 1012/L and 6.6 × 1012/L, respectively; Table 1). One horse had a mild neutropenia (2.05 × 109/L), and 1 had mild thrombocytosis (456 × 109/L) in Phase 1, both of which returned to within RI in Phase 2. Five horses in Phase 1 and 3 horses in Phase 2 had mild hyperfibrinogenemia according to our laboratory RI (2.46 to 3.40 g/L and 3.24 to 3.56 g/L, respectively). Two of these horses had increased fibrinogen in both phases, the remaining 3 horses in Phase 1 and 2 horses in Phase 2 showed increases in fibrinogen in only 1 of the experimental phases. On biochemistry, 6 horses in Phase 1 and all 8 horses in Phase 2 had mild to moderately elevated aspartate aminotransferase (AST) levels (range of increase 405 to 1245 U/L, RI: 180 to 350 U/L). Creatine kinase (CK) was increased concurrently in 3 of these 6 horses in Phase 1, and in 4 of 8 horses in Phase 2. Gamma-glutamyl transferase (GGT) was mildly to moderately increased in 5 horses in Phase 2 only (range of increase 38 to 86, RI: 1.0 to 35 U/L), 4 of which had concurrent increases in AST and CK and only 1 with a concurrent sole increase in AST. Lactate dehydrogenase (LDH) was mildly increased in 2 horses in Phase 2, both of which had concurrent increases in AST, and 1 in CK.
Table 1.
Baseline blood test results (minimum and maximum values) for study horses obtained at the beginning of Phases 1 and 2
| Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Parameter | Minimum | Maximum | Minimum | Maximum | Reference interval |
| Red blood cell count (×1012/L) | 6.3 | 8.5 | 6.6 | 9.5 | 7.0 to 12.0 |
| Hemoglobin (g/L) | 116 | 141 | 120 | 154 | 110 to 170 |
| Hematocrit (%) | 33 | 40 | 35 | 45 | 30 to 44 |
| Platelets (×109/L) | 102 | 158 | 97 (clumping) | 456 | 100 to 400 |
| White blood cell count (×109/L) | 4.1 | 8.4 | 5.5 | 11.1 | 5.5 to 12.5 |
| Neutrophils (×109/L) | 2.05 | 5.54 | 3.08 | 6.33 | 2.6 to 7.5 |
| Fibrinogen (g/L) | 1.71 | 3.45 | 1.87 | 3.56 | 0.76 to 2.3 |
| Total protein (g/L) | 61 | 68 | 60 | 75 | 56 to 80 |
| AST (U/L) | 234 | 621 | 447 | 1245 | 180 to 350 |
| LDH (U/L) | 224 | 530 | 228 | 592 | 150 to 450 |
| GGT (U/L) | 11 | 16 | 26 | 86 | 1 to 35 |
| Total bilirubin (μmol/L) | 17.9 | 35.8 | 12.3 | 23.6 | 13.7 to 54.7 |
| BUN (mmol/L) | 3.5 | 6.4 | 3.7 | 6.8 | 2.86 to 9.28 |
| Creatinine (μmol/L) | 90 | 130 | 77 | 94 | 88 to 194 |
| Creatine kinase (U/L) | 215 | 583 | 180 | 519 | 100 to 300 |
AST — aspartate aminotransferase; LDH — lactic dehydrogenase; GGT — gamma-glutamyl transferase; BUN — blood urea nitrogen.
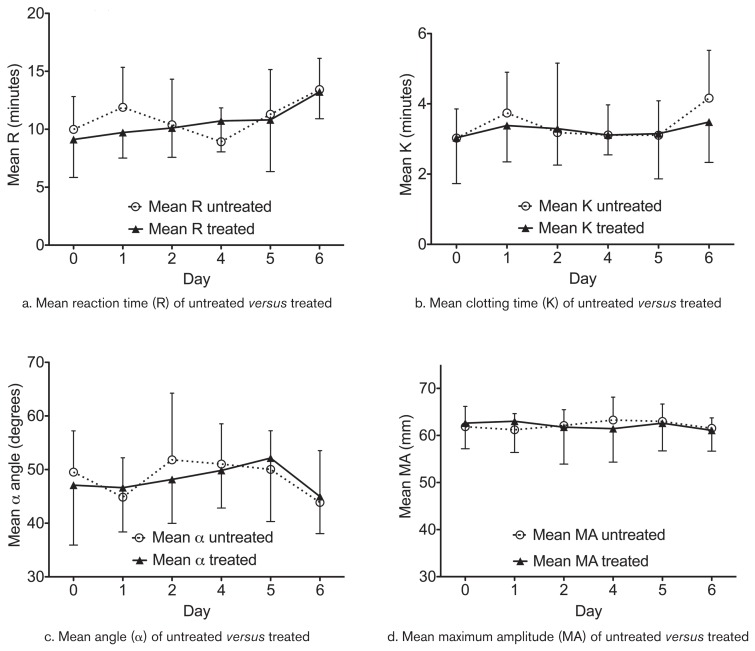
All data for all TEG parameters were normally distributed. Data from Days 3 and 7 were removed from the two-way ANOVA due to missing data points (operator error). There were no significant differences between treated and untreated horses in any of the 4 TEG parameters evaluated (Figure 1). There were no significant differences within the treated or untreated groups over time compared with baseline values.
Figure 1.
Average thromboelastography (TEG) parameters for R (min) a — K (min), b — α-angle (degrees), c — MA (maximum amplitude, mm), d — ± standard deviation (SD) for horses (n = 8) receiving 0.05 mg/kg body weight dexamethasone (n = 4) or an equivalent volume of saline (n = 4) IM daily for 7 d. After a washout period of 7 wk, the groups were switched and the experiment repeated. Day 0 represents baseline. The narrower error bars (|) represent SD of the control group while the wider error bars (⊥) represent SD of the dexamethasone group.
Our TEG results do not support development of a hypercoagulable state post dexamethasone administration (0.05 mg/kg BW) to healthy horses. These results may be indicative of a true lack of an effect of dexamethasone on coagulation in equine blood at the dose administered, or may be due to lack of analytical power due to small sample size. To our knowledge, there have been no previous studies investigating the effect of dexamethasone administration on TEG parameters in horses. Prior TEG studies looking at the effects of immunosuppressive doses of prednisone in healthy dogs revealed an increase in MA in treated animals (2,3). However, TEG analyses in these studies were performed on citrated whole blood without use of an activator. In addition to species differences, these studies are difficult to compare to our kaolin activated citrated TEG samples, given the effects TEG activators have on pre- and intra-analytical variation (6,8). One canine study evaluating citrated-kaolin samples in dogs with naturally occurring ACTH-dependent hyperadrenocorticism found evidence for hypercoagulability based on shorter K times, increased α angles and increased MA (10), but similar findings were not detected in our dexamethasone treated horses.
Humans given dexamethasone demonstrated evidence of increased levels of clotting factors and fibrinogen (1). Although a good proportion of the horses in our study showed evidence of mild hyperfibrinogenemia in their baseline blood test results, there were no corresponding significant changes in any of the TEG parameters measured, and more specifically, no significant increases in MA values. Dogs with naturally occurring hyperadrenocorticism were found to have increased fibrinogen concentrations that were associated with increased MA and positively correlated with MAfibrin (fibrin portion of MA as detected by TEG platelet mapping) (10), but we did not detect similar changes in MA in our study horses. The hyperfibrinogenemia in our horses was consistently mild, with fibrinogen values never exceeding 4 g/L, which has been reported as the upper limit of reference ranges in healthy horses (11), thus were not likely of sufficient concentration to have affected MA values.
Previous canine studies (2,3) demonstrated a decrease in fibrinolytic parameters (LY60) in dogs administered prednisone compared with their baseline values. Due to time constraints within our sampling protocol, we were not able to consistently obtain values for fibrinolytic parameters in our study horses, but these would be worth including in similar future studies.
It is possible that the dose of dexamethasone used in our study played a role in the inability to demonstrate a hypercoagulable state. The 0.05 mg/kg BW, q24h dose of dexamethasone was chosen for this study because it is a commonly used therapeutic protocol for inflammatory conditions in clinical practice (10). Considering that laminitis has been identified anecdotally as a consequence of steroid therapy, a dose > 0.05 mg/kg BW, q24h was avoided. It is possible that a higher dexamethasone dose on a mg/kg basis may have resulted in changes in TEG tracings.
A major limitation in this study was sample size. We were unable to use more than 10 horses due to budgetary and logistic constraints. It is possible that repeating the study with a greater number of horses would reduce type II error and result in a significant difference between treated and untreated groups.
There was variability within our TEG parameter results in both treated and untreated groups (Figure 1). This variability could have been due to a combination of effects from pre-analytic factors, breed or gender variations, or potentially the use of multiple machines for analysis. Measures were taken to help decrease this variability: all machines underwent electronic quality control (via E-testing) daily, biologic controls were performed daily prior to running any samples, analyses were run in duplicate to decrease imprecision, and an activator (kaolin) was used to help decrease the impact of pre-analytic factors as recommended by Laursen et al (8). Nonetheless, some degree of variability remained in our results. Future studies with a larger number of animals may increase accuracy of the results and potentially investigate any breed and/or gender differences that may be present.
The mild decrease in RBC count in 1 horse in both phases was not considered clinically significant based on concurrent hemoglobin concentration and hematocrit within reference intervals, and a normal physical examination in this individual. The single isolated mild neutropenia and mild thrombocytosis in 2 separate horses in Phase 1 were also not considered to be clinically significant given their mild degree of change, and their subsequent resolution in Phase II baseline CBC results.
In baseline biochemistry results, the concurrent increases in AST and CK were most likely associated with some degree of muscle injury (12). This could be associated with blood sampling or with horses adapting to new social pairings within their pens; running and/or chasing likely occurred as part of the establishment of social hierarchy prior to blood collection. Blood samples for serum biochemistry were shipped to a local laboratory for analysis, and any delay in separation of serum from cells before submission could have contributed to the increases in AST (12). Increases in GGT and LDH are more difficult to explain. There was investigation into the possibility of hepatotoxic plant exposure during the 7-week washout period while horses were on pasture, but no evidence for this was found. Brief physical examinations were performed daily on each horse prior to sampling and no abnormalities were noted for any of these horses during the study. Steroid hepatopathy could have also been possible (13), but less likely given the 7-week washout period between dexamethasone injections and biochemistry analyses.
In conclusion, based on these preliminary results, the administration of IM dexamethasone at a dose of 0.05 mg/kg BW, SID to healthy horses did not alter TEG parameters, between treated and untreated groups, or at any time point during the course of administration of dexamethasone. Studies involving larger numbers of animals are required to further investigate potential effects of steroids on coagulation parameters in horses. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Brotman DJ, Girod JP, Posch A, et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res. 2006;118:247–252. doi: 10.1016/j.thromres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Flint SK, Abrams-Ogg ACG, Kruth SA, Bersenas AM, Wood RD. Independent and combined effects of prednisone and acetylsalicylic acid on thromboelastography variables in healthy dogs. Am J Vet Res. 2011;72:1325–1332. doi: 10.2460/ajvr.72.10.1325. [DOI] [PubMed] [Google Scholar]
- 3.O’Kell AL, Grant DC, Panciera DL, Troy GC, Weinstein NM. Effects of oral prednisone administration with or without ultralow-dose acetylsalicylic acid on coagulation parameters in healthy dogs. Am J Vet Res. 2012;73:1569–1576. doi: 10.2460/ajvr.73.10.1569. [DOI] [PubMed] [Google Scholar]
- 4.Jacoby RC, Owings JT, Ortega T, Gosselin R, Feldman EC. Biochemical basis for the hypercoagulable state seen in Cushing syndrome. Arch Surg. 2001;136:1003–1007. doi: 10.1001/archsurg.136.9.1003. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LR, Lappin MR, Baker DC. Pulmonary thromboembolism in 29 dogs: 1985–1995. J Vet Intern Med. 1999;13:338–345. doi: 10.1892/0891-6640(1999)013<0338:ptid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Wiinberg B, Jensen AL, Kjelgaard-Hansen M, et al. Study on biological variation of haemostatic parameters in clinically healthy dogs. Vet J. 2007;174:62–68. doi: 10.1016/j.tvjl.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee A, Blois SL, Wood RD. Comparing citrated native, kaolin-activated, and tissue-factor-activated samples and determining intraindividual variability for feline thromboelastography. J Vet Diagn Invest. 2011;23:1109–1113. doi: 10.1177/1040638711425595. [DOI] [PubMed] [Google Scholar]
- 8.Laursen SH, Andersen PH, Kjelgaard-Hansen M, Wiinberg B. Comparison of components of biological variation between 3 equine thromboelastography assays. Vet Clin Pathol. 2013;42:443–450. doi: 10.1111/vcp.12079. [DOI] [PubMed] [Google Scholar]
- 9.Robinson NE, Jackson C, Jefcoat A, Berney C, Peroni D, Derksen FJ. Efficacy of three corticosteroids for the treatment of heaves. Equine Vet J. 2002;34:17–22. doi: 10.2746/042516402776181105. [DOI] [PubMed] [Google Scholar]
- 10.Park FM, Blois SL, Abrams-Ogg RD, Wood DG, Nykamp SG, Downie A. Hypercoagulability and ACTH-dependant hyperadrenocorticism in dogs. J Vet Intern Med. 2013;27:1136–1142. doi: 10.1111/jvim.12162. [DOI] [PubMed] [Google Scholar]
- 11.Crisman MV, Scarratt WK, Zimmerman KL. Blood proteins and inflammation in the horse. Vet Clin Equine. 2008;24:285–297. doi: 10.1016/j.cveq.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames, Iowa: Blackwell Publishing; 2008. [Google Scholar]
- 13.Cohen ND, Cartter GK. Steroid hepatopathy in a horse with glucocorticoid-induced hyperadrenocorticism. J Am Vet Med Assoc. 1992;200:1682–1684. [PubMed] [Google Scholar]