Abstract
Two young dogs were evaluated for an acute onset of abnormal head posture and eye movement. Neurological examination was characterized mostly by permanent neck extension, abnormalities of pupils, and eye movement. A mesencephalic mass lesion was detected on magnetic resonance imaging in both cases. Neurophysiological pathways likely responsible for this peculiar clinical presentation are discussed.
Résumé
Syndrome du mésencéphale dorsal associé à une extension du cou persistante : résultats de l’évaluation clinique et de l’imagerie diagnostique chez 2 chiens. Deux jeunes chiens ont été évalués suite à l’apparition soudaine d’une posture de tête et d’un mouvement des yeux anormaux. Un examen neurologique a été caractérisé surtout par une extension permanente du cou, des anomalies des pupilles et un mouvement des yeux. Une masse mésencéphalique a été détectée à l’imagerie par résonance magnétique dans les deux cas. Les voies neurophysiologiques, qui étaient probablement responsables de cette présentation clinique, sont discutées.
(Traduit par Isabelle Vallières)
Although relatively uncommon, lesions confined to the mesencephalon are well-described in small animals (1–3). Clinical signs frequently reported in these cases are abnormal mental status, postural reaction deficits, spastic paresis or plegia, propulsive pacing or circling, mydriasis unresponsive to light stimulation, strabismus either ventrolateral or extorsional, and menace deficits without visual impairment (1,4). This combination of signs is sometimes referred to as midbrain syndrome. The most common causes are cranial trauma with midbrain compression and/or hemorrhage, thiamine deficiency, and granulomatous meningoencephalitis (1,2,5,6). Clinical presentation can vary in severity and lateralization depending on the extent, location, and etiology of the lesion (1,4).
In human medicine, lesions affecting the dorsal part of the mesencephalon are responsible for a cluster of abnormalities concerning eye movement, eyelid position, and pupil dysfunction. This condition is called dorsal midbrain syndrome or Parinaud’s syndrome. The most common etiologies are tumors of the pineal gland or dorsal midbrain, multiple sclerosis, strokes, and obstructive hydrocephalus (7–9). However, any other lesion in this region can cause this syndrome (7).
This report describes a peculiar clinical presentation of 2 young dogs diagnosed with a dorsal midbrain lesion. Both cases presented with abnormal pupils, abnormal eye movement, and permanent neck extension.
Case descriptions
Case 1
A 3-month-old intact male German shepherd dog was presented with a 2-week history of abnormal posture of the head (persistently tilted upward) and mild divergent strabismus. In addition, an acute onset of bilateral mydriasis had been noticed 2 d prior to the referral. The dog had been treated with prednisone and amoxicillin, without any improvement in clinical signs.
On presentation, general physical examination was unremarkable. Neurological examination revealed a depressed and disorientated mental status and retrocollis (head persistently held upward) (10). Circling to both sides, tendency to stumble over obstacles and front limb hypermetria were seen at gait analysis. Proprioception was decreased in all 4 limbs. The vestibulo-ocular reflex (VOR) was bilaterally decreased, movements of the eyes were disconjugated and the right eye tended to tilt downward when the head was moved toward the right. Menace response was inconsistent and bilaterally decreased. Pupils were normal in size with bilaterally slowly responsive pupillary light reflex (PLR). Dazzle reflex and vision were judged normal as was the rest of the neurological examination (Video 1 — https://www.youtube.com/watch?v=6-rw4Pr8_58&feature=em-upload_owner). Based on the clinical findings, a brainstem lesion, predominantly involving the mesencephalon, was suspected. The main differential diagnoses were malformation/congenital, inflammatory (infectious or immune-mediated meningoencephalitis), metabolic (electrolyte imbalances, thiamine deficiency), and neoplastic (primary or secondary) diseases.
Complete blood (cell) count (CBC) and dynamic bile acids were within normal ranges and the serum biochemical profile was unremarkable except for elevated aspartate aminotransferase [70 U/L; reference range (RR): 10 to 62 U/L] and alkaline phosphatase (394 U/L; RR: 0 to 90 U/L) activities and a mild hyperphosphatemia (2.28 mmol/L; RR: 0.81 to 1.62 mmol/L). All these abnormalities were considered to be related to age and to previous treatment with corticosteroids.
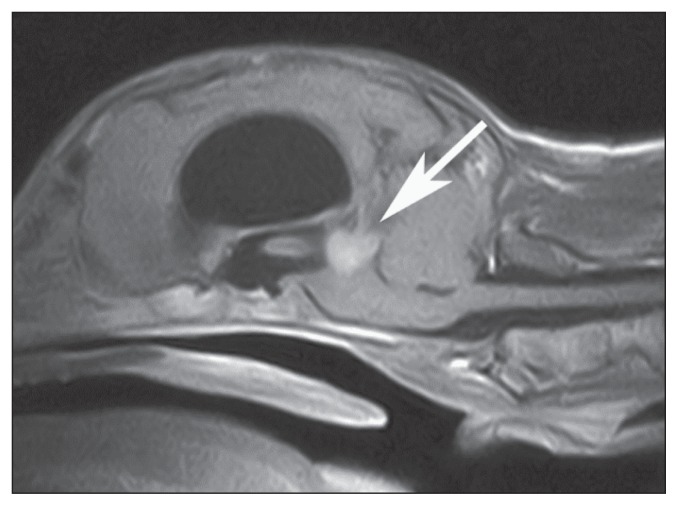
Magnetic resonance imaging (MRI) of the brain was performed with a 0.22 T unit (MrV; Paramed, Genoa, Italy). Spin Echo (SE) T1-weighted (T1W), post-contrast [0.1 mmol/kg body weight (BW) gadoteric acid IV, (Dotarem, Guerbet Laboratories, Milan, Italy)] SE T1W, and FSE T2-weighted (T2W) images were acquired in transverse, dorsal, and sagittal planes. Additionally fluid attenuated inversion recovery (FLAIR) images were acquired in transverse and dorsal planes. A rounded lesion (1.0 × 1.0 × 0.7 cm) was seen at the level of the middle fossa, just caudal to the third ventricle, involving the dorsal part of the mesencephalon. The mass appeared hypointense to gray matter with a hyperintense rim on T2W images, and homogeneously isointense to slightly hyperintense on pre-contrast T1W images. On post-contrast T1W images, the mass was homogenously enhanced (Figure 1). It was considered to be intra-axial in location; however, an extra-axial location could not be excluded. Ventriculomegaly of all 4 ventricles was evident (Figure 1). A periventricular hyper-intensity on T2W and FLAIR images was noticed at the level of lateral and third ventricles. Moreover the lesion produced a mass effect compressing and caudally displacing the cerebellum.
Figure 1.
Postcontrast midsagittal SE T1-weighted magnetic resonance image of the brain of dog 1. Notice the homogeneous enhancement of the rounded mass occupying the dorsal region of the mesencephalon (arrow). Severe dilation of the lateral and third ventricles is evident. A marked mass effect, displacing and compressing ventrally the brainstem and caudally the cerebellum, is evident. (Reprinted from: Thalamic astrocytic hamartoma and associated meningoangiomatosis in a German shepherd dog. In: Pasquali P, ed. Research in Veterinary Science. Vol 94. Philadelphia, Pennsylvania: Elsevier, 2013:644–647, with permission).
Differential diagnoses for the mass included neoplasia and granuloma. Additionally, perilesional edema and obstructive hydrocephalus were seen. Given the severity of signs and the location of the lesion, biopsy was not performed. Based on the poor prognosis, the owners declined any further diagnostic tests and elected euthanasia. Upon histopathological examination of the brain, an astrocytic thalamic hamartoma associated with dorsal mesencephalic meningoangiomatosis was diagnosed (11).
Case 2
A 14-month-old intact male Bordeaux mastiff dog was presented with a 1-week history of bilateral ventrolateral strabismus, abnormal posture of the head (persistently tilted upward), and abnormal mental status. An acute onset of bilateral mydriasis was also noticed some days prior to the referral.
On presentation, general physical examination was unremarkable. Neurological examination revealed a disoriented mental status, retrocollis, and ventrolateral strabismus in both eyes. Front limb hypermetria and a tendency to circle to the right were seen at gait analysis. Proprioception was decreased in both hind limbs and slightly decreased in the left front limb. Vestibulo-ocular reflex was abnormal with disconjugate eye movement: the right eye tended to tilt slightly downward when the head was moved toward the right, and did not abduct when the head was moved to the left. Menace response was bilaterally decreased. Mydriasis, absence of PLR, and dazzle reflex were noticed in both eyes. The rest of the neurological examination was normal (Video 2 — https://www.youtube.com/watch?v=X3FbHfI5jZ4&feature=em-upload_owner). Neurolocalization and main differential diagnoses were the same as for case 1. Routine hematologic and serum biochemical examinations were within normal ranges.
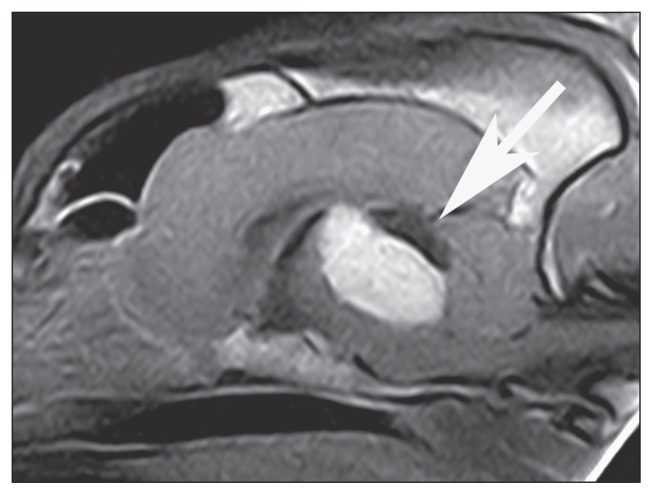
An MRI of the brain was performed with a 0.20 T unit (Vet MR; Esaote, Genoa, Italy). Spin Echo T1-weighted, post-contrast [0.2 mmol/kg gadopentetic acid, (Magnevist; Bayer Schering Pharma, Milan, Italy)] SE T1W, FSE T2W and FLAIR images were acquired in transverse planes. Additionally post-contrast T1W images were acquired in transverse, dorsal, and sagittal planes. A large (2.6 × 2.4 × 2.3 cm), ovoid, well-defined mass was detected in the middle fossa, protruding to some extent into the cranial fossa. It was located at the level of the dorsal portion of the mesencephalon, extending from the rostral part of the cerebellum to the thalamus. The mass had a fairly homogeneous structure, a bright hyperintense appearance in T2W images (signal not suppressed in FLAIR images), and a hypointense appearance in pre-contrast T1W images compared to the gray matter. On post-contrast T1W images, the mass was homogenously enhanced. It caused a severe mass effect, displacing and compressing the cerebellum caudally and the diencephalic structures cranially (Figure 2). It was thought to be intra-axial in location; however, an intraventricular location could not be excluded. Ventriculomegaly of the lateral and third ventricles was detected. Periventricular hyperintensity, likely due to edema, was detected at the level of lateral and third ventricles on T2W and FLAIR images. Differential diagnoses for the mass included neoplasia and granuloma. The owner declined further diagnostics. A symptomatic treatment with amoxicillin and clavulanate (Synulox; Pfizer, Rome, Italy), 20 mg/kg BW, PO, q12h, prednisone (Vetsolone, Bayer), 1 mg/kg BW, PO, q24h, and furosemide (Diuren, Teknofarma, Torino, Italy), 1 mg/kg BW, PO, q12h, was started. Despite medical therapy, the dog’s neurological condition rapidly deteriorated during the following week. A new neurological examination revealed stuporous mental status, non-ambulatory tetraparesis, decreased proprioception in all 4 limbs, absence of menace response, PLR, dazzle reflex and VOR in both eyes, reduction of the gag reflex and voluntary movement of the tongue. Due to the rapid progression of the clinical signs and the poor prognosis the owner elected euthanasia. A postmortem examination was declined.
Figure 2.
Postcontrast midsagittal SE T1-weighted magnetic resonance image of the brain of dog 2. Notice the homogeneous enhancement of the ovoid mass located at the level of the dorsal portion of mesencephalon (arrow). The mass extends from the level of the thalamus to the level of the medulla. A marked mass effect is evident, displacing and compressing the cerebellum caudally, the brainstem ventrally, and the diencephalic structures cranially.
Discussion
To our knowledge, the present report is the first to document this unusual group of clinical signs secondary to a discrete lesion located within the dorsal midbrain. Both cases (Videos 1,2) presented with retrocollis (10), a condition that has to be distinguished from opisthotonus, which is defined as a spasticity of dorsal neck and head muscles and extension of the neck associated with back arching (12). Opisthotonus is usually caused by extensive lesions of the mesencephalon and rostral cerebellum (4). In addition, in cases of extensive mesencephalic lesion, affected patients are usually recumbent and display severe clinical signs, such us extensor rigidity of all 4 limbs, and severe impairment of mental status (4). Indeed the 2 dogs reported here, were ambulatory, mental status was only mildly compromised at the onset of the clinical signs, and the back was not arched.
Retrocollis is described in cats and monkeys with experimentally induced, bilateral lesions of the interstitial nucleus of Cajal (INC) (13–15). This nucleus is located in the tegmentum of the rostral midbrain, lateral to the Edinger-Westphal nucleus, adjacent to the periaqueductal gray matter (13,14).
Experimentally induced lesions rostral to the INC did not cause impairment of head position, justifying the crucial role of this nucleus (13). The exact mechanism by which a bilateral INC lesion causes this abnormal posture is still unknown. Experimental studies on primates show that INC sends signals to a multitude of targets implicated in the control of head and neck movement (14,15). Fibers originating from this nucleus deploy terminal fields in the first 4 cervical segments of the spinal cord forming the interstitiospinal tract (13–16). Experimental studies on monkeys demonstrate that these fibers target the medial portion, near the border between gray and white matter, but also more laterally in more ventral portions of the anterior horn (Rexed laminae VII and VIII) (14). In the cat these regions of the spinal cord have been shown to contain motoneurons supplying long dorsal muscles of the neck (i.e., splenius, complexus, and biventer cervicis) (16,17). It seems also possible that the INC sends inhibitory projections to other brainstem regions, directly or indirectly, which tonically excite dorsal neck muscles (13,14). Consequently bilateral INC lesions result in hypertonia of these antigravity neck muscles (13,14). Furthermore, Fukushima et al (16) observed that retrocollis remains unchanged in darkness, demonstrating that it also occurs without visual inputs. It appeared soon after the induction of bilateral INC lesions and lasted consistently whether cats were sitting, standing, or walking. Moreover, when cats with bilateral INC lesions drank water from a basin on the floor, the neck remained extended and was not flexed ventrally (13). These observations indicate that head movement is also impaired in INC-lesioned cats (13). In our 2 cases retrocollis was consistently present both at rest and during movement (Videos 1,2).
In case 1 the histopathological examination confirmed the lesion seen at MRI extending from the region of the pretectal diencephalic nuclei to the dorsal portion of the mesencephalon, involving the periaqueductal gray matter and the nearby structures. Given the anatomical location of the INC (13), we assume that it is involved in the disease process. In case 2, bilateral involvement of this nucleus was presumed based on the strong resemblance of the clinical picture and the diagnostic imaging features to case 1, although lacking the histopathological confirmation. Since the INC is described in dogs (18), we hypothesize that the abnormal posture of the neck and the head in both our cases was due to its impairment.
Moreover, cats with experimental INC lesions displayed abnormal gait (13). Although vision was not affected, they tended to stumble over obstacles placed in front of them or to fall from the edge of a table. All these anomalies may be explained by gaze disturbances consequent to the abnormal posture of the head. Both cases showed similar deficits. Additionally, hypermetria, most notable in the front limbs, was seen at gait analysis.
Front limb hypermetria is reported in cerebellar lesions. In both our cases MRI revealed severe compression of cerebellar parenchyma with caudal displacement. However, cerebellar hypermetria is characterized by inability to regulate the rate, range, or force of a movement. In addition, during the stride the limb is usually raised higher than expected by excessive joint flexion due to inhibition of antigravity muscle activity. Additionally, spasticity and intentional tremors are frequently seen during movement in patients with cerebellar lesions (19). Moreover, bilateral INC lesions cause disturbances of head and neck position, and of eye movements (13,16). As well, these deficits may disturb vertical gaze, cause visual impairment, and consequently gait disturbances such as tendency to stumble over objects, falling backwards, and gait disturbances of front limbs (13). The absence of spasticity and intentional tremors at gait analysis in both cases sustains our hypothesis that hypermetria is due to gaze disturbances rather than to cerebellar involvement.
In human medicine, lesions affecting the dorsal part of the mesencephalon produce a specific clinical syndrome characterized by abnormalities of eye movement and eyelid position, and pupil dysfunction. The most common conditions associated with this syndrome are pineal region neoplasms, obstructive hydrocephalus, multiple sclerosis, dorsal midbrain infection, arteriovenous malformations, mesencephalic hemorrhages, and infarction (7,8). The clinical picture, referred to as dorsal midbrain or Parinaud’s syndrome, is classically characterized by ocular signs such as light-near dissociation of pupils, eyelid retraction, and vertical gaze disturbances (9). Pupils are usually dilated and PLR is absent or decreased (7–9).
In light-near dissociation, pupils constrict when the patient focuses on a near object (accommodation), but do not constrict when exposed to bright light (20). The functional role of the pupillary constriction during accommodation is poorly understood. Arguably, it may increase the depth of field by reducing the aperture of the eye, and thus reduce the amount of accommodation needed to bring the image in focus on the retina (21). To the best of our knowledge pupil constriction during accommodation has never been demonstrated in veterinary ophthalmology; consequently, light-near dissociation has never been described in dogs and no attempts of evaluation were made in the 2 patients. In case 2 pupils were dilated, while PLR was reduced in case 1 and absent in case 2. A bilateral involvement of the parasympathetic nucleus of the third cranial nerve is thought to be responsible for these anomalies (22).
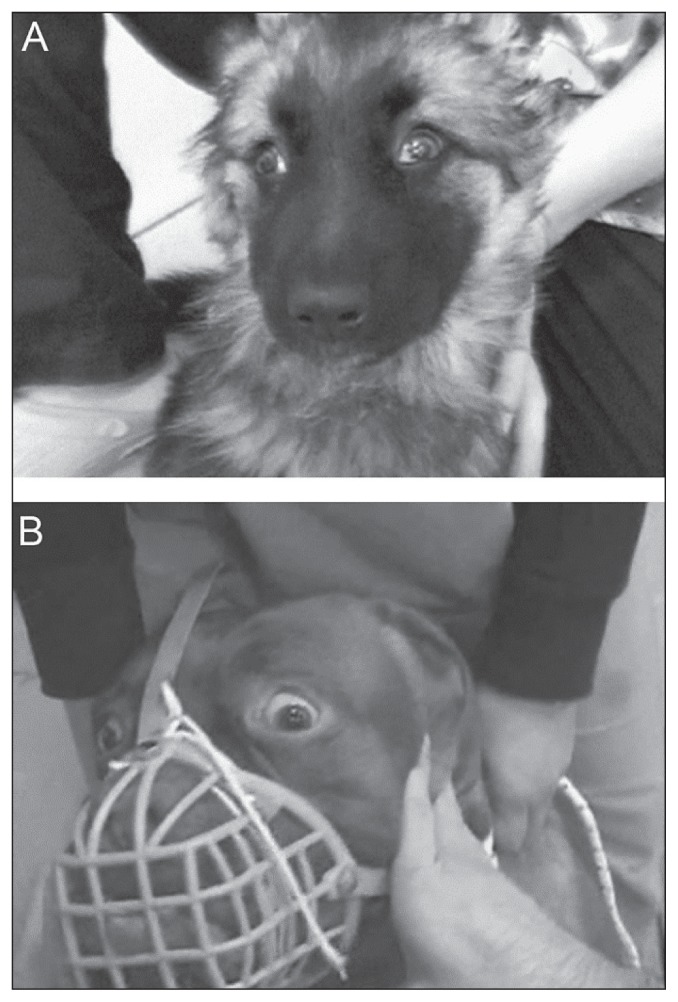
Upper eyelid retraction, also called Collier’s sign, is well-described in human ophthalmology. In this eyelid anomaly, the sclera can be seen above the cornea with the eyes in the primary position and to a greater extent during upward eye movements (23). This is an accepted medical sign of a midbrain lesion, thought to be due to damage to the posterior commissure levator inhibitory fibers, which originate in the M-group of neurons (24). Collier’s sign has never been described in veterinary medicine. During a careful, retrospective evaluation of eyelid position in videotapes, this sign was recognized in both our cases (Figure 3).
Figure 3.
Video frames of case 1 (A) and case 2 (B). Upper eyelid retraction (Collier’s sign). Note the bilateral abnormal retraction of the upper eyelid in both cases. The abnormal eyelid position leads the sclera to be seen above the iris and the eyelid opening is enlarged.
Video 1. (https://www.youtube.com/watch?v=6-rw4Pr8_58&feature=em-upload_owner) Case 1: Retrocollis was observed both with the dog in the sitting position and during movement. Tendency to stumble over obstacles and front limb hypermetria were seen at gait analysis. Menace response was inconsistent and bilaterally decreased. Vestibulo-ocular reflex was bilaterally decreased, movements of the eyes were disconjugate and decreased; the right eye tended to tilt downward when the head was moved toward the right.
Video 2. (https://www.youtube.com/watch?v=X3FbHfI5jZ4&feature=em-upload_owner) Case 2: Retrocollis and front limb hypermetria were observed during movement. Right circling was seen during movement without leash. Ventrolateral strabismus, decreased and inconsistent menace response and mydriasis were evident in both eyes. Vestibulo-ocular reflex was abnormal with disconjugate eye movement: the right eye tended to tilt downward when the head was moved toward the right, and did not abduct when the head was moved to the left. Pupillary light reflex was absent bilaterally.
In Parinaud’s syndrome, vertical gaze is impaired due to the lesion in the INC. It is well-documented that the INC is implicated in the control of eye movements in animals (13,14,25). Experimental studies in monkeys and cats show evidence of connection between INC and ipsilateral and contralateral oculomotor and trochlear nuclei (13,14,25). Unfortunately, vertical gaze abnormalities were not investigated in either of our cases.
It is demonstrated that fibers originating from the INC descend within the ipsilateral medial longitudinal fasciculus (MLF) to distribute terminal fields in the ipsilateral vestibular complex (mainly dorsal and medial vestibular nuclei) (13,14,26). Both our cases showed impairment of the horizontal VOR, not described in Parinaud’s syndrome. It is reasonable to suppose that in our cases the clinical presentation was different because of the extent of both lesions and mass effect causing involvement of surrounding parenchymal structures. Indeed, we believe that in both cases this deficit could be the consequence of impairment of multiple structures involved in this reflex (INC, oculomotor and trochlear nuclei, and MLF fibers) rather than dysfunction of a discrete center or pathway.
The main limitation of the current study is the incomplete neuro-ophthalmological examination report in both cases, given the retrospective nature of the report. Pupil dysfunction and further subtle abnormalities of spontaneous eye movement could have been better characterized and documented, integrating the clinical picture and consequently making a better comparison with the human counterpart. This is partially justified by the lack of detailed description in veterinary literature of peculiar signs, such as Collier’s sign and saccadic movements, due to discrete lesion in the dorsal mesencephalon.
Although not previously reported in dogs, retrocollis associated with eye movement disorders and pupil dysfunction should be considered strong indicators of a dorsal mesencephalic syndrome and should alert clinicians to promptly localize the lesion and perform the most appropriate diagnostic imaging procedure. CVJ
Footnotes
Case 1 was presented as an oral communication at 73rd Annual International Symposium of the Italian Society of Small Animal Practitioners (SCIVAC), Rimini, Italy, 2012.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Braund KG. Neurological syndromes. In: Vite CH, Braund KG, editors. Braund’s Clinical Neurology in Small Animals: Localization, Diagnosis and Treatment. 2nd ed. Philadelphia, Pennsylvania: IVIS; 2003. pp. 23–24. [Google Scholar]
- 2.Garosi LS, Dennis R, Platt SR, Corletto F, de Lahunta A, Jakobs C. Thiamine deficiency in a dog: Clinical, clinicopathologic, and magnetic resonance imaging findings. J Vet Intern Med. 2003;17:719–723. [PubMed] [Google Scholar]
- 3.Garosi LS, McConnell JF, Platt SR, et al. Clinical and topographic magnetic resonance characteristics of suspected brain infarction in 40 dogs. J Vet Intern Med. 2006;20:311–321. doi: 10.1892/0891-6640(2006)20[311:catmrc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.de Lahunta A, Glass E. The neurologic examination. In: de Lahunta A, Glass E, editors. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. W. B. St. Louis, Missouri: Saunders; 2009. pp. 487–501. [Google Scholar]
- 5.Schatzberg SJ. Idiopathic granulomatous and necrotizing inflammatory disorders of the canine central nervous system. Vet Clin North Am Small Anim Pract. 2010;40:101–120. doi: 10.1016/j.cvsm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Beltran E, Platt SR, McConnell JF, Dennis R, Keys DA, De Risio L. Prognostic value of early magnetic resonance imaging in dogs after traumatic brain injury: 50 cases. J Vet Intern Med. 2014;28:1256–1262. doi: 10.1111/jvim.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AG, Brown DG, Diaz PJ. Dorsal midbrain syndrome due to mesencephalic hemorrhage: Case report and serial imaging. J Neuro-Ophthal. 1996;16:281–285. [PubMed] [Google Scholar]
- 8.Allmer DM, Golis TA. Dorsal midbrain syndrome secondary to a pineocytoma. J Am Optom Assoc. 2001;72:234–238. [PubMed] [Google Scholar]
- 9.Perkin GD. Neuro-ophthalmological syndromes for neurologists. J Neurol Neurosurg Psych. 2004;75:iv20–iv23. doi: 10.1136/jnnp.2004.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papapetropoulos S, Baez S, Zitser J, Sengun C, Singer C. Retrocollis: Classification, clinical phenotype, treatment outcomes and risk factors. Eur Neurol. 2008;59:71–75. doi: 10.1159/000109265. [DOI] [PubMed] [Google Scholar]
- 11.Sebastianelli M, Mandara MT, Pavone S, Canal S, Bernardini M. Thalamic astrocytic hamartoma and associated meningoangiomatosis in a German Shepherd dog. Res Vet Sci. 2013;94:644–647. doi: 10.1016/j.rvsc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz MD, Coates JR, Kent M. Disorders of involuntary movement. In: Lorenz MD, Coates JR, Kent M, editors. Saunders Handbook of Veterinary Neurology. 5th ed. Philadelphia, Pennsylvania: Saunders; 2011. pp. 307–329. [Google Scholar]
- 13.Fukushima-Kudo J, Fukushima K, Tashiro K. Rigidity and dorsiflexion of the neck in progressive supranuclear palsy and the interstitial nucleus of Cajal. J Neurol Neurosurg Psych. 1987;50:1197–1203. doi: 10.1136/jnnp.50.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokkoroyannis T, Scudder CA, Balaban D, Highstein SM, Moschovakis AK. Anatomy and physiology of the primate interstitial nucleus of Cajal. I. Efferent projections. J Neurophysiol. 1996;75:725–739. doi: 10.1152/jn.1996.75.2.725. [DOI] [PubMed] [Google Scholar]
- 15.Farshadmanesh F, Chang P, Wang H, Yan X, Corneil BD, Crawford JD. Neck muscle synergies during stimulation and inactivation of the interstitial nucleus of Cajal (INC) J Neurophysiol. 2008;100:1677–1685. doi: 10.1152/jn.90363.2008. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima K, Pitts NG, Peterson BW. Direct excitation of neck motoneurons by interstitiospinal fibers. Exp Brain Res. 1978;33:565–581. doi: 10.1007/BF00235575. [DOI] [PubMed] [Google Scholar]
- 17.Abrahams VC, Keane J. Controlateral, midline and commissural motoneurons of neck muscle: A retrograde HRP study in the cat. J Comp Neurol. 1984;223:448–456. doi: 10.1002/cne.902230309. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher TF, Beitz AJ. The brain. In: Evans HE, de Lahunta A, editors. Miller’s Anatomy of the Dog. 4th ed. St. Louis, Missouri: Saunders; 2013. p. 670. [Google Scholar]
- 19.de Lahunta A, Glass E. Cerebellum. In: de Lahunta A, Glass E, editors. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St. Louis, Missouri: Saunders; 2009. pp. 348–388. [Google Scholar]
- 20.Thompson HS, Kardon RH. The Argyll Robertson pupil. J Neuro-Ophthal. 2006;26:134–138. doi: 10.1097/01.wno.0000222971.09745.91. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Ciuffreda KJ. Depth-of-focus of the human eye: Theory and clinical implications. Surv Ophthalmol. 2006;51:75–85. doi: 10.1016/j.survophthal.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 22.de Lahunta A, Glass E. Visual System. In: de Lahunta A, Glass E, editors. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St. Louis, Missouri: Saunders; 2009. pp. 389–428. [Google Scholar]
- 23.Karatas M. Internuclear and supranuclear disorders of eye movements: Clinical features and causes. Euro J Neurol. 2009;16:1265–1277. doi: 10.1111/j.1468-1331.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- 24.Larner AJ. Collier’s sign. In: Larner AJ, editor. A dictionary of neurological signs. 3rd ed. Manhattan, New York: Springer; 2011. p. 88. [Google Scholar]
- 25.Anderson JH. Ocular torsion in the cat after lesions of the interstitial nucleus of Cajal. Ann N Y Acad Sci. 1981;374:865–887. doi: 10.1111/j.1749-6632.1981.tb30927.x. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima K, Ohno M, Takahashi K, Kato M. Location and vestibular responses of interstitial and midbrain reticular neurons that project to the vestibular nuclei in the cat. Exp Brain Res. 1982;45:303–312. doi: 10.1007/BF00235791. [DOI] [PubMed] [Google Scholar]