Abstract
Illness and death from diseases caused by contaminated food are a constant threat to public health and a significant impediment to socio-economic development worldwide. To measure the global and regional burden of foodborne disease (FBD), the World Health Organization (WHO) established the Foodborne Disease Burden Epidemiology Reference Group (FERG), which here reports their first estimates of the incidence, mortality, and disease burden due to 31 foodborne hazards. We find that the global burden of FBD is comparable to those of the major infectious diseases, HIV/AIDS, malaria and tuberculosis. The most frequent causes of foodborne illness were diarrheal disease agents, particularly norovirus and Campylobacter spp. Diarrheal disease agents, especially non-typhoidal Salmonella enterica, were also responsible for the majority of deaths due to FBD. Other major causes of FBD deaths were Salmonella Typhi, Taenia solium and hepatitis A virus. The global burden of FBD caused by the 31 hazards in 2010 was 33 million Disability Adjusted Life Years (DALYs); children under five years old bore 40% of this burden. The 14 subregions, defined on the basis of child and adult mortality, had considerably different burdens of FBD, with the greatest falling on the subregions in Africa, followed by the subregions in South-East Asia and the Eastern Mediterranean D subregion. Some hazards, such as non-typhoidal S. enterica, were important causes of FBD in all regions of the world, whereas others, such as certain parasitic helminths, were highly localised. Thus, the burden of FBD is borne particularly by children under five years old–although they represent only 9% of the global population–and people living in low-income regions of the world. These estimates are conservative, i.e., underestimates rather than overestimates; further studies are needed to address the data gaps and limitations of the study. Nevertheless, all stakeholders can contribute to improvements in food safety throughout the food chain by incorporating these estimates into policy development at national and international levels.
An overview of foodborne diseases worldwide; part of the World Health Organization’s investigation into the incidence, mortality, and disease burden of foodborne hazards.
Summary Points
Thirty-one foodborne hazards caused 600 (95% uncertainty interval [UI] 420–960) million foodborne illnesses and 420,000 (95% UI 310,000–600,000) deaths in 2010.
The global burden of FBD caused by the 31 hazards studied was 33 (95% UI 25–46) million DALYs in 2010.
The most frequent causes of foodborne illness were diarrheal disease agents; particularly norovirus and Campylobacter spp.
Foodborne diarrheal disease agents, particularly non-typhoidal Salmonella enterica, caused 230,000 (95% UI 160,000–320,000) deaths
Other major causes of FBD deaths were Salmonella Typhi, Taenia solium, hepatitis A virus and aflatoxin.
40% of the FBD burden was among children under 5 years old.
The African (AFR), South-East Asian (SEAR) and Eastern Mediterranean (EMR) D subregions had the highest FBD burden.
Diarrheal disease agents were the leading cause of FBD burden in most subregions, and non-typhoidal Salmonella enterica caused an important burden in all subregions, particularly in the subregions in Africa.
Other main causes of diarrheal FBD burden were enteropathogenic Escherichia coli, enterotoxigenic Escherichia coli and Vibrio cholerae in low-income subregions, and Campylobacter spp. in high-income subregions.
The burden of aflatoxin was high in the AFR D, Western Pacific (WPR) B and SEAR B subregions, whereas dioxins caused the highest burden in SEAR D, EMR D and European (EUR) A and C subregions.
In the South-East Asian subregions, there was a considerable burden of Salmonella Typhi; the burden of Opisthorchis spp. was concentrated in the SEAR B region, where the seafoodborne trematodes Paragonimus spp. and Clonorchis sinensis were also important.
In Central and South American (AMR B and AMR D) subregions, T. solium and Toxoplasma gondii contributed significantly to the FBD burden.
These estimates should inform policy development at national and international levels to improve food safety throughout the food chain.
Introduction
Illness and death from diseases caused by contaminated food are a constant threat to public health and a significant impediment to socio-economic development worldwide. Foodborne disease (FBD) outbreaks are common and often cause considerable morbidity and mortality. These diseases may be caused by infectious agents, such as the Escherichia coli O104:H4 outbreak attributed to contaminated fenugreek sprouts, which caused 386 cases of illness and 54 deaths in Germany in 2011 [1]. They can also be caused by chemical contamination, for example the melamine contamination of infant milk formula in China in 2008, which resulted in 294,000 cases of illnesses, 50,000 hospitalizations and at least 6 deaths [2]. Such incidents, although they capture widespread attention, constitute only a fraction of the global FBD burden.
Despite the evident importance of FBDs to public health, the full extent of chemical and biological contamination of food, and its cost to society, is still unknown. Contaminants in food were not even included as risk factors in previous studies of the global burden of all diseases [3]. Foodborne contaminants are numerous, however; they include viruses and bacteria, parasites, chemicals, toxins and allergens that cause a wide range of conditions. Many are pathogens that cause neglected tropical diseases, for which data is extremely limited. Indeed, epidemiological data on FBDs generally, particularly in the developing world, remain scarce. Outbreaks often go unrecognized, unreported or uninvestigated and may be visible only if they have a major public health or economic impact. Moreover, many foodborne hazards are also transmitted by other routes: through water, soil, or air; by direct contact between people, or by contact between people and animals. Determining the proportion of any illness that is foodborne can be difficult. Thus, estimating the global and regional burden of any FBD is complex.
Recognizing the need for global and regional estimates of FBDs to guide public health policy, in 2006 the World Health Organization (WHO) launched the ‘Initiative to Estimate the Global Burden of Foodborne Diseases’ [4]. The primary goal of this initiative is to enable policy makers and other stakeholders to set appropriate, evidence-based priorities in the area of food safety. To this end, the initiative aims to: improve the ability of countries to assess their burden of FBD; increase the number of countries who undertake these assessments; estimate the global burden, according to age, sex, and region, of FBDs caused by a defined list of biological and chemical agents; increase awareness of FBDs among WHO Member States and improve their commitment to implement food safety standards; and to encourage countries to use estimates of FBD to inform their policy making. To implement the initiative, in 2007 the WHO established the Foodborne Disease Burden Epidemiology Reference Group (FERG), an advisory group of external experts. The findings of this group are now being published in a collection of research papers in PLOS Medicine and PLOS ONE [5]. Here, we provide a summary of their findings, including estimates of the global and regional FBD burden obtained by the FERG; further details about the specific hazard groups, attribution methods, computational methods and country studies can be found in the accompanying collection of papers. A WHO Technical Report [6] also provides a comprehensive overview of methods, results, a description of the process and activities relating to capacity building in WHO member states.
The FERG Task Forces
Several reviews have already described the background to the FERG and the approach it is taking [7–9]. In brief, the FERG consists of a Core (or Steering) Group to coordinate and oversee the scientific work, six 'task forces' advancing the work in specific areas, and external resource and technical advisers who are invited on an ad hoc basis to provide specific expertise. Three of the Task Forces are hazard-based (the Enteric Diseases Task Force, Parasitic Diseases Task Force, and Chemicals and Toxins Task Force), see Box 1. For each hazard that also has non-foodborne transmission pathway(s), a Source Attribution Task Force is specifically concerned with determining the proportion of the disease burden that is attributable to the consumption of contaminated food, see Box 2. The Country Studies Task Force fosters national FBD burden studies in selected countries (Albania, Japan, Thailand and Uganda) by piloting a specific protocol that was developed partly by adapting the National Burden of Disease Studies protocol published by the WHO [10]. The knowledge translation subgroup of this task force also provides tools to translate data on burden of disease into food safety policy. Finally, the Computational Task Force converts estimates from the hazard-based task forces into estimates of the global and regional burden of FBD, expressed in Disability Adjusted Life Years (DALYs), see Box 3.
Box 1. Choice of Foodborne Hazards
After reviewing the epidemiology of all the disease-causing agents potentially transmitted in food, the FERG hazard-based task forces identified 31 hazards (Table 1) to be included in the study, based on high incidence and/or mortality, and data availability. These included viruses, bacteria and protozoa causing predominantly acute diarrheal diseases (11 hazards); bacteria and protozoa causing invasive infectious diseases (seven hazards) and helminths (three cestodes, two nematodes and five trematodes including the broad group of ‘intestinal flukes’); and diseases induced by 3 chemical hazards. Analyses of the burden of arsenic, cadmium, lead and methylmercury in foods are ongoing and will be reported separately. Estimates of mortality for Mycobacterium bovis infections and morbidity and mortality for invasive non-typhoidal Salmonella enterica infections excluded illnesses attributed to HIV infection [11]. Incidence data for diseases caused by peanut allergens and four bacterial toxins (Bacillus cereus, Clostridium botulinum, Clostridium perfringens and Staphylococcus aureus) were not available for low-income countries and so were excluded from this global overview. Hazards occurring in particular regions, such as cyanide from cassava-based foods and foodborne trematodes, were included in the global overview.
Box 2. Attributing Illnesses to Food
The Codex Alimentarius Commission (CAC) [12] defines food as: any substance, whether processed, semi-processed or raw, which is intended for human consumption, and includes drinks, chewing gum and any substance that has been used in the manufacture, preparation or treatment of food, but does not include cosmetics or tobacco or substances used only as drugs. The definition includes all bottled drinks. Food can be contaminated at many steps along the chain from farm/factory to plate so a precise definition of the point of attribution is crucial. Food can be contaminated at many steps along the chain from farm/factory to plate and in accordance with the CAC definition, FERG estimated the proportion of cases that are foodborne as those where food is contaminated just prior to consumption. Twelve of the 31 hazards were judged by the FERG hazard-based task forces to be 100% foodborne. To estimate the FBD burden for the remaining 19 hazards, a structured expert elicitation using Cooke’s Classical Method [13] was undertaken to distinguish the foodborne transmission pathway from environmental, human-to-human, and animal-to-human transmission pathways collectively. The study provided hazard-specific attribution estimates for each of the 14 subregions (see Table 2 for the countries in each subregion). Details on the FERG expert elicitation can be found elsewhere [14].
Box 3. Measures of Disease Burden
The task forces estimated the incidence and duration of diseases caused by each hazard as well as their associated mortality in 2010 based on systematic reviews, complemented with other literature sources, surveillance data and expert inputs. For each hazard, the impact of all disease outcomes attributed to the primary exposure to a hazard is represented by a disease model. Two general approaches to estimate disease incidence were applied, guided by availability of data. The first approach aimed to identify all disease outcomes associated with a hazard and then establish probabilities for each outcome to occur. Any conditional dependencies between outcomes (based on the natural progression of the disease) were accounted for in this step. The second approach aimed to obtain data on the incidence of a specific outcome, and then to attribute a proportion of that incidence to a hazard. This hazard- and incidence-based approach is also used by the European Centre for Disease Prevention and Control to estimate the burden of a wide range of infectious diseases [16] and estimates the current and projected future burden of disease due to exposures occurring in 2010 or earlier. The chosen approach is well suited to illustrate the diversity of health impacts of FBD hazards, and highly relevant for identification of priorities for prevention, as reducing current exposure will also lead to prevention of future sequelae.
When incidence data were not available for all countries in a subregion, incidence rates were extrapolated from available data using a Bayesian log-normal random effects model, specifying the subregion as random effect. The predictive value of this model was explored and compared with other possible imputation models by McDonald et al. [17]. Detailed descriptions of methods to estimate incidence and mortality of all 31 hazards can be found in papers in the collection on enteric diseases [11], parasitic diseases [18], and chemicals and toxins [19].
The FERG studies used the DALY as a measure of population health to assess and compare the relative impact of different diseases and injuries on populations. The DALY measure combines the years of life lost (YLL) due to premature death and the years lived with disability (YLD) from a disease or condition, for varying degrees of severity, making time the common metric for death and disability [20]. One DALY is equivalent to one year of healthy life lost. As proposed by the Institute for Health Metrics and Evaluation in the Global Burden of Disease (GBD) 2010 study [21], and adopted by the WHO [22], we applied neither age-weighting nor time discounting. We used WHO life tables for YLL calculation. For YLD calculations, we used disability weights from the GBD 2010 study [23] with modifications as proposed by the WHO [22], and the GBD 2013 study [24]. DALY estimates were made at country level (194 countries) by 5-year age groups and gender for the base year 2010. Because of the level of uncertainty associated with estimates for each subpopulation, results are presented at subregional level (Table 2) for both sexes combined and for two age groups (<5 and ≥ 5 years of age). Both the population burden and the individual burden are presented. Statistical uncertainties in incidence and burden estimates were propagated by Monte Carlo simulation methods in the statistical programming environment R [25]. Full details of computational methods can be found in Devleesschauwer et al. [26]
Table 1. Hazards and outcomes included in estimates of the global burden of foodborne disease.
| Hazards | Outcomes |
|---|---|
| Diarrheal disease agents | |
| Viruses | |
| Norovirus | diarrheal disease |
| Bacteria | |
| Campylobacter spp. | diarrheal disease, Guillain-Barré syndrome |
| Enteropathogenic Escherichia coli | diarrheal disease |
| Enterotoxigenic E. coli | diarrheal disease |
| Shiga toxin-producing E. coli | diarrheal disease, hemolytic uremic syndrome, end-stage renal disease |
| Non-typhoidal Salmonella enterica | diarrheal disease, invasive salmonellosis |
| Shigella spp. | diarrheal disease |
| Vibrio cholerae | diarrheal disease |
| Protozoa | |
| Cryptosporidium spp. | diarrheal disease |
| Entamoeba histolytica | diarrheal disease |
| Giardia spp. | diarrheal disease |
| Invasive infectious disease agents | |
| Viruses | |
| Hepatitis A virus | hepatitis |
| Bacteria | |
| Brucella spp. | acute brucellosis, chronic brucellosis, orchitis |
| Listeria monocytogenes | perinatal: sepsis, CNS 1 infection, neurological sequelae |
| acquired: sepsis, CNS infection, neurological sequelae | |
| Mycobacterium bovis | tuberculosis |
| Salmonella Paratyphi A | paratyphoid fever, liver abcesses and cysts |
| Salmonella Typhi | typhoid fever, liver abcesses and cysts |
| Protozoa | |
| Toxoplasma gondii | congenital: intracranial calcification, hydrocephalus, CNS abnormalities, chorioretinitis early in life, chorioretinitis later in life |
| acquired: chorioretinitis, acute illness, post-acute illness | |
| Helminths | |
| Cestodes | |
| Echinococcus granulosus | pulmonary, hepatic, CNS cystic echinococcosis |
| Echinococcus multilocularis | abdominopelvic problems due to alveolar echinococcosis |
| Taenia solium | epilepsy |
| Nematodes | |
| Ascaris spp. | ascariasis, ascariasis-related mild abdominopelvic problems, ascariasis-related severe wasting |
| Trichinella spp. | acute clinical trichinellosis |
| Trematodes | |
| Clonorchis sinensis | abdominopelvic problems due to heavy clonorchiasis |
| Fasciola spp. | abdominopelvic problems due to heavy fascioliasis |
| Intestinal flukes 2 | abdominopelvic problems due to heavy intestinal fluke infection |
| Opisthorchis spp. | abdominopelvic problems due to heavy opistorchiasis |
| Paragonimus spp. | pulmonary problems due to heavy paragonimiasis, cerebral paragonimiasis |
| Chemicals and toxins | |
| Aflatoxin | hepatocellular carcinoma |
| Cassava cyanide | konzo |
| Dioxins | infertility, hypothyroidy due to prenatal and postnatal exposure |
1 CNS: central nervous system
2 Includes selected species of the families Echinostomatidae, Fasciolidae, Gymnophallidae, Heterophyidae, Nanophyetidae, Neodiplostomidae and Plagiorchiidae (depending on data availability)
Global Disease Burden
Of the approximately 600 million cases of illness caused by the 31 foodborne hazards in 2010 (see Box 1 and Table 3), infectious agents that cause diarrheal diseases accounted for the vast majority (550 million), in particular norovirus (120 million cases) and Campylobacter spp. (96 million cases). Among other hazards, hepatitis A virus, the helminth Ascaris spp. and the typhoid bacterium Salmonella Typhi were frequent causes of foodborne illness, causing 14, 12 and 7.6 million cases, respectively.
Table 3. Median global number of foodborne illnesses, deaths, Years Lived with Disability (YLDs), Years of Life Lost (YLLs) and Disability Adjusted Life Years (DALYs), with 95% uncertainty intervals, 2010.
| HAZARD | FOODBORNE ILLNESSES | FOODBORNE DEATHS | FOODBORNE YLDs | FOODBORNE YLLs | FOODBORNE DALYs |
|---|---|---|---|---|---|
| TOTAL | 600,652,361 (417,646,804–962,834,044) | 418,608 (305,128–598,419) | 5,580,028 (4,780,374–8,195,314) | 27,201,701 (19,655,451–38,922,210) | 32,841,428 (24,809,085–46,274,735) |
| Diarrheal disease agents | 548,595,679 (369,976,912–888,528,014) | 230,111 (160,039–322,359) | 839,463 (644,924–1,123,907) | 16,821,418 (11,700,916–23,579,652) | 17,659,226 (12,458,675–24,516,338) |
| Viruses | 124,803,946 (70,311,254–251,352,877) | 34,929 (15,916–79,620) | 91,357 (51,047–174,130) | 2,403,107 (1,102,397–5,387,672) | 2,496,078 (1,175,658–5,511,092) |
| Norovirus | 124,803,946 (70,311,254–251,352,877) | 34,929 (15,916–79,620) | 91,357 (51,047–174,130) | 2,403,107 (1,102,397–5,387,672) | 2,496,078 (1,175,658–5,511,092) |
| Bacteria | 349,405,380 (223,127,469–590,002,559) | 187,285 (131,742–254,037) | 685,212 (521,848–921,335) | 13,795,606 (9,688,221–18,893,580) | 14,490,808 (10,303,551–19,681,271) |
| Campylobacter spp. | 95,613,970 (51,731,379–177,239,714) | 21,374 (14,604–32,584) | 442,075 (322,192–587,072) | 1,689,291 (1,141,055–2,652,483) | 2,141,926 (1,535,985–3,137,980) |
| Enteropathogenic E. coli | 23,797,284 (10,750,919–62,931,604) | 37,077 (19,957–61,262) | 22,977 (9,662–66,211) | 2,908,551 (1,574,520–4,833,325) | 2,938,407 (1,587,757–4,865,590) |
| Enterotoxigenic E. coli | 86,502,735 (49,136,952–151,776,173) | 26,170 (14,887–43,523) | 70,567 (40,134–119,017) | 2,011,635 (1,132,331–3,407,273) | 2,084,229 (1,190,704–3,494,201) |
| Shiga toxin-producing E. coli | 1,176,854 (754,108–2,523,007) | 128 (55–374) | 3,486 (1,741–6,996) | 9,454 (4,140–27,208) | 12,953 (5,951–33,664) |
| Non-typhoidal S. enterica 1 | 78,707,591 (31,843,647–211,154,682) | 59,153 (36,341–89,045) | 78,306 (35,961–185,179) | 3,976,386 (2,410,953–6,180,921) | 4,067,929 (2,486,092–6,271,290) |
| Shigella spp. | 51,014,050 (20,405,214–118,927,631) | 15,156 (6,839–30,072) | 51,613 (21,184–114,267) | 1,181,231 (519,372–2,445,834) | 1,237,103 (554,204–2,520,126) |
| Vibrio cholerae | 763,451 (310,910–1,567,682) | 24,649 (10,304–50,042) | 2,721 (1,019–6,020) | 1,719,381 (718,642–3,487,195) | 1,722,312 (720,029–3,491,997) |
| Protozoa | 67,182,645 (35,794,977–120,556,797) | 5,558 (2,593–11,958) | 57,536 (30,526–102,608) | 432,316 (195,372–960,910) | 492,354 (239,400–1,034,790) |
| Cryptosporidium spp. | 8,584,805 (3,897,252–18,531,196) | 3,759 (1,520–9,115) | 8,155 (3,598–17,355) | 287,690 (114,012–711,990) | 296,156 (119,456–724,660) |
| Entamoeba histolytica | 28,023,571 (10,261,254–68,567,590) | 1,470 (453–5,554) | 20,851 (7,431–53,080) | 115,740 (32,070–476,144) | 138,863 (47,339–503,775) |
| Giardia spp. | 28,236,123 (12,945,655–56,996,454) | 0 (0–0) | 26,270 (11,462–53,577) | 0 (0–0) | 26,270 (11,462–53,577) |
| Invasive infectious disease agents | 35,770,163 (18,604,754–70,045,873) | 117,223 (54,789–243,482) | 1,098,675 (729,530–1,796,607) | 6,960,656 (3,128,316–14,882,637) | 8,065,581 (3,983,949–16,557,714) |
| Viruses | 13,709,836 (3,630,847–38,524,946) | 27,731 (7,169–77,320) | 85,885 (22,118–250,641) | 1,258,812 (325,409–3,509,844) | 1,353,767 (383,684–3,672,726) |
| Hepatitis A virus | 13,709,836 (3,630,847–38,524,946) | 27,731 (7,169–77,320) | 85,885 (22,118–250,641) | 1,258,812 (325,409–3,509,844) | 1,353,767 (383,684–3,672,726) |
| Bacteria | 10,342,042 (3,506,116–27,627,480) | 85,269 (37,573–196,544) | 225,792 (108,092–604,162) | 5,472,374 (2,283,968–12,803,285) | 5,697,913 (2,394,245–13,384,811) |
| Brucella spp. | 393,239 (143,815–9,099,394) | 1,957 (661–45,545) | 13,324 (4,095–315,952) | 110,971 (37,470–2,583,081) | 124,884 (43,153–2,910,416) |
| Listeria monocytogenes | 14,169 (6,112–91,175) | 3,175 (1,339–20,428) | 2,255 (843–14,981) | 116,109 (48,693–740,357) | 118,340 (49,634–754,680) |
| Mycobacterium bovis | 121,268 (99,852–150,239) | 10,545 (7,894–14,472) | 50,733 (38,441–68,052) | 556,998 (417,711–761,851) | 607,775 (458,364–826,115) |
| Salmonella Paratyphi A | 1,741,120 (536,650–4,310,983) | 12,069 (3,784–29,521) | 26,987 (7,610–72,811) | 829,136 (259,990–2,028,112) | 855,730 (268,879–2,100,120) |
| Salmonella Typhi | 7,570,087 (2,333,263–18,743,406) | 52,472 (16,454–128,350) | 117,334 (33,086–316,571) | 3,604,940 (1,130,390–8,817,876) | 3,720,565 (1,169,040–9,130,956) |
| Protozoa | 10,280,089 (7,403,516–14,904,324) | 684 (333–1,300) | 763,326 (511,314–1,175,619) | 62,899 (30,575–119,512) | 829,071 (561,297–1,264,567) |
| Toxoplasma gondii | 10,280,089 (7,403,516–14,904,324) | 684 (333–1,300) | 763,326 (511,314–1,175,619) | 62,899 (30,575–119,512) | 829,071 (561,297–1,264,567) |
| Helminths | 12,928,944 (8,957,617–24,008,256) | 45,226 (34,143–59,035) | 3,367,987 (2,840,638–4,358,741) | 2,428,929 (1,869,610–3,173,545) | 5,810,589 (4,864,518–7,367,619) |
| Cestodes | 430,864 (334,389–774,703) | 36,500 (25,652–50,063) | 1,220,578 (941,084–1,576,600) | 1,932,154 (1,387,290–2,664,120) | 3,158,826 (2,411,585–4,122,032) |
| Echinococcus granulosus | 43,076 (25,881–371,177) | 482 (150–3,974) | 12,121 (5,515–99,213) | 27,626 (8,577–227,715) | 39,950 (16,996–322,953) |
| Echinococcus multilocularis | 8,375 (656–17,005) | 7,771 (243–15,896) | 8,749 (856–22,576) | 303,039 (8,102–622,954) | 312,461 (9,083–640,716) |
| Taenia solium | 370,710 (282,937–478,123) | 28,114 (21,059–36,915) | 1,192,236 (916,049–1,522,267) | 1,586,288 (1,170,461–2,177,848) | 2,788,426 (2,137,613–3,606,582) |
| Nematodes | 12,285,286 (8,292,732–22,984,630) | 1,012 (388–2,783) | 518,451 (351,732–1,211,907) | 80,021 (30,652–220,274) | 605,738 (411,113–1,301,619) |
| Ascaris spp. | 12,280,767 (8,287,414–22,980,491) | 1,008 (384–2,781) | 518,096 (351,418–1,211,691) | 79,800 (30,426–220,154) | 605,278 (410,668–1,301,114) |
| Trichinella spp. | 4,472 (2,977–5,997) | 4 (2–5) | 342 (149–646) | 210 (116–306) | 550 (285–934) |
| Trematodes | 218,569 (167,886–281,872) | 7,533 (6,383–8,845) | 1,616,785 (1,257,657–2,062,782) | 403,884 (342,815–473,423) | 2,024,592 (1,652,243–2,483,514) |
| Clonorchis sinensis | 31,620 (21,515–45,059) | 5,770 (4,728–6,988) | 219,637 (149,514–312,718) | 302,160 (247,586–366,036) | 522,863 (431,520–635,232) |
| Fasciola spp. | 10,635 (6,888–24,100) | 0 (0–0) | 90,041 (58,050–209,097) | 0 (0–0) | 90,041 (58,050–209,097) |
| Intestinal flukes 2 | 18,924 (14,498–24,200) | 0 (0–0) | 155,165 (118,920–198,147) | 0 (0–0) | 155,165 (118,920–198,147) |
| Opisthorchis spp. | 16,315 (11,273–22,860) | 1,498 (1,230–1,813) | 102,705 (70,849–143,938) | 85,364 (70,123–103,317) | 188,346 (151,906–235,431) |
| Paragonimus spp. | 139,238 (95,610–195,078) | 250 (160–371) | 1,033,097 (730,118–1,423,031) | 15,535 (9,971–23,035) | 1,048,937 (743,700–1,438,588) |
| Chemicals and toxins | 217,632 (172,024–1,140,463) | 19,712 (8,171–51,664) | 247,920 (196,490–1,410,260) | 650,157 (283,769–1,617,168) | 908,356 (506,112–2,714,588) |
| Aflatoxin | 21,757 (8,967–56,776) | 19,455 (7,954–51,324) | 3,945 (1,551–10,667) | 632,901 (265,578–1,606,493) | 636,869 (267,142–1,617,081) |
| Cassava cyanide | 1,066 (105–3,016) | 227 (22–669) | 2,521 (249–7,142) | 15,694 (1,514–46,304) | 18,203 (1,769–53,170) |
| Dioxins | 193,447 (155,963–1,085,675) | 0 (0–0) | 240,056 (192,608–1,399,562) | 0 (0–0) | 240,056 (192,608–1,399,562) |
1 Diarrheal and invasive disease
2 Includes selected species of the families Echinostomatidae, Fasciolidae, Gymnophallidae, Heterophyidae, Nanophyetidae, Neodiplostomidae and Plagiorchiidae (depending on data availability).
Foodborne diarrheal disease agents also caused 230,000 of the 420,000 deaths due to foodborne hazards (Table 3). Of these, non-typhoidal S. enterica accounted for 59,000, enteropathogenic E. coli (EPEC) for 37,000, norovirus for 35,000, and enterotoxigenic E. coli (ETEC) for 26,000 deaths. Of the 59,000 global deaths due to non-typhoidal S. enterica, 32,000 were in the two African subregions, and included 22,000 deaths due to invasive disease by this bacterium. The major non-diarrheal causes of foodborne deaths were due to Salmonella Typhi (52,000), the helminth Taenia solium (28,000) hepatitis A virus (28,000) and aflatoxin with 20,000 (95% UI 8,000–51,000) deaths.
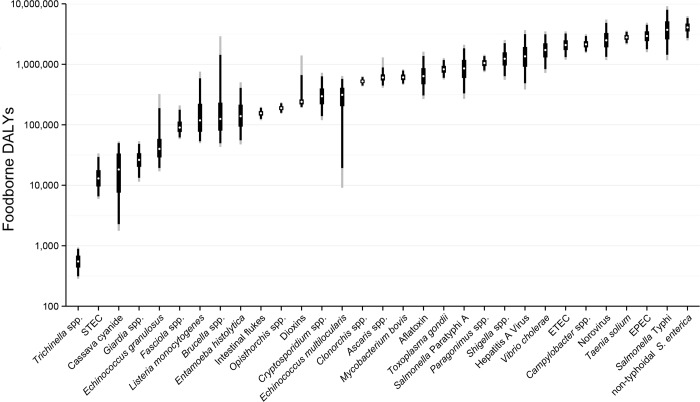
The global burden of FBD caused by the 31 hazards (including sequelae) in 2010 was 33 million DALYs (Table 3). Eighteen million DALYs, or 54%, of the total burden was attributed to diarrheal disease agents, particularly to non-typhoidal S. enterica, which was responsible for 4.0 million DALYs (Fig 1). Six diarrheal disease agents (norovirus, Campylobacter spp., EPEC, ETEC, Vibrio cholerae and Shigella spp.) each caused a foodborne burden of 1–3 million DALYs. Other foodborne hazards that contributed substantially to the global burden included Salmonella Typhi (3.7 million DALYs), T. solium (2.8 million DALYs), hepatitis A virus (1.4 million DALYs) and Paragonimus spp. (1.0 million DALYs). By contrast, the global burden of trichinellosis was estimated at only 550 DALYs. For full details of the numbers of cases of foodborne illness, deaths, DALYs, YLLs and YLDs for all 31 hazards in this study, see S1 Table, tabs 1–13. The Supporting Information also includes the data for total illnesses, deaths, DALYs, YLLs and YLDs by all exposure pathways for all hazards that were included in the source attribution expert elicitation (see S1 Table, tabs 14–18).
Fig 1. Ranking of foodborne hazards globally for 2010, expressed as Disability Adjusted Life Years.
White dots indicate the median burden, black boxes the inter-quartile range (50% UI), black lines the 5 and 95 percentiles (90%UI) and grey lines the 2.5 and 97.5 percentiles (95% UI). Note the y-axis is on a logarithmic scale. Abbreviations: EPEC: Enteropathogenic Escherichia coli; ETEC: Enterotoxigenic E. coli; STEC: Shiga toxin-producing E. coli.
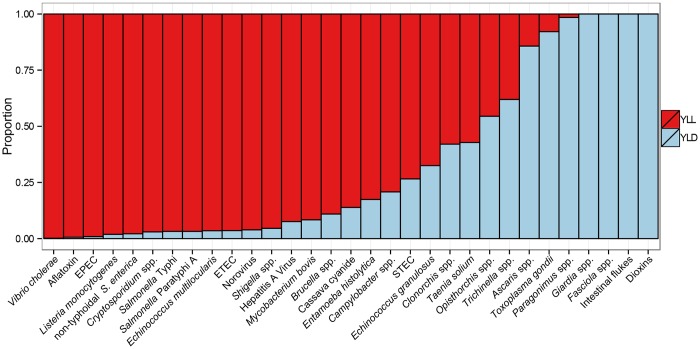
The relative contribution of mortality (measured as YLL) and morbidity (measured as YLD) to the total burden of disease varied widely between hazards (Fig 2). For 18 foodborne hazards, more than 75% of the total burden was due to premature mortality (red columns in Fig 2). These mainly include hazards leading to diseases with known high case-fatality ratios (non-typhoidal S. enterica, EPEC, ETEC, Shigella spp. and V. cholerae, Listeria monocytogenes, Salmonella Typhi and Salmonella Paratyphi, Echinococcus multilocularis and aflatoxin). At the other extreme, more than 75% of the total burden due to morbidity (blue columns in Fig 2) were accounted for by seven foodborne hazards, of which four (Giardia spp., Fasciola spp., intestinal flukes, and dioxins) were not assumed to cause fatal illnesses.
Fig 2. Relative contribution of years of life lost (YLL) due to premature mortality and years lived with disability (YLD) to the global burden of 31 hazards in food for 2010.
For abbreviations, see Fig 1.
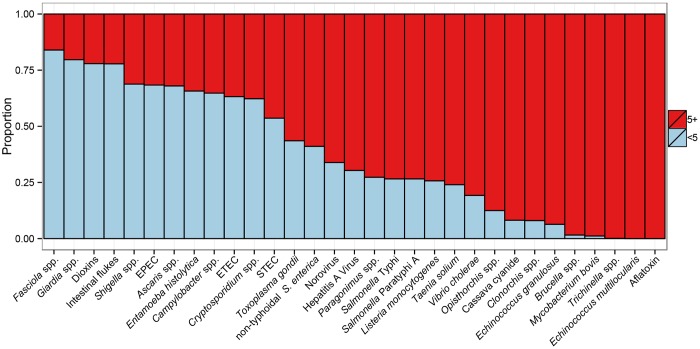
The FERG studies show that children under five years old bear 40% of the foodborne disease burden (including, for some hazards, the life-long burden of sequelae). More than 75% of the burden of four hazards (Fasciola spp., Giardia spp., dioxins, and intestinal flukes) occurred among children under five (Fig 3). Prenatal infections accounted for 21% of the burden of L. monocytogenes [27] and for 32% of the burden of Toxoplasma gondii [18]. By contrast, more than 75% of the burden of 11 hazards occurred among people over five years old.
Fig 3. Age-distribution of Disability Adjusted Life Years for 31 hazards contributing to the global burden of foodborne disease for 2010.
For abbreviations, see Fig 1.
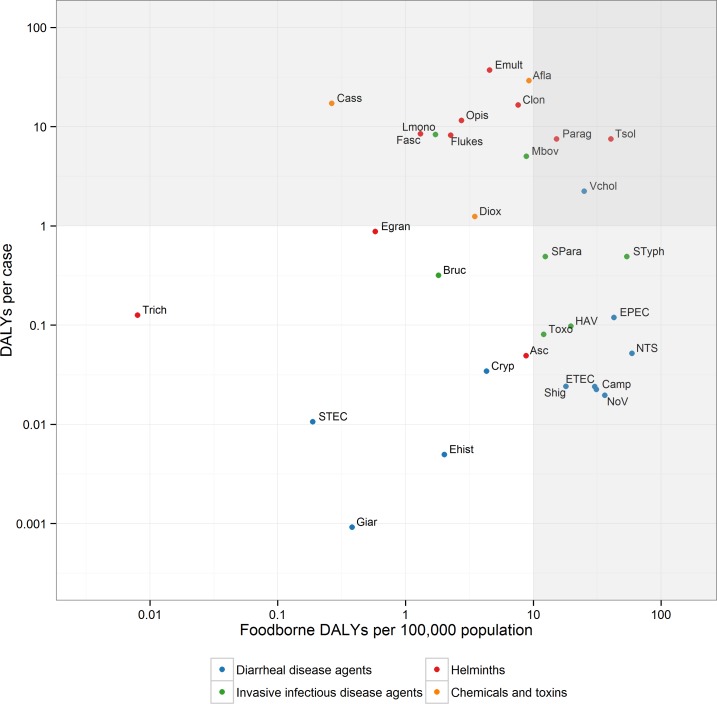
Fig 4 presents a scatterplot of the burden at individual level (DALYs per case, a measure for disease severity) and the burden at population level (foodborne DALYs per 100,000 population, also accounting for disease incidence). On the basis of this plot, hazards were divided by two criteria with arbitrary cut-offs as indicated by grey-shaded areas in the figure. V. cholerae, T. solium and Paragonimus spp. were in the high/high category. All other diarrheal disease agents were in the high/low category, except STEC, E. histolytica and Giardia spp. (low/low). The L/L category further included Trichinella spp. The low/high category contained agents that are of relatively low global impact but have a high impact on affected individuals. These included different parasites, particularly E. multilocularis, the invasive bacteria Brucella spp., L. monocytogenes and M. bovis. In subregions where the burden is higher than the global average, these agents are of specific relevance to policy makers.
Fig 4. Scatterplot of the global burden of foodborne disease per 100,000 population and per case.
The grey-shaded areas indicate arbitrary cut-offs between high (H) or low (L) population burden (> or ≤ 10 DALYs per 100,000 population) and high or low individual burden (> or ≤ 1 DALY per case). Abbreviations: NoV: Norovirus; Camp: Campylobacter spp.; EPEC: Enteropathogenic Escherichia coli; ETEC: Enterotoxigenic E. coli; STEC: Shiga toxin-producing E. coli; NTS: non-typhoidal Salmonella enterica; Shig: Shigella spp.; Vchol; Vibrio cholerae Ehist: Entamoeba histolytica; Cryp: Cryptosporidium spp.; Giar: Giardia spp.; HAV: Hepatitis A virus; Bruc: Brucella spp.; Lmono: Listeria monocytogenes; Mbov: Mycobacterium bovis; SPara: Salmonella Paratyphi A; STyph: Salmonella Typhi; Toxo: Toxoplasma gondii; Egran: Echinococcus granulosus; Emult: E. multilocularis; Tsol: Taenia solium; Asc: Ascaris spp.; Trich: Trichinella spp.; Clon: Clonorchis sinensis; Fasc: Fasciola spp.; Flukes: Intestinal flukes; Opis: Opisthorchis spp.; Parag: Paragonimus spp.; Diox: Dioxins; Afla: Aflatoxin.
Regional Differences
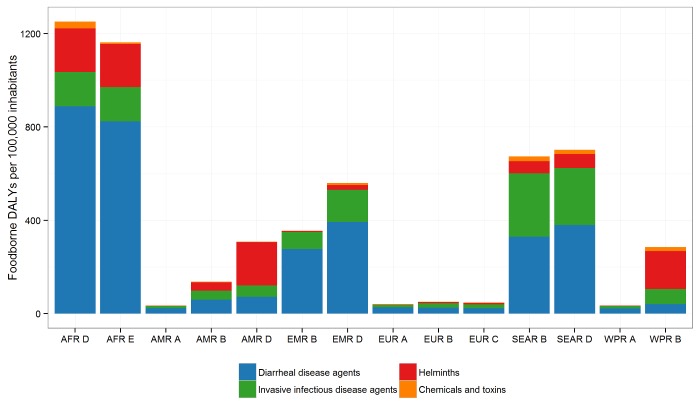
The studies found considerable regional differences in the burden of FBD (Table 4 and Fig 5). The highest burden per 100,000 population was observed in the two African subregions: 1,300 DALYs per 100,000 population in AFR D and 1,200 DALYs per 100,000 population in AFR E. In the South-East Asian subregions, SEAR B and SEAR D, the burden was 690 and 710 DALYs per 100,000 population, respectively, and in the Eastern Mediterranean subregion, EMR D, 570 DALYs per 100,000 population. The lowest burden was observed in the North American subregion AMR A (35 DALYs per 100,000 population), followed by the three European subregions EUR A, EUR B and EUR C, and the Western Pacific subregion WPR A, which were all in the range of 40–50 DALYs per 100,000 population. Other subregions (AMR B and AMR D, EMR B and WPR B) had intermediate burdens, all in the range of 140–360 DALYs per 100,000 population (see Table 2 for a full list of the countries in each subregion).
Table 4. Median rates of foodborne Disability Adjusted Life Years (DALYs) per 100,000 population, by subregion, with 95% uncertainty intervals, 2010.
| HAZARD | AFR D | AFR E | AMR A | AMR B | AMR D | EMR B | EMR D | EUR A | EUR B | EUR C | SEAR B | SEAR D | WPR A | WPR B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOTAL | 1,276 (459–2,263) | 1,179 (726–1,764) | 35 (23–49) | 140 (97–274) | 315 (243–575) | 362 (205–582) | 571 (325–954) | 41 (29–64) | 52 (33–136) | 49 (33–77) | 685 (360–1,291) | 711 (343–1,335) | 36 (23–170) | 293 (219–406) |
| Diarrheal disease agents | 889 (196–1,731) | 824 (447–1,326) | 23 (13–33) | 60 (36–94) | 72 (40–117) | 277 (153–460) | 393 (217–644) | 28 (17–39) | 25 (14–37) | 24 (13–35) | 330 (154–576) | 380 (159–717) | 23 (14–32) | 41 (21–65) |
| Viruses | 75 (6–222) | 76 (0–225) | 3 (0.6–8) | 12 (0–38) | 13 (0–43) | 28 (0.5–79) | 33 (0.4–90) | 4 (0–11) | 3 (0.08–9) | 3 (0.2–9) | 55 (0–224) | 69 (0.8–263) | 3 (0.09–7) | 4 (0–19) |
| Norovirus | 75 (6–222) | 76 (0–225) | 3 (0.6–8) | 12 (0–38) | 13 (0–43) | 28 (0.5–79) | 33 (0.4–90) | 4 (0–11) | 3 (0.08–9) | 3 (0.2–9) | 55 (0–224) | 69 (0.8–263) | 3 (0.09–7) | 4 (0–19) |
| Bacteria | 787 (186–1,482) | 712 (393–1,160) | 19 (10–28) | 45 (26–68) | 54 (28–87) | 237 (124–403) | 347 (190–576) | 24 (14–32) | 21 (11–32) | 20 (10–29) | 247 (107–429) | 285 (119–506) | 19 (11–27) | 34 (17–54) |
| Campylobacter spp. | 71 (35–119) | 70 (33–117) | 9 (5–14) | 15 (8–23) | 15 (8–26) | 75 (40–109) | 97 (54–143) | 10 (6–14) | 8 (4–13) | 8 (4–12) | 37 (14–87) | 33 (2–84) | 9 (5–14) | 10 (4–17) |
| Enteropathogenic E. coli | 136 (11–329) | 138 (6–327) | 0.006 (0.001–0.02) | 7 (0.4–21) | 9 (0.9–26) | 46 (9–114) | 60 (8–151) | 0.006 (0.001–0.02) | 0.004 (0–0.01) | 0.004 (0–0.01) | 64 (3–144) | 65 (1–162) | 0.006 (0.001–0.02) | 5 (0.3–13) |
| Enterotoxigenic E. coli | 107 (26–245) | 105 (17–240) | 0.003 (0–0.009) | 7 (1–19) | 9 (2–25) | 29 (6–78) | 37 (5–102) | 0.004 (0–0.01) | 0.004 (0–0.01) | 0.004 (0–0.01) | 42 (3–106) | 42 (2–111) | 0.003 (0–0.01) | 4 (0.3–11) |
| Shiga toxin-producing E. coli | 0.009 (0.002–0.03) | 0.08 (0.02–0.2) | 0.2 (0.05–0.3) | 0.4 (0.09–1) | 0.5 (0.2–1) | 0.2 (0.09–0.4) | 0.2 (0.1–0.5) | 0.6 (0.2–1) | 0.07 (0.02–0.2) | 0.1 (0.03–0.4) | 0.2 (0.02–1) | 0.2 (0.007–1) | 0.4 (0.1–1) | 0.01 (0.002–0.04) |
| Non-typhoidal S. enterica 1 | 338 (94–612) | 193 (44–336) | 9 (4–16) | 11 (2–20) | 14 (4–26) | 50 (17–82) | 67 (26–112) | 12 (7–18) | 12 (6–21) | 11 (5–19) | 59 (22–154) | 58 (0–162) | 9 (5–14) | 9 (4–16) |
| Shigella spp. | 37 (0–156) | 37 (0–148) | 0.2 (0–1) | 2 (0–8) | 2 (0–9) | 27 (0–109) | 37 (0–145) | 0.09 (0–0.9) | 0.1 (0–1) | 0.2 (0–1) | 25 (0.6–84) | 25 (0.7–90) | 0.2 (0–1) | 4 (0.1–11) |
| Vibrio cholerae | 70 (2–197) | 143 (4–383) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.2 (0.009–0.5) | 28 (0.9–96) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 2 (0.2–3) | 36 (0.2–133) | 0 (0–0) | 0.1 (0.005–0.4) |
| Protozoa | 20 (0–74) | 21 (5–66) | 0.2 (0.01–1) | 2 (0.4–6) | 3 (0.5–8) | 6 (0.9–27) | 7 (1–33) | 0.2 (0–0.8) | 0.2 (0–0.9) | 0.2 (0–0.9) | 10 (2–35) | 10 (2–39) | 0.2 (0–0.9) | 1 (0.2–4) |
| Cryptosporidium spp. | 12 (0–44) | 12 (0–45) | 0.2 (0.01–0.9) | 0.7 (0.08–3) | 1 (0.1–5) | 3 (0–21) | 3 (0–24) | 0.1 (0–0.8) | 0.1 (0–0.8) | 0.1 (0–0.8) | 6 (0–28) | 6 (0–32) | 0.1 (0–0.8) | 0.3 (0–3) |
| Entamoeba histolytica | 5 (0–41) | 5 (0–41) | 0 (0–0) | 0.4 (0–2) | 0.4 (0–3) | 2 (0–12) | 2 (0–17) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 2 (0–17) | 2 (0–18) | 0 (0–0) | 0.3 (0–1) |
| Giardia spp. | 0.7 (0–3) | 0.7 (0–3) | 0.03 (0–0.1) | 0.4 (0–2) | 0.5 (0.01–3) | 0.4 (0–2) | 0.6 (0.005–2) | 0.03 (0–0.1) | 0.03 (0–0.1) | 0.03 (0–0.1) | 0.1 (0–0.9) | 0.1 (0–1) | 0.03 (0–0.1) | 0.3 (0–1) |
| Invasive infectious disease agents | 146 (46–342) | 147 (55–343) | 10 (6–14) | 38 (16–76) | 49 (19–144) | 73 (32–148) | 137 (38–334) | 10 (7–15) | 19 (9–61) | 16 (10–29) | 272 (71–721) | 244 (38–623) | 10 (5–132) | 65 (19–145) |
| Viruses | 27 (4–77) | 18 (3–55) | 0.5 (0.07–2) | 1 (0.1–4) | 2 (0.2–7) | 2 (0.2–5) | 32 (2–102) | 0.8 (0.03–2) | 1 (0.2–3) | 1 (0.3–4) | 5 (0.6–15) | 58 (6–182) | 1 (0.07–3) | 5 (0.3–17) |
| Hepatitis A virus | 27 (4–77) | 18 (3–55) | 0.5 (0.07–2) | 1 (0.1–4) | 2 (0.2–7) | 2 (0.2–5) | 32 (2–102) | 0.8 (0.03–2) | 1 (0.2–3) | 1 (0.3–4) | 5 (0.6–15) | 58 (6–182) | 1 (0.07–3) | 5 (0.3–17) |
| Bacteria | 93 (31–259) | 104 (40–277) | 4 (2–7) | 16 (4–47) | 19 (5–65) | 50 (16–121) | 82 (22–241) | 3 (3–5) | 8 (3–39) | 5 (3–10) | 251 (59–696) | 165 (27–490) | 3 (1–126) | 50 (12–124) |
| Brucella spp. | 2 (0.2–53) | 0.3 (0.007–18) | 0.07 (0.02–0.6) | 1 (0.3–16) | 2 (0.2–38) | 23 (3–83) | 4 (0.6–68) | 0.3 (0.07–1) | 4 (0.7–35) | 0.8 (0.07–6) | 0.8 (0.004–112) | 0.7 (0.003–92) | 0.6 (0.02–125) | 0.6 (0.09–9) |
| Listeria monocytogenes | 1 (0–21) | 1 (0–21) | 3 (2–5) | 2 (0.2–17) | 1 (0–21) | 1 (0–21) | 1 (0–21) | 3 (2–4) | 0.3 (0.2–0.8) | 0.6 (0.3–2) | 1 (0–21) | 1 (0–21) | 1 (0.7–2) | 1 (1–4) |
| Mycobacterium bovis | 25 (15–39) | 34 (21–48) | 0.03 (0.01–0.06) | 0.4 (0.2–0.8) | 2 (0.8–4) | 1 (0.5–3) | 13 (6–25) | 0.08 (0.06–0.1) | 0.6 (0.5–1) | 3 (2–5) | 11 (4–27) | 14 (6–27) | 0.1 (0.08–0.2) | 3 (1–5) |
| Salmonella Paratyphi A | 11 (0–39) | 12 (0–43) | 0.1 (0–0.4) | 2 (0–6) | 2 (0.006–7) | 3 (0–12) | 10 (0–36) | 0.02 (0–0.1) | 0.3 (0–2) | 0.01 (0–0.06) | 42 (7–120) | 26 (0.6–80) | 0.1 (0–0.5) | 8 (1–22) |
| Salmonella Typhi | 47 (0–169) | 52 (0–187) | 0.4 (0–2) | 7 (0–27) | 8 (0.03–29) | 14 (0–51) | 45 (0–158) | 0.09 (0–0.6) | 2 (0–9) | 0.04 (0–0.3) | 184 (32–522) | 113 (3–347) | 0.6 (0–2) | 36 (6–95) |
| Protozoa | 21 (8–41) | 20 (9–37) | 5 (2–8) | 20 (9–33) | 27 (10–84) | 20 (10–35) | 18 (9–31) | 6 (3–9) | 10 (5–23) | 10 (5–18) | 13 (6–22) | 9 (2–19) | 5 (3–8) | 9 (4–14) |
| Toxoplasma gondii | 21 (8–41) | 20 (9–37) | 5 (2–8) | 20 (9–33) | 27 (10–84) | 20 (10–35) | 18 (9–31) | 6 (3–9) | 10 (5–23) | 10 (5–18) | 13 (6–22) | 9 (2–19) | 5 (3–8) | 9 (4–14) |
| Helminths | 186 (125–308) | 184 (141–240) | 1 (0.9–4) | 36 (27–134) | 185 (149–229) | 5 (2–15) | 21 (12–40) | 0.4 (0.2–1) | 6 (3–27) | 6 (4–15) | 52 (42–64) | 60 (45–80) | 2 (1–3) | 162 (131–202) |
| Cestodes | 172 (112–289) | 178 (136–235) | 0.4 (0.3–0.6) | 25 (19–34) | 71 (53–95) | 1 (0.2–10) | 0.7 (0.1–19) | 0.2 (0.05–0.5) | 4 (2–25) | 4 (2–12) | 3 (2–5) | 46 (34–61) | 0.03 (0.007–0.8) | 45 (25–65) |
| Echinococcus granulosus | 0.4 (0.06–21) | 0.8 (0.2–16) | 0.01 (0.002–0.03) | 0.3 (0.02–5) | 2 (0.4–8) | 0.9 (0.2–10) | 0.6 (0.1–19) | 0.1 (0.02–0.4) | 2 (0.5–6) | 0.5 (0.09–1) | 0.001 (0–0.1) | 0.8 (0.2–3) | 0.02 (0.001–0.8) | 0.3 (0.08–0.9) |
| Echinococcus multilocularis | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.03 (0.005–0.06) | 0.005 (0–0.05) | 0.03 (0.008–0.06) | 2 (0.5–21) | 2 (0.5–11) | 0 (0–0) | 0.007 (0–0.04) | 0.008 (0.001–0.02) | 18 (0–37) |
| Taenia solium | 170 (110–283) | 176 (134–229) | 0.4 (0.3–0.6) | 25 (19–32) | 69 (51–91) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.9 (0.6–2) | 3 (2–5) | 45 (33–60) | 0 (0–0) | 27 (20–35) |
| Nematodes | 13 (2–28) | 5 (1–11) | 0.6 (0.3–0.9) | 11 (3–106) | 12 (3–24) | 3 (1–7) | 13 (4–20) | 0.04 (0.02–0.07) | 1 (0.3–2) | 1 (0.4–2) | 8 (2–15) | 13 (4–26) | 0.004 (0.001–0.007) | 11 (3–22) |
| Ascaris spp. | 13 (2–28) | 5 (1–11) | 0.6 (0.3–0.9) | 11 (3–106) | 12 (3–24) | 3 (1–7) | 13 (4–20) | 0 (0–0) | 1 (0.3–2) | 1 (0.3–2) | 8 (2–15) | 13 (4–26) | 0 (0–0) | 11 (3–22) |
| Trichinella spp. | 0.001 (0–0.002) | 0.001 (0–0.002) | 0.009 (0.005–0.01) | 0.009 (0.005–0.01) | 0.009 (0.005–0.01) | 0 (0–0) | 0 (0–0) | 0.04 (0.02–0.07) | 0.04 (0.02–0.07) | 0.04 (0.02–0.07) | 0 (0–0.001) | 0 (0–0.001) | 0.004 (0.001–0.007) | 0.004 (0.001–0.007) |
| Trematodes | 0.06 (0.02–0.2) | 0.02 (0.008–0.07) | 0.2 (0.04–3) | 0.1 (0.04–0.5) | 101 (74–135) | 0.3 (0.2–0.5) | 7 (4–10) | 0.2 (0.05–0.6) | 0.2 (0.05–0.6) | 1 (0.8–1) | 40 (32–50) | 0.7 (0.2–2) | 2 (1–2) | 106 (85–131) |
| Clonorchis sinensis | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.04 (0.03–0.04) | 0.01 (0.003–0.04) | 0.04 (0.01–0.2) | 0.05 (0.01–0.2) | 31 (26–38) |
| Fasciola spp. | 0.02 (0.008–0.07) | 0.01 (0.005–0.04) | 0.04 (0.001–2) | 0.04 (0.02–0.1) | 46 (27–75) | 0.2 (0.1–0.3) | 7 (4–10) | 0.07 (0.02–0.2) | 0.06 (0.02–0.2) | 0.04 (0.01–0.1) | 0.02 (0.008–0.05) | 0.05 (0.02–0.1) | 0.07 (0.01–0.4) | 0.9 (0.1–8) |
| Intestinal flukes 2 | 0.01 (0.005–0.04) | 0 (0–0) | 0.1 (0.04–0.5) | 0.06 (0.02–0.2) | 0 (0–0) | 0.06 (0.02–0.2) | 0.08 (0.03–0.2) | 0.03 (0.009–0.09) | 0.05 (0.02–0.2) | 0.09 (0.03–0.2) | 0.2 (0.1–0.5) | 0.1 (0.03–0.4) | 1 (0.9–2) | 9 (7–11) |
| Opisthorchis spp. | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.07 (0.02–0.3) | 0.05 (0.01–0.3) | 0.9 (0.6–1) | 40 (32–50) | 0.4 (0.1–1) | 0 (0–0) | 3 (2–4) |
| Paragonimus spp. | 0.03 (0.008–0.08) | 0.008 (0.002–0.02) | 0.04 (0.004–0.6) | 0.04 (0.01–0.1) | 53 (38–73) | 0 (0–0) | 0.02 (0.008–0.07) | 0 (0–0) | 0 (0–0) | 0.03 (0.01–0.1) | 0.05 (0.008–0.5) | 0.06 (0.02–0.2) | 0.05 (0.02–0.2) | 60 (43–83) |
| Chemicals and toxins | 30 (8–85) | 7 (3–21) | 0.4 (0.2–3) | 3 (0.7–16) | 2 (0.09–159) | 0.8 (0.3–14) | 9 (4–66) | 2 (1–22) | 0.9 (0.4–25) | 2 (2–9) | 20 (4–75) | 18 (13–52) | 0.3 (0.06–13) | 18 (3–71) |
| Aflatoxin | 28 (7–78) | 3 (1–8) | 0.04 (0.006–0.2) | 3 (0.6–9) | 2 (0.07–137) | 0.7 (0.2–3) | 5 (1–17) | 0.3 (0.1–0.7) | 0.6 (0.3–1) | 0.5 (0.2–2) | 18 (3–52) | 4 (0.6–15) | 0.2 (0.04–0.8) | 17 (3–69) |
| Cassava cyanide | 1 (0.1–3) | 3 (0.3–9) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Dioxins | 0.2 (0.05–6) | 0.2 (0.09–9) | 0.3 (0.1–3) | 0.1 (0.03–11) | 0.2 (0.01–23) | 0.09 (0.004–11) | 3 (2–56) | 2 (1–22) | 0.3 (0.09–24) | 2 (1–8) | 0.2 (0.005–45) | 14 (12–40) | 0.1 (0.02–12) | 0.06 (0.006–5) |
1 Diarrheal and invasive disease.
2 Includes selected species of the families Echinostomatidae, Fasciolidae, Gymnophallidae, Heterophyidae, Nanophyetidae, Neodiplostomidae and Plagiorchiidae (depending on data availability).
Fig 5. The global burden of foodborne disease (DALYS per 100,000 population) by hazard groups and by subregion for 2010.
See Table 2 for the countries in each subregion.
Table 2. World Health Organization (WHO) Member States by subregion.
| Subregions 1 | WHO member states |
|---|---|
| AFR D | Algeria; Angola; Benin; Burkina Faso; Cameroon; Cape Verde; Chad; Comoros; Equatorial Guinea; Gabon; Gambia; Ghana; Guinea; Guinea-Bissau; Liberia; Madagascar; Mali; Mauritania; Mauritius; Niger; Nigeria; Sao Tome and Principe; Senegal; Seychelles; Sierra Leone; Togo. |
| AFR E | Botswana; Burundi; Central African Republic; Congo; Côte d'Ivoire; Democratic Republic of the Congo; Eritrea; Ethiopia; Kenya; Lesotho; Malawi; Mozambique; Namibia; Rwanda; South Africa; Swaziland; Uganda; United Republic of Tanzania; Zambia; Zimbabwe. |
| AMR A | Canada; Cuba; United States of America. |
| AMR B | Antigua and Barbuda; Argentina; Bahamas; Barbados; Belize; Brazil; Chile; Colombia; Costa Rica; Dominica; Dominican Republic; El Salvador; Grenada; Guyana; Honduras; Jamaica; Mexico; Panama; Paraguay; Saint Kitts and Nevis; Saint Lucia; Saint Vincent and the Grenadines; Suriname; Trinidad and Tobago; Uruguay; Venezuela (Bolivarian Republic of). |
| AMR D | Bolivia (Plurinational State of); Ecuador; Guatemala; Haiti; Nicaragua; Peru. |
| EMR B | Bahrain; Iran (Islamic Republic of); Jordan; Kuwait; Lebanon; Libyan Arab Jamahiriya; Oman; Qatar; Saudi Arabia; Syrian Arab Republic; Tunisia; United Arab Emirates. |
| EMR D | Afghanistan; Djibouti; Egypt; Iraq; Morocco; Pakistan; Somalia; South Sudan 2 ; Sudan; Yemen. |
| EUR A | Andorra; Austria; Belgium; Croatia; Cyprus; Czech Republic; Denmark; Finland; France; Germany; Greece; Iceland; Ireland; Israel; Italy; Luxembourg; Malta; Monaco; Netherlands; Norway; Portugal; San Marino; Slovenia; Spain; Sweden; Switzerland; United Kingdom. |
| EUR B | Albania; Armenia; Azerbaijan; Bosnia and Herzegovina; Bulgaria; Georgia; Kyrgyzstan; Montenegro; Poland; Romania; Serbia; Slovakia; Tajikistan; The Former Yugoslav Republic of Macedonia; Turkey; Turkmenistan; Uzbekistan. |
| EUR C | Belarus; Estonia; Hungary; Kazakhstan; Latvia; Lithuania; Republic of Moldova; Russian Federation; Ukraine. |
| SEAR B | Indonesia; Sri Lanka; Thailand. |
| SEAR D | Bangladesh; Bhutan; Democratic People's Republic of Korea; India; Maldives; Myanmar; Nepal; Timor-Leste. |
| WPR A | Australia; Brunei Darussalam; Japan; New Zealand; Singapore. |
| WPR B | Cambodia; China; Cook Islands; Fiji; Kiribati; Lao People's Democratic Republic; Malaysia; Marshall Islands; Micronesia (Federated States of); Mongolia; Nauru; Niue; Palau; Papua New Guinea; Philippines; Republic of Korea; Samoa; Solomon Islands; Tonga; Tuvalu; Vanuatu; Viet Nam. |
1 The subregions are defined on the basis of child and adult mortality as described by Ezzati et al. [15]. Stratum A: very low child and adult mortality, Stratum B: low child mortality and very low adult mortality, Stratum C: low child mortality and high adult mortality, Stratum D: high child and adult mortality, and Stratum E: high child mortality and very high adult mortality. The use of the term ‘subregion’ here and throughout the text does not identify an official grouping of WHO Member States, and the “subregions” are not related to the six official regions. AFR = African Region; AMR = Region of the Americas; EMR = Eastern Mediterranean Region; EUR = European Region; SEAR = South-East Asia Region; WPR = Western Pacific Region.
2 South Sudan was reassigned to the African Region in May 2013. As this study relates to time periods prior to this date, estimates for South Sudan were included in the Eastern Mediterranean Region.
The contribution of individual hazards to the burden of FBD differed markedly between subregions (Fig 5). In both African subregions, nearly 70% of the burden was due to diarrheal disease agents, particularly to non-typhoidal S. enterica (including invasive salmonellosis), EPEC, and ETEC; additionally, V. cholerae caused an important burden of diarrheal disease in the AFR E subregion, and T. solium caused a high burden in both African subregions (see Table 4 for the detailed data for all hazards and all subregions). In the SEAR D and SEAR B subregions, diarrheal disease agents contributed approximately 50% of the total disease burden, mainly caused by a range of hazards including EPEC, norovirus, non-typhoidal S. enterica, ETEC and Campylobacter spp. In both of these subregions, there was also a considerable burden of Salmonella Typhi (180 DALYs per 100,000 population in SEAR B and 110 DALYs per 100,000 population in SEAR D). The burden of disease due to the fluke Opisthorchis spp. was almost exclusively concentrated in SEAR B (40 DALYs per 100,000 population). In EMR D, diarrheal disease agents were responsible for approximately 70% of the total burden of FBD, with Campylobacter spp. the leading cause in the region, followed by EPEC, non-typhoidal S. enterica, Shigella spp. and ETEC. Other important hazards in this region were Salmonella Typhi, aflatoxin and hepatitis A virus.
In the WPR B subregion, diarrheal disease agents accounted for approximately 14% of the FBD burden, with Campylobacter spp. the leading cause. In this region, the seafoodborne trematodes Paragonimus spp. and Clonorchis sinensis were important contributors to the FBD burden. In the AMR B and AMR D subregions, the contribution of diarrheal disease agents to the total burden was smaller than in other subregions (approximately 40% and 20%, respectively), with Campylobacter spp., norovirus and non-typhoidal S. enterica causing most burden. In the AMR B region, important causes of FBD burden were T. solium (25 DALYs per 100,000 population) and T. gondii (20 DALYs per 100,000 population). In the AMR D region, the burden of T. solium was particularly high at 69 DALYs per 100,000 population; the trematodes Paragonimus spp. and Fasciola spp. contributing 53 and 46 DALYs per 100,000 population, respectively to the overall disease burden.
The burden due to chemical hazards was also highly localized. Aflatoxin caused the highest burden in AFR D, WPR B and SEAR B, whereas dioxins caused the highest burden in SEAR D, EMR D and EUR A and C. The burden of cassava cyanide was limited to the AFR regions, and was similar to that of aflatoxin in AFR D.
In the three European subregions, diarrheal disease agents contributed to 49–68% of the total burden of FBD, with non-typhoidal S. enterica and Campylobacter spp. being the most important hazards. Other important hazards included T. gondii in all European subregions, Brucella spp. in the EUR B and Mycobacterium bovis in the EUR C subregions. In the WPR A region, 65% of the burden was caused by diarrheal disease agents, with T. gondii and hepatitis A virus also contributing. Finally, in the AMR A region, diarrheal disease agents contributed approximately 67% of the total burden, with non-typhoidal S. enterica and Campylobacter spp. the most important hazards; T. gondii and L. monocytogenes were also relatively important.
Previous Estimates
Several high-income countries have published national estimates of FBD. Estimates of food-related illnesses and deaths in the USA were reported in the late 1990s [28] and updated to cover the period 2000–2008 [29,30]. Similar studies are available from the UK [31], Australia [32], France [33] and Canada [34]. Some countries have extended this work to estimate DALYs, including New Zealand [35], Greece [36], the Netherlands [37], and the USA [38]. While the range of hazards covered in these previous studies differed from those of the FERG studies, the focus was on enteric diseases and a limited number of invasive and parasitic diseases. The FERG data, by contrast, cover numerous countries across the globe and provide a more complete picture of FBD.
Comparisons of our estimate of the burden of FBD with other estimates, such as those of the Institute for Health Metrics and Evaluation's GBD 2010 study [21], must be made with care because of differences in the methodology and data used. For example, the GBD 2010 study used prevalence-based DALYs, whereas our study used incidence-based DALYs. As a consequence, the impact of sequelae such as Guillain-Barré syndrome (due to Campylobacter spp.), hemolytic uremic syndrome (due to Shiga toxin-producing E. coli) and invasive disease (due to non-typhoidal S. enterica) were attributed to the diarrheal disease agents in our estimates whereas in the GBD 2010 study they were recorded in different disease categories. Furthermore, the GBD 2010 study used a different life table than FERG and more extensive mathematical modeling to account for data gaps, which smoothed the data considerably, resulting in narrower uncertainty intervals than in our study. The GBD 2010 and FERG studies used the same set of disability weights, but the FERG included some updates as recommended by WHO. Neither study applied time-discounting or age-weighting in their baseline estimates.
The GBD 2010 study, which looked at all sources of disease, found that the key hazards and risk factors for disease burden were dietary risk factors (254 million DALYs), unimproved water and sanitation (211 million DALYs), HIV/AIDS (82 million DALYs), malaria (82 million DALYs), air pollution (76 million DALYs) and tuberculosis (49 million DALYs). Recently published findings from WHO [39] for 2012 were: HIV/AIDS (92 million DALYs); malaria (55 million DALYs) and tuberculosis (44 million DALYs). Hence, the burden of FBD (33 million DALYs) is of a similar order of magnitude as each of the 'big three' infectious diseases (HIV/AIDS, malaria and tuberculosis) and air pollution, but clearly lower than the burden of dietary risk factors or unimproved water and sanitation.
Our estimate of 29,000 deaths due to foodborne transmission of invasive non-typhoidal S. enterica only included infections in non-HIV infected individuals. Ao et al. [40] estimated there were approximately 680,000 deaths due to invasive non-typhoidal salmonellosis in 2010. Of these, approximately 350,000 would be due to foodborne transmission, assuming 52% of all non-typhoidal salmonellosis cases is transmitted by food [14]. Even though this high number of deaths among HIV infected people is not included in the FERG estimates of the burden of FBD, they would be preventable by food safety interventions.
Limitations
Our study is subject to several limitations, notably due to uncertainties in the data limitations on burden estimates and attribution estimates. For most hazards (25 of the 31 studied), the 95% DALY uncertainty interval (UI) ranged from one-fourth to four times the median. The uncertainty was markedly greater for E. multilocularis (because of uncertainty in the attribution estimates), E. granulosus and L. monocytogenes (because of uncertainties in the imputation results). In low-income countries, where the burden is highest, data availability was generally most problematic. Furthermore, in these countries, the proportions of diseases transmitted by food, water and the environment are difficult to disentangle, as contaminated water may also result in contamination of foods. Due to these limitations, we were not able to present reliable estimates at country level, and elected to present results at subregion level.
For some hazards (e.g., M. bovis and E. multilocularis, aflatoxin and dioxins), incident illness is related to past exposures due to long incubation times of disease. For such hazards, the estimated burden reflects exposure dating back to the average incubation period of the disease rather than current exposure. For some hazards (e.g., dioxins), the impact on the child depends on the lifelong exposure of the mother.
Our estimates of the FBD burden are probably conservative, i.e., underestimates rather than overestimates. Limited resources and data obliged us to focus on only a subset of more than 100 hazards of potential relevance [41]. In particular, we did not include burden estimates for several chemicals (arsenic, cadmium, lead and methylmercury), because methods for estimation of the fraction of illnesses attributed to foodborne exposure to these chemicals are not readily available. Even for the hazards we have studied, it was not always possible to include all relevant disease outcomes in our estimates of burden. For example, we did not include functional bowel disorders as potential outcomes for enteric infections [42]. Inclusion of these outcomes would likely considerably increase the burden of enteric infections [43]. Aflatoxin burden was estimated using a counterfactual approach, estimating population attributable fractions from exposure assessment estimates and cancer potency factors, and applying these to WHO estimates for incidence and mortality by hepatocellular carcinoma. Risk assessment, as used to assess the burden of dioxins [19], has been proposed as an alternative basis for estimating this particular burden, and would result in considerably higher estimates of the burden of aflatoxin [44].
A further limitation of this study is that DALYs do not quantify the full societal impact of FBD. The economic burden (cost-of-illness, losses in the agricultural and food sectors and trade impacts) is also an important factor to consider in national and international decision-making. Also, the process of food production can cause human diseases by mechanisms other than direct transmission of pathogens through food. For example, animal husbandry is an important source of zoonotic disease agents that spread from pigs, poultry, cattle, etc, by direct contact or through the environment, and may also affect livestock health. It is increasingly necessary to consider holistically all aspects of food-related disease in a One Health Framework [45].
Enforcing Food Safety Standards
Despite its data gaps and assumptions, this study presents the first ever estimates of the global burden of FBD and should serve as an important resource to focus activities that will reduce this burden. A sustainable, multi-sectoral response is needed from governments and international organizations to reduce the visible and ‘hidden’ burden; this includes enforcement of food safety standards and effective surveillance networks at country, regional and global levels. This will require a concerted effort by all stakeholders in the food chain, from primary production to consumers. The diversity of foodborne hazards suggests the need for a multi-faceted strategy, with priorities tailored to each region. While national studies may further refine these priorities and are highly recommended, the current findings could already be a basis for developing strategies at the global, regional and national levels.
The diversity of foodborne hazards and regional differences in their importance suggest the need for consideration of these estimates at the national or even subnational level. As one of its aims, the FERG has fostered national studies of the burden of FBD, and pilot studies have been conducted in Albania, Japan, Thailand and Uganda. The tools and protocols developed by the FERG to support such national studies emphasize the collation of local data to validate its regional estimates, the consideration of local hazards that may not have been addressed at a global level, and the translation of burden estimates into food safety policy. The estimates developed by this WHO initiative will be invaluable for countries where local data gaps prevent the development of a full picture of FBD.
The considerable difference in the burden of foodborne disease between low- and high-income regions suggests that a major proportion of the current burden is avoidable. The WHO is working with governments and partners, including food producers, caterers and consumers, to reduce food contamination throughout the food chain, and particularly at the point of consumption, to levels at which the exposure to pathogens and contaminants does not pose significant risks for human health. There is, therefore, an urgent need to develop cost-effective food hygiene interventions that can be implemented in resource-poor settings. This research and development should be informed by estimates of the burden of specific food vehicles, taking all hazards into account.
General principles for strengthening food safety systems have been suggested by the WHO; they include integrating food safety into nutrition and food security policies and programs, and fostering closer collaboration between the various sectors involved (agriculture, human health, animal health, trade, tourism, etc.). The WHO recommends governments put in place risk-based food control systems and implement international food safety standards as established by the Codex Alimentarius Commission [12]. Food handlers and consumers should handle and prepare food safely, practicing the WHO's 'Five Keys to Safer Food' and grow fruits and vegetables using the WHO's 'Five Keys to Growing Safer Fruits and Vegetables' to decrease microbial contamination [46].
FBDs are closely linked to poverty in developing countries but they are also a global public health issue because growing international trade increases the risk of contamination in transported foods; also, migration and travel can expose populations to new hazards. Achievement of the internationally agreed Millennium Development Goals and the proposed Sustainable Development Goals, including the overarching goals of poverty reduction, achieving food security and ensuring healthy lives, will depend in part on successful reduction of the burden of FBD.
Supporting Information
Estimates of the global burden of foodborne disease, 2010.
(XLSX)
Acknowledgments
FERG members included all co-authors, and (in alphabetical order): Gabriel O. Adegoke, Reza Afshari, Deena Alasfoor, Janis Baines, Kalpana Balakrishnan, Wan Mansor Bin Hamza, Robert E. Black, P. Michael Bolger, Wanpen Chaicumpa, Alejandro Cravioto, Dörte Döpfer, John E. Ehiri, Aamir Fazil, Catterina Ferreccio, Eric M. Fèvre, Gillian Hall, Fumiko Kasuga, Karen H. Keddy, Claudio F. Lanata, Haicho Lei, Xiumei Liu, Ben Manyindo, George Nasinyama, Pierre Ongolo-Zogo, John I. Pitt, Mohammad B. Rokni, Banchob Sripa, Rolaf van Leeuwen, Philippe Verger, Arve Lee Willingham, Xiao-Nong Zhou. Resource advisers included Willy Aspinall, Robert Buchanan, Christine Budke, Marisa L. Caipo, Hélène Carabin, Dana Cole, Roger M. Cooke, John A. Crump, Fadi El-Jardali, Christa Fischer-Walker, Thomas Fürst, Juanita A. Haagsma, Aron J. Hall, Olga Henao, Sandra Hoffmann, Helen Jensen, Nasreen Jessani, Marion P.G. Koopmans, Myron M. Levine, Charline Maertens de Noordhout, Shannon Majowicz, Scott A. McDonald, Sara Pires, Elaine Scallan, Banchob Sripa, M. Kate Thomas, Linda Verhoef, Felicia Wu, Marco Zeilmaker.
We would like to acknowledge the assistance of the WHO Secretariat over the course of the FERG initiative, particularly Tim Corrigan, Tanja Kuchenmüller, Yuki Minato, and Kurt Straif (International Agency for Research on Cancer, Lyon, France). We acknowledge the Institute for Health Metrics and Evaluation—Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (Seattle, WA, USA) for providing data on the global burden of selected diseases. The European Centre for Disease Control and Prevention (Solna, Sweden) supported the work by providing their outcome trees and sponsoring a study on disability weights.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention, Department of the Navy, the US Government or other institutions listed. CFL and FJA are employees of the US Government.
Abbreviations
- DALYs
Disability Adjusted Life Years
- EPEC
Enteropathogenic Escherichia coli
- ETEC
Enterotoxigenic Escherichia coli
- FBD
Foodborne disease
- FERG
Foodborne Disease Burden Epidemiology Reference Group
- FOS
Department of Food Safety and Zoonoses (WHO)
- GBD
Global Burden of Disease
- SMPH
Summary measure of population health
- STEC
Shiga-toxin producing E. coli
- YLD
Years Lived with Disability
- YLL
Years of Life Lost
Funding Statement
This study was commissioned and paid for by the World Health Organization (WHO). Copyright in the original work on which this article is based belongs to WHO. The authors have been given permission to publish this article.
Footnotes
Provenance: Not commissioned; part of a Collection; not externally peer reviewed
References
- 1. Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365(19):1771–80. 10.1056/NEJMoa1106483 [DOI] [PubMed] [Google Scholar]
- 2. Ingelfinger JR. Melamine and the global implications of food contamination. N Engl J Med. 2008;359(26):2745–8. 10.1056/NEJMp0808410 [DOI] [PubMed] [Google Scholar]
- 3. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO consultation to develop a strategy to estimate the global burden of foodborne diseases Geneva, Switzerland: World Health Organization, 2006. http://www.who.int/foodsafety/publications/burden_sept06/en/ [Google Scholar]
- 5. FERG. World Health Organization Estimates of the Global Burden of Foodborne Disease. PLOS Collections. http://collections.plos.org/ferg-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. The World Health Organization Estimates of the Global Burden of Foodborne Dieseases: FERG Project Report. http://www.who.int/foodsafety/areas_work/foodborne-diseases/ferg/en/, 2015.
- 7. Kuchenmüller T, Hird S, Stein C, Kramarz P, Nanda A, Havelaar AH. Estimating the global burden of foodborne diseases—a collaborative effort. Euro Surveill. 2009;14(18). [DOI] [PubMed] [Google Scholar]
- 8. Lake RJ, Havelaar AH, Kuchenmüller T. New research on estimating the global burden of foodborne disease In: Sofos J, editor. Advances in microbial food safety. 1 Oxford: Woodhead Publishing; 2013. p. 260–71. [Google Scholar]
- 9. Havelaar AH, Cawthorne A, Angulo F, Bellinger D, Corrigan T, Cravioto A, et al. WHO Initiative to Estimate the Global Burden of Foodborne Diseases. Lancet. 2013;381:S59. [Google Scholar]
- 10. World Health Organization. National burden of disease studies: a practical guide Geneva, Switzerland: World Health Organization, 2001. http://www.who.int/healthinfo/nationalburdenofdiseasemanual.pdf [Google Scholar]
- 11. Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001921 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Codex Alimentarius Commission. Procedural manual Rome: Food and Agriculture Organization of the United Nations, 2014. http://www.codexalimentarius.org/procedures-strategies/procedural-manual/en/ [Google Scholar]
- 13. Cooke RM. Experts in uncertainty Oxford: Oxford University Press; 1991. [Google Scholar]
- 14. Hald T, Aspinall W, Devleesschauwer B, Cooke RM, Corrigan T, Havelaar AH, et al. World Health Organization estimates of the relative contributions of food to the burden of disease due to selected foodborne hazards: a structured expert elicitation. PLoS ONE. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–60. [DOI] [PubMed] [Google Scholar]
- 16. Kretzschmar M, Mangen MJ, Pinheiro P, Jahn B, Fevre EM, Longhi S, et al. New methodology for estimating the burden of infectious diseases in europe. PLoS Med. 2012;9(4):e1001205 10.1371/journal.pmed.1001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald SA, Devleesschauwer B, Speybroeck N, Hens N, Praet N, Torgerson PR, et al. Data-driven methods for imputing national-level incidence rates in global burden of disease studies. Bull World Health Organ. 2015;93:228–36. 10.2471/BLT.14.139972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ. 2013;91(7):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibb H, Devleesschauwer B, Bolger PM, Bellinger D, Wu F, Ezendam J, et al. WHO estimates of the global and regional burden from chemicals and toxins in food, 2010. F1000Research. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349(9063):1436–42. [DOI] [PubMed] [Google Scholar]
- 21. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability Adjusted Life Years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. WHO methods and data sources for global burden of disease estimates 2000–2011. Geneva, Switzerland: World Health Organization, 2013 WHO/HIS/HSI/GHE/2013.4. http://www.who.int/healthinfo/statistics/GlobalDALYmethods_2000_2011.pdf
- 23. Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salomon JA, Haagsma JA, Davis A, Maertens de Noordhout C, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–-23 [DOI] [PubMed] [Google Scholar]
- 25. R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 26. Devleesschauwer B, Haagsma JA, Angulo FJ, Bellinger D, Cole D, Döpfer D, et al. Methodological Framework for World Health Organization Estimates of the Global Burden of Foodborne Disease. PLoS ONE 10:e0142498 10.1371/journal.pone.0142498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maertens de Noordhout C, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(11):1073–82. 10.1016/S1473-3099(14)70870-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. 10.3201/eid1701.091101p1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. Foodborne illness acquired in the United States—unspecified agents. Emerg Infect Dis. 2011;17(1):16–22. 10.3201/eid1701.091101p2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adak GK, Long SM, O'Brien SJ. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut. 2002;51(6):832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, Millard G, et al. Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis. 2005;11(8):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaillant V, de Valk H, Baron E, Ancelle T, Colin P, Delmas MC, et al. Foodborne infections in France. Foodborne Pathog Dis. 2005;2(3):221–32. [DOI] [PubMed] [Google Scholar]
- 34. Thomas MK, Murray R, Flockhart L, Pintar K, Pollari F, Fazil A, et al. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog Dis. 2013;10(7):639–48. 10.1089/fpd.2012.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lake RJ, Cressey PJ, Campbell DM, Oakley E. Risk ranking for foodborne microbial hazards in New Zealand: burden of disease estimates. Risk Anal. 2010;30:743–52. 10.1111/j.1539-6924.2009.01269.x [DOI] [PubMed] [Google Scholar]
- 36. Gkogka E, Reij MW, Havelaar AH, Zwietering MH, Gorris LG. Risk-based estimate of effect of foodborne diseases on public health, Greece. Emerg Infect Dis. 2011;17(9):1581–90. 10.3201/eid1709.101766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Havelaar AH, Haagsma JA, Mangen MJ, Kemmeren JM, Verhoef LP, Vijgen SM, et al. Disease burden of foodborne pathogens in the Netherlands, 2009. Int J Food Microbiol. 2012;156:231–8. 10.1016/j.ijfoodmicro.2012.03.029 [DOI] [PubMed] [Google Scholar]
- 38. Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect. 2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Global Health Observatory Data Repository. http://apps.who.int/gho/data/node.main.DALYNUMWORLD?lang=en. Accessed July 22, 2014.
- 40. Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal salmonella disease, 2010. Emerg Infect Dis. 2015;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization. WHO initiative to estimate the global burden of foodborne diseases: First formal meeting of the Foodborne Disease Burden Epidemiology Reference Group (FERG): implementing strategy, setting priorities and assigning the tasks Geneva, Switzerland: World Health Organization, 2008. http://www.who.int/foodsafety/publications/burden_nov07/en/ [Google Scholar]
- 42. Porter CK, Gormley R, Tribble DR, Cash BD, Riddle MS. The Incidence and gastrointestinal infectious risk of functional gastrointestinal disorders in a healthy US adult population. Am J Gastroenterol. 2011;106(1):130–8. 10.1038/ajg.2010.371 [DOI] [PubMed] [Google Scholar]
- 43. Haagsma JA, Siersema PD, De Wit NJ, Havelaar AH. Disease burden of post-infectious irritable bowel syndrome in The Netherlands. Epidemiol Infect. 2010;138:1650–6. 10.1017/S0950268810000531 [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118(6):818–24. 10.1289/ehp.0901388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From "one medicine" to "one health" and systemic approaches to health and well-being. Prev Vet Med. 2011;101(3–4):148–56. 10.1016/j.prevetmed.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. World Health Day 2015: Food safety—the global view. 2105. http://www.who.int/campaigns/world-health-day/2015/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimates of the global burden of foodborne disease, 2010.
(XLSX)