Abstract
Introduction:
The current study aimed to elucidate the role of preparatory cognitive control in decision making and its neural correlates using functional Magnetic Resonance Imaging (fMRI). To this effect, by employing a series of new cognitive tasks, we assessed the role of preparatory cognitive control in monetary (risky) decision making.
Methods:
The participants had to decide between a risky and a safe gamble based on their chance of winning (high or low). In the 2-phase gambling task (similar to Cambridge gambling task), the chance and the gamble were presented at the same time (i.e. in a single phase), but in a new 3-phase gambling task, the chance is presented before the gamble. The tasks ended with a feedback phase.
Results:
In the 3-phase task, holding the chance in memory to guide their decision enabled the participants to have more control on their risk taking behaviors as shown by activation in a network of brain areas involved in the control and conflict, including dorsal Anterior Cingulate Cortex (dACC), indexed by faster reaction times and better performance in the gambling task, and the temporal lobe, which has a role in holding contextual information.
Discussion:
Holding information in memory to guide decision presumably enables the participants to have more control on their risk taking behaviors. The conflict and uncertainty resulting from this risky decision was indexed by the activation of dACC, known to be activated in conflict and cognitive control.
Keywords: Decision making, Memory, Control, Conflict, Anterior cingulate cortex, Functional magnetic resonance imaging
1. Introduction
In recent years, many methods have been invented for studying the neural bases of cognitive processes, from sensory-motor to higher levels (Walton et al., 2004; Krug and Carter, 2010; Cohen and Lieberman, 2010; Coutlee and Huettel, 2012; Daliri, 2012; Morsheddost, Asemani, and Shalchy, 2015), especially decision making (Krug and Carter, 2010; William et al., 2004; Keri et al., 2004; Bhanji, Beer, & Bunge, 2010; Miyapuram, Tobler, Gregorios-Pippas, & Schultz, 2012). In this regard, gambling tasks provide objective measures to study decision processes in normal people as well as individuals with brain lesions (Bechara et al., 1998; Delgado et al., 2000; Breiter, 2001; Bush et al., 2002; Knutson, 2001; Rogers et al., 1999, 2004). In most studies, hypothetical monetary rewards are used; studies have shown that it can activate BOLD signals as good as real rewards (Miyapuram, Tobler, Gregorios-Pippas, & Schultz, 2012), even when it is illusory (Sohrabi, Smith, West, & Cameron, 2007).
In most neuroimaging studies using gambling tasks, the participants can see the gamble and outcome together and the imaging results do not separate the brain activations related to the decision and the outcome. However, Rogers et al. (2004) employed a two-phase gambling task (Rogers et al., 1999) with two phases; decision and outcome, and event-related functional Magnetic Resonance Imaging (fMRI) and showed the role of the upper part of Prefrontal Cortex (PFC; superior PFC and caudal Anterior Cingulate Cortex; ACC) in decision phase and lower parts of PFC (Orbitofrontal Cortex; OFC and subgenual ACC) in outcome phase. Some of the problems with the task used in Rogers et al. (1999) were mentioned in the following paper by these researchers (Rogers et al., 2004). Especially, the role of preparation by further separating the chance to win and the gamble itself demand more investigations.
Therefore, in the present study, we employed fMRI to elucidate the neural bases of monetary Risky Decision Making (RDM) and non-monetary Perceptual Decision Making (PDM) and to investigate the role of preparation using a modified version of Rogers et al. task, also known as Cambridge gambling task. Here we separated decision and outcome phases as in Rogers and colleagues’ task but we also, in one condition, separated observing the chance and the amount to gamble. Moreover, we compared these two risky (i.e. RDM) conditions with a control condition using a perceptual task (i.e. PDM). The goal was to reveal the role of seeing the chance to win before facing the gamble options. Then, any behavioral and brain activation results could be attributed to the role of being prepared and knowing the likelihood of winning before the risky decision.
2. Methods
2.1. Material and procedure
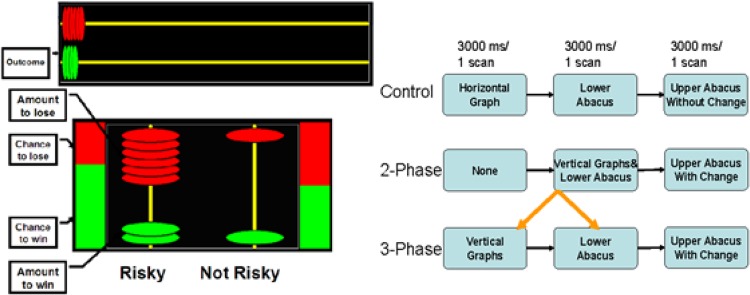
In this study, participants had to decide between a risky and a safe betting option based on the information about the risk of each option. The information for the task was presented on a screen using a projector and could be seen through a mirror mounted to the MRI scanner. As shown in Figure 1, in the 3-phase task, two graphs were presented showing the risk for two betting options that were not yet presented. The graphs were then removed and two options for betting were presented (with a small abacus). The risk (chance of winning) and the betting information were displayed for three seconds each.
Figure 1.
An example of the tasks (here, 2-phase task), left panel, and the design, right panel.
Participants had to choose their bet while the betting information was displayed. Next, there was a three second display giving feedback on the outcome of the bet (with a horizontal abacus on the top). In order to use the risk information to choose between the two betting options, participants must retain the risk information in memory.
In the 2-phase task, participants were presented with a blank display for three seconds followed by the risk information and the betting options displayed together for three seconds. During this time, they chose their bet with index or middle finger of their right hand. This was followed by the three second feedback display. Participants completed 24 trials in each condition. The conditions were alternated in blocks of four trials each. The control or perceptual task was similar to the 3-phase task but the screen did not change during outcome phase. Participant simply compared the ratios of the colored parts with the number of relevant colored disks.
So, they were instructed to select the column with a ratio of green to red disks correlated with the ratio of green and red in the graph, by pressing the related button with right index finger and the right middle finger, for the column at left and right, respectively. These two columns appeared randomly at the right and the left side. When the participant pressed the button, the related column was illuminated by a white rectangle. In the control condition, no feedback (including correctness) was provided but the horizontal abacus remained throughout the trial for all three conditions.
The task was programmed in Microsoft Visual Basic 6 (Microsoft Corp.) with millisecond time precision using Class and Thread Priority procedure (Chambers & Brown, 2003) and was run on a laptop running Microsoft Windows. The stimuli were back projected (using an SVGA projector) onto a screen to be viewed through a mirror attached to the standard coil of the MRI machine. Participants responded by the index or middle finger of their right hand using a fiber optic response device.
2.2. Participants
Participants were 8 right-handed normal volunteers (4 males; mean age 26) with normal or corrected to normal vision (using contact lenses). They all signed an informed consent and an MRI safety form at the Ottawa Hospital.
2.3. fMRI design
The imaging was performed using a 1.5-T Siemens MAGNETOM Symphony MR scanner with the quantum gradient set. Participants lay supine with their head secured in a standard head holder. Whole brain echo planar fMRI, based on the BOLD signal, was performed using a gradient echo pulse sequence (TR/TE 3000/40 ms, flip angle 90, slice thickness 5 mm, 27 axial slices).
The fMRI data were analyzed with Statistical Parametric Mapping analytic package (SPM8, Wellcome Department of Cognitive Neurology). For each participant, the images were realigned (with re-slicing and co-registering), normalized, and finally spatially smoothed with 10 mm FWHM isotropic Gaussian kernel. Then, all data were analyzed (except the first four images of the initial extra trials included for hemodynamic T1 equilibration purpose), taking into account the hemodynamic response and the global effect using a block design (Behroozi, Daliri, and Boyaci, 2011; Behroozi and Daliri, 2012, for a review).
The designs and stimuli in the experiment are shown in Figure 1. The experiment contained 72 trials of 9-second duration. During each trial, 3 images were taken of the whole brain. Each 4 trials (each having one specific option) made a 36s block. Therefore, each condition included 6 repeated blocks (three 1/3 chance and three 2/3 chance). The brain structures were labeled using Talairach and Tournoux (1988), taking into account the difference between MNI and Talairach atlases (Brett et al., 2002).
3. Results
3.1. Behavioral analysis results
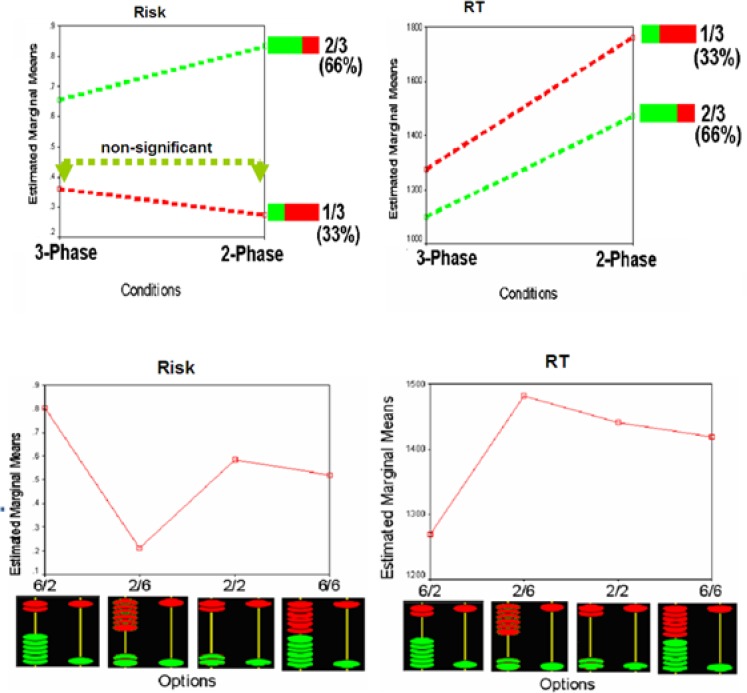
Reaction times (RTs) and risk (choosing from columns other than one with 1 green and 1 red disk) were analyzed by two separate 2×2×4 repeated measure ANOVAs (RDM tasks by chance by options), using SPSS. In both analyses, the main factors and interactions were significant. In general, participants took more risks and had longer RTs in 2-phase compared to 3-phase trials. In both conditions, participants showed the highest risks and lowest RTs in 6 greens/2 reds option and/or 2/3 chance. Whereas they showed the lowest risks and longest RTs in 2 greens/6 reds option and/or 1/3 chance (Figure 2). These results showed the effects of preparation on decision making, by the new treatment employed in the 3-phase condition. The behavioral data of the perceptual task were not analyzed, but the descriptive results showed that participants had understood the task instruction in all three tasks.
Figure 2.
Behavioral results for the two risky tasks with two chance conditions, top, and four option conditions, bottom.
3.2. Imaging results
As mentioned above, in this experiment each 4 trials (having one specific option of disk ratios) made a 36s block. Therefore, each condition included 6 repeated blocks (three with 1/3 chance and three with 2/3 chance). The effects of the two different chances are not reported here for the sake of simplicity, and the fact that they involved different amount of reward, affected fMRI results (Elliot et al., 2000).
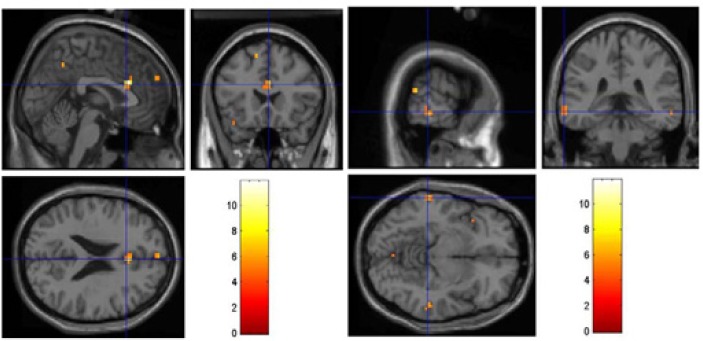
Random effect (i.e. second level) analyses (RFX) were performed with the P<0.001, uncorrected, and cluster size of at least 4 voxels. Only 3-phase – 2-phase contrast was significant. As shown in Figure 3, by comparing the new 3-phase gambling task with the 2-phase, we found higher activation in dACC and temporal lobe when participants had enough time to memorize their chance in the gamble in the first phase (not found for the 2-phase and perceptual tasks).
Figure 3.
The result of 3-phase – 2-phase contrast, showing activation in Dorsal Anterior Cingulate (dACC), left panel, and temporal lobe, right panel (other contrasts were not significant).
4. Discussion
In this study, we employed a new task and fMRI to investigate the role of control and conflict in decision making. In the two-phase gambling task, the chance and the gamble are presented at the same time, while in the three-phase one, the chance is presented before the gamble. In the latter case, holding the chance in memory to guide their decision presumably enable the participants to have more control on their risk taking behaviors as shown by activation in a network of brain areas; including dACC, indexed by faster reaction times and better performance in the gambling task, and the temporal lobe, which has a role in holding contextual information. This pattern of activation was also supported by comparing the two gambling conditions with a control perceptual decision task, as only the 3-phase condition showed this activation pattern in the brain.
The conflict and uncertainty resulting from this risky decision was indexed by the activation of dACC, an area well known to be activated in conflict (Carter et al., 1998; Walton et al., 2004; Krug and Carter, 2010), cognitive control (Walton et al., 2004; Krug and Carter, 2010; Cohen and Lieberman, 2010; Coutlee and Huettel, 2012), value (e.g. gain/loss) processing (William et al., 2004), and mental effort awareness (Naccache et al., 2005), and uncertainty (Keri et al., 2004; Bhanji, Beer, & Bunge, 2010).
Moreover, A higher activation in temporal lobe was not found for the 2-phase and perceptual tasks when the participants had time to memorize their chance in the gamble in the first phase. The temporal lobe is important for memory to help PFC in maintaining instructions (Ranganath et al., 2004) and working memory of relational visual information (Olson et al., 2006). However, this only happened when participants knew the chance of winning before facing the betting options. Therefore, it may be argued that, as dACC got activated by cognitive control and conflict, temporal lobe was activated by working memory when participants had time to employ it later in deciding on the gamble.
Using another method, Walton et al. (2004) found that the dACC is activated in conflict monitoring of voluntary (controlled) actions, not in externally directed ones. William et al. (2004) were able to measure the activity of the human dACC, before and after its ablation, when they opened the participants’ skull for surgical cingulotomy. They showed the role of the dACC in monitoring of the task set and, especially, of the reward reduction.
In a lesion study by Naccache et al. (2005), a patient with dACC lesion had problem in reporting mental effort in a cognitive control task, while had a normal performance in doing the task. The normal performance of patients with dACC in non-value-based (non-monetary) tasks such as Stroop and Go/NoGo has also been shown in other lesion studies (Fellows and Farah, 2005). However, the activation of a given area (s) only implies necessity, but not sufficiency for a function (Sohrabi and Brook, 2005), and the area(s) must be considered only as a part of a distributed network of brain areas involved in the decision process.
Acknowledgements
Authors thank the neurologists and technicians at MRI unit, Ottawa General Hospital, for their help in conducting the experiment.
References
- Bechara A., Damasio H., Tranel D., Anderson S. W. (1998). Dissociation of working memory from decision making within the human prefrontal cortex. The Journal of Neuroscience, 18(1), 428– 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozi M., Daliri M. R., Boyaci H. ( 2011). Statistical analysis methods for the fMRI data. Basic and Clinical Neuroscience, 2(4), 67–74. [Google Scholar]
- Behroozi M., Daliri M. R. ( 2012). Software tools for the analysis of functional magnetic resonance imaging. Basic and Clinical Neuroscience, 3(5), 71–83. [Google Scholar]
- Bhanji J. P., Beer J. S., Bunge S. A. (2010). Taking a gamble or playing by the rules: Dissociable prefrontal systems implicated in probabilistic versus deterministic rule-based decisions. NeuroImage, 49( 2), 1810– 1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H. C., Aharon I., Kahneman D., Dale A., Shizgal P. (2001). Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron, 30( 2), 619– 639. [DOI] [PubMed] [Google Scholar]
- Brett M., Johnrude I. S., Owen A. M. (2002). The problem of functional localization in the human brain. Nature Reviews Neuroscience, 3( 3), 243– 249. [DOI] [PubMed] [Google Scholar]
- Bush G., Vogt B. A., Holmes J., Dale A. M., Greve D., Jenike M. A., et al. (2002). Dorsal anterior cingulate cortex: A role in reward based decision making. Proceedings of National Academy of Science of the United States of America, 99(1), 523– 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S., Braver T. S., Barch D., Botvinick M. M., Noll D., Cohen J. D. (1998). Anterior cingulate cortex error detection and the on-line monitoring of performance. Science, 280( 5364), 747– 749. [DOI] [PubMed] [Google Scholar]
- Chambers C. D., Brown M. (2003). Timing accuracy under Microsoft Windows revealed through external chronometry. Behaviour Research Methods, Instruments, and Computers, 35 (1), 96– 108. [DOI] [PubMed] [Google Scholar]
- Cohen J. R., Lieberman M. D. (2010). The common neural basis of exerting self-control in multiple domains. Self-control Society, Mind, and Brain, 1, 141– 160. [Google Scholar]
- Coutlee C. G., Huettel S. A. (2012). The functional neuroanatomy of decision making: Prefrontal control of thought and action. Brain Research, 1428, 3– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliri M. R. (2012). Predicting the cognitive states of the subjects in functional magnetic resonance imaging signals using the combination of feature selection strategies. Brain Topography, 25(2), 129– 134. [DOI] [PubMed] [Google Scholar]
- Delgado M. R., Nystrom L. E., Fissell C., Noll D. C., Fiez J. A. ( 2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072–3077. [DOI] [PubMed] [Google Scholar]
- Elliott R., Friston K. J., Dolan R. J. (2000). Dissociable neural responses associated with reward, punishment and risk-taking behavior. Journal of Neuroscience, 20, 6159– 6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows L. K., Farah M. J. (2005). Is anterior cingulate cortex necessary for cognitive control? Brain, 128(4), 788–796. [DOI] [PubMed] [Google Scholar]
- Keri S., Decety J., Roland P. E., Gulyas B. ( 2004). Feature uncertainty activates anterior cingulate cortex. Human Brain Mapping, 21 (1), 26– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G. W., Adams C. M., Varner J. L., Hommer D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport, 12( 17), 3683– 3687. [DOI] [PubMed] [Google Scholar]
- Krug M. K., Carter C. S. (2010). Anterior cingulate cortex contributions to cognitive and emotional processing: A general purpose mechanism for cognitive control and self-control. Self-control in Society, Mind, and Brain, 1, 3– 27. [Google Scholar]
- Miyapuram K. P., Tobler P. N., Gregorios-Pippas L., Schultz W. (2012). BOLD responses in reward regions to hypothetical and imaginary monetary rewards. NeuroImage, 59( 2), 1692– 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsheddost H., Asemani D., Shalchy M. A. ( 2015). Evaluation of hemodynamic response function in vision and motor brain regions for the young and elderly adults. Basic and Clinical Neuroscience, 6(1), 58–68. [PMC free article] [PubMed] [Google Scholar]
- Naccache L., Dehaene S., Cohen L., Habert M., Guichart-Gomez E., Galanaud D., et al. (2005). Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia, 43( 9), 1318– 1328. [DOI] [PubMed] [Google Scholar]
- Olson I. R., Page K., Moore K. S., Chatterjee A., Verfaellie M. (2006). Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience, 26(17), 4596– 4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Yonelinas A. P., Cohen M. X., Dy C. J., Tom S. M., D’Esposito M. D. ( 2004). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia, 42(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Rogers R. D., Owen A. M., Middleton H. C., Pickard J., Robbins T. W. (1999). Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience, 19( 20), 9029– 9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. D., Ramnani N., Mackay C., Wilson J. L., Jezzard P., Carter C. S., et al. (2004). Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry, 55( 6), 594– 602. [DOI] [PubMed] [Google Scholar]
- Sohrabi A., Brook A. (2005). Functional Neuroimaging and its Implications for Cognitive Science: Beyond Phrenology and Localization. Proceedings of the 27th annual meeting of the Cognitive Science Society (pp. 2044–2049). Stresa Italy. [Google Scholar]
- Sohrabi A., Smith A. M., West R. L., Cameron I. (2007). Uncertainty, Risk, and Illusion in Reward Prediction: Evidence from fMRI. In Hardy-Vallée B. (Ed.), Cognitive Decision-Making: Empirical and Foundational issues (pp. 79–94). Cambridge: Cambridge Scholars Press Ltd. [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Walton M. E., Devlin J. T., Rushworth M. F. S. (2004). Interactions between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience, 7( 11), 1259– 1265. [DOI] [PubMed] [Google Scholar]
- Williams Z. M., Bush G., Rauch S. L., Cosgrove G. R., Eskandar E. N. (2004). Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nature Neuroscience, 7(12), 1370– 1375. [DOI] [PubMed] [Google Scholar]