Abstract
Introduction:
Various neuroregenerative procedures have been recently employed along with neurorehabilitation programs to promote neurological function after Spinal Cord Injury (SCI), and recently most of them have focused on the acute stage of spinal cord injury. In this report, we present a case of acute SCI treated with neuroprotective treatments in conjunction with conventional rehabilitation program.
Methods:
A case of acute penetrative SCI (gunshot wound), 40 years old, was treated with intrathecal bone marrow derived stem cells and parenteral Granulocyte-Colony Stimulating Factor (G-CSF) along with rehabilitation program. The neurological outcomes as well as safety issues have been reported.
Results:
Assessment with American Spinal Injury Association (ASIA), showed neurological improvement, meanwhile he reported neuropathic pain, which was amenable to oral medication.
Discussion:
In the acute setting, combination therapy of G-CSF and intrathecal Mesenchymal Stem Cells (MSCs) was safe in our case as an adjunct to conventional rehabilitation programs. Further controlled studies are needed to find possible side effects, and establish net efficacy.
Keywords: Mesenchymal stem cells, Granulocyte-colony stimulating factor, Spinal cord injury
1. Introduction
Various neuroregenerative procedures have been recently employed along with neurorehabilitation programs to promote neurological function after spinal cord injury; recently most of them have focused on the acute stage of Spinal Cord Injury (SCI) (Guest et al., 2012).
These procedures include various pharmacological and cellular interventions, which may be employed systemically, intrathecally, or intralesionally (Saberi H., Derakhshanrad N., & Yekaninejad M., 2014). They may be administered at the time of surgical decompression and fusion, or shortly thereafter.
Of these interventions, two have been widely studied so far: 1) Granulocyte-Colony Stimulating Factor (G-CSF) administration (Inada et al., 2014), and 2) intrathecal bone marrow mesenchymal stem cells (Saito et al., 2012). Both of them have been employed experimentally (Jia et al., 2014; Kadota et al., 2012), and clinically (Takahashi et al., 2012) (Karamouzian, Nematollahi-Mahani, Nakhaee, & Eskandary, 2012). Combination of these two methods may be a promising method, which requires not only safety appraisal (Lammertse et al., 2007), but also a treatment protocol, with the aim of observing synergistic activity. In this case, we report co-administration of intrathecal cultured mesenchymal stem cells, and subcutaneous G-CSF (Derakhshanrad et al., 2013) in a patient with acute penetrative SCI and in the follow up, the neurological and functional outcomes (Steeves et al., 2007) as well as the observed adverse effects are reported.
2. Case presentation
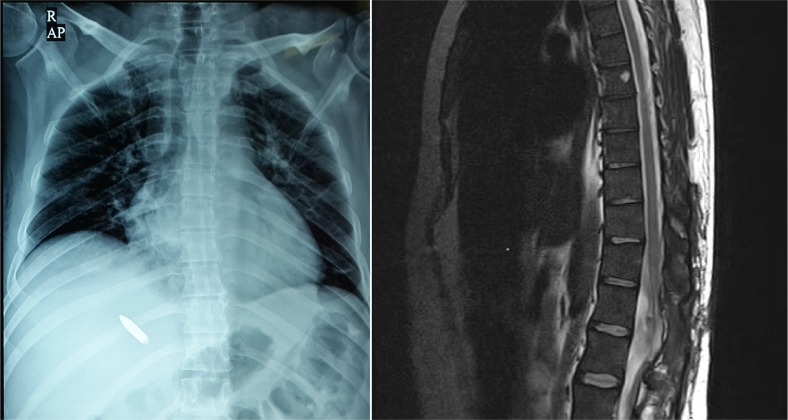
A 40-year-old man was referred to our center due to complete paraplegia, following gunshot to the left flank region since 15 days ago. In the emergency evacuation center, he had been admitted in shock and hypovolemic state. Emergency laparotomy was performed and revealed retroperitoneal hematoma on the left side due to the left kidney rupture, accordingly left nephrectomy was performed at that time. After hemodynamic stabilization, a laminectomy for dural repair was done. Upon arrival to our center the spinal X-rays revealed stable burst fractures of T12 and L1 vertebrae associated with lateral mass fracture. There was also a bullet in the right hemithorax subcutaneously (Figure 1).
Figure 1.
Left: Chest X-ray, Note the Bullet in right hemithorax, and Right: Magnetic Resonance Imaging of the thoracolumbar junction upon admission, not the spinal cord is not anatomically disconnected.
Removal of the metallic bullet was easily accomplished under C-arm control before MRI examination on the following day. Actually the bullet entry was through the left flank and it had passed through the posterior elements of T12-L1 vertebral bodies, contusing but not passing the spinal cord. The patient was paraplegic at T12 sensory level (AIS=A). On T2 weighted MRI, the rostrocaudal length of signal change area was about 20 mm (Figure 1). Written informed consent was obtained for both intrathecal MSCs injection and subcutaneous G-CSF administration from the patient.
2.1. Isolation and culture of Bone Marrow-derived Mesenchymal Stem Cells (BM-MSCs):
The technical aspects of cell preparation are discussed in detail to elucidate the biological character of the injected solution. Eighty milliliter of bone marrow aspirate was harvested from the right iliac crest in the operation theatre, aseptically through a posterior iliac crest puncture using Jamshidi needle. Bone marrow was transferred to the current Good Manufacturing Practice (cGMP) facility clean room.
All cell manufacturing procedures were performed under laminar vertical air flow cabinets with the relevant quality assurance and GMP guidelines. Each 7 mL of bone marrow was carefully layered over 3 mL of Ficoll-Paque TM PREMIUM (GE Healthcare Life Sciences, USA) solution and centrifuged at 450×g for 30 minutes at 20°C. The upper layer was carefully aspirated and discarded leaving the mononuclear cells (MNCs) layer intact. Mononuclear cells were collected and transferred to 50 mL falcon tubes (all from TPP, Switzerland). Consequently, collected cells were washed by adding up to 45 mL of phosphated buffer saline (PBS, CliniMACS®, Miltenyi Biotec, Germany) and centrifuged at 300×g for 5 minutes and then at 200×g for another 5 minutes.
Then, supernatant was removed gently and cell pellet was transferred into filter cap culture flasks containing complete culture media (DMEM supplemented with 10% fetal bovine serum) (FBS, Pharma grade, Australian origin and gamma irradiated, PAA, Austria). The culture flasks were incubated at 37°C, 5% CO2. After 48 hours, culture medium containing non-adherent cells were removed, discarded, and then complete culture medium was added and renewed every 72 hours regularly. Subcultures were performed at 80% confluency, using TrypLE select (Life Technologies, USA).
2.2. Characterization of BM-MSCs
BM-MSCs were negative for CD11b, 19, 34, 45, HLA-DR, and positive for CD105, 44, and CD73.
2.3. Differentiation potential
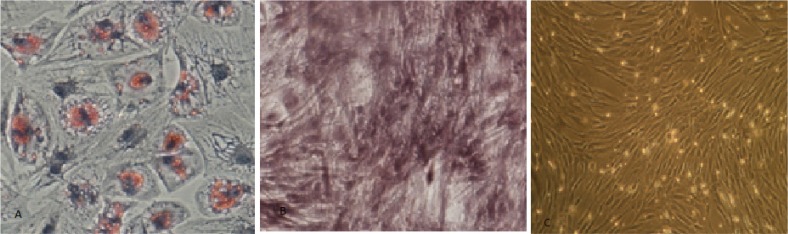
We evaluated the differentiation potential of isolated BM-MSCs by staining with oil red (adipogenic), and alizarin red (osteogenic) (Figure 2). Microbiological tests were performed for aerobic, anaerobic, and fungi before, during, and after cell culture procedure, and all of them were negative. Finally, 41 million BM-MSCs were obtained from the third passage (after 28 days) with 98% purity and 93% viability, which were suspended in 3 mL injectable Normal Saline and transferred to the operating room, for intrathecal injection. Figure 2c shows inverted microscope view of BM-MSCs with 200X magnification.
Figure 2.
Photomicrograph of the cultured cells, staining with Oil Red (adipogenic), and Alizarin Red (osteogenic). Adipogenic (a) osteogenic (b), and differentiation of BM-MSCs (c).
2.4. Cell delivery procedure and drug administration protocol
About 45 days after trauma, with the signal of the cell preparation team, the patient was prepared and scheduled for the cell transplantation. He was taken to operation theatre and 3 mL isobaric cell suspension was injected intrathecally through lumbar puncture with gauge 20 spinal needle under sterile condition in lateral decubitus position. The patient was kept recumbent in the bed, and instructed to roll in the bed for 48 hours, and followed up for any complications.
2.5. G-CSF administration
Three weeks after trauma, 5μg/kg/d of G-CSF (Filgrastim, Neupogen®; Amgen, Thousand Oaks, CA, USA) was administered subcutaneously for 7 consecutive days. The peak white blood cell count was 36000/mm3. This regimen was repeated after 3 months, and the peak cell count was 32000.
2.6. Rehabilitation program
Active rehabilitation program (6 sessions per week) was resumed shortly after each treatment, for a six months period. He had a problem in sitting position for doing activity of daily living (ADL) just for less than 1 minute because of poor trunk control and postural hypotension at the beginning. Client was able to participate in ADL for at least 2 hours after 14 days. During these sessions, he participated in transfer activities and wheelchair propulsion. Good trunk control exercises, especially external oblique and rectus abdominis, were done to facilitate bend down and move from side to side without fear of falling forward. Upper extremity strengthening exercises were done to facilitate using walker and to compensate for weakness in lower extremities. Lower extremity braces were prescribed with the walker to facilitate ambulation in short distances only.
2.7. Periodic examination
American Spinal Injury Association (ASIA), Spinal Cord Independence Measure (SCIM III), International Association of Neurorestoratology-Spinal Cord Injury Functional Rating Scale (IANR-SCIFRS), Modified Ashworth Scale (MAS), Visual Analog Scale (VAS) pain score, and digital gait analysis were examined as baseline and thereafter during the next 12 months period by independent observers. Also possible untoward complications were scrutinized and recorded.
After six months, to evaluate kinematics of lower extremity and obtaining desired data from kinematics angles, the subject was asked to walk across the laboratory on a force plate (Kistler Multi-component Force Plate for Biomechanics, Type 9281E, Switzerland). The walkway domain was calibrated using laser calibration in order to construct proper local and global coordinate systems. In order to track the changes in the angles during walking, reflective markers were set on the right and left feet of the subject.
At least 2 cameras are needed to analyze in 3-D space. So to reach 3-D analysis and obtaining more accurate data, 6 video cameras (Manfrotto, 190XPROB.I) were used to shoot a film during walking. Data were filtered using the fourth order low-pass Butterworth in SIMI motion analysis software. Then, filtered data were collected and imported into the Excel to compare results by representation in curves.
There was a low-grade fever in the next day after intrathecal cell delivery. The changes in ASIA, SCIM III, IANR-SCIFRS, MAS, and VAS (VAS=70 after 3 months) have been reported in Table 1. Also digital gait analysis was performed, which has been diagrammatically depicted in Figure 3.
Table 1.
ASIA, SCIM III, IANR-SCIFRS scores, MAS and VAS pre and post-Treatment.
| Variables | Pre intervention | Post intervention (after 6 months) | Post intervention (after 1 year) |
|---|---|---|---|
| Motor (Lower extremities) | 10 | 29 | 37 |
| Light touch | 80 | 93 | 94 |
| Pin prick | 80 | 86 | 90 |
| SCIM III | 21 | 58 | 67 |
| IANR-FRS | 18 | 34 | 41 |
| MAS | 0 | 0 | 0 |
| VAS | 20 | 70 | 70 |
Ranges for score categories are as follows. Motor: minimum 0, maximum 100; light touch: minimum 0, maximum 112; pinprick: minimum 0, maximum 112; IANR-SCIFRS: minimum 0, maximum 48;SCIM III: minimum 0, maximum 100.
Abbreviations. IANR-SCIFRS, International Association of Neurorestoratology-Spinal Cord Injury Functional Rating Scale; SCIM III, Spinal Cord Independence Measure III; MAS, Modified Ashworth Scale; VAS, Visual Analog Scale.
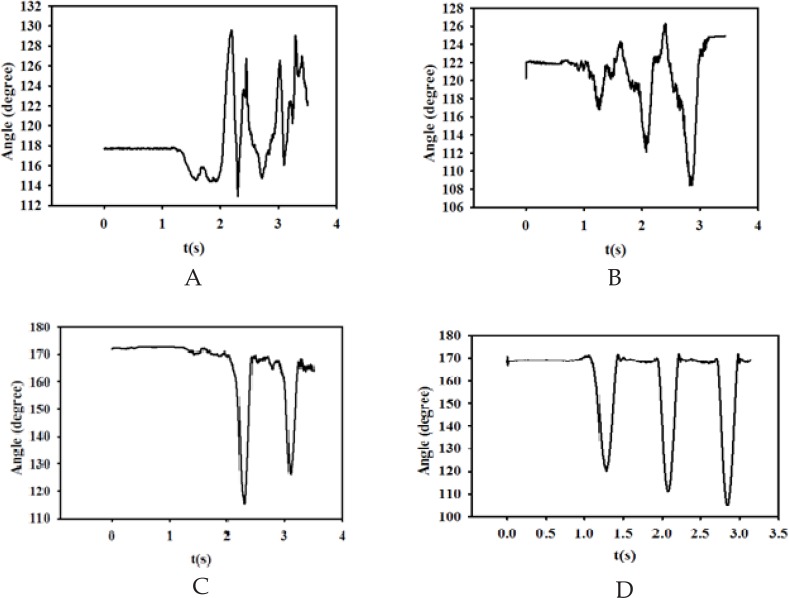
Figure 3.
Figures 3A to 3D illustrate the left and right knee angles during gait (angle between ankle- knee- hip markers), respectively. Figure 3A. Left Foot angle during gait (angle between Toe- Ankle- knee markers), Figure 3B. Right Foot angle during gait (angle between Toe- Ankle- knee markers), Figure 3C. Left Knee angle during gait (angle between Ankle- Knee- Hip markers), Figure 3D. Right Knee angle during gait (angle between Ankle- Knee- Hip markers).
Figure 3a shows the left foot angle during walking, which is assumed to be the angle between toe-ankle-knee markers. As apparent, this angle varies approximately from 112 to 140 degrees. The subject is initially in double-support position and starts moving with right foot plantar, flexion and therefore, the ankle angle increases and then decreases in dorsiflexion. Figure 3b demonstrates the right foot angle during walking. The figure illustrates that the angle changes between 108 to 126 degrees, which is significantly lower than that of left foot. Furthermore, the shapes of signals, which can describe the pattern of walking in ankle angle point of view, are not the same. Such differences between these angles may be related to the severity of the paralysis on the left side.
Figures 3c and 3d illustrate the left and right knee angles during gait (angle between ankle-knee-hip markers), respectively. As seen from these figures, although there is not considerable difference between ranges of changing angles (120–170 degrees), but they are different from signal shape point of view. In other words, the pattern of walking in right foot is more stable than that of left foot, which may be related to the greater weakness in the left side of subject’s body.
3. Results
The ASIA Impairment Scale (AIS) changed from A to C and the SCIM III score rose from 21 to 67. The IANR-SCIFRS improved from 18 to 41. Detailed ASIA examination has been tabulated in Table 1.
4. Discussion
Mesenchymal stem cells have been used for the treatment of SCI with various administration routes (intramedullary, intrathecally, or intravenously). We employed lumbar puncture as a less invasive method for cell delivery and limit the cell culture passages to three steps, for prevention of changes in nuclear ploidy (Bernardo et al., 2007). Delivery route is of outmost importance, because lumbar puncture may be repeated if necessary, and one would be able to re-treat the case in future study protocols. Also subcutaneous route for G-CSF delivery was chosen as a minimally invasive and repeatable route (Saberi H. et al., 2014).
Neurosurgically, our case was AIS-A, although most authors recommend neuroregenerative treatments for Non-Frankel A cases (Saberi H., Derakhshanrad N., & Yekaninejad M. S., 2014) to see efficacy, Frankel A patients may be good candidates for safety consideration.
Therapeutic responses in the acute stage may be not only due to intervention, but also because of autorecovery (6–13%) (Harrop et al., 2012). Therefore a randomized double-blind clinical trial to assess the net effect of treatment and autorecovery on the obtained outcomes, is a methodological necessity (Lammertse et al., 2007) to clarify the net effect of treatment or any synergism in three limbs of the study. In the cell processing stage, authors have adhered to clinical grade clean room facility for human use (Bernardo et al., 2007).
The major shortcoming of the lumbar puncture is that, if there is already a CSF block and/or adhesive arachnoiditis, the injected cells may not reach the lesion site. On the other hand, the injected cells may reach only the outer layer of spinal cord pia mater. As it may be seen in the pre-treatment MRI, there was no block in CSF pathways in this case. Other studies have used intrathecal cell therapy with the same precautions (Callera & do Nascimento, 2006). Nevertheless using labeled cells to ensure local incorporation may be mandatory.
For safety precautions, the surveillance sessions were scheduled more frequently, at weekly intervals. Close follow-ups were scheduled, for detection of any possible complications, and clinical team was ready for necessary interventions.
The treatment was performed in the acute phase of disease, with the hope that scar formation in the spinal cord would be minimal at this stage. Also in 2010, the number of acute neuroprotective interventions for SCI has increased (Saberi H. et al., 2014). The employed assessment techniques are the standard recommended methods by ICCP panel guideline (Fawcett et al., 2007; Lammertse et al., 2007; Steeves et al., 2007; and Tuszynski et al., 2007).
The observed effect size, compared to the mean effect size reported elsewhere, is promising (Motor 37 vs. 16.29, Pinprick 10 vs. 13.46, and Light touch 14 vs. 17.08) (Saberi H. et al., 2014). There was a major motor improvement, although the changes in Pinprick and Light touch were less significant. The location of the lesion may be an explanation for this finding.
Documentation of motor function by ASIA method is more or less subjective and observer dependent. In order to make our findings more objective and assess the walking ability automatically, we employed digital gait analysis. As it can be seen in Figure 3, all the lower extremity joints were active and contribute to locomotion.
Neuropathic pain has been reported to be a known complication of cellular treatments and in our case there was a new onset of neuropathic pain with subjective increment, however the high incidence of pain (70%) in SCI cases (Harrop et al., 2012) may also be an explanation for the new onset of painful syndrome. Further studies maybe needed to determine the net effect of cell therapy in the creation of neuropathic pain. Increased spasticity, although a known complication of cellular treatments, was not a problem in our case, possibly because of the level of the lesion. He is now receiving gabapentin 300mg twice daily, to relieve neuropathic pain and Methoral 25mg daily for arterial hypertension.
Assessment of the outcome of injected stem cells in the lesion site and throughout the neuraxis is a major clue for establishment of intrathecal cell delivery. So absence of meticulous cell tracing and post injection viability assessment is an important issue. Most of these assessment methods are invasive and their application in clinical setting may not be warranted in human subjects. In our case, pan- spinal and brain MRI with Gadolinium contrast was performed one year after treatment and did not show any evidence of cellular seeding and/or new growth throughout the neuraxis.
In the acute setting, combination therapy of G-CSF and intrathecal MSCs could be a safe adjunct to the conventional rehabilitation programs. Further studies are needed to find possible side effects, and establish the efficacy.
Acknowledgements
We are indebted to our patient for his participation in the study and adherence to study protocol.
References
- Bernardo M. E., Zaffaroni N., Novara F., Cometa A. M., Avanzini M. A., Moretta A., et al. (2007). Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Research, 67( 19), 9142– 9149. [DOI] [PubMed] [Google Scholar]
- Callera F., do Nascimento R. X. (2006). Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: a pre-liminary safety study. Experimental Hematology, 34( 2), 130– 131. [DOI] [PubMed] [Google Scholar]
- Derakhshanrad N., Saberi H., Yekaninejad M. S., Eskandari G., Mardani A., Rahdari F., et al. (2013). Safety of granulocyte colony-stimulating factor (G-CSF) administration for postrehabilitated motor complete spinal cord injury patients: an open-label, phase I study. Cell Transplantation, 22( Suppl 1), S139– 146. [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., Curt A., Steeves J. D., Coleman W. P., Tuszynski M. H., Lammertse D., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord, 45( 3), 190– 205. [DOI] [PubMed] [Google Scholar]
- Guest J., Harrop J. S., Aarabi B., Grossman R. G., Fawcett J. W., Fehlings M. G., et al. (2012). Optimization of the decision-making process for the selection of therapeutics to undergo clinical testing for spinal cord injury in the North American Clinical Trials Network. Journal of Neurosurgery: Spine, 17( 1 Suppl), 94– 101. [DOI] [PubMed] [Google Scholar]
- Harrop J. S., Hashimoto R., Norvell D., Raich A., Aarabi B., Grossman R. G., et al. (2012). Evaluation of clinical experience using cell-based therapies in patients with spinal cord injury: a systematic review. Journal of Neurosurgery: Spine, 17( 1 Suppl), 230– 246. [DOI] [PubMed] [Google Scholar]
- Inada T., Takahashi H., Yamazaki M., Okawa A., Sakuma T., Kato K., et al. (2014). Multicenter prospective nonrandomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor for acute spinal cord injury: analyses of follow-up cases after at least 1 year. The Spine Journal (Phila Pa 1976), 39( 3), 213– 219. [DOI] [PubMed] [Google Scholar]
- Jia Y., Wu D., Zhang R., Shuang W., Sun J., Hao H., et al. (2014). Bone marrow-derived mesenchymal stem cells expressing the Shh transgene promotes functional recovery after spinal cord injury in rats. Neuroscience Letters, 573, 46– 51. [DOI] [PubMed] [Google Scholar]
- Kadota R., Koda M., Kawabe J., Hashimoto M., Nishio Y., Mannoji C., et al. (2012). Granulocyte colony-stimulating factor (G-CSF) protects oligodendrocyte and promotes hindlimb functional recovery after spinal cord injury in rats. The Public Library of Science (PLoS One), 7(11), e50391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamouzian S., Nematollahi-Mahani S. N., Nakhaee N., Eskandary H. (2012). Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clinical Neurology and Neurosurgery, 114( 7), 935– 939. [DOI] [PubMed] [Google Scholar]
- Lammertse D., Tuszynski M. H., Steeves J. D., Curt A., Fawcett J. W., Rask C., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord, 45( 3), 232– 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi H., Derakhshanrad N., Yekaninejad M. (2014). Review of recently documented clinical neuroprotective and cellular treatment for spinal cord injury: an analysis of outcomes. Journal of Neurorestoratology, 2, 15– 24. [Google Scholar]
- Saberi H., Derakhshanrad N., Yekaninejad M. S. (2014). Comparison of neurological and functional outcomes after administration of granulocyte-colony stimulating factor (G-CSF) in motor complete vs. motor incomplete post-rehabilitated, chronic spinal cord injuries: a phase I/II study . Cell Transplantation , 23 ( Suppl 1 ), S19– 23, doi: 10.3727/096368914X684943 . [DOI] [PubMed] [Google Scholar]
- Saito F., Nakatani T., Iwase M., Maeda Y., Murao Y., Suzuki Y., et al. (2012). Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restorative Neurology and Neuroscience, 30( 2), 127– 136. [DOI] [PubMed] [Google Scholar]
- Steeves J. D., Lammertse D., Curt A., Fawcett J. W., Tuszynski M. H., Ditunno J. F., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord, 45( 3), 206– 221. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Yamazaki M., Okawa A., Sakuma T., Kato K., Hashimoto M., et al. (2012). Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. European Spine Journal, 21( 12), 2580– 2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski M. H., Steeves J. D., Fawcett J. W., Lammertse D., Kalichman M., Rask C., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord, 45( 3), 222– 231. [DOI] [PubMed] [Google Scholar]