Abstract
In social species, individuals contact members of the same group much more often than those of other groups, particularly for contacts that could directly transmit disease agents. This disparity in contact rates violates the assumptions of simple disease models, hinders disease spread between groups, and could decouple disease transmission from population density. Social behavior of white-tailed deer has important implications for the long-term dynamics and impact of diseases such as bovine tuberculosis and chronic wasting disease (CWD), so expanding our understanding of their social system is important. White-tailed deer form matrilineal groups, which inhabit stable home ranges that overlap somewhat with others—a pattern intermediate between mass-action and strict territoriality. To quantify how group membership affects their contact rates and document the spectrum of social affiliation, we analyzed location data from global positioning system (GPS) collars on female and juvenile white-tailed deer in 2 study areas: near Carbondale in forest-dominated southern Illinois (2002–2006) and near Lake Shelbyville in agriculture-dominated central Illinois (2006–2009). For each deer dyad (i.e., 2 individual deer with sufficient overlapping GPS data), we measured space-use overlap, correlation of movements, direct contact rate (simultaneous GPS locations < 10 m apart), and indirect contact rate (GPS locations < 10 m apart when offset by 1 or 3 days). Direct contact rates were substantially higher for within-group dyads than between-group dyads, but group membership had little apparent effect on indirect contact rates. The group membership effect on direct contact rates was strongest in winter and weakest in summer, with no apparent difference between study areas. Social affiliations were not dichotomous, with some deer dyads showing loose but positive affiliation. Even for obvious within-group dyads, their strength of affiliation fluctuated between years, seasons, and even days. Our findings highlight the poor fit between deer behavior and simple models of disease transmission and, combined with previous infection data, suggest that direct contact is the primary driver of CWD transmission among free-living female and juvenile white-tailed deer.
Key words: contact, disease, global positioning system, group, Illinois, landscape, Odocoileus virginianus, transmission, white-tailed deer
Contact among animals is necessary for the establishment and spread of infectious diseases, and contact patterns can be influenced by a suite of intrinsic and extrinsic ecological factors such as community structure (Dearing et al. 2015), social organization, and landscape structure. Many classical models of disease transmission (Anderson and May 1978; Swinton et al. 2001) treat hosts as if all individuals move and interact independently. The result is density-dependent transmission, characterized by force of infection (probability per unit time of an uninfected host becoming infected) increasing with the number of infectious hosts per unit area and the basic reproductive number of the disease (R 0) increasing with overall host density. In group-living animals, however, contacts within groups are much more frequent than between animals in separate groups (Altizer et al. 2003). If group structure and local spacing among animals are largely independent of overall population density, the concentration of contacts within social groups could decouple contact rates (and hence rate of disease spread) from density, leading to frequency-dependent disease transmission (Getz and Pickering 1983; De Jong et al. 2002; McCallum et al. 2002): force of infection increases with the fraction (“frequency”) of hosts that are infected (also called infection prevalence) and R 0 is not directly tied to host density. Early in an epizootic, infection prevalence and the density of infectious hosts increase similarly, so density- and frequency-dependent transmission yield similar results. During a die-off, however, infection prevalence can increase even as the density of infectious animals drops. Thus, frequency-dependent transmission implies that force of infection can remain high and even increase as a host population dies off, raising the potential of driving the host to local extinction and impairing attempts to control disease by maintaining low host density (May and Anderson 1978; Getz and Pickering 1983; Gross and Miller 2001; Schauber and Woolf 2003; de Castro and Bolker 2005; Potapov et al. 2012). On the other hand, compartmentalization of contacts hinders between-group transmission and generally makes widespread epizootics less likely, especially for directly transmitted pathogens with short infectious periods and hosts with small group sizes (Ball et al. 1997; Cross et al. 2005). Understanding the pattern of contacts within and among social groups, and how those patterns change over time, is important for understanding the potential effects of disease on host populations.
Jolles and Ezenwa (2015) highlight characteristics that make ungulates useful as model species for studying disease, including high population densities, extensive knowledge base, and well-developed management programs. One additional dimension that could be added to their list is that ungulates display a wide range of levels of sociality, from huge herds (e.g., wildebeest, Connochaetes taurinus, and American bison, Bison bison) to extended matrilines (e.g., red deer, Cervus elaphus) to solitary territoriality (e.g., Japanese serow, Capricornis crispus). Thus, this group provides a rich opportunity to assess how social behaviors influence the transmission and population-level impact of disease.
White-tailed deer (Odocoileus virginianus) are an important species ecologically and recreationally, and the implications of their social structure on disease transmission have received increasing attention due to the emergence of bovine tuberculosis and chronic wasting disease (CWD) in free-ranging populations (McCarty and Miller 1998; Gross and Miller 2001; Schauber and Woolf 2003; Grear et al. 2010; Habib et al. 2011; Magle et al. 2013). White-tailed deer exhibit an intermediate level of sociality, with females and their recent offspring forming relatively stable, matrilineal groups and males forming loose bachelor groups (Hawkins and Klimstra 1970; Hirth 1977; Nixon et al. 1991, 1994; Comer et al. 2005). Except during summer parturition period, when females become solitary (Schwede et al. 1993; Bertrand et al. 1996), female offspring often remain associated with their mother's social group, leading to extended matrilines in areas where survival is high (Severinghaus and Cheatum 1956; Hawkins and Klimstra 1970; Nixon et al. 1991, 1992). Older female offspring often separate from the matriline but maintain home ranges that overlap their mothers', as described by the “rose-petal hypothesis” (Porter et al. 1991). Hawkins and Klimstra (1970) studied social organization in a high-density population of white-tailed deer (63 deer/km2) and found that the most common social group size was 4 individuals consisting of an adult female, her 1–2-year-old daughter, and 2 offspring < 1 year old of the older female. Within social groups, the strongest associations are observed between females and their young and between sibling juveniles. Allogrooming is common among deer in social groups (Marchinton and Hirth 1984) and represents a probable route of transmission for many pathogens. During late winter and early spring, white-tailed deer are often seen feeding in large groups that comprise several social groups; however, these unrelated groups congregate only temporarily to feed and often do not bed together (Hawkins and Klimstra 1970). Porter et al. (1991) studied 8 deer family groups comprised of 3–9 individuals each on their study area (deer densities were < 13 deer/km2). Although deer core areas overlapped extensively with those of their group members, they tended not to overlap with those of deer in other groups, indicating that between-group contact among individuals is limited (Porter et al. 1991). Matrilineal home ranges in white-tailed deer tend to be very stable in space even over multiple generations (Nelson and Mech 1999). In some cases, neighboring deer have been slow to reoccupy the home ranges of matrilines removed by culling (McNulty et al. 1997; Kilpatrick et al. 2001), although Henderson et al. (2000) found that neighboring females partially compensate by increased home range size. Unrelated male white-tailed deer > 1 year old form loose groups (Marchinton and Hirth 1984) and tend to be segregated in space and habitat type from female groups in the nonbreeding season (Kie and Bowyer 1999). This social structure places white-tailed deer in an awkward transition zone between individualized behavior amenable to modeling with a mass-action framework (Anderson and May 1978) and formation of discrete herds, which would be amenable to a metapopulation framework (Fulford et al. 2002). Thus, this species represents a challenging scenario for epidemiological modeling.
The stability of group structure and the degree of familiarity and relatedness an individual animal is likely to share with neighbors, particularly among females, can be affected by landscape structure. White-tailed deer thrive in ecotones between forest and other habitats, feeding on a broad range of herbaceous and woody vegetation, including ornamental plants and crops. In agriculture-dominated landscapes, deer have been observed to increase use of fields with standing crops, only to move back to woody cover after crop harvest (Nixon et al. 1991; Vercauteren and Hygnstrom 1998). This seasonal shift in space use could alter the degree of group integrity and intergroup familiarity. Also, woody cover tends to be highly concentrated and linear (e.g., along riparian corridors) in agriculture-dominated landscapes, a pattern that likely concentrates deer activity as well. Such crowding within patches of woody cover could either suppress or increase contact between groups. Finally, dispersal rates and distances of both male and female white-tailed deer are elevated in more agricultural landscapes (Nixon et al. 1991, 2007; Long et al. 2005; Skuldt et al. 2008), and Hirth (1977) observed large mixed-sex aggregations of white-tailed deer more commonly in open landscapes. Frequent encounters with unfamiliar animals could either reduce the compartmentalization of contacts within groups as dispersers join existing groups (albeit more weakly affiliated than the original members), or intensify it as groups attempt to resist interlopers.
We had 2 objectives. Our 1st objective was to quantify the effect of group membership on direct and indirect contact rates among female and juvenile white-tailed deer and test the hypothesis that the effect of group membership on contact rates differed between 2 very disparate landscapes and between seasons. We define direct contact as 2 deer being in close physical proximity and indirect contact as 1 deer visiting a location previously visited by another deer. Our analysis for objective 1 treats affiliation as dichotomous (within or between groups), so our 2nd objective was to evaluate support for dichotomous affiliation against the alternative hypothesis that affiliation is a continuously variable characteristic of 2 animals. We characterized the individual variability and temporal pattern of social affiliation among female and juvenile white-tailed deer to address 3 specific hypotheses: that social affiliation is restricted to group members, that interactions between groups are brief and incidental, and that affiliation strength of within-group dyads would decrease over years (as older daughters become more independent and leave their mother's home range).
Materials and Methods
Study area.
We monitored movements and quantified contact rates among deer (mainly females) inhabiting 2 disparate landscapes in Illinois: an exurban area ~4 km SE of Carbondale (centered around 37°42ʹ14ʺN, 89°9ʹ2ʺE), where high-quality habitat is essentially contiguous (Fig. 1a), and an agriculture-dominated area in and around the Lake Shelbyville State Fish and Wildlife Area (centered around 39°32ʹ48ʺN, 88°39ʹ7ʺE), where woody cover is concentrated along riparian corridors and lakeshores (Fig. 1b). The climate of both areas is characterized by moderate winters and hot, humid summers, with Carbondale and Lake Shelbyville having (respectively) mean January low temperatures of −6.2°C and −6.7°C and mean July high temperature of 31°C and 30°C (Midwest Regional Climate Center 2007). A rectangle containing > 95% of locations for all collared deer in the Carbondale study area covered ~1,900 ha, mostly composed of oak-hickory forest (57%), with some hay fields and other grasslands (26%) and row crop agriculture (12%; primarily planted in soybeans), plus minor components of urban land use and old fields. The Lake Shelbyville study area was much larger, ~18,900 ha, dominated by agriculture (mainly corn and soybean fields; 45%), with lesser coverage by grassland (18%), forest (18%; restricted to the lakeshore and riparian areas), open water (10%), and minor contributions of wetland and human-developed areas. Estimated survival rates of female deer are high in both study areas (87% in the Carbondale area—Storm et al. 2007; 85% in the Lake Shelbyville area—Anderson 2010).
Fig. 1.
Study areas near a) Carbondale and b) Lake Shelbyville, Illinois, for studies of contact and social affiliations among white-tailed deer (Odocoileus virginianus). Rectangles contain nearly all deer locations in each study area; no individual deer used in this study was located outside the rectangle > 5% of the time.
Deer capture.
We captured and handled deer in accordance with the guidelines of the American Society of Mammalogists (Sikes et al. 2011), and our procedures were approved by the Southern Illinois University Carbondale Institutional Animal Care and Use Committee. Deer were captured during October–March, from 2002 to 2006 near Carbondale and from 2006 to 2009 near Lake Shelbyville. We caught deer by using tranquilizer darting (Pneu-dart, Inc., Williamsport, Pennsylvania), modified Clover traps (Clover 1954; Thompson et al. 1989), drop nets (Wildlife Capture Services LLC, Flagstaff, Arizona), and rocket nets (Hawkins et al. 1968) at sites baited with corn and apples. Captured deer were immobilized with an intramuscular injection (3 cc) of a 2:1 mix of Telazol (Tiletamine HCl, 2mg/kg and Zolazepam HCl, 4mg/kg; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) and Rompun (Xylazine HCl, 2mg/kg; Mobay Corporation, Shawnee, Kansas) for darting (Murray et al. 2000) and a 9:1 mix of Ketaset (Ketamine HCl, 10mg/kg; Fort Dodge Laboratories, Inc.) and Rompun for all other methods. Age at capture was determined as juvenile (~0.5 years old), yearling (~1.5 years old), or adult (> 2 years old) based on tooth emergence and wear (Severinghaus 1949). Each captured deer was marked with a uniquely numbered ear tag and either a VHF ear-tag transmitter (Advanced Telemetry Systems, Inc., Isanti, Minnesota; 13g), a GPS collar (Telonics, Inc., Mesa Arizona; 700g), or a VHF radiocollar (Advanced Telemetry Systems, Inc.; 500g). We deployed GPS collars on juveniles and older females because we were focusing on contacts within and among matrilineal social groups.
Collars deployed in 2002 and 2003 recorded locations hourly and we programmed their release mechanisms to drop off on 1 June, after 4–5.5 months. In subsequent years, we programmed collars to record deer locations every 2h (except every 1h during November and December) and to drop off after 12–17 months. We programmed all collars to determine their locations within the same 3-min windows. These collars in a stationary position under closed canopy yielded a median position error of 8.8 m (Schauber et al. 2007).
For this paper, we used GPS data from 27 female deer and 1 male juvenile from the Carbondale study area, which were monitored for periods of 1–16 months between October 2002 and May 2006, providing between 310 and 10,493 locations per deer (Fig. 2a). In the Lake Shelbyville area, we used data from 19 females and 1 male juvenile equipped with GPS collars. These deer were monitored for periods of 2 to > 26 months from January 2006 until May 2009, providing between 455 and 8,596 locations per deer (Fig. 2b). We excluded data from 6 additional GPS-collared female deer in the Lake Shelbyville area, due to collar malfunction, very short periods of data collection, or spatial isolation from all other GPS-collared deer. Also, we excluded data from the first 3 days after capture and any locations with estimated elevations > 100 m different from the known elevation of the study area.
Fig. 2.
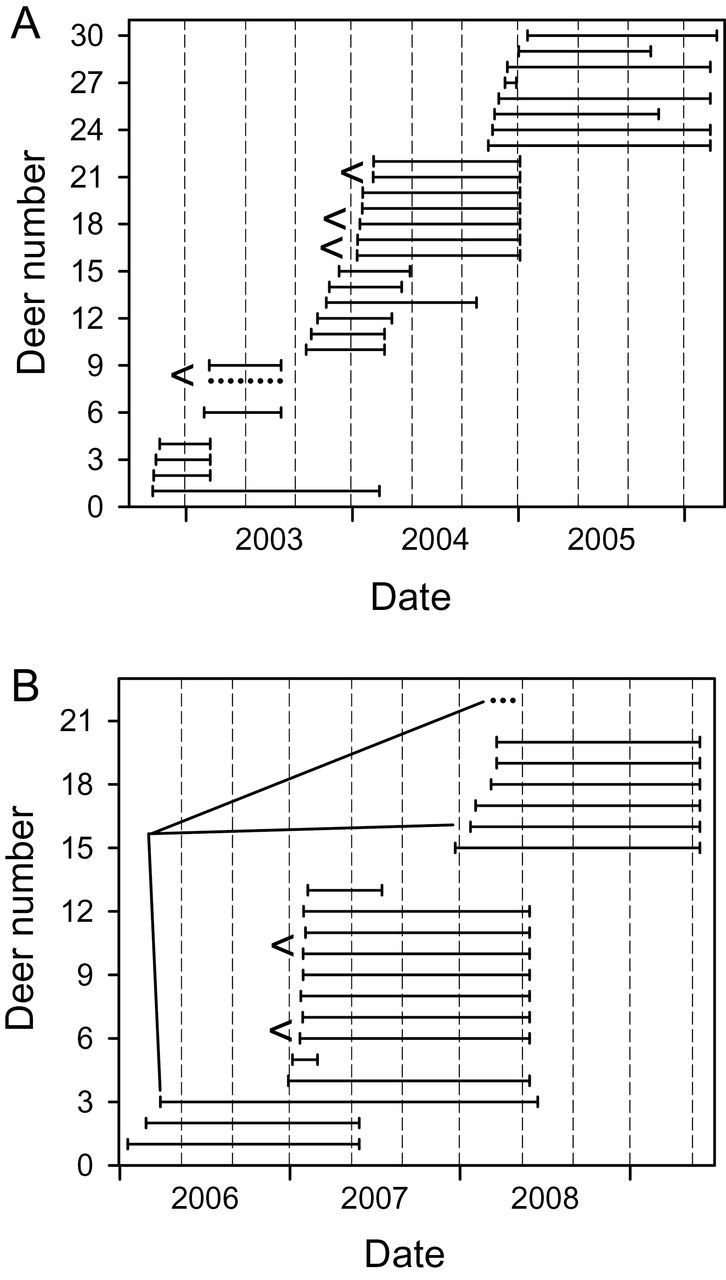
Timeline of data collection for female and juvenile white-tailed deer (Odocoileus virginianus; females = solid lines, juvenile males = dotted lines) equipped with GPS collars in 2 study areas and periods: a) near Carbondale, Illinois, 2002–2006 and b) near Lake Shelbyville, Illinois, 2006–2009. Timelines for deer in the same social group are connected by slanting lines “<”). Vertical dashed lines demarcate 3 seasons per year (winter–spring, summer, and autumn) for analysis.
Objective 1.
To quantify the effect of social group membership on contact rate and test whether that effect differed between study areas and seasons, we based our approach on that of Schauber et al. (2007). Our unit of analysis was a dyad, consisting of 2 deer with sufficient overlapping data (≥ 200 simultaneous GPS locations) in a seasonal period. From n deer with overlapping GPS data in a seasonal period, the maximum number of possible dyads is (n 2–n)/2. However, as detailed below, we generally included fewer than that maximum in our analyses. We tested for main and interactive effects of group status, study area, and season on dyadwise direct and indirect contact rates, after accounting for overlap of space use and temporal autocorrelation. We broke the year up into 3 biologically relevant seasons: summer (15 May–31 August, when females are solitary for parturition and neonatal care), autumn (1 September–31 December, when female groups re-form and mating behavior occurs), and winter–spring (1 January–14 May, when breeding is completed and feeding congregations occur). It is important to note that we use the term “season” to indicate the time of year in general (e.g., autumn) and “seasonal period” to indicate time in a particular year (e.g., autumn 2004).
We then defined within-group dyads (i.e., 2 members of the same group) based on overlapping home ranges and highly correlated movements. To do so, we summed the Universal Transverse Mercator x and y coordinates for each deer (i) at each time (t) to give a single value for each location (i.e., z i[t] = x i[t] + y i[t]) and calculated the Pearson correlation coefficient (r) of those summed coordinates for each dyad over a given seasonal period (i.e., correlation between z i and z j for all t in that seasonal period). Within-group dyads had exceptionally high correlation values (r > 0.45; Fig. 3) and all others were considered between-group dyads. It is important to note that 2 animals do not need to be close to each other for their movements to be highly correlated, so testing whether group membership (defined by movement correlation) influences contact rates is not logically circular. For example, adding any constant value to x-coordinates of 1 deer will change their distance to the locations of another deer but will not change the movement correlation coefficient for the dyad. Our criterion for group membership is admittedly arbitrary but reflects the apparent line between “typical” and outlying correlation values. If a dyad met this criterion in 1 seasonal period, we considered it to be a within-group dyad in all seasonal periods because groups break up during summer, but we assume that the members remain familiar with each other and are not likely to respond to each other the same as to nonmembers. We identified 4 within-group dyads representing 4 separate groups in the Carbondale area and 5 within-group dyads representing 3 separate groups (1 group contained 3 collared deer, providing 3 possible dyads) in the Lake Shelbyville area. One within-group dyad in Carbondale continued to act as group members during October 2004–January 2005 (when together), even though 1 member moved approximately monthly between 2 home ranges separated by approximately 2 km. For this deer with 2 home ranges, we included only data before autumn 2004 in contact analyses.
Fig. 3.
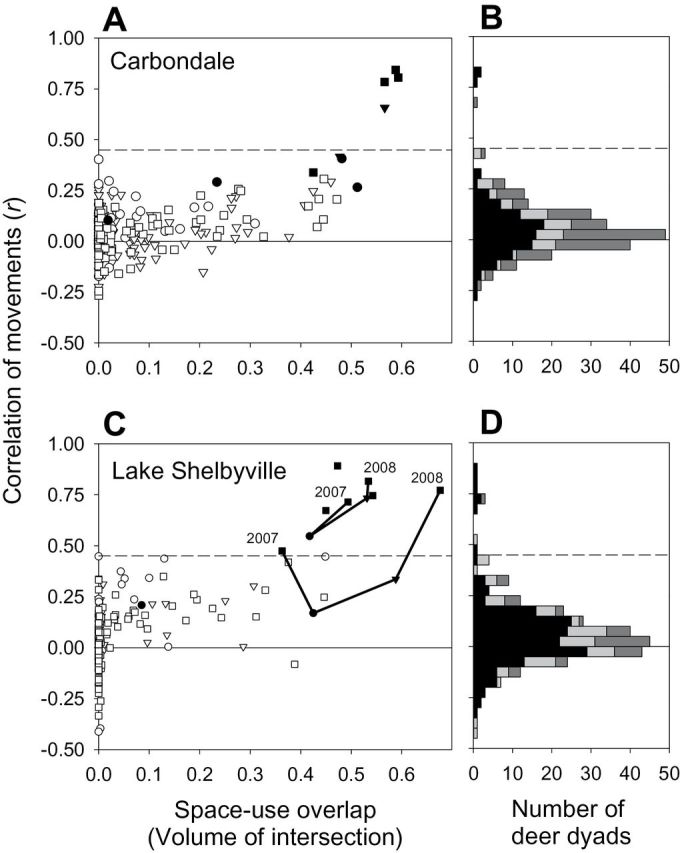
Movement and space-use criteria used to assess group membership status of GPS-collared white-tailed deer (Odocoileus virginianus) from study areas near a and b) Carbondale, Illinois, (October 2002–June 2006) and b) and c) Lake Shelbyville, Illinois (January 2006–June 2009). a and c) Relationship between correlation of movements and space-use overlap (measured by volume of intersection of fixed-kernel utilization distributions). Each symbol represents 1 deer dyad and seasonal period (triangle = autumn, square = winter–spring, circle = summer). Symbol fill indicates the group status of the dyad (filled = within-group, open = between-group). Note that the same dyad may be present in > 1 seasonal period. Two within-group dyads from Lake Shelbyville site provided data from February 2007 to June 2008; sequential data points for each of these dyads are connected by lines, allowing comparison between years. b and d) Frequency distribution of correlation coefficients for seasonal movement (black = winter–spring, light gray = summer, dark gray = autumn), indicating the rarity of coefficients > 0.45 (dashed line indicates the criterion for within-group status).
High within-group contact rates could simply be due to group members sharing the same home range. Also, our 2 study areas differed greatly in spatial extent, so an apparently lower contact rate in 1 study area could simply be due to collared deer being more widely separated. Therefore, we quantified the overlap of space use for each dyad and seasonal period by the volume of intersection (VOI—Millspaugh et al. 2004) of their fixed-kernel utilization distributions, fitted to a random sample of 200 locations per deer and seasonal period via least-squares cross-validation in the Home Range Tools (Rodgers et al. 2005) extension of ArcGIS (ESRI 2006). Two deer with identical space use (i.e., equal probability of being found in each point in space) would have VOI = 1, whereas completely disjoint home ranges result in VOI = 0.
We measured direct and indirect contact rates for each dyad and seasonal period (Table 1) by the fraction of the temporally paired (simultaneous or offset 1 or 3 days) GPS locations of that dyad that were < 10 m apart in space. Schauber et al. (2007) examined proximity criteria ranging from 10 to 100 m, and time offsets for indirect contacts from 1 to 30 days. For this analysis, we focused on the 10-m criterion because we expected it to be more indicative of potential disease transmission than greater distances would, whereas a smaller criterion would decrease sample size of observed contact events and likely yield little additional information because of imprecision of the GPS locations. We considered indirect contact to occur by 1 deer (“donor”) leaving behind pathogens that could infect another deer (“recipient”) that visits the same location at a later time. We calculated indirect contact rate was calculated by the fraction of locations of recipient deer i that were < 10 m from a prior location (1 or 3 days before) of a potential donor deer j. We chose these time offsets because Schauber et al. (2007) found little difference in the pattern of indirect contacts for offsets > 3 days. Because indirect contact rates are not reciprocally identical, we randomly selected 1 member of each dyad to serve as the recipient.
Table 1.
Number of white-tailed deer (Odocoileus virginianus) dyads (2 deer with sufficient GPS location data) from study areas near Carbondale and Lake Shelbyville, Illinois, 2002 to 2009, used in analyses of contact rate. Within-group dyads were identified by extensive overlap of space use and highly correlated movements, as described in text. Note that the same dyad may be present in > 1 seasonal period.
| Year | Season | Study area | Within-group dyads | Between-group dyads |
|---|---|---|---|---|
| 2002 | Autumn | Carbondale | 0 | 6 |
| 2003 | Winter–spring | Carbondale | 1 | 9 |
| Summer | Carbondale | 1 | 3 | |
| Autumn | Carbondale | 0 | 21 | |
| 2004 | Winter–spring | Carbondale | 3 | 51 |
| Summer | Carbondale | 3 | 10 | |
| Autumn | Carbondale | 2 | 47 | |
| 2005 | Winter–spring | Carbondale | 0 | 21 |
| Summer | Carbondale | 0 | 21 | |
| Autumn | Carbondale | 0 | 21 | |
| 2006 | Winter–spring | Carbondale | 0 | 10 |
| 2006 | Winter–spring | Lake Shelbyville | 0 | 0 |
| Summer | Lake Shelbyville | 0 | 0 | |
| Autumn | Lake Shelbyville | 0 | 0 | |
| 2007 | Winter–spring | Lake Shelbyville | 2 | 55 |
| Summer | Lake Shelbyville | 2 | 28 | |
| Autumn | Lake Shelbyville | 2 | 21 | |
| 2008 | Winter–spring | Lake Shelbyville | 5 | 66 |
| Summer | Lake Shelbyville | 1 | 15 | |
| Autumn | Lake Shelbyville | 0 | 15 | |
| 2009 | Winter–spring | Lake Shelbyville | 0 | 15 |
For each measure of contact rate, we used generalized linear mixed models (Cross et al. 2012) to test for main and interactive effects of group status (within-group or between-group), study area, and season (winter–spring, summer, or autumn). Specifically, we used mixed-model logistic regression (PROC GLIMMIX in SAS; SAS Institute 2008) and set α = 0.05. Each deer dyad was treated as a statistical subject, and only 1 member of each social group was selected for consideration of between-group contacts (i.e., between-group dyads including other members of that group were not included) because behaviors of members of the same group are not independent. We used a total of 111 between-group dyads from Carbondale and 128 between-group dyads from Lake Shelbyville used in analyses (Table 1). To account for nonindependence of data, intercept and the effect of seasonal period (e.g., autumn 2002) were random effects, varying among dyads (SAS syntax: “Random intercept period/Subject = dyad”). To factor out the effects of overlap of space use and temporal autocorrelation, we also included the fixed-effect covariates VOI, VOI2 (to allow for nonlinearity), and a binary variable (Contactt−1) indicating whether the most recent locations for that dyad constituted a contact. We used the “DDFM = BETWEENWITHIN” option for assigning denominator degrees of freedom for tests of fixed effects: VOI, VOI2, Contactt−1, season (winter–spring, summer, or autumn), and any interactions involving season were treated as within-subject effects (i.e., can take multiple values for each dyad), whereas group status and study area were treated as between-subject effects (only 1 value for each dyad). Starting with the full model, we sequentially removed interactions with P > 0.1, beginning with the 3-way interaction (group status × season × study area). All main effects remained in the statistical model.
Objective 2.
To characterize the individual variability and temporal pattern of social affiliation among female and juvenile white-tailed deer, we plotted the relationship between VOI and correlation of movement at the time scale of a seasonal period and also examined affiliation at a finer temporal scale by measuring the distance between the deer in each dyad at each GPS location time and by measuring the correlation of movements within a 3-day moving window. We used these data to address (by seeking counter examples) 3 a priori hypotheses: 1) that only deer dyads that are clearly members of the same group or that have extensive space-use overlap would show statistically significant positive correlation of movements; 2) that encounters between deer of different social groups would be brief and incidental; and 3) that affiliation strength of within-group dyads would decrease over years.
Results
Objective 1.
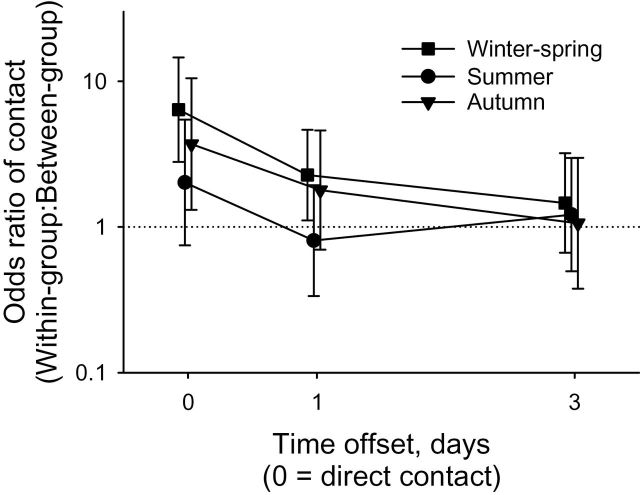
Not surprisingly, dyadwise contact rates (both direct and indirect) were higher when the 2 deer had been in contact at the previous time step (i.e., Contactt−1 = 1), and contact rates increased with increasing overlap of space use (VOI; Table 2). All interaction terms involving study area were dropped from the statistical models because they had high P-values (> 0.1); the main effect of study area was also statistically nonsignificant for all contact rates, direct and indirect (Table 2). After accounting for effects of VOI and Contactt−1, we found direct contact rates were higher for within-group than between-group dyads and we found marginal evidence that the effect of group status differed among seasons (Table 2). The effect of group status on direct contact rate (i.e., odds ratio within-group:between-group) appeared to be greatest in winter–spring (odds ratio = 6.4) and weakest in summer (odds ratio = 1.5 with confidence interval including 1.0; Fig. 4). We found no statistically significant main or interactive effects of group status or study area on indirect contacts, although the season × group status was marginally nonsignificant for indirect contacts with a 1-day offset (Table 2). In qualitative agreement with earlier analyses based on only part of the Carbondale data (Schauber et al. 2007), the effect of group status was substantially (2.5- to 3.2-fold) greater for direct than indirect contacts (Fig. 4), with only weak evidence for an effect of group status on indirect contacts with 1-day offset and no evidence for an effect of group status on 3-day indirect contacts (Table 2).
Table 2.
Results of mixed-model logistic regression testing factors hypothesized to affect dyadwise direct and indirect contacts rates (simultaneous or offset locations < 10 m apart) among GPS-collared white-tailed deer (Odocoileus virginianus) at 2 Illinois study areas: near Carbondale (2002–2006) and near Lake Shelbyville (2006–2009).
| Explanatory variable | Response variable (contact rate) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Direct (no offset) | Indirect (1-day offset) | Indirect (3-day offset) | |||||||
| d.f. | F | P | d.f. | F | P | d.f. | F | P | |
| VOIa | 1,178 | 139.01 | < 0.0001 | 1,178 | 112.53 | < 0.0001 | 1,178 | 103.1 | < 0.0001 |
| VOI2 | 1,178 | 39.75 | < 0.0001 | 1,178 | 51.94 | < 0.0001 | 1,178 | 37.75 | < 0.0001 |
| Contactt−1 b | 1,45 | 331.46 | < 0.0001 | 1,38 | 52.39 | < 0.0001 | 1,38 | 51.47 | < 0.0001 |
| Study area | 1,219 | 0.01 | 0.91 | 1,219 | 0.48 | 0.49 | 1,219 | 0.12 | 0.73 |
| Season | 2,178 | 0.01 | 0.99 | 2,178 | 1.99 | 0.14 | 2,180 | 3.28 | 0.040 |
| Group statusc | 1,219 | 10.85 | 0.0012 | 1,219 | 1.44 | 0.23 | 1,219 | 0.58 | 0.45 |
| Season × (group status) | 2,178 | 2.95 | 0.055 | 2,178 | 2.48 | 0.086 | |||
a VOI = volume of intersection between fixed-kernel utilization distributions of a deer dyad in season.
b Binary variable indicating whether a deer dyad's most recent pair of locations constituted a contact.
c Within-group or between-group.
Fig. 4.
Estimated effect of group status (within-group or between-group) of white-tailed deer (Odocoileus virginianus) dyads on odds of contact (GPS locations for a deer dyad < 10 m apart, direct = simultaneous locations [0 days], indirect = 1 or 3 days apart), as a function of season and study area in Illinois. Odds ratio of 1.0 indicates no effect of group status. Error bars are 95% confidence intervals.
Objective 2.
A stereotypical within-group dyad showed consistently close proximity (simultaneous locations mostly < 100 m apart) and high correlation of movements during winter, gradual weakening of affiliation during late spring, and an abrupt separation around June, the typical time of parturition. Our most cohesive dyad, in which both deer had been collared as yearlings, resumed close affiliation by the end of July and maintained it through the autumn (Fig. 5A). However, some within-group dyads did not resume close affiliation until late autumn or winter (Fig. 5B). By contrast, deer in a stereotypical between-group dyad rarely showed close proximity or strong correlation of movements, even those dyads with moderate to high VOI (Fig. 6A). However, we found a wide range of variation between these stereotypical patterns, including dyads that neatly straddled the dichotomy, showing a moderate tendency to remain in close proximity but spending a substantial amount of time apart, with moderate positive correlations of movement (Fig. 6B). Such dyads did not meet our criteria for being categorized as within-group, but they clearly were not behaving independently.
Fig. 5.

Affiliation strength for 2 within-group dyads of GPS-collared female white-tailed deer (Odocoileus virginianus) in Illinois, measured by distances (m) between simultaneous locations (black line indicates 3-day running median) and correlations of movements over a 3-day moving window. Seasons for analysis are demarcated by dashed vertical lines. A) The most strongly affiliated dyad we observed, both collared as yearlings at the Lake Shelbyville study area, with only a brief separation during summer parturition. B) An adult dyad near Carbondale showing high cohesion in winter–spring followed by separation during summer parturition, but which did not resume strong affiliation until late autumn–early winter.
Fig. 6.
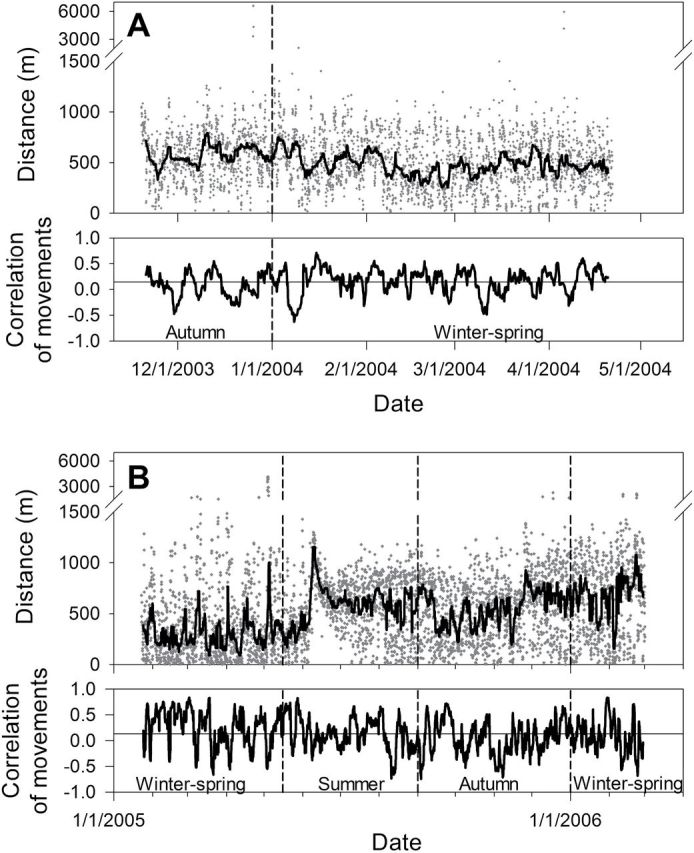
Affiliation strength for 2 between-group dyads (seasonal correlation of movements < 0.45) of GPS-collared female white-tailed deer (Odocoileus virginianus) near Carbondale, Illinois, measured by distances (m) between simultaneous locations (black line indicates 3-day running median) and correlations of movements over a 3-day moving window. Seasons for analysis are demarcated by dashed vertical lines. A) An adult pair showing a stereotypical pattern of independent movement, with the 2 deer rarely in proximity. B) An adult dyad showing intermediate level of affiliation, with substantial, but not overwhelming amount of time spent in close proximity and moderate correlation of movements, particularly during winter–spring 2005.
Our data did not support the hypothesis that social affiliation is restricted to group members. Even for dyads that were clearly members of separate groups, seasonal movement correlation coefficients were not centered about zero but had an excess of positive values (Fig. 3b and 3d). Given the large sample sizes, even a slight positive correlation can be statistically significant (e.g., with n = 1,000, r > 0.07 will yield P < 0.05). This excess of positive correlations of movement was apparent even for deer dyads whose home ranges overlapped only slightly, as with VOI values as low as 0.1 near Carbondale and 0.03 near Lake Shelbyville (Fig. 3a and 3c). In both study areas, movement correlations increased dramatically for VOI > 0.4, suggesting a threshold separating within-group from between-group dyads (Fig. 3a and 3c). Therefore, although strongly correlated movements were indicative of group membership, deer from neighboring groups also showed weakly but positively correlated movements.
Our data also did not support the hypothesis that direct contacts for between-group dyads would be brief and incidental. Close examination of proximity and correlation data from between-group dyads often showed periods when the 2 deer moved in concert and in close proximity, sometimes remaining together for days. One dyad, for example, exhibited a repeated, almost cyclic pattern of spending 1–3 days in close affiliation, separating for several days, and then resuming close affiliation (Fig. 7). In this dyad, 1 member had a larger home range that almost completely encompassed the home range of the other, and the periods of affiliation occurred when the wider ranging individual occupied the zone of home range overlap.
Fig. 7.
Short-term variation in affiliation strength for 1 between-group (seasonal correlation of movements < 0.45) dyad of GPS-collared female white-tailed deer (Odocoileus virginianus) during January–April 2005 near Carbondale, Illinois, measured by distances (m) between simultaneous locations (black line indicates 3-day running median) and correlations of movements over a 3-day moving window.
Finally, our data did not support the hypothesis that affiliation strength of within-group dyads would decrease over years. We collected > 12 months of simultaneous data only for 2 within-group dyads (one shown in Fig. 5A), both in the Lake Shelbyville study area, with repeat data collected during February–June of 2007 and 2008. Both dyads showed stronger affiliation in the 2nd year than the 1st year: greater VOI and stronger correlation of movements (Fig. 3c), plus lower median distance between simultaneous locations (30.6 m in 2007 to 18.4 m in 2008 for yearling–yearling dyad; 268.7 m in 2007 to 78.0 m in 2008 for adult–adult dyad). Another piece of evidence against this hypothesis was a dyad in the Carbondale study area that started with moderate VOI and affiliation strength in winter–spring 2004, but 1 deer moved to a new home range > 2 km away during May 2004, presumably to give birth. This dispersing deer returned in October, and then alternated approximately monthly between these 2 disjunct home ranges during October–December 2004. Normally, within-group pairs showed stronger affiliation in winter–spring than in autumn, but when this dyad were together in autumn they were more strongly affiliated than they had been in the previous winter–spring.
Discussion
The strength and pattern of social affiliation among animals can influence the prevalence of infectious diseases as well as the impact of disease at the population level. Group-living species often have higher risk of parasitism by a more diverse suite of parasites than solitary species (Altizer et al. 2003). On the other hand, strong compartmentalization of infectious contacts within groups can reduce mean infection prevalence and the population-level impact of disease, despite rapid within-group spread (Blower and McLean 1991; Cross et al. 2005). Culling of European badgers (Meles meles) to control bovine tuberculosis has shown that disruption of stable groups can increase between-group transmission even as overall host density drops (Tuyttens et al. 2000; Vicente et al. 2007). Therefore, understanding the strength and stability of social affiliations among mammals provides key information to understanding and predicting the spread and impact of infectious disease.
Relating social contact patterns to infection patterns can help shed light on fundamental questions of wildlife disease dynamics. For example, CWD can be transmitted by both direct and indirect routes and indirect transmission of CWD appears to be common in captive cervids; however, the relative importance of direct and indirect contact for free-living cervid populations is unknown (Miller and Williams 2003; Miller et al. 1998, 2000, 2004, 2006). Grear et al. (2010) examined patterns of CWD infection in female white-tailed deer harvested in south-central Wisconsin and found that the presence of a closely related female deer infected with CWD in close proximity increased the odds of being infected by > 100-fold relative to the presence of an unrelated infected female (ln odds ratio [β] = 4.93 for related and 0.09 for unrelated, exp[4.93−0.09] = 126). Their findings indicate that CWD transmission occurs much more readily between members of the same matrilineal social group than between groups or from males to females. Combining that result with our data indicating that the distinction between within- and between-group contact rates is much stronger for direct than indirect contacts, we find that the evidence is most consistent with the hypothesis that direct transmission is the dominant mode of CWD spread among free-living female white-tailed deer, at least at the present stage of the epizootic. Because prions that cause CWD can persist for long periods in the environment (Miller et al. 2004; Pedersen et al. 2006), the relative importance of indirect transmission could increase as time progresses and infection prevalence increases (Almberg et al. 2011). However, recent research suggests that prion infectivity is substantially higher via inhalation of aerosol than via ingestion (Denkers et al. 2013), again pointing to a key role for close physical proximity in CWD transmission.
Grear et al. (2010) estimated a much larger effect of group membership (as indicated by genetic relatedness) on CWD infection odds than we found for odds of GPS locations being < 10 m apart, which suggests that our approach underestimates the effect of group membership on direct contact rates relevant to disease transmission. Indeed, it seems reasonable that if 2 deer are < 10 m apart, they would be more likely to come into actual physical contact if they are members of the same group than if they are members of different groups. Such an underestimate is supported by preliminary results from deer carrying both GPS collars and proximity loggers that detect only very close contacts (< 1 m), which suggest that deer from neighboring groups are frequently found < 10 m apart but rarely come into close physical contact (Tosa et al. 2015). Future work, incorporating direct visual observations and close-range proximity detectors, will enable us to test this expectation.
Both our study and that of Grear et al. (2010) suffer from limitations related to indentifying social group membership. We used movement behavior of each pair of deer to assess group membership. However, social affiliations did not fall neatly into 2 categories—some deer dyads exhibited occasional periods of highly correlated movements in close proximity interspersed with periods of independent movements. This lack of a clear dichotomy is likely the reason our estimates of the effect of group membership on direct contact rates based on this full data set from 2 study areas are lower than estimates (up to 22-fold higher odds of direct contact for within-group dyads) based on part (up to January 2005) of the Carbondale data set (Schauber et al. 2007). Several of the between-group dyads collared in 2005–2006 exhibited clear evidence of familiarity and temporary affiliation (as in Fig. 7) but never met our criteria for behaving as a group. Conversely, some dyads we identified as within-group only exhibited strong affiliation during winter–spring. Grear et al. (2010) used genetic relatedness as an indicator of potential social interaction, but closely related deer may not necessarily behave as a group and not all members of a group may be relatives. We are beginning genetic analysis of samples collected from Illinois deer, which will enable us to directly compare behavioral observations from the deer in our study with the degree of genetic relatedness and to assess the level of concordance provided by these 2 approaches to identifying groups.
Deer in our study with high levels of overlap in space use had the highest contact rates and were the most likely to be part of the same group, as we have reported previously (Schauber et al. 2007; Kjær et al. 2008). This finding is eminently sensible and agrees with the recent results of Robert et al. (2012), who found that VOI is strongly related to contact frequency among raccoons (Procyon lotor), and Magle et al. (2013), who found that mean VOI is considerably greater for closely related white-tailed deer dyads than for dyads with lower genetic relatedness. However, it is important to note that VOI (and contact patterns) are strongly seasonal and deer that form a highly cohesive group in winter may act independently during autumn. Also, data reported by Magle et al. (2013) show a substantial amount of scatter around this relationship, such that some close relatives have little overlap of space use, and some apparently unrelated dyads have VOI > 0.8. Thus, genetic relatedness and space use are correlated but not interchangeable indices of potential contact.
We found no evidence that overall contact rates or the effect of group membership on contact rates differed between the Lake Shelbyville and Carbondale study areas, despite the prominent differences in spatial extent and landscape characteristics. Upon initial look, this finding contrasts with Habib et al. (2011), who found that between-group contact rates for pairs of white-tailed deer were negatively related to the percentage of forest cover in the landscape. However, they found that this reduction in contact rates was tied to a reduction in home range size and between-group home range overlap. Our analysis statistically removes the effects of overlap when quantifying the effects of group membership, so the 2 results are not actually contradictory. Removing the effects of overlap allows us to focus specifically on interactions based on relationship status, whereas analyzing gross contact rates (without removing the effect of overlap) provides a more direct look at transmission potential. The consistency we observed provides confidence that the same relationships between contact rates and space use overlap, season, and group membership hold across a wide range of habitat conditions.
Similar to Schauber et al. (2007) and Kjær et al. (2008), we found that the effect of group membership on direct contact rates was strongest in winter–spring and weakest in summer. The weak effect of group membership on contact rates during summer undoubtedly stems from the territorial behavior of female white-tailed deer near and after parturition toward familiar and unfamiliar deer alike (Schwede et al. 1993; Bertrand et al. 1996). The stronger compartmentalization of contacts within groups during winter seems contrary to the common observation that female and juvenile white-tailed deer can be found in large multigroup aggregations in winter and early spring (Hawkins and Klimstra 1970; Hirth 1977; Lingle 2003). Hawkins and Klimstra (1970) indicated that even though groups may feed with other groups at this time of year, they retain group identity and cohesion, which could account for our results. Autumn is the breeding season for white-tailed deer, which are polygynous. We previously reported that rates of direct contact between female deer from different groups in the Carbondale study area were greatest in autumn compared with other seasons, which we had attributed to increased activity associated with breeding behavior (Kjær et al. 2008). However, another explanation for our results is our decision to treat a dyad as within-group in all seasonal periods if it met our criteria for group membership in at least 1 period. Our rationale for doing so is that being members of the same group is likely to confer a degree of familiarity that colors behavioral interactions at other times (e.g., during the summer social breakdown). Several within-group dyads in our study maintained relatively independent movements during autumn before exhibiting high space-use overlap and tightly correlated movements in winter. Thus, we may have labeled a dyad “within-group” that only became associated during the winter period of larger aggregations.
Close examination of pairwise movement patterns of deer in our study revealed the difficulty of distinguishing within-group from between-group interactions. We observed a nearly continuous distribution of movement correlations, and even dyads that were clearly affiliated did so with a strength that varied substantially over time scales ranging from years down to days. The most consistent pattern was a breakup of social structure during summer, as is typical for the species (Bertrand et al. 1996), but previously affiliated deer sometimes did not reestablish strong affiliation until late autumn or winter. Hawkins and Klimstra (1970) provided detailed observations of social affiliations among female and juvenile white-tailed deer and reported that females and their young show strongest affiliations, but pairs of females typically separate by the time the younger member reach 3 years of age. Therefore, we expected that within-group dyads would show a general pattern of weakening affiliation as years passed (i.e., group dynamics would be dominated by fission rather than a balance of fission and fusion). Our data to address this expectation were limited to 2 dyads but both showed the opposite pattern, with stronger affiliation in the 2nd year. Therefore, a dyad with relatively weak affiliation one year could turn out to be members of the same group in later years. Several deer dyads showed strong social affiliation during the times when they shared space, but 1 individual alternated its space use between shared and unshared space at time scales ranging from days to months. Because we did not collar all deer in the study areas, we do not know if these alternating deer acted as members of other groups in the other portions of their use areas.
As is common in science, this analysis raises more questions than it answers: how do the familial relationships among these deer map onto patterns of social affiliation? For example, were deer dyads that showed moderate levels of affiliation but did not meet our criterion for “within-group” status composed of close relatives? Magle et al. (2013) observed that some deer dyads with high VOI are unrelated. This begs the question of whether, at a given level of space-use overlap, variation in social affiliation among dyads is explained by genetic relatedness. An additional question is whether deer that alternate between separate home ranges or parts of their home range maintain separate sets of social affiliations. The answers have important implications for the rate and pattern of disease spread within and among deer groups. For example, even if maintaining separate home ranges is a rare behavior for female white-tailed deer, individuals that do so could be disproportionately important for between-group transmission and geographic spread, particularly if they have strong affiliations in > 1 area.
Our results indicate that white-tailed deer show a clear distinction in their direct contact patterns based on group affiliations, but the strength of those group affiliations can fluctuate on time scales ranging from years to days. We did not see an overall tendency toward reduced social affiliations between years, and temporary dispersal appeared to increase social affiliation when the wayward deer returned to its original home range. In the context of disease ecology, our findings imply that direct disease transmission among deer is likely to be compartmentalized within groups, whereas indirect transmission of disease (e.g., via environmental contamination or sessile vectors) is probably determined by space-use overlap rather than social affiliation. Our results also indicate that the same pair of deer may move largely independently 1 year and then show greater affiliation in the next, providing avenues for between-group transfer of long-lived infections such as CWD and bovine tuberculosis. As Cross et al. (2005) emphasized, the importance of social structuring on disease dynamics depends on the frequency of between-group contact (especially joining other groups) and group size relative to the typical duration of infectiousness. Strong compartmentalization of contacts within small groups can greatly reduce the chances of successful establishment and large-scale epizootics of “fast” diseases (those with short infectious period), but chronic diseases with extended infectious periods are much less impeded by group structure. CWD and bovine tuberculosis are both “slow” diseases of deer with infectious periods > 1 year and the potential for indirect transmission (Clifton-Hadley and Wilesmith 1991; Miller et al. 2000; Williams et al. 2002), suggesting a smaller role for social structure and the potential utility of simpler disease models. However, the much higher transmission of CWD between related than unrelated female white-tailed deer (Grear et al. 2010) and the fact that white-tailed deer typically live in smaller and more stable groups than mule deer (O. hemionus—Lingle 2003) point to the strong possibility that social structure may affect dynamics of even chronic diseases. Disparate transmission within groups compared to between groups is a condition that could promote frequency-dependent transmission; however, it is not a sufficient condition, as between-group transmission is necessary for the disease to persist and is more likely to depend on local population density. Overall, female and juvenile white-tailed deer show compartmentalization of contacts within groups, but group membership is not always clear and is sometimes temporary. Mechanistic models of group formation and between-group contact are likely to be key in predicting the long-term dynamics and impacts of disease on their white-tailed deer populations.
Acknowledgments
Primary funding for this research was provided by the Illinois Department of Natural Resources through the Federal Aid in Wildlife Restoration Program, Project W-87-R, with additional support from the Southern Illinois University, Carbondale Graduate School, and the Cooperative Wildlife Research Laboratory. We thank the United States Army Corps of Engineers, Illinois Department of Natural Resources, and cooperating landowners for property access and logistical support. We thank the myriad field assistants, graduate students, and volunteers who helped conduct these studies. We appreciate R. Ostfeld inviting us to be part of the symposium “Interactions between Mammals and their Pathogens” at the 92nd Annual Meeting of the American Society of Mammalogists in 2012, which gave rise to this Special Feature.
Literature Cited
- Almberg E. S., Cross P. C., Johnson C. J., Heisey D. M., Richards B. J. 2011. Modeling routes of chronic wasting disease transmission: environmental prion persistence promotes deer population decline and extinction. PLoS One 6:e19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S., et al. 2003. Social organization and parasite risk in mammals. Annual Review of Ecology, Evolution, and Systematics 34:517–547. [Google Scholar]
- Anderson C. W. 2010. Ecology and management of white-tailed deer in an agricultural landscape: analyses of hunter efficiency, survey methods, and ecology. Ph.D. dissertation, Southern Illinois University, Carbondale. [Google Scholar]
- Anderson R. M., May R. M. 1978. Regulation and stability of host-parasite population interactions: I. Regulatory processes. Journal of Animal Ecology 47:219–247. [Google Scholar]
- Ball F., Mollison D., Scalia-Tomba G. 1997. Epidemics with two levels of mixing. Annals of Applied Probability 7:46–89. [Google Scholar]
- Bertrand M. R., DeNicola A. J., Beissinger S. R., Swihart R. K. 1996. Effects of parturition on home ranges and social affiliations of female white-tailed deer. Journal of Wildlife Management 60:899–909. [Google Scholar]
- Blower S. M., McLean A. R. 1991. Mixing ecology and epidemiology. Proceedings of the Royal Society of London, B. Biological Sciences 245:187–192. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley R. S., Wilesmith J. W. 1991. Tuberculosis in deer - a review. Veterinary Record 129:5–12. [DOI] [PubMed] [Google Scholar]
- Clover M. R. 1954. A portable deer trap and catch-net. California Fish and Game 40:367–373. [Google Scholar]
- Comer C. E., Kilgo J. C., D'Angelo G. J., Glenn T. C., Miller K. V. 2005. Fine-scale genetic structure and social organization in female white-tailed deer. Journal of Wildlife Management 69:332–344. [Google Scholar]
- Cross P. C., Lloyd-Smith J. O., Johnson P. L. F., Getz W. M. 2005. Duelling timescales of host movement and disease recovery determine invasion of disease in structured populations. Ecology Letters 8:587–595. [Google Scholar]
- Cross P. C., Creech T. G., Ebinger M. R., Heisey D. M., Irvine K. M., Creel S. 2012. Wildlife contact analysis: emerging methods, questions, and challenges. Behavioral Ecology and Sociobiology 66:1437–1447. [Google Scholar]
- De Castro F., Bolker B. M. 2005. Mechanisms of disease-induced extinction. Ecology Letters 8:117–126. [Google Scholar]
- De Jong M. C. M., Bouma A., Diekmann O., Heesterbeek H. 2002. Modelling transmission: mass action and beyond. Trends in Ecology and Evolution 117:64. [Google Scholar]
- Dearing M. D., Clay C., Lehmer E., Dizney L. 2015. The roles of community and contact rates on pathogen prevalence. Journal of Mammalogy 96: 29–36. [Google Scholar]
- Denkers N, et al. 2013. Aerosol transmission of chronic wasting disease in white-tailed deer. Journal of Virology 87:1890–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI. 2006. ArcGIS desktop. Release 9.2. Environmental Systems Research Institute, Redlands, California. [Google Scholar]
- Fulford G. R., Roberts M. G., Heesterbeek J. A. P. 2002. The metapopulation dynamics of an infectious disease: tuberculosis in possums. Theoretical Population Biology 61:15–29. [DOI] [PubMed] [Google Scholar]
- Getz W. M., Pickering J. 1983. Epidemic models: thresholds and population regulation. American Naturalist 121:892–898. [Google Scholar]
- Grear D. A., Samuel M. D., Scribner K. T., Weckworth B. V., Langenberg J. A. 2010. Influence of genetic relatedness and spatial proximity on chronic wasting disease infection among female white-tailed deer. Journal of Applied Ecology 47:532–540. [Google Scholar]
- Gross J. E., Miller M. W. 2001. Chronic wasting disease in mule deer: disease dynamics and control. Journal of Wildlife Management 65:205–215. [Google Scholar]
- Habib T., Merrill E., Pybus M., Conner M. M. 2011. Modelling landscape effects on density-contact rate relationships of deer in eastern Alberta: implications for chronic wasting disease. Ecological Modelling 222:2722–2732. [Google Scholar]
- Hawkins R. E., Klimstra W. D. 1970. A preliminary study of the social organization of the white-tailed deer. Journal of Wildlife Management 34:407–419. [Google Scholar]
- Hawkins R. E., Klimstra W. D., Montgomery G. G. 1968. Cannon-netting deer. Journal of Wildlife Management 32:191–195. [Google Scholar]
- Henderson D. W., Warren R. J., Cromwell J. A., Hamilton R. J. 2000. Responses of urban deer to a 50% reduction in local herd density. Wildlife Society Bulletin 28:902–910. [Google Scholar]
- Hirth D. H. 1977. Social behavior of white-tailed deer in relation to habitat. Wildlife Monographs 53:1–55. [Google Scholar]
- Jolles A. E., Ezenwa V. O. 2015. Ungulates as model systems for the study of disease processes in natural populations. Journal of Mammalogy 96:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kie J. G., Bowyer R. T. 1999. Sexual segregation in white-tailed deer: density-dependent changes in use of space, habitat selection, and dietary niche. Journal of Mammalogy 80:1004–1020. [Google Scholar]
- Kilpatrick H. J., Spohr S. M., Lima K. K. 2001. Effects of population reduction on home ranges of female white- tailed deer at high densities. Canadian Journal of Zoology 79:949–954. [Google Scholar]
- Kjær L. J., Schauber E. M., Nielsen C. K. 2008. Spatial and temporal analysis of contact rates in female white-tailed deer. Journal of Wildlife Management 72:1819–1825. [Google Scholar]
- Lingle S. 2003. Group composition and cohesion in sympatric white-tailed and mule deer. Canadian Journal of Zoology 81:1119–1130. [Google Scholar]
- Long E. S., Diefenbach D. R., Rosenberry C. S., Wallingford B. D., Grund M. R. D. 2005. Forest cover influences dispersal distance of white-tailed deer. Journal of Mammalogy 86:623–629. [Google Scholar]
- Magle S. B., Samuel M. D., Van Deelen T. R., Robinson S. J., Mathews N. E. 2013. Evaluating spatial overlap and relatedness of white-tailed deer in a chronic wasting disease management zone. PLoS One 8:e56568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchinton R. L., Hirth D. H. 1984. Behavior. Pp. 129–168 in Ecology and management of white-tailed deer (Halls L. K., ed.). Stackpole Publishing Co, Harrisburg, Pennsylvania. [Google Scholar]
- May R. M., Anderson R. M. 1978. Regulation and stability of host-parasite population interactions: II. Destabilizing processes. Journal of Animal Ecology 47:249–267. [Google Scholar]
- McCallum H., Barlow N., Hone J. 2002. Modelling transmission: mass action and beyond. Trends in Ecology & Evolution 17:64–65. [Google Scholar]
- McCarty C. W., Miller M. W. 1998. A versatile model of disease transmission applied to forecasting bovine tuberculosis dynamics in white-tailed deer populations. Journal of Wildlife Diseases 34:722–730. [DOI] [PubMed] [Google Scholar]
- McNulty S. A., Porter W. F., Mathews N. E., Hill J. A. 1997. Localized management for reducing white-tailed deer populations. Wildlife Society Bulletin 25:265–271. [Google Scholar]
- Midwest Regional Climate Center. 2007. 2007 Illinois weather archive http://mcc.sws.uiuc.edu/prod_serv/prodserv.htm Accessed 1 February 2007.
- Miller M. W., Williams E. S. 2003. Horizontal prion transmission in mule deer. Nature 425:35–36. [DOI] [PubMed] [Google Scholar]
- Miller M. W., Williams E. S., Hobbs N. T., Wolfe L. L. 2004. Environmental sources of prion transmission in mule deer. Emerging Infectious Diseases 10:1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. W., Wild M. A., Williams E. S. 1998. Epidemiology of chronic wasting disease in captive Rocky Mountain elk. Journal of Wildlife Diseases 34:532–538. [DOI] [PubMed] [Google Scholar]
- Miller M. W., Hobbs N. T., Tavener S. J. 2006. Dynamics of prion disease transmission in mule deer. Ecological Applications 16:2208–2214. [DOI] [PubMed] [Google Scholar]
- Miller M. W., et al. 2000. Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. Journal of Wildlife Diseases 36:676–690. [DOI] [PubMed] [Google Scholar]
- Millspaugh J. J., Gitzen R. A., Kernohan B. J., Larson M. A., Clay C. L. 2004. Comparability of three analytical techniques to assess joint space use. Wildlife Society Bulletin 32:148–157. [Google Scholar]
- Murray S. S., Monfort S. L., Ware L., McShea W. J., Bush M. 2000. Anesthesia in female white-tailed deer using telazol and xylazine. Journal of Wildlife Diseases 36:670–675. [DOI] [PubMed] [Google Scholar]
- Nelson M. E., Mech L. D. 1999. Twenty-year home-range dynamics of a white-tailed deer matriline. Canadian Journal of Zoology 77:1128–1135. [Google Scholar]
- Nixon C. M., Hansen L. P., Brewer P. A., Chelsvig J. E. 1991. Ecology of white-tailed deer in an intensively farmed region of Illinois. Wildlife Monographs 118:1–77. [Google Scholar]
- Nixon C. M., Hansen L. P., Brewer P. A., Chelsvig J. E. 1992. Stability of white-tailed doe parturition ranges on a refuge in east-central Illinois. Canadian Journal of Zoology 70:968–973. [Google Scholar]
- Nixon C. M., et al. 1994. Behavior, dispersal, and survival of male white-tailed deer in Illinois. Illinois Natural History Survey Biological Notes 139:1–29. [Google Scholar]
- Nixon C. M., et al. 2007. White-tailed deer dispersal behavior in an agricultural environment. American Midland Naturalist 157:212–220. [Google Scholar]
- Pedersen J. A., McMahon K. D., Benson C. H. 2006. Prions: novel pathogens of environmental concern? Journal of Environmental Engineering 132:967–969. [Google Scholar]
- Porter W. F., Mathews N. E., Underwood H. B., Sage R. W., Behrend D. F. 1991. Social organization in deer: implications for localized management. Environmental Management 15:809–814. [Google Scholar]
- Potapov A., Merrill E., Lewis M. A. 2012. Wildlife disease elimination and density dependence. Proceedings of the Royal Society Series B 279:3139–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert K., Garant D., Pelletier F. 2012. Keep in touch: does spatial overlap correlate with contact rate frequency? Journal of Wildlife Management 76:1670–1675. [Google Scholar]
- Rodgers A. R., Carr A. P., Smith L., Kie J. G. 2005. HRT: home range tools for ArcGIS. Ontario Ministry of Natural Resources, Center for Northern Forest Ecosystem Research, Ontario, Canada. [Google Scholar]
- SAS Institute Inc. 2008. SAS version 9.2. SAS Institute Inc, Cary, North Carolina. [Google Scholar]
- Schauber E. M., Woolf A. 2003. Chronic wasting disease in deer and elk: a critique of current models and their application. Wildlife Society Bulletin 31:610–616. [Google Scholar]
- Schauber E. M., Storm D. J., Nielsen C. K. 2007. Effects of joint space use and group membership on contact rates among white-tailed deer. Journal of Wildlife Management 71:155–163. [Google Scholar]
- Schwede G., Hendrichs H., McShea W. J. 1993. Social and spatial organization of female white-tailed deer, Odocoileus virginianus, during the fawning season. Animal Behaviour 45:1007–1017. [Google Scholar]
- Severinghaus C. W. 1949. Tooth development and wear as criteria of age in white-tailed deer. Journal of Wildlife Management 13:195–216. [Google Scholar]
- Severinghaus C. W., Cheatum E. L. 1956. Life and times of the white-tailed deer. Pp. 57–186 in The deer of North America (Taylor W. P., ed.). The Wildlife Management Institute, Washington, D.C. [Google Scholar]
- Sikes R. S., Gannon W. L., and the Animal Care and Use Commit tee of the American Society of Mammalogists. 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 92:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuldt L. H., Mathews N. E., Oyer A. M. 2008. White-tailed deer movements in a chronic wasting disease area in south-central Wisconsin. Journal of Wildlife Management 72:1156–1160. [Google Scholar]
- Storm D. J., Nielsen C. K., Schauber E. M., Woolf A. 2007. Space use and survival of white-tailed deer in an exurban landscape. Journal of Wildlife Management 71:1170–1176. [Google Scholar]
- Swinton J, et al. 2001. Microparasite transmission and persistence. Pp. 83–101 in The ecology of wildlife diseases (Hudson P. J., Rizzoli A., Grenfell B. T., Heesterbeek H., Dobson A. P., eds.). Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Thompson M. J., Henderson R. E., Lemke T. O., Sterling B. A. 1989. Evaluation of a collapsible Clover trap for elk. Wildlife Society Bulletin 17:287–290. [Google Scholar]
- Tosa, M. I., E. M. Schauber, and C. K. Nielsen. 2015. Familiarity breeds contempt: combining proximity loggers with GPS reveals female white-tailed deer (Odocoileus virginianus) avoiding close contact with neighbors. Journal of Wildlife Diseases 51:79–88. [DOI] [PubMed] [Google Scholar]
- Tuyttens F. A. M., Delahay R. J., Macdonald D. W., Cheeseman C. L., Long B., Donnelly C. A. 2000. Spatial perturbation caused by a badger (Meles meles) culling operation: implications for the function of territoriality and the control of bovine tuberculosis (Mycobacterium bovis). Journal of Animal Ecology 69:815–828. [DOI] [PubMed] [Google Scholar]
- Vercauteren K. C., Hygnstrom S. E. 1998. Effects of agricultural activities and hunting on home ranges of female white-tailed deer. Journal of Wildlife Management 62:280–285. [Google Scholar]
- Vicente J., Delahay R. J., Walker N. J., Cheeseman C. L. 2007. Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high-density badger Meles meles population. Journal of Animal Ecology 76:348–360. [DOI] [PubMed] [Google Scholar]
- Williams E. S., Miller M. W., Kreeger T. J., Kahn R. H., Thorne E. T. 2002. Chronic wasting disease of deer and elk: a review with recommendations for management. Journal of Wildlife Management 66:551–563. [Google Scholar]