Abstract
Objective
Accurate discrimination of asthma episodes increases the likelihood they will be managed effectively. The purpose of the study was to examine the effect of feedback in a signal detection task on perception of increased airflow obstruction in children with persistent asthma.
Methods
The effect of feedback training on the perception of resistive loads was evaluated in 155 children with persistent asthma between 8 and 15 years of age. Each child participated in four experimental sessions that occurred about once every 2 weeks, an initial session followed by three training sessions. During the initial session, the threshold resistance to breathing was determined for each child. Subsequently, each child was randomly assigned to one of two resistive load training conditions in a signal detection paradigm: training with immediate performance feedback or training with no performance feedback.
Results
The threshold resistance to breathing, determined in the initial session, was equivalent between groups. Children in the feedback condition discriminated more accurately between both the presence and absence of increases in the resistance to breathing (206 ± 48 vs. 180 ± 39 correct responses, p < .001), and differences over time between groups increased reliably as a function of training (139 ± 34 vs. 120 ± 29 correct responses, p < .001). Response times and confidence ratings were equivalent between groups, and no differences in breathing patterns were observed between groups.
Conclusion
Feedback training results in improved perception of respiratory sensations in children with asthma, a finding with implications for strategies of asthma self-management.
Keywords: asthma, children, discrimination (psychology), resistive loads, respiration, signal detection
There is no more essential element in the management of asthma than monitoring the control of asthma to determine that the goals of therapy are met (1,2). For example, it is widely assumed that early recognition of an acute asthma episode increases the likelihood it will be treated early, and for this reason it is valued (1–3). Accurate assessment of asthma symptoms permits the patient to select an effective management strategy from among possible asthma countermeasures: when it is sufficient to rest and stay vigilant; when and in what quantity to take medication; and when to seek medical assistance. Delaying asthma control measures until after asthma symptoms escalate may precipitate significant future exacerbations (4,5).
Appropriate recognition of acute asthma is most commonly accomplished by monitoring symptoms and signs, and normally asthma patients rely on symptoms and signs to assess acute episodes and to guide asthma control measures. However, asthma symptoms are not always closely correlated with underlying physiological changes in the airways (6–8), and studies in which systematic symptom monitoring was compared with medical management resulted either in a small benefit for symptom monitoring (9) or no benefit at all (10). Methods to overcome the weaknesses of symptom monitoring alone include the use of portable meters that measure some aspect of airflow obstruction, most commonly peak expiratory flow. As an objective quantity, peak flow provides data that enhances symptom monitoring in estimating changes in levels of asthma control. But purported benefits of peak flow in assisting symptom monitoring have been either largely absent (11), modest (10,12) or inconsistent (13–16). Moreover, convenient access to the meter is not always possible.
Improving patient perception of respiratory sensations is an alternative to either symptom or peak flow monitoring for gathering information for managing developing asthma. This line of inquiry represents an application of the study of detection of added respiratory loads pioneered by Campbell and colleagues (17–19), research with significant clinical implications (e.g., 20–22). To date, asthma control efforts focused on improving recognition of airflow obstruction have involved use of feedback for correct judgments of added resistive loads, and have demonstrated improved sensitivity to airflow obstruction in adults with asthma (23–24). Those investigations were limited, however, by exclusive use of young adults with mild asthma and by small sample size. In the present study we extended use of the procedure to a large group of children with persistent asthma and hypothesized that children given feedback would improve in their perception of inspiratory airflow obstruction. We did not set out to address the impact of feedback training on asthma control; adequate testing of that relationship depends first on evidence of feedback effects on the perception of respiratory sensations.
Method
Research Setting
Participants in this study were enrolled in a large-scale pediatric asthma education research program (Project On TRAC: Taking Responsibility for Asthma Control). The program consisted of three sessions of asthma education; home monitoring of asthma symptoms for 30 days; four resistive load detection sessions (the first of which established the threshold resistance to breathing); and six-month follow-up. This report is limited to the responses from children who completed the four resistive load detection sessions and were assigned at random to one of two signal detection training conditions: training with feedback (n = 78) or training without feedback (n = 77).
The complete design of Project On TRAC included three resistive load detection conditions, including signal detection training with feedback and signal detection training without feedback. In a third condition, we tested the reliability of the threshold resistance to breathing. Specifically, in a third group of children (n = 75) the threshold resistance to breathing was determined on multiple occasions - in each of four, consecutive laboratory sessions - in the same individuals. Those results will be published separately.
Children were diagnosed with asthma at least two years prior to their entry into the study; used controller medications daily; and reported at least occasional asthma symptoms and/or nighttime cough. Children and their families were advised about all aspects of the research at an initial enrollment meeting. The child’s caregiver (parent or legal guardian) provided written informed consent and the child provided written informed assent. Institutional review boards at both UNC Charlotte (#09-09-03) and Ohio University (#03F024) approved the research protocol.
Apparatus
In all four sessions, participants breathed on a circuit with a nose clip in place (as depicted in Figure 1) that consisted of a rubber mouthpiece attached to a pneumotachograph (Hans Rudolph, model R3813) and a two-way non-rebreathing Hans Rudolph J valve (model R2600). Airflow was measured using a Grass volumetric pressure transducer that was attached to the pneumotachograph. Inspiratory pressure was measured with a second PT5 transducer connected to the mouthpiece. Airflow and pressure signals were monitored continuously, amplified with Grass DC preamplifiers (model 7P1G), and displayed on separate channels of a Grass polygraph (model 7D).
Figure 1.
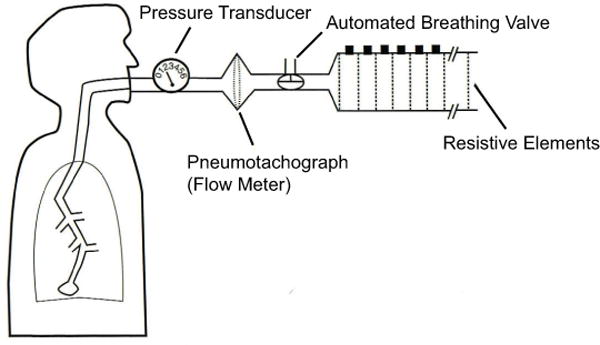
A two-way, automated breathing valve located in a control room was attached to the inspiratory side of the circuit with rubber tubing (3 cm ID and 1.5 m in length). The automated breathing valve was normally ‘open’ to room air; children breathed continuously through the open circuit except during inspiration on specific (target) breaths. During the threshold task, resistive loads were exchanged as needed and attached manually to the other port of the two-way valve (once every 3 to 6 breaths); during the signal detection task a single load was attached to the circuit, which remained in place throughout the session. On specific (target) breaths in both tasks, a computer-operated relay closed the valve open to room air and opened the limb of the breathing circuit containing the resistive load at the onset of inspiration (when rising inspiratory pressure was sustained for 100 msec). The computer switched the valve back to room air when peak inspiratory pressure was detected. Resistive loads were constructed of nylon screens varying in mesh size enclosed in PVC pipe tubing, 2.5 cm in diameter and 17 cm in length.
Procedure
Children completed four laboratory sessions and in each session, the children made repeated judgments as to whether a resistive load had been placed in the breathing circuit on specific (target) breaths. We did not constrain in any way, or attempt to alter through instruction or coaching, the breathing pattern of patients.
Each child was seated in an armchair in a separate testing room and monitored with a video camera and two-way intercom. Children were instructed to pay attention to breaths that “may or may not feel different than normal.” Two warning cues were presented to each child during the breath immediately before a trial: a 1 KHz auditory tone, and the words "Next Breath" displayed on a computer monitor placed 1.5 m in front of the participant. On the breath after each stimulus presentation the child was asked to indicate whether or not there was a change in the resistance to breathing on the preceding breath by pressing keys on a response board. Prompts for the participants’ keyboard responses were displayed on the computer monitor. In addition, we recorded inspiratory pressure and inspiratory time on every trial for two breaths, the breath immediately preceding the warning cues (control breath) and the target breath.
Children completed the four laboratory sessions – one about every two 2 weeks – over the course of 6–7 weeks (M = 48 days) between October, 2004 and September, 2009; each session lasted about 60–90 minutes. At enrollment, demographic information was collected and self-reported asthma severity was rated from mild to severe, from very well managed to not managed at all, and from hardly noticeable to very troublesome. Anthropometrics and spirometry were recorded at the start of Session 2. Lung function was recorded with a dry rolling-seal spirometer (Vacuumed, model 1618). Children selected a small prize (book, puzzle, board game, etc) from a toy box at the end of Sessions 1 and 3; received a $10 gift card at the end of Session 2; and accepted a “Certificate of Completion” at the end of Session 4.
Session One
The first session was designed to determine the threshold resistance to breathing. Threshold levels were determined using the method of constant stimuli, a widely accepted procedure for determining sensory thresholds (25). In the task, a constant number of stimuli are presented to participants for a fixed number of presentations (possibly 50 to 200 times each), and a psychometric function plotting the percentage of correct detections as a function of stimulus intensity is determined. We used ten resistive loads (linear for flow rates to 1 l/sec) including a (imperceptible) "catch" load consisting of a section of PVC pipe without nylon mesh in the threshold resistance task. On each target breath, one of the ten loads was added to the circuit for a single inspiration. The loads ranged in intensity from 0.20 (the “catch” load) to 7.58 cmH20/l/s. Each load was presented ten times in random order (for a total of 100 load presentations), once every 3 to 6 breaths with the restriction that each intensity was presented only once in any ten-load sequence. Sensory thresholds derived from classic psychophysical procedures such as the method of constant stimuli do not take into account false alarms, i.e., the rate at which subjects indicate the presence of a stimulus when in fact none was presented. Accordingly, we used a conservative approach for estimating the threshold resistance for each child: threshold was defined as the stimulus intensity detected on 50% of the trials after correcting for false alarms. The proportion of “yes” responses to the ten presentations of each resistive load was corrected for false alarms (i.e., for the proportion of “yes” responses to the 10, “catch” trials) using the following formula:
Following the initial session children we formed two groups by randomly assigning children either to (1) feedback training condition in which children were given immediate feedback after each trial as to the accuracy of their responses or (2) no feedback training condition in which children were given no information regarding the accuracy of their responses.
Sessions Two, Three, and Four
Children were asked to determine if an increase in the resistance to breathing occurred during specific (target) breaths. Each session consisted of five blocks of 20 trials each (10 signal and 10 non-signal); within each block the order of signal and non-signal trials was random. There was a short rest period (50 sec) between each set of blocks. Each block of trials was started by a 1 KHz warning tone 2 sec in duration. The trial number (range, 1 to 20) in each block was displayed on a computer monitor placed before the participants. After each stimulus presentation the child was asked to indicate whether or not there was a change in the resistance to breathing on the preceding breath by pressing keys on a response board. After responding all children were next asked to select the level of confidence in their judgment on a five-point scale (1 = very confident, 2 = mostly confident, 3 = somewhat confident, 4 = not really confident, 5 = not at all confident) by pressing keys on the response board. Immediately thereafter, the children assigned to the feedback condition were given feedback regarding the accuracy of their responses. Specifically, a message displayed on the computer monitor reminded the child about the decision they made (“change in resistance” or “no change in resistance”) and provided the child feedback as to the accuracy of their response (“correct” or “incorrect”). The children assigned to the no feedback condition were kept uninformed about their performance; no messages were displayed to remind them either about their decision or as to the accuracy of their response.
The resistive load used as the stimulus for the ‘signal’ trials in the three signal detection sessions varied between participants based on performance in the threshold resistance breathing task (Session One). In previous experiments, healthy young adults and young adults with asthma reliably detected a resistive load in a signal detection task with feedback selected a priori as one-half the magnitude of a threshold load determined by the method of constant stimuli (23). Accordingly, in the present study involving children we adopted the same approach: each participant was assigned a priori a resistive load (i.e., ‘signal’) as close as possible, within the limits of the breathing circuit (9 possible levels), to one-half the magnitude of the threshold resistance to breathing determined in the first session. The resistive load assigned to each participant was used in each of the three signal detection sessions.
Data Reduction and Statistical Analysis
Data are presented as mean ± standard deviation (s.d.) or N (%) as appropriate. We subsequently characterize the treatment of data separately for Session One and for Sessions Two-Four and later we report only significant effects that also satisfied the Greenhouse-Geisser adjustment for repeated measures. We used SPSS version 17.0 for all analyses (SPSS Inc., Chicago, IL).
Session One
The threshold resistance to breathing was determined for each participant by regressing the added resistance of the loads against the proportion of “yes” responses (adjusted for false alarm rates) and predicting the load detected 50% of the time (25). Average threshold resistance levels, and the average intensity of loads used in the signal detection task derived from these levels, were compared between children assigned at random to feedback conditions with separate unpaired t tests.
Sessions Two, Three, and Four
On each trial children were asked to determine if an increase in the resistance to breathing occurred yielding four possible signal detection outcomes including: a ‘hit’ (a yes response on a signal trial); a ‘miss’ (a no response on a signal trial); a ‘false alarm’ (a yes response on a no signal trial); or a ‘correct rejection’ (a no response on a no signal trial). Our primary analysis focused on the differential effects of training on the cumulative number of correct responses (i.e., hits plus correct rejections) between sessions. In our case the number of correct responses, which has been used to index perception in various physiological detection tasks (e.g., 26–28), reflected the ability of individuals to accurately discriminate between both the presence and absence of increased resistance to breathing. We conducted an analysis of variance (ANOVA) testing for effects due to “training condition” (2 levels), “session” (3 levels), and “blocks of trials” (5 levels), with repeated measures on the last two factors. We also conducted exploratory analyses to examine effects of age, gender, race, asthma severity and duration, and lung function on training.
Our secondary analysis focused on the effects of training on signal detection outcomes: “sensitivity,” the ability of individuals to accurately discriminate ‘signal’ (the presence of a load) from ‘noise,’ and “response bias” (the criterion used to judge the presence or absence of a signal) (29,30). To accomplish this, hit rates (percent “yes” responses to the 50 signal trials) and false alarm rates (percent “yes” responses to the 50 non-signal trials) were determined for each child, and used to compute non-parametric signal detection indices of both sensitivity (A′) and response bias (B′) for each session (31). Values for A′ vary between 0.5 and 1; an A′ value of 0.5 indicates chance levels of discrimination whereas a value of 1 indicates perfect discrimination. Values for B′ vary between − 1 and 1; increasing negativity reflects an increasingly lax or risky criterion and increasing positivity reflects an increasingly cautious or strict criterion. We estimated baseline sensitivity and response bias measures during Session One (a no feedback task for all participants) by calculating hit rates to the load that was later assigned to each child in Sessions Two-Four and false alarm rates to the 10, “catch” trials. Average A′ and B′ baseline estimates were compared between children with separate unpaired t tests. Additionally, sensitivity and response bias were analyzed with separate ANOVAs testing effects due to “training condition” (2 levels) and “session” (4 levels), with repeated measures on the latter factor.
Finally, we put in place analyses to demonstrate the reliability of our training task between feedback conditions and between sessions. Response times for keyboard presses and confidence ratings were recorded on each trial to examine potential variations in attention, motivation, or similar factors that may have emerged between feedback conditions to inadvertently affect task-oriented behavior. Differences in response times and confidence ratings were evaluated with separate ANOVAs testing for effects due to “training condition” (2 levels), “session” (3 levels), and “behavioral decision” (4 levels) with repeated measures on the latter three factors. We recorded peak inspiratory pressure and inspiratory time continuously on every trial for two breaths (the breath immediately preceding the warning cues (control breath) and the target breath) to examine breathing patterns between feedback groups and further document the reliability of our experimental setup. Differences in levels of inspiratory pressures and times in the signal detection task were evaluated with separate ANOVAs testing for effects due to “training condition” (2 levels), “breath” (control or target breath; 2 levels) and “session” (3 levels) with repeated measures on the latter two factors, for each behavioral decision (hit, miss, false alarm, and correct rejection).
Results
Participants
The number of children that completed training in the feedback and no feedback conditions was 78 and 77 respectively. The sample numbered 97 boys and 58 girls; 75 were non-Hispanic Black and 68 were non-Hispanic White. Their age, on average, was 10.1 ± 1.7 years of age, and they had been diagnosed with asthma for 6.7 ± 2.9 years prior to their entry into the program. Their average age at diagnosis was 3.3 ± 2.6 years. The children exhibited some symptoms of asthma over the course of the previous year. They reported an average of 20 asthma attacks during the previous 12 months, and missed approximately 4–5 days of school. The proportion of children assigned to each experimental condition did not vary reliably either by gender (p = 0.35) or by race (p = 0.44). Demographics, measures of self-reported asthma severity, and anthropometric data were collected from all participants when they enrolled in the program and are shown in Table 1, separated by experimental group. Average lung function values recorded at the start of Session 2 are also shown in Table 1. Statistical differences were observed between the two groups for actual forced vital capacity (FVC) values (higher in the no feedback children) and the forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC ratio) (higher in the feedback children). Lung function values (percent predicted values and the FEV1/FVC ratio) were within normal limits, however, for all participants.
Table 1.
Characteristics of participants (n = 155) in each signal detection condition
| Variable | Condition | |
|---|---|---|
| Feedback (n=78) |
No Feedback (n=77) |
|
| Sex | ||
| Boys (n) | 46 | 51 |
| Girls (n) | 32 | 26 |
| Race | ||
| Black (n) | 35 | 40 |
| White (n) | 37 | 31 |
| Age (yrs) | 9.9 ± 1.6* | 10.2 ± 1.9 |
| Age at asthma diagnosis (yrs) | 3.6 ± 2.8 | 3.1 ± 2.4 |
| School missed the previous 12 months due to asthma (days) | 4.9 ± 5.8 | 3.9 ± 4.8 |
| Asthma attacks the past 12 months (number) | 24.2 ± 70.9 | 19.2 ± 46.8 |
| Self-reported asthma severity1 | ||
| Severe | 2.7 ± 1.2 | 2.4 ± 1.1 |
| Managed | 2.8 ± 1.1 | 2.7 ± 1.1 |
| Troublesome | 2.9 ± 1.2 | 2.7 ± 1.1 |
| Anthropometrics | ||
| Height (cm) | 144.8 ± 12.1 | 147.3 ± 12.9 |
| Weight (kg) | 43.8 ± 15.9 | 47.9 ± 17.9 |
| BMI2 | 20.4 ± 4.9 | 21.5 ± 5.3 |
| Lung function | ||
| FEV13 (l) | 1.9 ± 0.5 | 1.9 ± 0.5 |
| FEV1 percent predicted | 99.9 ± 23.1 | 99.6 ± 22.7 |
| FVC4 (l)** | 2.2 ± 0.6 | 2.4 ± 0.8 |
| FVC percent predicted | 99.7 ± 19.5 | 104.7 ± 24.1 |
| FEV1/FVC** | 85.9 ± 9.5 | 81.5 ± 14.6 |
Asthma severity was rated from mild (“1”) to severe (“5”), from very well managed (“1”) to not managed at all (“5”), and from hardly noticeable (“1”) to very troublesome (“5”)
BMI = body mass index
FEV1 = forced expiratory volume in one second
FVC = forced vital capacity..
Averages are mean ± SD. Differences between conditions were examined with separate unpaired t tests.
Difference between conditions, p < .05.
Session One
Children randomly assigned to the feedback condition did not differ in the threshold resistance to breathing from those assigned to the no feedback condition: 7.81 ± 6.0 cmH20/l/s versus 6.33 ± 5.3 cmH20/l/s, respectively. This comparison was limited, however, to children whose threshold values were judged a priori to be interpretable based on the correlation between resistive load levels and “yes” responses.
Children were subsequently assigned a training load for Sessions Two to Four about one-half the magnitude of the threshold resistance to breathing based on performance in Session One; those who did not exhibit an interpretable level of performance in the threshold task {feedback condition (n = 21); no feedback condition (n = 18)} were assigned a priori the largest load in the circuit (7.58 cmH20/l/s). Ultimately, the average level of resistance of the training load used for children in the feedback condition (M = 5.3 ± 2.5 cmH20/l/s) was statistically equivalent to the average level of resistance of the training load (M = 4.9 ± 2.6 cmH20/l/s) used for children in the no feedback condition {t(153) = −1.29, p = 0.20}.
Sessions Two, Three, and Four
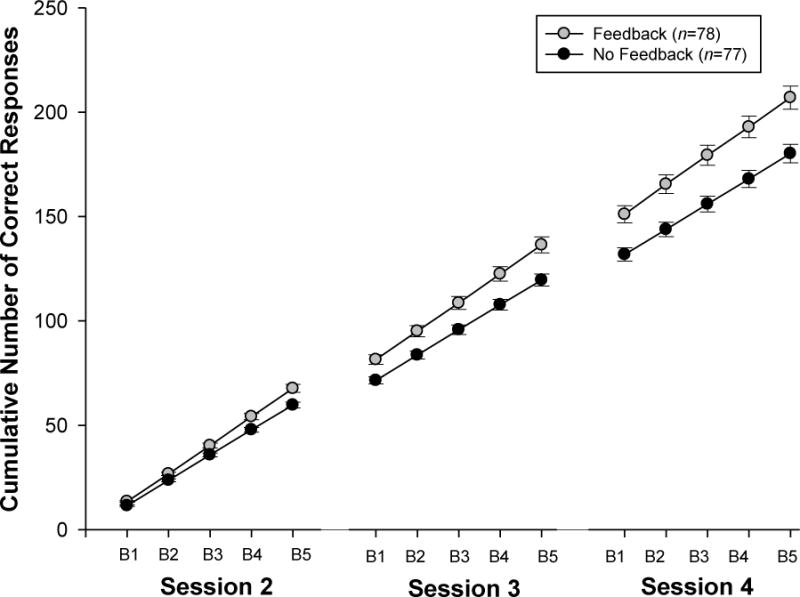
Overall, children in the feedback condition discriminated more accurately between both the presence and absence of increased resistance to breathing (i.e., evidenced more "correct responses") than children in the no feedback condition {F(1, 153) = 13.5, p < .001}. Moreover, differences between groups increased both within sessions and between sessions as a function of training: both the feedback condition × block {F(4, 153) = 11.9, p < .001} and feedback condition × session interactions {F(2, 153) = 13.7, p < .001} were reliable (Figure 2). One reliable effect, for self-rated asthma severity, emerged from the results of exploratory analyses {F(1, 151) = 6.2, p = .014}. Children who rated their asthma as mild or moderate evidenced more correct responses (M = 202 ± 50) compared to those who rated their asthma as moderate to severe (M = 185 ± 39; t(153) = −2.34, p = 0.021).
Figure 2.
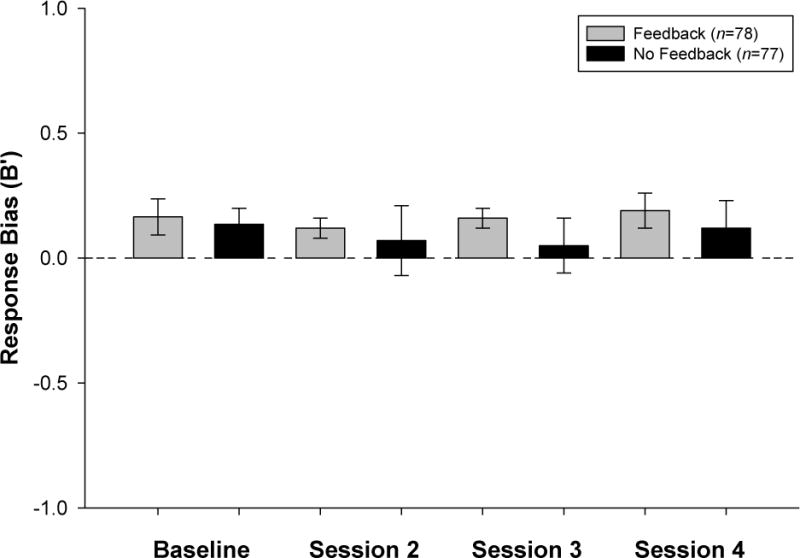
Before training, average A′ and B′ baseline estimates were equivalent between children in the two feedback conditions {t(153) = −0.41, p = 0.68 and t(153) = −0.31, p = 0.76, respectively}. Children in the feedback condition were more sensitive to increases in the resistance to breathing (i.e., evidenced significantly greater A′ values) than children in the no feedback condition {F(1, 153) = 8.9, p = .003} but the feedback condition × session interaction did not reach statistical significance {F(3, 153) = 1.7, p = 0.17} (Figure 3). No differences in response bias were evident either between training conditions {F(1, 153) = 0.83, p = 0.36} or between training sessions {F(3, 153) = 0.29, p = 0.82} (Figure 4).
Figure 3.
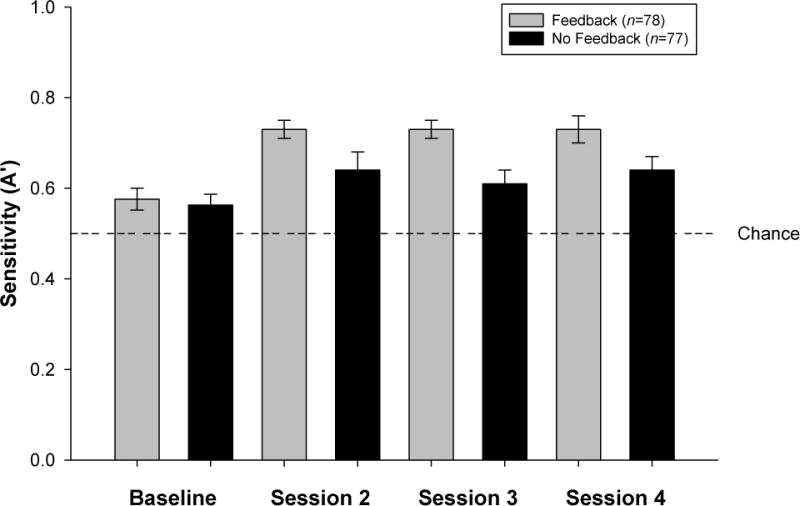
Figure 4.
Behavioral Responses
Response times did not vary between groups {F(1, 153) = 1.59, p = 0.21} but decreased reliably between sessions (median and IQR = 1162 (807–1534), 645 (467–1019, and 615 (369–787)) msec respectively, in Session Two, Three, and Four) {F(2, 306) = 101.35, p < .001}. No differences in confidence ratings were observed between training conditions {F(1, 153) = 0.07, p = 0.78}. Average ratings observed in Sessions Two (M = 1.86 ± 0.6) and Three (M = 1.83 + 0.7), however, were less confident than those evident in Session Four (M = 1.70 ± 0.7) {F(2, 153) = 7.86, p < .001}.
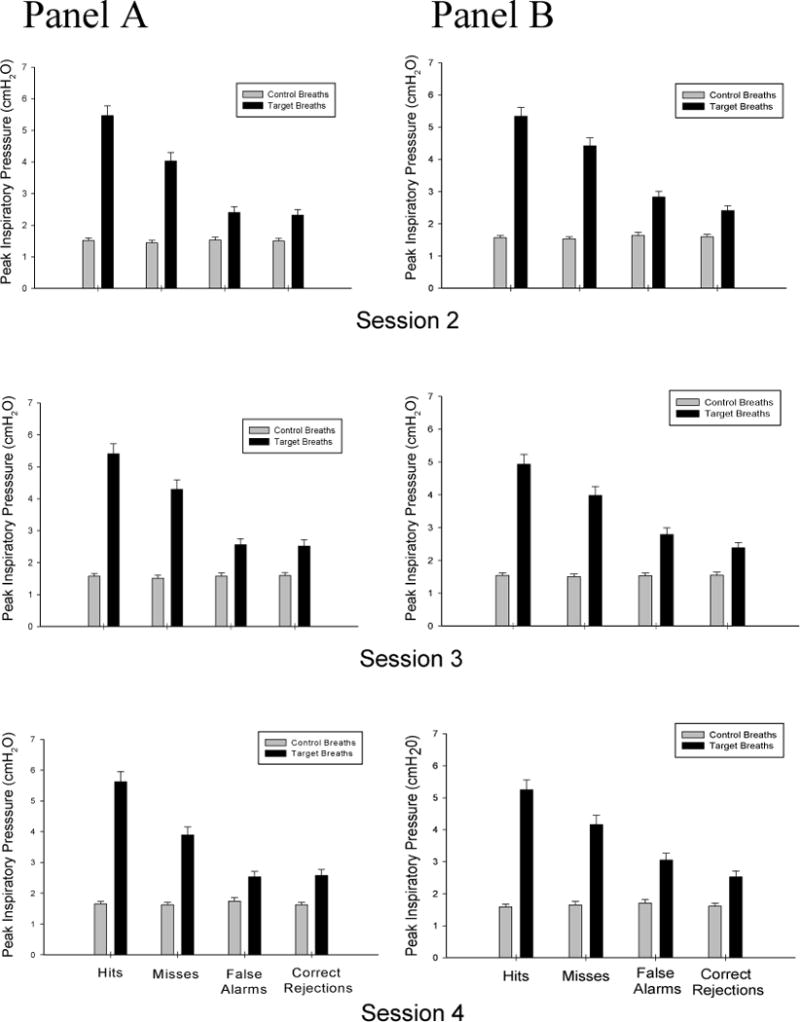
Physiological Responses
No differences in inspiratory pressures were observed between feedback groups {F(1, 153) = 0.02, p = 0.87}but inspiratory pressures on target breaths were significantly greater than those on control breaths. The analyses for inspiratory pressures yielded no other reliable terms (Figure 5). No differences in inspiratory times were observed between feedback groups {F(1, 153) = 0.01, p = 0.97}, but inspiratory times for “target” breaths (553 ± 187 msec) were significantly longer than those for “control” breaths (456 ± 146 msec) {F(1, 153) = 91.72, p < .001. Inspiratory times in Session Two (M = 545 ± 277 msec) were longer than those in Sessions Three (M = 488 ± 178 msec) and Four (M = 481 ± 160 msec) {F(2, 153) = 6.31, p = .004}.
Figure 5.
Discussion
We demonstrated that (1) children in the feedback condition discriminated more accurately between the presence and absence of increased resistance to breathing than children in the no feedback condition; (2) discrimination between the presence and absence of resistive loads increased both within sessions and between sessions as a function of feedback training; and (3) children in the feedback condition evidenced greater sensitivity (greater A′ values) to increased airflow obstruction although such differences did not vary reliably as a function of feedback training session. The effect of feedback on the ability of children to more accurately discriminate between both the presence and absence of increased resistance to breathing emerged during the initial training session, and persisted throughout the balance of training. Feedback often leads to a gradual acquisition of discrimination, a phenomenon similar to the fairly robust acquisition of discrimination between feedback and no feedback conditions that we observed. The effects of feedback suggested that perception of respiratory sensations may be subject to modification.
Health care decisions are influenced by the visibility of symptoms and by the frequency of their occurrence. However, application of methodologies to improving patient recognition of the presence or absence of relevant physiological warning signs through reinforcement is not common. We have demonstrated that in children with asthma perception of internal state improves as a function of feedback experience. Feedback for correct responses (reinforcement) may yield adaptive self-regulatory behaviors through improved appraisal of respiratory sensations. Increases in discriminative capacities (for example, when to continue with an activity or when to stop and rest) are relevant not only to those patients who might underestimate the presence of airflow obstruction but also to those patients who might overestimate the presence of airflow obstruction. Stimulus discrimination is an important ingredient in self-management programs that have been effective in improving outcome measures in asthma (1).
The training effects we observed raised questions concerning the factors that might facilitate perception of resistive loads. One likely factor relates to the highly structured laboratory protocol we employed. For example, equivalences between groups for behavioral responses (response times and confidence ratings) suggest that participants were engaged or otherwise highly occupied with the task. For improved perception of respiratory sensations to matter clinically the parameters and goals of training must be more clearly delineated. In terms of approach, we recommend close examination of the magnitude of the training stimulus. We derived a stimulus intensity based on the threshold resistance to breathing; such a labor-intensive decision might be streamlined by simply selecting a resistance about equivalent to a patient’s intrinsic airway resistance. On the other hand, larger training loads may both rapidly increase discrimination of load and no-load trials and contribute to its utility as a self-monitoring tool. Finally, in the current project the resistive load assigned to each participant was used in each of the three signal detection sessions. As the effects of feedback emerge it would be interesting to consider the effects of varying resistive load intensities on performance, either within sessions or between sessions.
The resistive load assigned to most children for use in the signal detection task was based on the threshold resistance to breathing - and in the proportion of children whose threshold resistance values were judged to be interpretable in Session One (75%) - our threshold and background resistance measures were consistent with those reported elsewhere. For threshold resistance to breathing, our values were similar to those reported by McQuaid and colleagues (32) and by Fritz and colleagues (33). In terms of background resistance, detection thresholds have been shown to vary consistently as a function of intrinsic airway resistance according to Weber’s law (e.g., 34). Unfortunately we were unable to record airway resistance in our participants. However, we subsequently used reference values provided by Koopman and colleagues (35) to estimate airway resistance of each child measured by the interrupter method (M = 4.22 cmH2O/l/s) and by body plethysmography (M = 6.44 cmH2O/l/s). Our estimated resistance values yielded Weber fractions equivalent, respectively, to those reported by Davenport and Kifle (36) and by Fritz, McQuaid, and colleagues (32,33,37). We judged our recording procedures comparable to those of others.
Patients with asthma vary widely in their ability to perceive airway obstruction and some asthmatics seem more aware of symptoms than others despite similar degrees of airway obstruction. A reflection of that variability was evident, in part, in the number of children whose threshold resistance values were judged to be uninterpretable in Session One. The proportion of children who did not exhibit an interpretable level of performance in the threshold task in our study (25%) was similar, however, to previous experiments involving school-aged children (e.g., 29% in McQuaid et al. (32) and 32% in children with asthma in Fritz et al. (33)). These and similar observations raise issues over the factors that contribute to reliable perception of increased airflow obstruction in children. Koinis-Mitchell et al. (38) recently characterized the role of attentional-based mechanisms in symptom perception. As a field of study, however, attention has focused far more on the clinical significance of reduced perception of airflow obstruction in the control of asthma rather than on the attributes of “good perceivers” of asthma. A spectrum of cognitive and physiological variables are worthy of future consideration (39).
Reduced perception of declines in lung function may lead to symptom under-recognition, underestimation of the seriousness of acute asthma, and undertreatment. Among the factors that influence reduced symptom perception, one often considered is sensory adaptation (3). Sensory adaptation is evident in findings that the symptom-peak flow relationship declines during asthma exacerbation (40) and that the sudden bronchoconstriction of early asthma is detected more easily than the relatively more gradual bronchoconstriction that occurs in late asthma (41). In our exploratory analyses, children who rated their asthma as mild or moderate evidenced more correct responses as a function of feedback training compared to those who rated their asthma as moderate to severe. Although these outcomes were dependent on subjective ratings and not necessarily changes in lung function, the experience of more severe asthma alone may serve to interfere with accurate self-monitoring of asthma control.
The design of our pediatric asthma education research program precluded testing of the independent effects of feedback of training on asthma-related outcomes; sensitivity to patient burden precluded additional signal detection sessions including those that might have involved withholding feedback from both groups. Also as evidenced in our approach, we did not set out to evaluate “poor” or “good” asthma symptom perception. However, of the procedures that afford accurate discrimination of asthma episodes, added load training appears to have potential. It is objective and focuses solely on airflow obstruction, a measure closely related to an asthma exacerbation. Unlike peak flow measurement, it does not require use of a meter or necessitate implementation of a recording procedure. Therefore, patients may find the results of added load training simpler to execute – and therefore of greater value - than peak flow monitoring.
On the other hand, several issues pose difficulty for added load training. First, it is not clear that added loads are similar to intrinsic resistive loads associated with asthma although they have been used extensively to identify neural pathways and brain regions involved in respiratory control and respiratory perception (42). The question is controversial, and evidence has been marshaled both for (43–44) and against (37,45–47) the relationship between perception of various physiological events. Resolving discrepancies among subjective experiences, however, may not be critical if resistive load training was worthwhile within the context of asthma management. Second, it is not clear that added load training will generalize to either environments or to periods beyond those unique to the training setting. For example, children were cued to attend to specific, target breaths as part of the protocol. In addition, a robust literature relates to emotional and contextual factors that affect perception of asthma symptoms (e.g., 48) and it is unknown how these factors might influence patient decision criterion. Third, a practical matter may discourage patients from seeking added load training: as the parameters of training become more clearly delineated it could require an experience more involved and of longer duration than either symptom or peak flow monitoring. Only resolution of these various questions will establish whether resistive load training can take its place in the arsenal of approaches to asthma self-management.
Acknowledgments
We are grateful to John C. Baird for review of earlier versions of the manuscript. Professor Baird passed away at his home in Vermont on June 8, 2011.
The project described was supported by Grant Number R01HL068706 from the National Heart, Lung, and Blood Institute (PI: Dr. Harver; Co-PI: Dr. Kotses). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
List of Abbreviations
- A′
a prime (a non-parametric alternative to d prime)
- ANOVA
analysis of variance
- B′
b prime (a non-parametric alternative to beta)
- cmH2O/l/sec
centimeters of water, per liter per second (1.36 cmH2O = 1 mmHg)
- FEV1
forced expiratory volume in one second (expiratory volume exhaled at the end of the first second of a forced expiratory maneuver)
- FVC
forced vital capacity (total expiratory volume exhaled at the end of a forced expiratory maneuver)
- IQR
inter-quartile range
- msec
millisecond
- PVC
polyvinyl chloride
- yrs
years
Footnotes
Drs. Harver and Kotses were involved in the conception and design of the study, and drafted initial versions of the manuscript. Dr. Harver has full access to the data and will vouch for the integrity of the data analysis. Drs. Humphries, Ashe, and Black coordinated patient recruitment activities, contributed to the interpretation of results, and provided substantial comments on the manuscript. Ms. Ersek participated fully in data acquisition and data analysis activities, constructed the Tables and Figures, and reviewed the final version of the manuscript.
Conflicts of Interest
We declare that all authors are free of any possible conflicts of interest in the manuscript, including financial, consultant, institutional or other relationships that might lead to bias or a conflict of interest.
References
- 1.Creer TL. Behavioral and cognitive processes in the self-management of asthma. J Asthma. 2008;45:81–94. doi: 10.1080/02770900701247236. [DOI] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2007. Aug, Available from: http://www.ncbi.nlm.nih.gov/books/NBK7232/ [Google Scholar]
- 3.Kotses H, Harver A, Humphries CT. Home monitoring in asthma self-management. J Asthma. 2006;43:1–7. doi: 10.1080/02770900600701309. [DOI] [PubMed] [Google Scholar]
- 4.Magadle R, Berar O, Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329–333. doi: 10.1378/chest.121.2.329. [DOI] [PubMed] [Google Scholar]
- 5.Restrepo RD, Peters J. Near-fatal asthma: Recognition and management. Curr Opin Pulm Med. 2008;14:13–23. doi: 10.1097/MCP.0b013e3282f1982d. [DOI] [PubMed] [Google Scholar]
- 6.McFadden ER, Jr, Kiser R, DeGroot WJ. Acute bronchial asthma: Relationships between clinical and physiologic manifestations. N Engl J Med. 1973;288:221–225. doi: 10.1056/NEJM197302012880501. [DOI] [PubMed] [Google Scholar]
- 7.Rubinfeld AR, Pain MCF. Perception of asthma. Lancet. 1976;93:883–884. doi: 10.1016/s0140-6736(76)92097-3. [DOI] [PubMed] [Google Scholar]
- 8.Davis SQ, Permutt Z, Permutt S, Naureckas ET, Bilderback AL, Rand CS, Stein BD, Krishnan JA. Perception of airflow obstruction in patients hospitalized for acute asthma. Ann Allergy Asthma Immunol. 2009;102:455–461. doi: 10.1016/S1081-1206(10)60117-2. [DOI] [PubMed] [Google Scholar]
- 9.Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, Fillion A, Lavalee M, Krusky M, Boulet LP. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med. 1997;155:1509–14. doi: 10.1164/ajrccm.155.5.9154850. [DOI] [PubMed] [Google Scholar]
- 10.Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest. 1997;112:1534–8. doi: 10.1378/chest.112.6.1534. [DOI] [PubMed] [Google Scholar]
- 11.Buist AS, Vollmer WM, Wilson SR, Frazier EA, Hayward AD. A randomized clinical trial of peak flow versus symptom monitoring in older adults with asthma. Am J Respir Crit Care Med. 2006;174:1077–1087. doi: 10.1164/rccm.200510-1606OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial comparing peak expiratory flow with symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med. 1998;157:540–546. doi: 10.1164/ajrccm.157.2.9703060. [DOI] [PubMed] [Google Scholar]
- 13.Adams RJ, Boath K, Campbell DA, Ruffin RE. A randomized trial of peak-flow and symptom-based action plans in adults with moderate-to-severe asthma. Respirolgy. 2001;6:297–304. doi: 10.1046/j.1440-1843.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 14.Charlton I, Charlton G, Broomfield J, Mullee MA. Evaluation of peak flow and symptoms-only self-management plans for control of asthma in general practice. BMJ. 1990;301:1355–9. doi: 10.1136/bmj.301.6765.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malo JL, L’Archeveque J, Trudeau RT, d’Aquino C, Cartier A. Should we monitor peak expiratory flow rates or record symptoms with a simple diary in the management of asthma. J Allergy Clin Immunol. 1993;91:702–709. doi: 10.1016/0091-6749(93)90189-m. [DOI] [PubMed] [Google Scholar]
- 16.Zemek RL, Bhogal SK, Ducharme FM. Systematic review of randomized controlled trials examining written action plans in children. Arch Pediatr Adolesc Med. 2008;162:157–163. doi: 10.1001/archpediatrics.2007.34. [DOI] [PubMed] [Google Scholar]
- 17.Bennett FD, Jayson MIV, Rubenstein D, Campbell EJM. The ability of man to detect added non-elastic loads the breathing. Clinical Science. 1962;23:155–162. [PubMed] [Google Scholar]
- 18.Campbell EJM. A being breathing thoughtful breaths. Am J Resp Crit Care Med. 2000;162:2027–2028. doi: 10.1164/ajrccm.162.6.10-00hh. [DOI] [PubMed] [Google Scholar]
- 19.Campbell EJM, Freedman S, Smith PS, Taylor ME. The ability of man to detect added elastic loads to breathing. Clinical Science. 1961;20:223–231. [PubMed] [Google Scholar]
- 20.Banzett RB, Dempsey JA, O’Donnell DE, Wamboldt MZ. Symptom perception and respiratory sensation in asthma. Am J Resp Crit Care Med. 2000;162:1178–1182. doi: 10.1164/ajrccm.162.3.9909112. [DOI] [PubMed] [Google Scholar]
- 21.Manning HL, Schwartzstein RM. Respiratory sensations in asthma: Physiological and clinical implications. J Asthma. 2001;38:447–460. doi: 10.1081/jas-100105865. [DOI] [PubMed] [Google Scholar]
- 22.Rubin BK, Pohanka V. Beyond the guidelines: Fatal and near-fatal asthma. Peadiatr Resp Rev. 2012;13:106–111. doi: 10.1016/j.prrv.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Harver A. The effects of feedback on the ability of asthmatic subjects to detect increases in the flow-resistive component to breathing. Health Psychol. 1994;13:52–62. doi: 10.1037//0278-6133.13.1.52. [DOI] [PubMed] [Google Scholar]
- 24.Stout C, Kotses H, Creer TL. Improving perception of air flow obstruction in asthma patients. Psychosom Med. 1997;59:201–206. doi: 10.1097/00006842-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Baird JC, Noma E. Fundamentals of scaling and psychophysics. New York: Wiley; 1978. [Google Scholar]
- 26.Ashton R, White KD, Hodgson G. Sensitivity to heart rate: A psychophysical study. Psychophysiol. 1979;16:463–466. doi: 10.1111/j.1469-8986.1979.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 27.Harver A, Katkin ES, Bloch E. Signal-detection outcomes on heartbeat and respiratory resistance detection tasks in male and female subjects. Psychophysiol. 1993;30:223–230. doi: 10.1111/j.1469-8986.1993.tb03347.x. [DOI] [PubMed] [Google Scholar]
- 28.Rouse CH, Jones GE, Jones KR. The effect of body composition and gender on cardiac awareness. Psychophysiol. 1988;25:400–407. doi: 10.1111/j.1469-8986.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- 29.Swets JA. Detection theory and psychophysics: A review. Psychometrika. 1961;26:49–63. doi: 10.1007/BF02289684. [DOI] [PubMed] [Google Scholar]
- 30.Swets JA. Is there a sensory threshold? Science. 1961;134:168–177. doi: 10.1126/science.134.3473.168. [DOI] [PubMed] [Google Scholar]
- 31.Boice R, Gardner RM. A computer program to generate parametric and nonparametric signal-detection parameters. Bull Psychonom Soc. 1988;26:365–367. [Google Scholar]
- 32.McQuaid EL, Fritz GK, Yeung A, Biros PA, Mansell A. Resistive-load detection in healthy school-aged children. Pediatr Pulmonol. 1996;22:357–63. doi: 10.1002/(SICI)1099-0496(199612)22:6<357::AID-PPUL4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 33.Fritz GK, McQuaid EL, Nassau JH, Klein RB, Mansell A. Thresholds of resistive load detection in children with asthma. Pediatr Pulmonol. 1999;28:271–276. doi: 10.1002/(sici)1099-0496(199910)28:4<271::aid-ppul6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 34.Stubbing DG, Killian KJ, Campbell EJ. Weber’s law and resistive load detection. Am Rev Respir Dis. 1983;127:5–7. doi: 10.1164/arrd.1983.127.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Koopman M, Zanen P, Kruitwagen CLJJ, van der Ent CK, Arets HGM. Reference values for paediatric pulmonary function testing: The Utrecht dataset. Respir Med. 2011;105:15–23. doi: 10.1016/j.rmed.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Davenport PW, Kifle Y. Inspiratory resistive load detection in children with life-threatening asthma. Pediatr Pulmonol. 2001;32:44–8. doi: 10.1002/ppul.1087. [DOI] [PubMed] [Google Scholar]
- 37.Fritz GK, Adams SK, McQuaid EL, Klein R, Kopel S, Nassau J, Mansell A. Symptom perception in pediatric asthma: Resistive loading and in vivo assessment compared. Chest. 2007;132:884–889. doi: 10.1378/chest.06-2140. [DOI] [PubMed] [Google Scholar]
- 38.Koinis-Mitchell D, McQuaid EL, Seifer R, Kopel SJ, Nassau JH, Klein RB, Feldman J, Wamboldt MZ, Fritz GK. Symptom perception in children with asthma: cognitive and psychological factors. Health Psychol. 2009;28:226–37. doi: 10.1037/a0013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweitzer C, Marchal F. Dyspnoea in children. Does development alter the perception of breathlessness? Respir Physiol Neurobiol. 2009;167:144–53. doi: 10.1016/j.resp.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Yoos HL, Kitzman H, McMullen A, Sidora K. Symptom perception in childhood asthma: how accurate are children and their parents? J Asthma. 2003;40:27–39. doi: 10.1081/jas-120017204. [DOI] [PubMed] [Google Scholar]
- 41.Turcotte H, Corbeil F, Boulet LP. Perception of breathlessness during bronchoconstriction induced by antigen, exercise, and histamine challenges. Thorax. 1990;45:914–9188. doi: 10.1136/thx.45.12.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans KC. Cortico-limbic circuitry and the airways: Insights from functional neuroimaging of respiratory afferents and efferents. Biol Psychol. 2010;84:13–25. doi: 10.1016/j.biopsycho.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near fatal asthma. New Engl J Med. 1994;330:1329–1334. doi: 10.1056/NEJM199405123301901. [DOI] [PubMed] [Google Scholar]
- 44.Julius SM, Davenport KL, Davenport PW. Perception of intrinsic and extrinsic respiratory loads in children with life-threatening asthma. Pediatr Pulmonol. 2002;34:425–33. doi: 10.1002/ppul.10199. [DOI] [PubMed] [Google Scholar]
- 45.Kelsen SG, Prestel TF, Cherniack NS, Chester EH, Deal EC. Comparison of the respiratory responses to external resistive loading and bronchoconstriction. Clinical Investigation. 1981;67:1761–1768. doi: 10.1172/JCI110215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moy ML, Weiss JW, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162:451–455. doi: 10.1164/ajrccm.162.2.9907138. [DOI] [PubMed] [Google Scholar]
- 47.Taguchi O, Kikuchi Y, Hida W, Iwase N, Satoh M, Chonan T, Takishima T. Effects of bronchoconstriction and external resistive loading on the sensation of dyspnea. J Appl Physiol. 1991;71:2183–2190. doi: 10.1152/jappl.1991.71.6.2183. [DOI] [PubMed] [Google Scholar]
- 48.Janssens T, Verleden G, De Peuter S, Van Diest I, van den Bergh O. Inaccurate perception of asthma symptoms: A cognitive-affective framework and implications for asthma treatment. Clin Psych Rev. 2009;29:317–327. doi: 10.1016/j.cpr.2009.02.006. [DOI] [PubMed] [Google Scholar]


