Abstract
The assembly of functional neural circuits requires the combined action of progressive and regressive events. Regressive events encompass a variety of inhibitory developmental processes, including axon and dendrite pruning, which facilitate the removal of exuberant neuronal connections. Most axon pruning involves the removal of axons that had already made synaptic connections, thus, axon pruning is tightly associated with synapse elimination. In many instances these developmental processes are regulated by the interplay between neurons and glial cells that act instructively during neural remodeling. Owing to the importance of axon and dendritic pruning, these remodeling events require precise spatial and temporal control, and this is achieved by a range of distinct molecular mechanisms. Disruption of these mechanisms results in abnormal pruning, which has been linked to brain dysfunction. Therefore, understanding the mechanisms of axon and dendritic pruning will be instrumental in advancing our knowledge of neural disease and mental disorders.
Keywords: axon retraction, synapse elimination, dendrite severing, circuit refinement, Wallerian degeneration, neurite remodeling
INTRODUCTION
Establishment of proper connections in the developing brain is essential for perception, language, thought, consciousness, learning and memory. Although neural circuits are initially assembled through progressive events that include cell migration, neuronal process growth, target recognition, and synaptogenesis, regressive events play crucial roles in neural circuit refinement. Regressive events encompass a variety of processes such as synapse elimination, dendritic spine constraint, and axon and dendrite pruning. Axon pruning events facilitate the removal of exuberant axonal branches, many of which have already formed functional synaptic connections prior to their removal (Liu et al 2005, Low et al 2008). Thus, synapse elimination usually precedes pruning. Disruption of normal pruning events during circuit maturation and refinement can lead to brain dysfunction and neurological disease. On the other hand, excessive cortical pruning during puberty is strongly correlated with the early onset of schizophrenia (Lewis & Levitt 2002). Furthermore, certain axon pruning events and pathological neural degeneration events show striking histological resemblance to one another, implying that the underlying molecular mechanisms might be similar. Therefore, uncovering mechanisms that direct pruning is instrumental for advancing our knowledge about normal brain development, mental disorders and neuronal regeneration. Here, we review the current understanding of axon pruning and related regressive events, and we reflect upon potential implications of these processes in the context of neural pathology.
AXON PRUNING AND SYNAPSE ELIMINATION: GENERAL CONSIDERATIONS
Regressive events occur during the development of many neural circuits and provide a means to refine preexisting circuits. Synapse elimination and axon pruning are widespread in the central nervous system (CNS) and have also been observed in the peripheral nervous system (PNS). While it is not clear why exuberant connections are made in the first place, one possible explanation is the limited availability of unique cues to establish a myriad of specific mature circuits. Given these limited resources, sequential steps of circuit establishment and refinement allow for fine tuning of connections. Another reason for the establishment of more connections than are ultimately needed is to allow for activity-dependent sculpting of neural circuits and refinement of connections through competition. Finally, these exuberant connections themselves may play functional physiological or structural roles during early development, and though important during larval or embryonic stages they must be refined or eliminated prior to adulthood.
There are two basic types of axon pruning: pruning of short axon terminal branches and stereotyped pruning of long axons. The former normally involves integration of environmental factors such as trophic support or neuronal activity, however, during stereotyped pruning elimination of axons is genetically predetermined. Several examples of the first class can be found in the PNS. Sensory and sympathetic axons compete for neurotrophic support provided by intermediate and final targets (Hamburger & Levi-Montalcini 1949, Harrington & Ginty 2013, Levi-Montalcini 1987). This competition is enhanced by the presence of punishment signals that further penalize the losing axons, ultimately resulting in their removal (Deppmann et al 2008). In most instances, pruning of sensory and sympathetic axons is accompanied by apoptosis (Harrington & Ginty 2013, Levi-Montalcini 1987). Motor axons also compete for occupancy of the neuromuscular junction (NMJ), and after a period of competition mediated by neuronal activity individual muscle fibers end up innervated by a single motor axon (Balice-Gordon & Lichtman 1994, Colman et al 1997, Walsh & Lichtman 2003). A classic example of short axon collateral pruning in the developing CNS is the refinement that occurs in the climbing fiber circuit of the cerebellum (Crepel et al 1976, Mariani & Changeux 1981). This process is mostly dependent on neuronal activity, similar to the NMJ. Initially, multiple climbing fibers innervate a single Purkinje cell. However, during the first postnatal weeks only one climbing fiber remains associated with each Purkinje cell and translocates to the Purkinje cell dendritic arbor, while CF axons that remain on the Purkinje cell soma are lost (Altman 1972, Chedotal & Sotelo 1992).
Many examples of large-scale stereotyped pruning are found throughout CNS development. These pruning events can be further subdivided into two classes based on anatomical and histological observations: (1) degenerative-like axon elimination, in which axons become fragmented during the course of pruning; and (2) retraction-like pruning, whereby axons draw back without shedding axon fragments. Examples falling into the first class include axon remodeling in the Drosophila mushroom body (MB) (Lee et al 1999), and in mammals the removal of visual cortical spinal tract projections (Stanfield et al 1982) and over extending retinocolicular projections (McLaughlin et al 2003). An example of retraction-like axon pruning in the rodent CNS is the pruning of the hippocampal infrapyramidal tract (IPT) during postnatal development (Bagri et al 2003). These and other examples of axon pruning and synapse elimination, and also the mechanisms that underlie these regressive events, will be discussed in detail below.
MOLECULAR MECHANISMS OF NEURONAL PROCESS PRUNING AND SYNAPSE ELIMINATION
Pruning of axons and synapses can be triggered by many intrinsic and extrinsic factors. However, these processes must be tightly regulated to generate precise circuit organization. What are the molecular mechanisms that underlie these developmental processes? Genetic and biochemical studies implicate activation of pro-apoptotic caspases, the proteosome-ubiquitin pathway, repulsive guidance cue signaling, neuronal activity, and growth-promoting signaling in developmental pruning events.
Self destruction pathways
Recent studies highlight the importance of the caspase cascade in axon pruning and synapse elimination. Caspases are well known integral regulators of apoptosis, and they can be activated by a variety of cell death pathways (Denault & Salvesen 2002). Extracellular triggers involve the activation of receptors such as Fas, TNFα receptor 1 (TNF-R1), and other death receptors (DRs) by their respective ligands (Haase et al 2008). One such receptor, the orphan-receptor DR6, binds to amyloid precursor protein (APP) to regulate degeneration-like pruning in the PNS and CNS (Nikolaev et al 2009, Olsen et al 2014). Activation of the same caspase-dependent signaling pathway is observed when both sensory and sympathetic axons are deprived of neurotrophic factors (Cusack et al 2013, Nikolaev et al 2009, Simon et al 2012). This type of degeneration-like pruning requires classical apoptotic signaling, including the apoptotic factor Bcl2-associated X protein (Bax) and also Caspase-9 activation of Caspase-6 and Caspase-3. However, in contrast to apoptosis, this signaling event does not require apoptotic protease activating factor 1 (APAF1) (Cusack et al 2013, Simon et al 2012). In the presence of neurotrophic support, axons are protected by the axon-specific translation of B cell lymphoma w (Bclw), a Bax inhibitor (Cosker et al 2013). Interestingly, although caspases are involved in cell death and degeneration-like pruning, they are dispensable for Wallerian degeneration (WD), the programmed degeneration of the axonal fragment distal to a PNS or CNS injury site (discussed below).
Competition for neurotrophic support is a key driving force for circuit assembly and refinement in the PNS. Seminal experiments done by Hamburger and Levi-Montalcini demonstrated the requirement for target derived neuron survival factors (Hamburger & Levi-Montalcini 1949, Levi-Montalcini 1987), establishing a foundation for the neurotrophic factor hypothesis (Oppenheim 1989). This hypothesis states that neurons compete for limited target-derived survival factors. The ‘winning’ neurons achieve a competitive advantage and survive, while neurons that fail to compete initially loose their axons by degeneration-like pruning and later die. Neurotrophins, the best characterized of these target-derived survival factors, signal through specific tyrosine-kinase receptors and have been long known to sculpt PNS and CNS circuits (Harrington & Ginty 2013). It is interesting to note that neurotrophin deprivation triggers axon pruning and cell death in a caspase-dependent manner (Cusack et al 2013, Simon et al 2012). Because neurotrophin deprivation-induced pruning usually accompanies cell death, it is often hard to distinguish between these two developmental events.
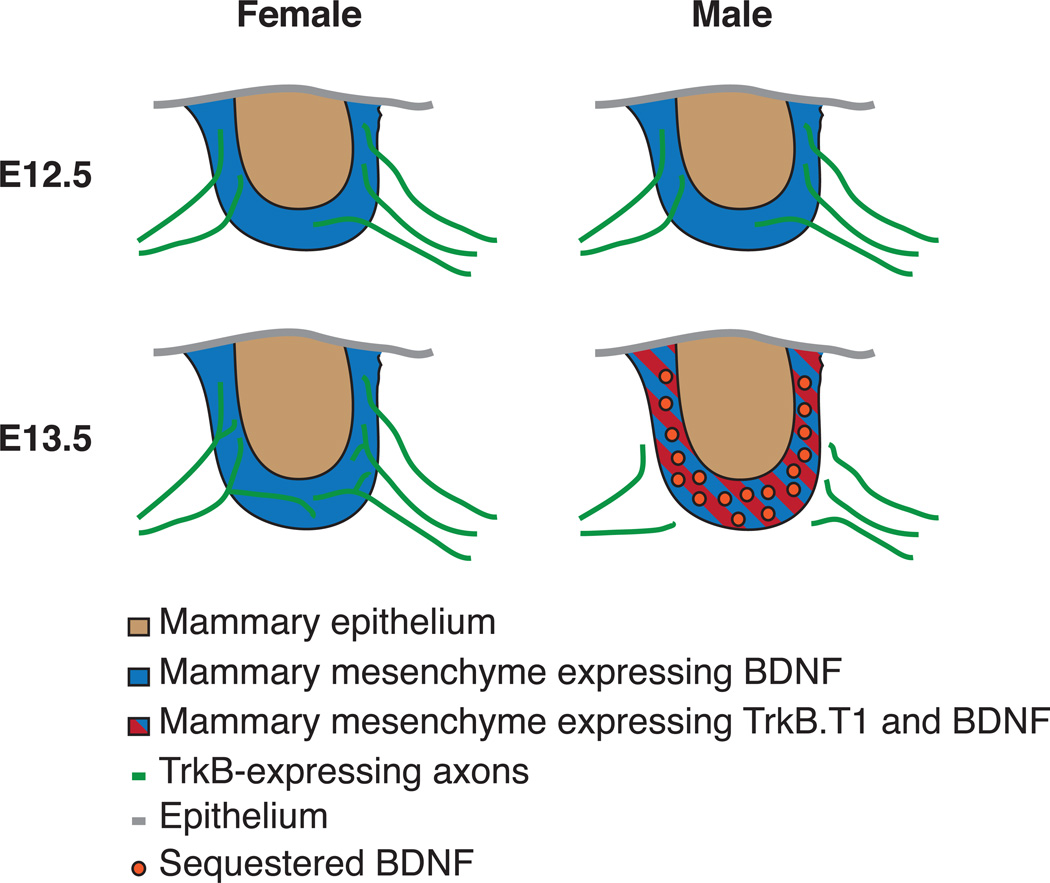
An interesting example of PNS circuit refinement that sheds light on this issue is the sexually dimorphic innervation of the murine mammary gland (Figure 1). The neurotrophin brain-derived neurotrophic factor (BDNF) is secreted by the mammary mesenchyme and is required for establishing mammary gland sensory innervation in both sexes at early embryonic developmental stages (Liu et al 2012). These effects of BDNF on sensory axon innervation of the developing mammary gland are mediated by the TrkB receptor tyrosine-kinase (RTK). Later in development, androgens promote expression of a truncated TrkB receptor isoform lacking its cytoplasmic signaling domain in the mammary mesenchyme of males, but not in females (Klein et al 1990, Liu et al 2012). This attenuates BDNF-TrkB signaling in the sensory axons of male embryos and leads to a rapid loss of mammary gland innervation that is independent of neuronal apoptosis. Thus, sex hormone regulation of BDNF-TrkB signaling directs sexually dimorphic refinement of sensory innervation, which ultimately results in the generation of a sex-specific neural circuit. Since formation of sexually dimorphic mammary gland sensory circuitry is not due to apoptotic loss of male sensory neurons, these data reveal a distinction between BDNF deprivation-mediated cell death and axon pruning.
Figure 1.
Sex hormone regulation of the neurotrophic signal BDNF directs sexually dimorphic axonal maintenance and pruning. During early mammary gland development in the mouse (E12.5), TrkB+ axons receive trophic support from BDNF-expressing mammary mesenchyme in both males and females. At E13 androgens in males induce expression of a truncated form of the TrkB receptor (TrkB.T1) by the mammary mesenchyme. TrkB.T1 sequesters BDNF, neutralizing BDNF signaling. As a result, sensory axons in males are deprived of neurotrophic support and are rapidly pruned (E13.5).
The ubiquitin proteosome system (UPS) provides another example of a self-destruction pathway that participates in axon and dendritic pruning (Hoopfer et al 2006, Kuo et al 2005, Watts et al 2003). Activation of the UPS is required for mushroom body (MB) remodeling in the brains of Drosophila pupae. After larval MB circuit establishment, two classes of MB neurons (α’/β’ and γ) elaborate axonal projections. However, in early pupae, γ neuron axons and dendrites are selectively pruned, while α’ and β’ projections remain intact through metamorphosis (Yu & Schuldiner 2014). MB pruning proceeds through local neurite degeneration, starting with dendrites and followed by axons (Watts et al 2003, Yu & Schuldiner 2014). The whole process of MB remodeling is ecdysone-dependent and requires a functional UPS, and in the absence of either the ubiquitin activating enzyme (E1), or two of the proteosome subunits, severe axon pruning defects occur. The ubiquitin E3 ligase complex, which includes the core proteins Cullin1 and Slimb, is also necessary for MB pruning (Wong et al 2013). This same molecular machinery is required during dendrite severing of dendritic arborization (da) neurons, which are sensory neurons that extend their dendrites along the epidermis. Interestingly, the substrate-recognition protein Slimb functions during da dendritic pruning by promoting ubiquitination of Akt, an activator of the PI3K/TOR signaling pathway. AKT ubiquitination leads to its degradation and also inactivation of the PI3K/Tor pathways. These results suggest a functional link between the UPS and PI3K/TOR signaling pathways during neural circuit remodeling.
Although many of the molecular effectors used during axon pruning are also required for dendrite pruning, there are some interesting differences. Inactivation of PI3K/Tor signaling pathways is required for da dendrite severing in the body of developing Drosophila pupae, but not for MB axon pruning (Wong et al 2013, Yu & Schuldiner 2014). Another example of a molecular distinction between axon and dendrite remodeling is the requirement for the flavoprotein oxidoreductase Mical during da dendritic pruning, but not during MB axon remodeling (Kirilly et al 2009). Although some of these differences can be attributed to intrinsic characteristics of MB and da neurons, fundamental differences in microtubule polarity between dendritic and axonal compartments likely underlie some of the signaling distinctions between the pruning of axons and dendrites. Electron microscopic ultrastructural analysis revealed that axons of mature vertebrate neurons contain microtubules that are oriented exclusively with plus-ends projecting away from the soma, whereas the dendrites contain microtubules with mixed polarity (Baas et al 1988). More recently, using dynamic imaging of MTs it was shown that polymerization of MTs in mature neurons occurs almost exclusively in the anterograde direction for axons, while both anterograde and retrograde polymerization was observed in dendrites (Kollins et al 2009). It is therefore not surprising that although many signaling events in dendrite and axon pruning are shared, there are differences that likely reflect unique cytoskeletal architecture and composition between axons and dendrites.
Repulsive guidance cue signaling and pruning
During early neural development axons navigate the extracellular milieu guided by attractive and repulsive cues. These attractive and repulsive guidance cues are reused throughout neural development and into adulthood, and they mediate a myriad of cellular guidance events ranging from cell migration to circuit refinement. Of particular interest is the function of repulsive cues during axon pruning and synapse elimination. Two families of repellents play major roles during the later stages of circuit refinement and maturation: semaphorins and ephrins.
The role of semaphorin (Sema) signaling during initial circuit assembly in vertebrates and invertebrates is well documented (Bashaw & Klein 2010, Koropouli & Kolodkin 2014, Pasterkamp & Giger 2009). Intriguingly, semaphorins are expressed in many regions of the CNS well after axons have arrived at their targets. This raises the possibility of these guidance cues playing later roles in circuit maturation and refinement. One member of the Class 3 secreted Semas, semaphorin 3F (Sema3F), participates in axon pruning and synapse constraint in several brain regions (Bagri et al 2003, Koropouli & Kolodkin 2014, Low et al 2008, Tran et al 2009). For example, Sema3F acts through its holoreceptor complex comprised of neuropilin-2 (Npn2) and plexin A3/plexin A4 (PlexA3/PlexA4) to direct the pruning of visual corticospinal tract axons in the mouse (Low et al 2008). During late embryogenesis and early postnatal development, axons extending from layer V pyramidal neurons in the visual cortex project along with layer V axons from the motor cortex into the spinal cord (Stanfield et al 1982). However, during the second postnatal week transient synapses previously formed by layer V visual cortical axons in the spinal cord are eliminated, and these axons are removed by Npn2-dependent degeneration-like stereotyped pruning (Low et al 2008).
Sema3F also participates in the stereotyped pruning of axons that originate in the hippocampal dentate gyrus (DG) and extend beneath the main mossy fiber bundle in the infrapyramidal tract (IPT), which is also known as the infrapyramidal bundle (Bagri et al 2003). The IPT forms synapses on basal dendrites of CA3 pyramidal neurons (Liu et al 2005). Stereotyped pruning of the IPT occurs during postnatal murine development by neurite retraction and superficially resembles axon repulsion (Bagri et al 2003). This retraction-like pruning event is accompanied by synapse elimination within the IPT (Liu et al 2005). Sema3F is required for IPT axon pruning and synapse elimination, in addition to dendritic spine remodeling and repulsion of DG axons (Bagri et al 2003, Liu et al 2005, Sahay et al 2003, Tran et al 2007). The underlying signaling events that regulate IPT pruning involve the inhibition of the small G-protein Rac1 by the Rac GTPase activating protein (GAP) β2-Chimaerin (β2Chn), an event that is essential for Sema3F-mediated IPT axon pruning (Riccomagno et al 2012). β2Chn selectively binds to the cytoplasmic domain of the Sema3F receptor neuropilin-2 (Npn-2), and activation of β2Chn by Sema3F is necessary for pruning both in vitro and in vivo. However, β2Chn is dispensable for axon repulsion and also for DG dendritic spine remodeling. This selective requirement for β2Chn in IPT pruning defines a mechanistic distinction among axon pruning, repulsion, and dendritic spine remodeling, all of which are mediated by the same chemorepellent, Sema3F. Taken together, these studies reveal the important roles of secreted semaphorins in postnatal axon pruning and circuit refinement.
A second family of repulsive cues, the ephrins and their Eph receptors, also plays key roles during axon pruning and refinement of CNS circuits. Ephrins act as ligands for Eph receptors (forward signaling), and also as receptors for Ephs (reverse signaling) (Klein 2012). Ephrin-Eph forward and reverse signaling play crucial roles in retinocollicular circuit establishment and refinement (Cang & Feldheim 2013, Feldheim et al 1998, McLaughlin & O'Leary 2005). Retinal ganglion cells (RGCs) are the only cell type in the retina that projects axons to the brain, and initially RGC axons extend along the length of the SC and sprout axon collaterals in their target area (Nakamura & O'Leary 1989, Simon & O'Leary 1990). Later during visual system development, an ephrinA-EphA counter-gradient in the SC acts instructively in the fragmentation-mediated degeneration-like pruning of overshooting RGC axons, setting up a template for topographic map formation. The roles of ephrins and Ephs in retinorecipient topographic mapping have been extensively reviewed and so will not be discussed here in detail (Cang & Feldheim 2013, McLaughlin & O'Leary 2005). Refinement of these rough topographic maps is provided by spontaneous waves of neural activity in the retina (Cang et al 2008). These retinal waves during the first postnatal week require cholinergic neurotransmission, and normal patterns of retinal activity are disrupted in the absence of the β2 subunit of the nicotinic acetylcholine receptor (Feller et al 1996, Picciotto et al 1995, Xu et al 1999). In β2−/− mice, several facets of dLGN and SC circuit remodeling are distorted, including retinotopy and segregation of inputs from each eye (Cang & Feldheim 2013, Cang et al 2005, Grubb et al 2003, McLaughlin et al 2003, Muir-Robinson et al 2002). This demonstrates a strong correlation between waves of neural activity and retinorecipient circuit remodeling. Thus, the combination of cholinergic waves and ephrinA signaling facilitates the establishment of topographic maps, highlighting the importance of the coordinated actions of neural activity and guidance-cue signaling during circuit refinement by axon pruning.
Ephrin signaling also participates in synapse elimination and refinement in several regions of the forebrain. Recent data suggest that reverse ephrinB signaling participates in hippocampal IPT remodeling (Xu & Henkemeyer 2009). EphB1, EphB2 and EphB3 act as redundant ligands for presynaptically expressed ephrin-B3 during IPT pruning. This process requires tyrosine phosphorylation-dependent reverse signaling mediated by the adaptor protein Grb4. Grb4, in turn, recruits effectors that activate Rac1 signaling. Ephrin-B3 also acts as a postsynaptic receptor in CA1 pyramidal neurons to mediate dendritic branch and spine constraint, and these effects are also mediated, in part, by Grb4-mediated activation of Rac1(Xu et al 2011). As described above, Sema3F acts instructively by activating a Rac-GAP, β2-Chimaerin, to direct IPT pruning (Riccomagno et al 2012). Given the opposing activities of Sema3F and Eph-reverse signaling on Rac1 activation, tight temporal and spatial regulation of Rac activity is likely important for IPT pruning. Rac activation has been shown to be a prerequisite for PlexinA1 activation during DRG axon guidance (Toyofuku et al 2005). Thus, one possibility is that Rac activation by EphB/Grb4 acts to keep PlexinA3 competent to signal. Subsequently, Sema3F expressed by interneurons in the distal infrapyramidal region triggers IPT pruning by binding to the Npn2/PlexA3 holoreceptor, activating β2Chn and inhibiting Rac1 activity.
Recent work in zebrafish highlights the importance of heparan sulfate proteoglycans (HSPGs) during the degeneration-like pruning events that eliminate mis-sorted axons in the optic tract during retinotectal development (Poulain & Chien 2013). HSPGs act non-cell-autonomously to correct mis-sorted axons, however whether HSPGs act directly on misrouted axons as a signaling cue or indirectly by modulating repulsive guidance cue signaling remains to be determined. It is intriguing that although HSPGs are known to modulate ephrin, slit and semaphorin signaling, guidance cue pathways with known roles in topographic mapping and retinal axon guidance (Cang & Feldheim 2013, Cho et al 2012, de Wit & Verhaagen 2007, McLaughlin & O'Leary 2005, Van Vactor et al 2006), HSPGs do not seem to be involved in ordering axonal projections along the optic tract (Poulain & Chien 2013).
Neuronal activity and pruning – molecular effectors
Neuronal activity plays a significant role in sculpting neural circuits. Neuronal activity can drive changes at single synapses, strengthening or weakening individual connections, but it can also act as a powerful regulator of overall circuit organization and function in the context of complex neural networks. A classical example of activity-dependent circuit maturation, pruning and refinement in the CNS is the postnatal development of the climbing fiber (CF) system in the cerebellum. Climbing fibers (CFs) originate in the inferior olive and make synaptic contacts with Purkinje Cells (PCs) in the cerebellum. Initially, each PC receives somatic innervation from multiple CFs with similar strength (Crepel et al 1976, Mariani & Changeux 1981). During the first weeks after birth, CFs undergo extensive axon pruning, and the synapses formed by the “weaker” CFs are progressively removed. By the end of this pruning period, a single “winning” CF will take over the PC dendritic field and completely displace the weaker CFs (Altman 1972, Chedotal & Sotelo 1992, Watanabe & Kano 2011). In the mature cerebellum, a single CF will form hundreds of synapse on the proximal dendrite of the PC by wrapping around it, making the CF-PC connection one of the strongest excitatory connections in the CNS (Ito 1984, Watanabe 2008). Depolarization of a single CF strongly depolarizes the PC that it innervates and triggers Ca2+ entry (Kano et al 1992, Regehr & Mintz 1994). CFs play a critical role in motor learning, and disruption of CF pruning has severe neuropathological consequences that include ataxia (Watanabe & Kano 2011).
Neural activity is the main driving force for sculpting connectivity in the CF system. Translocation/extension of CFs on to PC dendrites is an activity-dependent process: administration of tetrodotoxin or AMPA receptor blockers leads to CF atrophy even in adult rats and mice (Bravin et al 1999, Cesa et al 2007, Kakizawa et al 2005). CF presynaptic activity followed by Ca2+ influx into the PC and activation of Ca2+ -dependent signaling constitute key events during the early phases of CF synaptic pruning. Early postnatal alteration of normal CF activity patterns resulting from pharmacological application of harmaline, or genetic manipulation by transgenic overexpression of a chloride channel, results in multiple CFs innervating individual PCs (Andjus et al 2003, Lorenzetto et al 2009). Not surprisingly, PC-specific genetic ablation of CAv2.1, the pore forming subunit of a P/Q type voltage dependent Ca2+ channel that accounts for the major Ca2+ current in PCs (Mintz et al 1992, Stea et al 1994), results in a severe impairment of CF synapse elimination during the early pruning phase (Hashimoto et al 2011). How these changes in PC neural activity are communicated to the CF is discussed below. The end result of this activity-dependent CF homosynaptic competition is that only the CF with the strongest synaptic connection to the PC is allowed to extend axon branches to PC dendrites (Altman 1972, Chedotal & Sotelo 1992, Hashimoto et al 2009a).
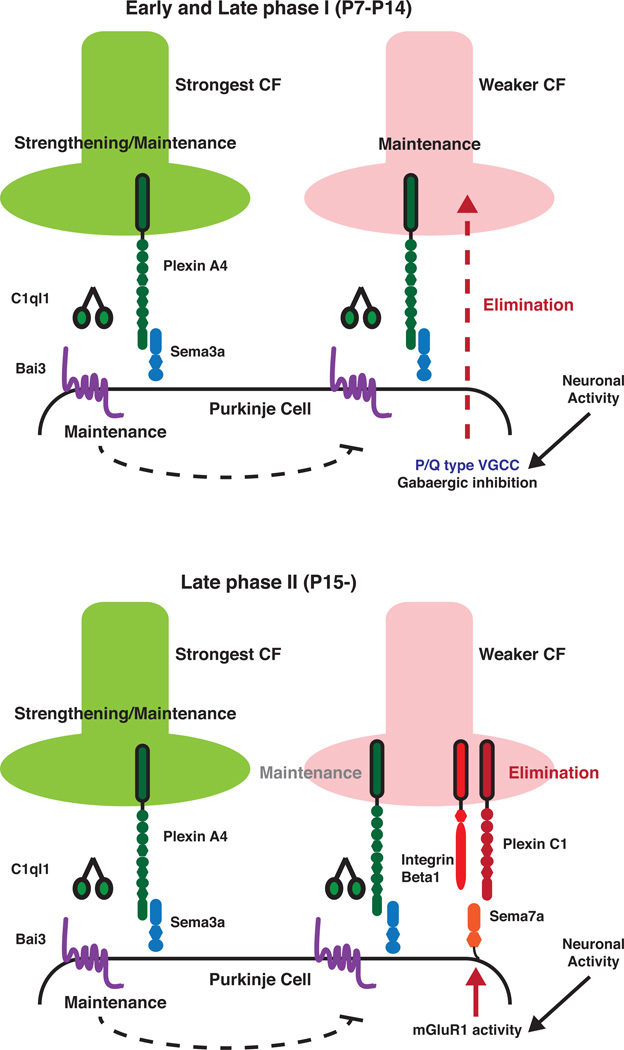
Following extension of CF axons along the dendrites of the PC to which it is most strongly connected, the early phase of synapse elimination begins in which somatic synapses formed by both stronger and weaker CFs are non-selectively eliminated. This is accompanied by further distal extension of axon branches from the stronger CF (Hashimoto et al 2009a, Hashimoto et al 2009b). As a result, weaker CFs that have only established synapses on to the PC soma are further weakened, while stronger CFs become comparatively stronger since they have already formed synapses on the PCs dendritic arbors. After the second postnatal week in the mouse, a second phase of CF synapse refinement that is dependent upon normal parallel fiber (PF) innervation of PCs begins (Crepel et al 1981). PFs are the bifurcated axons of cerebellar granule cells (GCs), and they innervate distal PC dendrites. Although each PC receives up to 106 PF synapses, individual PFs only make a few synapses onto each PC (Napper & Harvey 1988). CF elimination in this later phase is driven by heterotypic competition with PFs. Early genetic studies of spontaneous mutants revealed that normal formation of PF-PC synapses is essential for CF synapse elimination (Crepel & Mariani 1976, Mariani et al 1977, Watanabe & Kano 2011). If either GCs or PF-PC contacts are disrupted, CF synapse elimination does not proceed normally (Crepel & Delhaye-Bouchaud 1979, Hashimoto et al 2009b). PF-PC innervation acts in two ways to drive this later phase of CF pruning: first, it restricts CF innervation trans-synaptically through a cerebellin-1 (Cbln1)/glutamate receptor δ2 (GluRδ2)-dependent process; second, neural activity transmitted trough the PF-PC circuit activates a metabotropic glutamate receptor 1 (mGluR1) signaling cascade in PCs that drives the perisomatic elimination of CF synapses (Hashimoto et al 2001, Ichise et al 2000, Kakizawa et al 2000, Kano et al 1997). Thus, neural activity, first in the CF-PC pathway and later in the PF-PC pathway, is a powerful driving force for both phases of CF refinement (Figure 2).
Figure 2.
Antagonistic signals regulate climbing fiber refinement. C1ql1/Bai3, and Sema3A/PlexinA4 signaling promote maintenance and strengthening of synapses by acting through receptors expressed by the postsynaptic Purkinje Cell (PC) and presynaptic climbing fiber (CF), respectively. At earlier phases (P7 to P14), AMPA receptor-mediated PC neural activity, driven by CFs, promotes elimination of weaker climbing fibers by an as yet unclear mechanism involving P/Q-type Voltage Gated Calcium Channels (VGCC) that is modulated by gabaergic inhibition. Later (Late phase II, from P15 onward), activation of mGluR1 signaling by neural activity in the Parallel Fiber-PC circuit becomes the main driver for elimination of weaker CFs. This mGluR1 signaling cascade promotes the PC expression of Sema7A that, in turn, acts through presynaptic receptors Integrin β1 and also PlexinC1.
Although much emphasis has been placed on identifying factors acting in postsynaptic PCs that regulate CF pruning, very little is known about the molecular mechanisms that instruct the presynaptic CFs to prune. In this regard, a signal produced by the PC in response to mGluR1 activation has been proposed to trigger the nonselective elimination of perisomatic CF synapses during the second phase of pruning, but the identity of this signal/s has remained elusive (Watanabe & Kano 2011). One idea is that repulsive cues secreted by the PCs in an activity dependent manner contribute to the CF selection process. Recent work highlights the importance of Sema7a, a GPI-linked semaphorin, in this process (Uesaka et al 2014). Knock-down of Sema7A in PCs, or its receptors PlexinC1 and β1-Integrin in CFs, results in impaired CF elimination during the later phase of refinement. Another semaphorin secreted by PCs, Sema3A, apparently acts in an opposing fashion by stabilizing CF synapses (Uesaka et al 2014). Interestingly, Sema7A expression is reduced in the absence of mGluR1, and overexpression of Sema7A can rescue the effects of mGluR1 knock-down in PCs, suggesting that Sema7A acts downstream of mGluR1 during the second phase of CF pruning to influence CF innervation of PCs (Uesaka et al 2014) (Figure 2). Overall, these data reveal essential crosstalk between neural activity and repulsive guidance cues during cerebellar circuit refinement.
Transcriptional control of axon pruning
A well-studied example of degeneration-like pruning is the stereotyped pruning of Drosophila mushroom body (MB) γ neurons during metamorphosis (Yu & Schuldiner 2014). Although the final steps of γ neuron pruning events are executed by the UPS, as described above, the initiation of this process is tightly controlled by transcriptional cascades (Watts et al 2003, Yu & Schuldiner 2014). The TGF-β ligand myoglianin (Myo), acting through its receptor baboon (Babo), promotes the expression of ecdysone receptor isoform B1 (EcR-B1) in γ neurons, but not in α’/β’ neurons (Awasaki et al 2011, Zheng et al 2003). Recently, an immunoglobulin superfamily (IgSF) transmembrane protein called Plum was shown to facilitate Myo signaling upstream of Babo (Yu et al 2013). Plum, like Myo and Babo, is essential for EcR-B1 expression and pruning. EcR-B1 and ultraspiracle (USP) form a nuclear hormone receptor complex that, when bound by the molting hormone ecdysone, triggers the onset of pruning by activating downstream transcriptional pruning programs (Lee et al 2000). This results in the expression of the transcription factor Sox14, which in turn regulates expression of the aforementioned E3 ubiquitin ligase Cullin1 (Kirilly et al 2011, Wong et al 2013). A Myo/Plum/Babo/EcR-B1 pathway also participates during refinement of synaptic arborizations at the neuromuscular junction (NMJ) in Drosophila, but to what extent the same downstream effectors participate in MB pruning and NMJ remodeling is not known (Yu et al 2013).
A signaling cascade that results in transcriptional control of developmental axon pruning has also been described in mammals, albeit in the context of a different set of signaling components (Chen et al 2012). The kinase Gsk3β, which among its many functions participates in the regulation of transcriptional programs (Hur & Zhou 2010), is required for NGF deprivation–induced sensory DRG neuron degeneration in vitro and developmental pruning in vivo (Chen et al 2012). Using Campenot chambers it was shown that Gsk3β acts in sensory neuron somata and nuclei, but not in axons, to promote pruning in vitro. This effect is mediated in part by the regulation of gene expression. Two of the genes regulated by Gsk3β, the transcription factor t-box 6 (tbx6) and the long noncoding RNA deleted in lymphocytic leukemia 2 (dleu2), are also necessary for NGF-withdrawal–induced degeneration-like pruning of sensory neurons in vitro. Given that tbx6 is a transcription factor and that dleu2 encodes two miRNAs, it is possible that a transcriptional/post-transcriptional mechanism regulates pruning events remotely from the soma and nucleus. Whether any of these transcriptional cascades act instructively or only as necessary permissive players during these pruning events is an interesting subject for future investigation.
Cytoskeletal remodeling and pruning
The neuronal cytoskeleton is composed of three major components: actin, microtubules (MTs) and neurofilaments (NFs). Though neurofilaments serve well-characterized structural roles (Perrot et al 2008), actin filaments are highly dynamic during neural development (Andersen et al 2010, Gomez & Letourneau 2014). Actin remodeling has been indirectly implicated in retraction-like pruning, as exemplified by the opposing roles of EphB and Sema3 signaling in modulating Rac1 activity during IPT refinement (Riccomagno et al 2012, Xu & Henkemeyer 2009). MTs are also highly dynamic polymers, and they are essential for trafficking in neurons, neuronal structure, and certain aspects of growth cone steering (Kollins et al 2009, Prokop 2013). MT breakdown is one of the initial steps in axonal degeneration-like pruning, and MT fragmentation in both flies and mammalian neurons accompanies this class of pruning (Watts et al 2003, Zhai et al 2003). Moreover, MT destabilization is sufficient to induce axon fragmentation in vitro (Luduena et al 1986). The molecular machinery that participates in this process is beginning to be uncovered (Schuldiner & Yaron 2014). The kinesin superfamily protein 2a (Kif2A) is a key player in the breakdown of microtubules during axon pruning in mammals (Maor-Nof et al 2013). Kif2A is required in vitro for MT disassembly in DRG neurons upon trophic factor deprivation, and mice carrying mutations in Kif2A show skin hyperinnervation phenotypes suggestive of defects in competition-dependent pruning (Maor-Nof et al 2013). In Drosophila, the katanin-like molecule Kat60L plays similar roles in dendritic arborization (da) dendrite severing, but not in MB remodeling (Lee et al 2009). These observations suggest an overall conservation of the underlying mechanisms that mediate degeneration-like remodeling in both axons and dendrites.
Synapse Elimination and Axon Pruning: Are they Separable Events?
Histological studies in mouse neurological disease models reveal a strong correlation between axon pruning and defects in synapse elimination (Holmes et al 1999, Ivanco & Greenough 2002). For example, pruning of the IPT is aberrant in the mouse model for Fragile-X, the Fmr1−/− mouse, which also shows defects in the constraint of synapses and spines in several forebrain regions (Comery et al 1997). Axon pruning is almost always preceded by synapse elimination. For example, during IPT pruning distal infrapyramidal synapses are eliminated prior to IPT axonal retraction (Liu et al 2005). This was observed by detailed immunohistological and electron microscopic analysis of nescient IPT synapses during pruning. Similarly, synapses are eliminated just prior to pruning of the visual branch of the CST and pruning of retinocollicular axons that have overshot their SC targets (Cheng et al 2010, Low et al 2008). It remains to be seen, however, whether synapse elimination and axonal pruning within these axon tracts are distinct, sequential, cellular events, or if both developmental processes are intrinsically linked and controlled by the same molecular mechanisms. Since synapses are normally actively maintained, one likely mechanism underlying synapse elimination events that precede axon pruning is the inhibition of synapse-maintenance pathways, including actin cytoskeleton regulators, adhesion receptors and scaffolding proteins (Lin & Koleske 2010). Supporting this notion is the observation that signaling events with known roles in synapse maintenance, including activation of Rac1 and inhibition of Rho, are actively disrupted during axon pruning (Lin & Koleske 2010, Nakayama et al 2000). Is then axon pruning a by-product of synapse elimination? To answer this complex question will likely require a combination of molecular genetics, histology and careful time lapse imaging of these developmental processes. Androgen-dependent pruning of the male mammary gland is very likely not preceded by synapse formation and elimination (Liu et al 2012)), suggesting that in this system there is a mechanistic separation between these developmental processes. Importantly, a recent study shows that in the retinogeniculate system axon complexity remains stable while extensive synapse elimination is taking place (Hong et al 2014). Presynaptic boutons cluster and grow in size during this synapse remodeling process, and axon elimination only occurs after the completion of the critical period for eye-specific retinogeniculate axon sorting, highlighting a temporal separation between synapse elimination and axon pruning.
THE ROLE OF GLIA DURING PRUNING AND SYNAPSE ELIMINATION
The importance of glial cells acting as phagocytes for fragmented axons and cell debris during axon pruning is well established. For example, in the Drosophila mushroom body (MB) glial cells infiltrate the MB lobes that are undergoing degenerative-pruning, and inhibition of endocytosis specifically in glia results in axon clearance defects (Awasaki & Ito 2004, Watts et al 2004). In line with these observations, the glial cell surface engulfment receptor draper (an ortholog of Ced-1 in C. elegans) and the scavenger receptor Ced-6 are required for efficient clearance of axon fragments during developmental MB axon degeneration (Awasaki et al 2006, Hoopfer et al 2006). Surprisingly, the main glial cell type that participates in the clearance of MB degenerating axons in Drosophila pupae turns out to be astrocytes (Hakim et al 2014, Tasdemir-Yilmaz & Freeman 2014). The draper signaling pathway is required in astrocytes for MB pruning and acts in a partially redundant fashion with the Crk/Mbc/dCed-12 complex. Drosophila astrocytes serve widespread roles during neuronal pruning since they participate in clearing of axon fragments and the cell bodies of peptidergic neurons in the ventral nerve cord (Tasdemir-Yilmaz & Freeman 2014). In the mammalian PNS, perisynaptic Schwann cells (SCs) carry out similar roles during synapse elimination at the NMJ: they engulf and eliminate presynaptic terminals by a process known as “axosome” shedding (Bishop et al 2004, Smith et al 2013).
More recently, it has become clear that glial cells participate in an instructive fashion during axon and synapse pruning events. At least two different glial cell types, astrocytes and microglia, actively participate in synapse elimination and axon pruning in the mammalian CNS. One region of the CNS where the involvement of glial cells during pruning has been studied in some detail is the retinogeniculate system. During early postnatal development, retinal ganglion cell (RGC) axons form transient synapses with relay neurons in the dorsal lateral geniculate nucleus of the thalamus (Stevens et al 2007). However, many of these synapses are eliminated, and the remaining axons elaborate arbors before eye opening (P14 in the mouse). This refinement process is regulated by TGF-β secreted by retinal astrocytes (Bialas & Stevens 2013), drawing an interesting parallel with Drosophila MB pruning, which is also regulated by TGF-β secreted by astrocytes. In the mammalian retina, TGF-β promotes the expression of the complement cascade initiator C1q by RGCs. C1q translocates to distal axons in the thalamus, is secreted, and tags weak synapses by currently unknown mechanisms (Bialas & Stevens 2013, Stevens et al 2007). C1q then triggers the classical complement cascade and causes the activation of C3. C3-tagged synapses are recognized by microglia expressing the complement receptor CR3 and then engulfed, resulting in the elimination of weak retinogeniculate synapses (Schafer et al 2012). Genetic disruption of any of these steps results in eye-specific RGC axon arbor segregation deficits in the LGN caused by the retention of excess RGC inputs onto post-synaptic neurons. The mechanisms that facilitate the tagging of weaker synapses and the preservation of stronger synapses remain to be studied. Microglia may play a broader role during pruning, since disrupting the migration of microglia or their development results in synaptic pruning defects in the hippocampus and cortex, and these defects correlate with changes in social behavior (Paolicelli et al 2011, Zhan et al 2014). Interestingly, activation of microglia might also be essential in the adult to provide neuroprotection and to modulate synaptic plasticity through the regulation of AMPA receptor and dendritic spine number (Chen et al 2014, Ji et al 2013).
Microglia-dependent synapse elimination only accounts for some of the retinogeniculate circuit refinement that establishes eye-specific segregation of retinal input to the dLGN (Stevens et al 2007). Recently, astrocytes have been shown to actively engulf synapses in the dLGN (Chung et al 2013). This mode of circuit refinement is dependent upon neuronal activity and requires Megf10- and MERTK-dependent phagocytic pathways. Interestingly, Megf10 is an orthologue of Draper which, as described above, is essential for astrocyte-dependent MB pruning in Drosophila (Hakim et al 2014, Tasdemir-Yilmaz & Freeman 2014). Astrocytes deficient for Megf10 or MERTK show decreased engulfment activity in vitro. Disruption of these phagocytic pathways leads to an overabundance of functional synapses and also to defects in eye-specific segregation of retinal input to the dLGN. Whether these pathways act exclusively in an astrocyte-autonomous manner in vivo remains to be explored. In adults, astrocytes also engulf cortical synapses, suggesting that astrocyte-dependent synapse elimination might be a widespread event (Chung et al 2013). Overall, these studies highlight the importance of glial cells in sculpting neural circuits during development. In the mammalian CNS, the two cell types that play essential roles during axon remodeling and synapse refinement are astrocytes and microglia. Interestingly, astrocytes participate in axon refinement in both vertebrates and invertebrates, suggesting yet another mechanistic conservation of neuronal process pruning.
HOW IS PRUNING LOCALLY RESTRICTED?
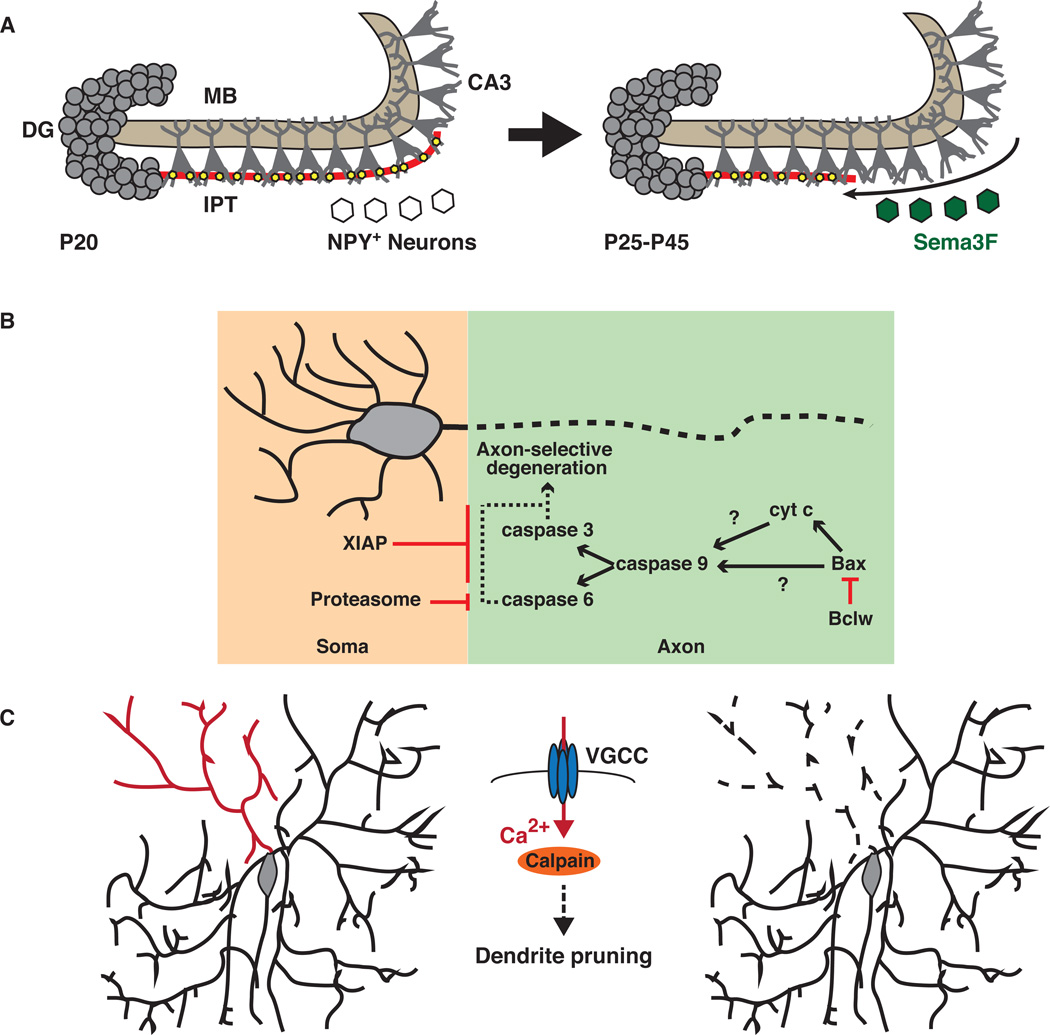
An interesting issue is how pruning is restricted to particular subsets of axons, branches, dendrites or synapses. Depending on the main driving force for a particular pruning event, the mechanisms that locally restrict pruning vary. If repulsive cues are the trigger for pruning, restricted expression of the ligand or its receptor could limit pruning to a few axons, axon branches, or dendrites. An example of this strategy is the pruning of the hippocampal IPT; in this system, only axons in the infrapyramidal bundle are pruned, sparing the main mossy fiber bundle. The local constraint of IPT pruning is achieved by restricting the expression of the ligand, Sema3F, to interneurons in the infrapyramidal area (Bagri et al 2003, Tran et al 2009). The timing of IPT pruning seems to be a consequence of the onset of Sema3F expression in these neurons, which commences around P25 (Figure 3A). This temporal control of axon pruning might be reinforced by an increase in the expression of the necessary cytoplasmic effector β2Chn, which also peaks between P25 and P35 (Riccomagno et al 2012).
Figure 3.
Spatiotemporal control of pruning. Three examples of spatiotemporal control strategies are presented. (a) Expression of the axonal repellent Sema3F (green) commences at P25 and is restricted to neuropeptide Y (NPY)+ interneurons in the infrapyramidal region, resulting in pruning of the infrapyramidal tract (IPT) alone while sparing the main bundle (MB) of the mossy fibers. (b) Caspase-dependent signaling is regulated at multiple levels so as to restrict its activity to the degenerating axon (blue box) without triggering cell death or dendritic pruning (yellow box). (c) Calcium influx in specific dendritic branches acts as a spatial and temporal cue to trigger pruning in Drosophila. Intrinsic excitability increases locally, activating calcium influx via voltage-gated calcium channels (VGCCs), which results in the activation of the calcium-activated protease calpain. Abbreviations: CA3, cornu ammonis 3; DG, dentate gyrus.
Other types of developmental strategies have been adopted to spatially restrict caspase-dependent pruning (Figure 3B). In the case of caspase signaling, it is critical to restrict these signaling events to the axonal compartment so as not to trigger apoptosis in the soma. One strategy employed to do this is the local release of cytochrome C within this cellular compartment. This is reinforced by the combined actions of the proteasome-mediated degradation and X-linked inhibitor of apoptosis Protein (XIAP) (Cusack et al 2013, Unsain et al 2013). The proteasome activity restricts caspase activation to axons during axon-specific degeneration, likely acting on Caspase-6, a known target of the proteasome degradation complex (Gray et al 2010, Tounekti et al 2004). Endogenous XIAP is a potent inhibitor of neural caspases and protects the soma from caspase activation during axon-specific degeneration in mammals, and also serves to confine caspase activity to sensory dendrites in Drosophila (Cusack et al 2013, Kuo et al 2006, Potts et al 2003, Unsain et al 2013, Williams et al 2006). Thus, a multilayered strategy is in place to restrict caspase activation during degeneration-like pruning to the axonal compartment.
An example of local activity-dependent pruning of dendritic branches is the control of this process by calcium transient activation of the protease calpain. Drosophila Class IV da neurons undergo dramatic pruning during metamorphosis. The UPS and caspases participate in these pruning events (Kuo et al 2005, Kuo et al 2006, Williams et al 2006), but how specific dendritic branches that undergo pruning are selected for removal was not known. Intriguing new data demonstrate that calcium transients restricted to particular dendritic branches spatially and temporally control pruning (Kanamori et al 2013) (Figure 3C). Calcium transients are reliable predictors of which dendritic branches will be pruned, and calcium entry is controlled by local increases in intrinsic excitability that in turn activate the opening of voltage-gated calcium channels (VGCCs) (Kanamori et al 2013). Given the role played by calcium entry and VGCCs in regulating climbing fiber pruning in the mammalian cerebellum (Hashimoto et al 2011), it is tempting to speculate that this defines an evolutionarily conserved mechanism capable of locally restricting neuronal process pruning to select neurons in specific locations. In da neurons in Drosophila, the calcium-activated protease calpain functions downstream of calcium transients and is required for dendritic pruning (Kanamori et al 2013). Calpain has also been shown to participate in axonal degeneration in mammals (George et al 1995, Ma et al 2013), and the calpain inhibitor calpastatin acts as a key checkpoint for axonal survival following injury and during development (Yang et al 2013). Thus, regulated expression and localization of calpastatin expression could provide an additional level of spatial and temporal control over pruning.
These examples of spatial and temporal control of neuronal process pruning illustrate the broad array of developmental strategies that have evolved to tightly control neural circuit refinement. Future studies will most certainly reveal additional phylogenetically conserved mechanisms, given the importance of restricting developmental pruning to specific cellular compartments. In this regard, two recent studies provide intriguing new evidence supporting a role for the endocytic machinery in down regulating inhibitory signals produced by the synapse to promote neuronal process pruning in Drosophila (Issman-Zecharya & Schuldiner 2014, Zhang et al 2014). This novel function for endocytosis during neural development highlights an additional mechanism for achieving spatiotemporal regulation of developmental pruning.
PRUNING VERSUS DEGENERATION
As we have discussed, there are two main classes of neuronal process pruning: retraction-like pruning and degeneration-like pruning. The later shows a striking histological similarity with Wallerian Degeneration (WD). WD is a mode of degeneration that occurs after an axon is severed in the periphery or in the CNS; it begins with a latent phase and continues with the extensive fragmentation of the axon cytoskeleton and disintegration of internal organelles distal to the injury site, concluding with the complete breakdown of the axon distal to the injury site (Coleman & Freeman 2010, Lee 1963, Thomas 1964, Thomas & Sheldon 1964). WD was initially thought to be the passive degradation of the distal axon due to the lack of soma-derived trophic support. However, with the discovery of a neomorphic spontaneous mutation in the mouse that delays WD following axotomy for weeks, called Wallerian Degeneration slow (Wlds), it became evident that WD is indeed an active process (Lunn et al 1989, Mack et al 2001). Wlds encodes a chimeric fusion of the E4 ubiquitin ligase Ube4b and the NAD+ scavenging enzyme nicotinamide mono-nucleotide adenylyltransferase 1 (Nmant1). Wlds acts cell-autonomously to protect axons in a dominant manner. Although the mechanisms by which Wlds provides axonal protection are not fully understood, its protective effects require an intact Nmant1 domain and are phylogenetically conserved across diverse species (Freeman 2014, Neukomm & Freeman 2014).
Though there are similarities between WD and degeneration-like pruning at the neuroanatomical level, there are both parallels and distinctions at the molecular level between these two processes. Calcium signaling is likely one of the earliest triggers for WD following axon injury. Calcium entry from the extracellular space is both required and sufficient to drive WD and acts by activating calpain family proteases (George et al 1995, Ma et al 2013). As we have seen, activation of calcium signaling in dendritic branches is predictive of select pruning events in Drosophila (Kanamori et al 2013) and is also a key step in climbing fiber pruning (Hashimoto et al 2011), suggesting mechanistic similarities between developmental pruning and WD. Another similarity is the involvement of the UPS system in both processes. As mentioned above, the UPS is an essential player during the developmental pruning of the MB axons (Watts et al 2003) and da dendrites (Kuo et al 2005), and the UPS also acts cell-autonomously to regulate WD. Inhibiting the UPS slows down WD in Zebrafish, mice, Drosophila and neural cultures (Martin et al 2010, Zhai et al 2003), demonstrating the importance of the UPS in both WD and developmental degeneration-like pruning.
On the other hand, Wlds is sufficient to delay WD for weeks, but it does not affect developmental pruning, highlighting an important molecular distinction between these two processes (Hoopfer et al 2006). A recently discovered scaffolding protein that is essential for WD in both Drosophila and mouse, dSARM/SARM1 (Drosophila sterile a/Armadillo/Toll-interleukin receptor homology domain protein), is dispensable for developmental pruning, providing additional genetic evidence that WD and pruning are distinct biological processes (Osterloh et al 2012). Another distinction between WD and axon pruning is the requirement for the E3 ubiquitin ligase highwire. Initially identified in Drosophila as a regulator of synapse elaboration (Wan et al 2000), both highwire and its mammalian homolog Phr1 (PAM, Highwire, RPM-1) are essential for WD, but are not required for developmental pruning (Babetto et al 2013, Watts et al 2003, Xiong et al 2012). Furthermore, although caspase signaling is important for developmental pruning induced by neutrophin deprivation, it is not required for injury-induced WD (Cusack et al 2013, Osterloh et al 2012, Simon et al 2012). These studies demonstrate that there are mechanistic similarities, and also clear distinctions, between developmental regulated pruning and WD (Figure 4).
Figure 4.
Parallels and distinctions between degeneration-like pruning and Wallerian degeneration. Despite broad similarities (blue box), as we investigate deeper into the mechanisms of these regressive events clear molecular distinctions arise (yellow box).
PRUNING IN HUMAN DEVELOPMENT AND DISEASE – THE EXAMPLE OF SCHIZOPHRENIA
In humans, axonal and synaptic pruning occurs in two main waves: the first during the first two years after birth, and the second during adolescence. Adolescence is a time of extensive CNS circuit refinement, coinciding with emotional reactivity, and it is a developmental period when symptoms of many psychiatric disorders, including schizophrenia and anxiety, become manifest (Casey et al 2008). Prefrontal cortex (PFC) development during puberty and adolescence plays an important role in the maturation of higher cognitive abilities such as decision-making and emotional control (Casey et al 2008). Experiments in animal models, from rodents to primates, establish that the prefrontal cortex is a brain area that undergoes massive refinement during adolescence. For example, in rats medial PFC dendrites undergo refinement, and axonal projections from the PFC to the amygdala undergo axon pruning during adolescence (Cressman et al 2010, Koss et al 2014). Adolescent hamsters also show extensive dendritic pruning in the amygdala (Zehr et al 2006). In the PFC of monkeys, pyramidal neurons that form intrinsic neural circuits within layer 3 undergo large-scale pruning of axonal arbors (Woo et al 1997). Consistent with animal model data, electron microscopy (EM) studies in postmortem human brains show that synapse number peaks between 2 and 4 years of age, and that most synapse elimination in the PFC occurs during puberty and adolescence (Huttenlocher & Dabholkar 1997). Data supporting these observations come from MRI studies that suggest the human brain reaches approximately 90% of its adult size by age six, but the gray and white matter subcomponents of the brain continue to undergo dynamic changes throughout adolescence (Giedd 2004, Gogtay et al 2004, Sowell et al 2003). Similar to what was observed through direct histological assessment, MRI analysis in humans revealed that brain regions involved in primary functions, such as motor and sensory systems, are refined prior to higher-order association areas, such as the PFC, that integrate these primary functions. Therefore, primary cortex is refined mainly during childhood, whereas higher-order association cortical areas are remodeled during puberty and adolescence. For example, there is a loss of grey matter in the somatosensory cortex before any loss is observed in the dorsal PFC (Gogtay et al 2004, Sowell et al 2004). This PFC refinement and reduction in grey matter during adolescence is consistent not only with EM analysis of synapse number in humans, but also with histological analyses in nonhuman primates (Bourgeois et al 1994, Huttenlocher 1979, Huttenlocher & Dabholkar 1997).
Schizophrenia is a severe and chronic brain disorder that occurs in 1% of the population and affects men and women equally. It usually produces a lifetime of disability and emotional distress for affected individuals (Lewis & Levitt 2002). The onset of symptoms such as hallucinations and delusions occurs between ages 16 and 30. Although, prenatal and perinatal traumatic stimuli might contribute to schizophrenia, the first symptoms are seen after adolescence. Since connections in the brain undergo major refinement during puberty, these changes could trigger symptom onset. Thus, abnormal pruning during adolescence has been proposed as a contributing factor to the onset of the disease (Feinberg 1982). This hypothesis is supported by the observation that the expression of numerous synaptic vesicle markers, including synaptophysin, synaptosomal-associated protein 5 and rab3, among others, is decreased in the brains of adult patients (Davidsson et al 1999, Glantz & Lewis 1997, Halim et al 2003, Honer et al 2002). Consistent with these data, neuropil volume is decreased, but the number of neurons remains intact, in the PFC of schizophrenics (Selemon & Goldman-Rakic 1999). Furthermore, dendritic spines, the sites of excitatory synaptic transmission, are decreased in number in PFC pyramidal neurons in patients with schizophrenia (Broadbelt et al 2002, Glantz & Lewis 2000, Kalus et al 2000). This reduction in spine number and in the expression of the presynaptic marker vesicular glutamate transporter 1 (vglut1) is evident in layer 3, but not layer 5, of the PFC in patients with schizophrenia, suggesting layer specificity is part of this synaptic pruning phenotype (Bitanihirwe et al 2009). Although these studies are informative, they were performed in the postmortem adult brains and do not indicate how such synaptic defects became prominent in the brains of these schizophrenia patients.
Advanced brain imaging techniques provide an excellent tool to understand these abnormal developmental changes as they occur in the nervous system of schizophrenia patients. A recent MRI longitudinal study demonstrates that the prefrontal lobes of both control and patients undergo significant surface contraction, consistent with a grey matter decline as a function of normal developmental pruning (Sun et al 2009). Interestingly, PFC surface contraction is significantly enhanced in schizophrenia patients, and it correlates with decreases in grey matter volume. These exaggerated regressive changes seen in these patients support the idea that there is an increased rate of developmental synaptic pruning that results in excessive loss of neuronal connections during the early stages of schizophrenia (Sun et al 2009). Using a similar approach, in this case structural Magnetic Resonance (sMR), Andreasen and colleagues have recently performed an extensive longitudinal study looking at brain volume changes in young adults after the patients had their first schizophrenic episode (Andreasen et al 2011). They found a significant decrease in grey and white matter, and this decrease was most severe during the first few years after symptom onset. This is remarkably coincident with the normal timing of extensive brain circuit refinement that occurs during adolescence. This idea is further supported by metabolic studies using magnetic resonance spectroscopy (MRS) on first episode schizophrenia patients that suggest an increase in membrane phospholipid catabolism/breakdown in the prefrontal cortex of these patients right after onset, yet no changes in membrane catabolism in older patients (Pettegrew et al 1991, Stanley et al 1995). Taken together, these studies suggest an enhanced period of refinement in the early stages of schizophrenia.
A complex neurological disease such as schizophrenia is likely to arise as the result of many collaborating causal factors, and a multitude of small developmental disturbances could converge to contribute to its etiology. These data described here highlight the strong correlation between pruning and schizophrenia. Although a direct association between schizophrenia and mutations in genes with known functions during pruning has been hard to establish, it is interesting that several genes important for calcium signaling and synapse development, stability and refinement have been linked to this neurological disease (Boksa 2012, Schizophrenia Working Group of the Psychiatric Genomics 2014). Taken together, these studies point to developmental circuit refinement as one of the contributing factors to be considered when analyzing the etiology of this debilitating disease.
CONCLUDING REMARKS
Regressive events such as axon pruning and synapse elimination are developmental processes that play essential roles in establishing brain circuitry and plasticity. These regressive events normally follow the initial assembly of neural circuits, and they provide additional opportunities for circuit refinement, allowing genetic programs and experience-mediated neuronal activity to be integrated to sculpt connections. While some interesting parallels exist between pruning and Wallerian degeneration, many of the molecular players that participate in pruning are unique to this type of regressive event. Future studies will likely reveal more distinctions, and perhaps similarities, between these events, emphasizing the need for continued intensive investigation in multiple neural systems directed toward understanding regressive process removal during neural development and following neuronal injury.
As we begin to understand the molecular mechanisms underlying axon pruning and circuit refinement, clear connections to neurological disease are starting to emerge. In this regard, mis-regulated axon pruning remains a likely contributing factor to developmental neurological disorders such as schizophrenia and, possibly, autism. As more information becomes available highlighting the importance of axon pruning and synapse elimination in the etiology of human disease, it is evident that we must gain further insight into the mechanisms that regulate these regressive developmental events.
ACKNOWLEDGEMENTS
We thank Marc Freeman, Roman Giger, and Oren Schuldiner for helpful comments on the manuscript. Work cited here from the authors’ laboratory is supported by the NIH/NIMH (RO1 MH59199 to A.L.K.). A.L.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
NOTE ADDED IN PROOF
A recent study (Kakegawa et al. 2015) sheds light on molecular mechanisms that underlie the later stages of CF elimination in the establishment of CF-PC connectivity. C1ql1, a component of the innate immunity system, is expressed by CFs, signaling in an anterograde fashion to establish and also maintain single winner CF connections to PCs. C1ql1 signals through the brain-specific angiogenesis inhibitor 3 (Bai3) receptor, which is expressed on PCs. Loss of either C1ql1 or Bai3 in vivo results in failure to eliminate less dominant CF inputs and defects in motor learning (Figure 2). This work provides interesting links between synapse elimination in the cerebellum and in other CNS circuits, most notably in the retinogeniculate system. It will be interesting to determine how the action of C1ql1 is coordinated with the other signaling events that contribute to CF pruning that we discuss in this review.
LITERATURE CITED
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Andersen E, Asuri N, Clay M, Halloran M. Live imaging of cell motility and actin cytoskeleton of individual neurons and neural crest cells in zebrafish embryos. J Vis Exp. 2010 doi: 10.3791/1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjus PR, Zhu L, Cesa R, Carulli D, Strata P. A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum. Neuroscience. 2003;121:563–572. doi: 10.1016/s0306-4522(03)00556-6. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Huang Y, O'Connor MB, Lee T. Glia instruct developmental neuronal remodeling through TGF-beta signaling. Nat Neurosci. 2011;14:821–823. doi: 10.1038/nn.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 2013;3:1422–1429. doi: 10.1016/j.celrep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo TU. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Abnormal synaptic pruning in schizophrenia: Urban myth or reality? J Psychiatry Neurosci. 2012;37:75–77. doi: 10.1503/jpn.120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bravin M, Morando L, Vercelli A, Rossi F, Strata P. Control of spine formation by electrical activity in the adult rat cerebellum. Proc Natl Acad Sci U S A. 1999;96:1704–1709. doi: 10.1073/pnas.96.4.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Wang L, Stryker MP, Feldheim DA. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. J Neurosci. 2008;28:11015–11023. doi: 10.1523/JNEUROSCI.2478-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa R, Scelfo B, Strata P. Activity-dependent presynaptic and postsynaptic structural plasticity in the mature cerebellum. J Neurosci. 2007;27:4603–4611. doi: 10.1523/JNEUROSCI.5617-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A, Sotelo C. Early Development of Olivocerebellar Projections in the Fetal Rat Using CGRP Immunocytochemistry. Eur J Neurosci. 1992;4:1159–1179. doi: 10.1111/j.1460-9568.1992.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Chen M, Maloney JA, Kallop DY, Atwal JK, Tam SJ, et al. Spatially coordinated kinase signaling regulates local axon degeneration. J Neurosci. 2012;32:13439–13453. doi: 10.1523/JNEUROSCI.2039-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun. 2014;5:4486. doi: 10.1038/ncomms5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TW, Liu XB, Faulkner RL, Stephan AH, Barres BA, et al. Emergence of lamina-specific retinal ganglion cell connectivity by axon arbor retraction and synapse elimination. J Neurosci. 2010;30:16376–16382. doi: 10.1523/JNEUROSCI.3455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Chak K, Andreone BJ, Wooley JR, Kolodkin AL. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012;26:2222–2235. doi: 10.1101/gad.193136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Pazyra-Murphy MF, Fenstermacher SJ, Segal RA. Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. J Neurosci. 2013;33:5195–5207. doi: 10.1523/JNEUROSCI.3862-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N. Distribution of climbing fibres on cerebellar Purkinje cells in X-irradiated rats. An electrophysiological study. J Physiol. 1979;290:97–112. doi: 10.1113/jphysiol.1979.sp012762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, Dupont JL. Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, x-irradiated and hypothyroid rats. Brain Res. 1981;227:59–71. doi: 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the Weaver Mutant Mouse. J Neurobiol. 1976;7:579–582. doi: 10.1002/neu.480070610. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, et al. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson P, Gottfries J, Bogdanovic N, Ekman R, Karlsson I, et al. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophr Res. 1999;40:23–29. doi: 10.1016/s0920-9964(99)00037-7. [DOI] [PubMed] [Google Scholar]
- de Wit J, Verhaagen J. Proteoglycans as modulators of axon guidance cue function. Adv Exp Med Biol. 2007;600:73–89. doi: 10.1007/978-0-387-70956-7_7. [DOI] [PubMed] [Google Scholar]
- Denault JB, Salvesen GS. Caspases: keys in the ignition of cell death. Chem Rev. 2002;102:4489–4500. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisen J, Lu Q, et al. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998;21:1303–1313. doi: 10.1016/s0896-6273(00)80650-9. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Freeman MR. Signaling mechanisms regulating Wallerian degeneration. Curr Opin Neurobiol. 2014;27:224–231. doi: 10.1016/j.conb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Letourneau PC. Actin dynamics in growth cone motility and navigation. J Neurochem. 2014;129:221–234. doi: 10.1111/jnc.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DC, Mahrus S, Wells JA. Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell. 2010;142:637–646. doi: 10.1016/j.cell.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Curr Opin Neurobiol. 2008;18:284–291. doi: 10.1016/j.conb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim Y, Yaniv SP, Schuldiner O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS One. 2014;9:e86178. doi: 10.1371/journal.pone.0086178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Levi-Montalcini R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool. 1949;111:457–501. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a "winner" climbing fiber to the Purkinje cell dendrite and subsequent elimination of"losers" from the soma in developing cerebellum. Neuron. 2009a;63:106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, et al. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21:9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, et al. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2011;108:9987–9992. doi: 10.1073/pnas.1101488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience. 2009b;162:601–611. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. The Journal of comparative neurology. 1999;404:537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Bayer TA, Xie J, Hu L, et al. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cereb Cortex. 2002;12:349–356. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- Hong YK, Park S, Litvina EY, Morales J, Sanes JR, Chen C. Refinement of the Retinogeniculate Synapse by Bouton Clustering. Neuron. 2014 doi: 10.1016/j.neuron.2014.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Issman-Zecharya N, Schuldiner O. The PI3K-class III complex promotes axon pruning by downregulating a Ptc derived signal via endosome-lysosomal degradation. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.10.013. In press. [DOI] [PubMed] [Google Scholar]
- Ito M. The modifiable neuronal network of the cerebellum. Jpn J Physiol. 1984;34:781–792. doi: 10.2170/jjphysiol.34.781. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. Altered mossy fiber distributions in adult Fmr1 (FVB) knockout mice. Hippocampus. 2002;12:47–54. doi: 10.1002/hipo.10004. [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Miyazaki T, Yanagihara D, Iino M, Watanabe M, Kano M. Maintenance of presynaptic function by AMPA receptor-mediated excitatory postsynaptic activity in adult brain. Proc Natl Acad Sci U S A. 2005;102:19180–19185. doi: 10.1073/pnas.0504359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kanamori T, Kanai MI, Dairyo Y, Yasunaga K, Morikawa RK, Emoto K. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013;340:1475–1478. doi: 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, et al. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Gu Y, Huang Y, Wu Z, Bashirullah A, et al. A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat Neurosci. 2009;12:1497–1505. doi: 10.1038/nn.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D, Wong JJ, Lim EK, Wang Y, Zhang H, et al. Intrinsic epigenetic factors cooperate with the steroid hormone ecdysone to govern dendrite pruning in Drosophila. Neuron. 2011;72:86–100. doi: 10.1016/j.neuron.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signalling during development. Development. 2012;139:4105–4109. doi: 10.1242/dev.074997. [DOI] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Kollins KM, Bell RL, Butts M, Withers GS. Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural Dev. 2009;4:26. doi: 10.1186/1749-8104-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropouli E, Kolodkin AL. Semaphorins and the dynamic regulation of synapse assembly, refinement, and function. Curr Opin Neurobiol. 2014;27:1–7. doi: 10.1016/j.conb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci U S A. 2005;102:15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci U S A. 2009;106:6363–6368. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC. Electron microscopy of Wallerian degeneration. J Comp Neurol. 1963;120:65–79. doi: 10.1002/cne.901200107. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. doi: 10.1016/s0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annual review of neuroscience. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annual review of neuroscience. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, et al. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338:1357–1360. doi: 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetto E, Caselli L, Feng G, Yuan W, Nerbonne JM, et al. Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2009;106:16475–16480. doi: 10.1073/pnas.0907298106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luduena RF, Anderson WH, Prasad V, Jordan MA, Ferrigni KC, et al. Interactions of vinblastine and maytansine with tubulin. Ann N Y Acad Sci. 1986;466:718–732. doi: 10.1111/j.1749-6632.1986.tb38454.x. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Ma M, Ferguson TA, Schoch KM, Li J, Qian Y, et al. Calpains mediate axonal cytoskeleton disintegration during Wallerian degeneration. Neurobiol Dis. 2013;56:34–46. doi: 10.1016/j.nbd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Maor-Nof M, Homma N, Raanan C, Nof A, Hirokawa N, Yaron A. Axonal pruning is actively regulated by the microtubule-destabilizing protein kinesin superfamily protein 2A. Cell Rep. 2013;3:971–977. doi: 10.1016/j.celrep.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. II. Spontaneous activity of inferior olivary neurons and climbing fibermediated activity of cerebellar Purkinje cells in developing rats. J Neurosci. 1981;1:703–709. doi: 10.1523/JNEUROSCI.01-07-00703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Crepel F, Mikoshiba K, Changeux JP, Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1977;281:1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- Martin SM, O'Brien GS, Portera-Cailliau C, Sagasti A. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137:3985–3994. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annual review of neuroscience. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J Neurosci. 2002;22:5259–5264. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, O'Leary DD. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci. 1989;9:3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274:168–177. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- Neukomm LJ, Freeman MR. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 2014;24:515–523. doi: 10.1016/j.tcb.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Olsen O, Kallop DY, McLaughlin T, Huntwork-Rodriguez S, Wu Z, et al. Genetic analysis reveals that amyloid precursor protein and death receptor 6 function in the same pathway to control axonal pruning independent of beta-secretase. J Neurosci. 2014;34:6438–6447. doi: 10.1523/JNEUROSCI.3522-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27–65. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991;48:563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Potts PR, Singh S, Knezek M, Thompson CB, Deshmukh M. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163:789–799. doi: 10.1083/jcb.200307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain FE, Chien CB. Proteoglycan-mediated axon degeneration corrects pretarget topographic sorting errors. Neuron. 2013;78:49–56. doi: 10.1016/j.neuron.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. The intricate relationship between microtubules and their associated motor proteins during axon growth and maintenance. Neural Dev. 2013;8:17. doi: 10.1186/1749-8104-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Mintz IM. Participation of multiple calcium channel types in transmission at single climbing fiber to Purkinje cell synapses. Neuron. 1994;12:605–613. doi: 10.1016/0896-6273(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Hurtado A, Wang H, Macopson JG, Griner EM, et al. The RacGAP beta2-Chimaerin Selectively Mediates Axonal Pruning in the Hippocampus. Cell. 2012;149:1594–1606. doi: 10.1016/j.cell.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6671–6680. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner O, Yaron A. Mechanisms of developmental neurite pruning. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-014-1729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, et al. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DK, O'Leary DD. Limited topographic specificity in the targeting and branching of mammalian retinal axons. Dev Biol. 1990;137:125–134. doi: 10.1016/0012-1606(90)90013-9. [DOI] [PubMed] [Google Scholar]
- Smith IW, Mikesh M, Lee Y, Thompson WJ. Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination. J Neurosci. 2013;33:17724–17736. doi: 10.1523/JNEUROSCI.3339-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield BB, O'Leary DD, Fricks C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature. 1982;298:371–373. doi: 10.1038/298371a0. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Williamson PC, Drost DJ, Carr TJ, Rylett RJ, et al. An in vivo study of the prefrontal cortex of schizophrenic patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch Gen Psychiatry. 1995;52:399–406. doi: 10.1001/archpsyc.1995.03950170073010. [DOI] [PubMed] [Google Scholar]
- Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, et al. Localization and functional properties of a rat brain alpha 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci U S A. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry. 2009;14:976–986. doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PK. Changes in the Endoneurial Sheaths of Peripheral Myelinated Nerve Fibres during Wallerian Degeneration. J Anat. 1964;98:175–182. [PMC free article] [PubMed] [Google Scholar]
- Thomas PK, Sheldon H. Tubular Arrays Derived from Myelin Breakdown during Wallerian Degeneration of Peripheral Nerve. J Cell Biol. 1964;22:715–718. doi: 10.1083/jcb.22.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tounekti O, Zhang Y, Klaiman G, Goodyer CG, LeBlanc A. Proteasomal degradation of caspase-6 in 17beta-estradiol-treated neurons. J Neurochem. 2004;89:561–568. doi: 10.1111/j.1471-4159.2004.02349.x. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annual review of cell and developmental biology. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, et al. Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science. 2014;344:1020–1023. doi: 10.1126/science.1252514. [DOI] [PubMed] [Google Scholar]
- Unsain N, Higgins JM, Parker KN, Johnstone AD, Barker PA. XIAP regulates caspase activity in degenerating axons. Cell Rep. 2013;4:751–763. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Molecular mechanisms governing competitive synaptic wiring in cerebellar Purkinje cells. The Tohoku journal of experimental medicine. 2008;214:175–190. doi: 10.1620/tjem.214.175. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. The European journal of neuroscience. 2011;34:1697–1710. doi: 10.1111/j.1460-9568.2011.07894.x. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- Wong JJ, Li S, Lim EK, Wang Y, Wang C, et al. A Cullin1-based SCF E3 ubiquitin ligase targets the InR/PI3K/TOR pathway to regulate neuronal pruning. PLoS Biol. 2013;11:e1001657. doi: 10.1371/journal.pbio.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Xiong X, Hao Y, Sun K, Li J, Li X, et al. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012;10:e1001440. doi: 10.1371/journal.pbio.1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nature neuroscience. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, Sun S, Gibson JR, Henkemeyer M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat Neurosci. 2011;14:1421–1429. doi: 10.1038/nn.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, et al. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, et al. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 2013;80:1175–1189. doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Yu F, Schuldiner O. Axon and dendrite pruning in Drosophila. Curr Opin Neurobiol. 2014;27:192–198. doi: 10.1016/j.conb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Gutman I, Mosca TJ, Iram T, Ozkan E, et al. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-beta signaling. Neuron. 2013;78:456–468. doi: 10.1016/j.neuron.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang Y, Wong JJ, Lim KL, Liou YC, et al. Endocytic pathways downregulate the L1-type cell adhesion molecule neuroglian to promote dendrite pruning in Drosophila. Dev Cell. 2014;30:463–478. doi: 10.1016/j.devcel.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang J, Haerry TE, Wu AY, Martin J, et al. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]