Abstract
This study reviews the findings from the Infant Development, Environment, and Lifestyle Study (IDEAL), a multisite, longitudinal, prospective study designed to determine maternal outcome and child growth and developmental findings following prenatal methamphetamine exposure from birth up to age 7.5 years. These findings are presented in the context of the home environment and caregiver characteristics to determine how the drug and the environment interact to affect the outcome of these children. No neonatal abstinence syndrome requiring pharmacologic intervention was observed but heavy drug exposure was associated with increased stress responses in the neonatal period. Poorer inhibitory control was also observed in heavy methamphetamine exposed children placing them at high risk for impaired executive function. Independent of methamphetamine exposure, children with more responsive home environments to developmental and emotional needs demonstrated lower risks for internalizing and externalizing behavior.
Introduction
Substance use among women of reproductive age is a concern worldwide. Children of drug abusing parents are at increased risk for child abuse and neglect, witnessing intimate partner violence, disrupted continuity of primary caregiving, high parental stress, caregiver depression and other co-occurring mental health disorders(Brown and Hohman 2006). Further, children of drug using parents often lack basic needs and resources, and experience negative life events (Brown and Hohman 2006). In the United States, approximately 6.5% of all females over the age of 12 and 5% of pregnant women 15 to 44 years of age reported current illicit drug use (Substance Abuse and Mental Health Services Administration 2012). Women account for a substantial subset of methamphetamine (MA) users; data from treatment centers in 2005 showed 46% of patients treated for amphetamine abuse were women (Substance Abuse and Mental Health Services Administration 2008), increasing to 47.5% in 2011 (Substance Abuse and Mental Health Services Administration 2013). Further, prevalence of MA abuse during pregnancy in women seeking treatment tripled from 1994 to 2006, rising to 24% of all pregnant women admitted to federally funded treatment centers (Terplan et al. 2009).
MA is a central nervous system stimulant that increases the experience of pleasure by blocking the reuptake of dopamine. Initially, increased dopamine in the brain's reward centers leads to intense euphoria. However, chronic methamphetamine abuse often leads to paranoia, delusions, hallucinations, insomnia, and extreme weight loss. These effects are especially of concern since poor maternal nutrition and increased blood pressure lead to vasoconstriction and a restriction of nutrients and oxygen to the baby as well as fetal hypertension(Plessinger 1998).
Methamphetamine use during pregnancy has been associated with an increased incidence of cardiac defects, cleft lip, biliary artesia, stillbirth, cerebral hemorrhage, mongolian spots, systolic murmur and undescended testes (Plessinger 1998) as well as adverse somatic growth effects(Oro and Dixon 1987; Little et al. 1988). These initial reports were limited by reliance on hospital records, retrospective analysis, small sample size, and lack of adjustment for confounding factors.
Studies in the preclinical rat model have demonstrated a range of physical, motor, neurotransmitter and behavioral effects in MA exposed offspring. These include increased maternal and offspring mortality, retinal eye defects (Acuff-Smith, Schilling, Fisher, & Vorhees, 1996; Acuff-Smith, George, Lorens, & Vorhees, 1992; Yamamoto, Yamamoto, Fukui, & Kurishita, 1992), cleft palate and rib malformations (Yamamoto et al. 1992) decreased rate of physical growth (Acuff-Smith et al., 1996; Cho, Lyu, Lee, Kim, & Chin, 1991), and delayed motor development (Acuff-Smith et al., 1996; Cho et al., 1991). Neurotoxic effects of prenatal MA exposure on serotogenetic neurons produce neurochemical alternations in the CNS (Cabrera et al. 1993; Weissman and Caldecott-Hazard 1995) thought to be associated with learning impairment (Acuff-Smith et al., 1996), behavioral deficits (Weissman and Caldecott-Hazard 1995), increased motor activity,(Acuff-Smith et al., 1992) and enhanced conditioned avoidance responses (Cho et al. 1991).
Studies in the preclinical model also suggest that the timing of methamphetamine exposure during gestation influences outcome. Neonatal rats exposed to methamphetamines, consistent with third trimester exposure in humans, causes spatial learning and memory deficits as adults (Williams et al. 2003) . Rats exposed to methamphetamine in the second half of gestation and then re-exposed as adults, demonstrate poorer cognitive function than those exposed during the first half of gestation(Hrebíčková et al. 2014). High dose, but not low dose, methamphetamine exposure consistent with the first half of human gestation has also been found to induce delays in behavioral development (McDonnell-Dowling et al. 2014). NMDA receptors in the hippocampus are altered by methamphetamine suggesting a mechanism of action for the observed alterations in behavior(Šlamberová et al. 2014). Further, epigenetic changes in the brain have been demonstrated following methamphetamine exposure in mice (Itzhak et al. 2014). These epigenetic changes may account for the adverse effects observed in the future generations of offspring exposed to methamphetamine during development(Šlamberová et al. 2007). Of critical importance, improvements in sensorimotor testing have been demonstrated by cross fostering exposed offspring to unexposed mothers, suggesting the postnatal environment is a critical factor for attenuating methamphetamine-induced changes in neural function(Pometlová et al. 2009) .
Investigators in Sweden followed a group of 65 children exposed to amphetamines prenatally through age 15. They found that at birth, one year, four years, and ten years of age, exposed females were significantly lighter and shorter, whereas there was no significant difference between the exposed and unexposed males (Eriksson and Zetterström 1994). By age 14, the exposed boys were significantly taller and heavier than the unexposed, and the girls were significantly shorter than the unexposed girls (Cernerud et al. 1996).
This same Swedish group reported neurodevelopmental findings in their amphetamine exposed cohort. They reported that exposed infants were more likely to be drowsy in the first few months of life (Billing et al. 1980), and exhibit speech problems, signs of wariness of strangers, and emotional characteristics of autism by age one (Billing et al. 1980). By age 4, exposed children had lower IQ scores than a normative group of Swedish children (Billing et al. 1988). At age 8, prenatal exposure predicted problems with peers and aggressive behavior (Billing et al. 1994), and by 14 years of age, prenatal exposure was associated with decreased school performance, particularly in math, language and physical fitness activities (Cernerud et al. 1996). They did however lack a matched control group, utilize a small sample size, include other prenatal drug use, and rely upon self-report for exposure. Lower scores on neurocognitive tests (Chang et al. 2004a) including attention, memory, spatial performance, IQ and executive function have also been reported (Lu et al. 2009; Sowell et al. 2010; Piper et al. 2011). However, these reports utilized a small sample, and often did not control for the impact of attention deficit disorder medication, which enhances cognitive abilities.
The Infant Development, Environment, and Lifestyle Study (IDEAL) took many of the previous study limitations into consideration and accounted for them in a multisite, longitudinal, prospective analysis of the effects of prenatal methamphetamine exposure on children. This article is a summary of the maternal and child outcomes published to date from the IDEAL study.
Review of Methods
All published studies reporting maternal and child outcome data from the IDEAL study were included in this review. A brief summary of the Methods for the IDEAL study follows with references provided for more in-depth review.
Study Design
Recruitment occurred over a 2-year period at four clinical sites (Los Angeles, CA; Des Moines, IA; Tulsa, OK; Honolulu, HI) with an elevated prevalence of MA use compared with other areas in the United States. The study was approved by the Institutional Review Boards at all participating sites, and informed consent was obtained from all participants. A federal Certificate of Confidentiality was obtained to ensure the confidentiality of maternal drug use and results of meconium drug testing, but any evidence of child abuse or neglect remained reportable.
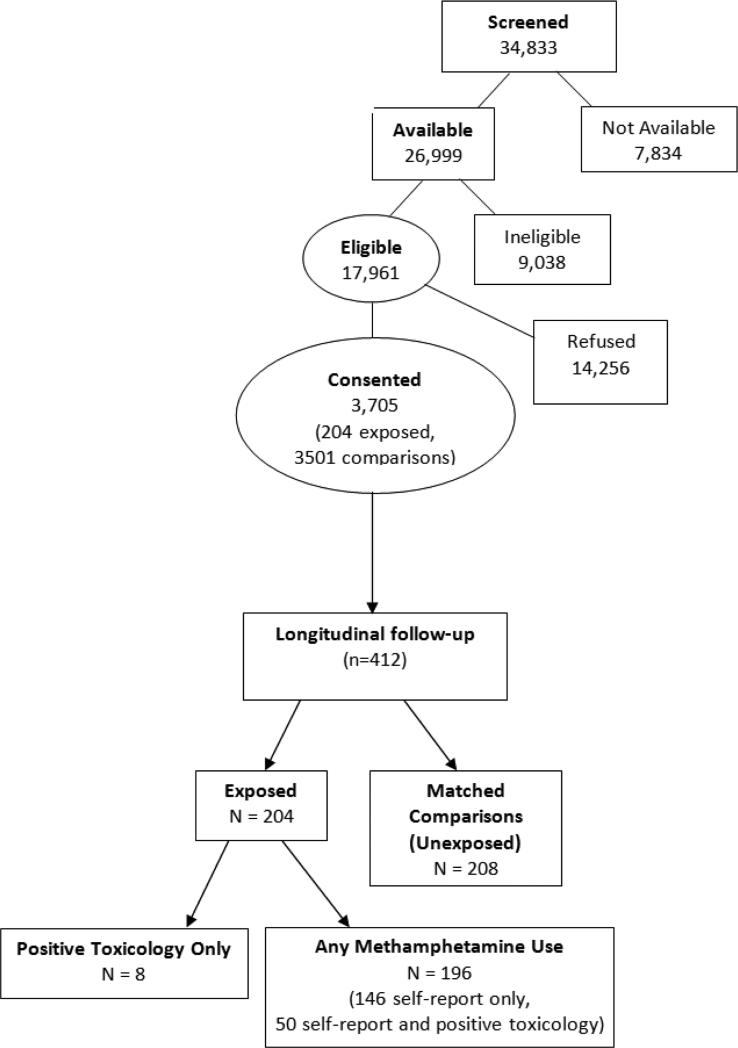
34,833 mother-infant pairs were screened at the time of the infant's birth, of which 26,999 were available and screened for eligibility (Figure 1). After screening for eligibility, 17,961 (66.5%) were eligible for the study. Mothers were excluded if they were under 18 years of age, used opioids, lysergic acid diethylamide (LSD), phencyclidine (PCP) or cocaine only during pregnancy, displayed low cognitive functioning, were overtly psychotic or had a documented history of psychosis, or were non-English speaking. Additional maternal exclusion factors included incarceration or institutionalized, having a child previously enrolled in the study, or distance from study site was prohibitive for follow-up. Exclusion criteria for infants included critical illness and unlikely to survive, multiple birth, major life threatening congenital anomaly or documented chromosomal abnormality associated with mental or neurological deficiency and overt clinical evidence of an intrauterine infection. Of these eligible subjects, 3,705 (21%) mother-infant pairs consented to participate in the study.
Figure 1.
Recruitment Flow Chart
Among the consented, only mothers with prenatal MA use (n=204) and their matched unexposed comparisons (n=208) were enrolled for longitudinal follow-up. All newborns with prenatal MA exposure, as identified by maternal self-report of MA use during this pregnancy and/or positive meconium toxicology were enrolled in the study. Of the 204 subjects in the MA group, eight subjects denied MA use but were identified as exposed by toxicology only; 196 subjects reported amphetamine use with 146 by self-report only (toxicology was negative) and 50 by self-report and positive toxicology. After the enrollment of an exposed child, the next unexposed child matching study criteria (race, birth weight category, maternal education, and type of insurance as a proxy for socioeconomic status) at the same recruitment site were enrolled as the control for the exposed child. Children born to women who denied MA use during pregnancy and had a negative meconium screen were considered unexposed. The study criterion for heavy use is based on the average frequency of MA use across pregnancy. Heavy use is defined as ≥3 days per week and some use is defined as <3 days per week.
Meconium from the first and/or earliest discharge of meconium were collected on all neonates. The samples were processed centrally (United States Drug Testing Laboratory in Des Plaines, IL) for analysis of the amphetamine class, cocaine metabolites, cannabinoids, opioids and cotinine. The specimen was initially screened with a sensitive enzyme multiplied immunoassay test (EMIT II; Dade-Behring, Cupertino, CA). If positive results were obtained, the specific drug analyte or metabolite was confirmed by gas chromatography-mass spectrometry (Smith et al. 2006).
After informed consent was obtained, a maternal interview (the Recruitment Lifestyle Interview) was conducted to determine the presence or absence of licit and illicit prenatal drug use, information regarding the course of pregnancy, number of prenatal care visits and sociodemographic information (Bauer et al. 2002; Lester et al. 2002). Education and occupation information was collected to calculate the 4-factor Hollingshead index of SES which has been adapted to single parent and non-nuclear families (Hollingshead 1975; LaGasse et al. 1999). For each drug used during pregnancy, a second interview, the Substance Use Inventory, assessed retrospectively the frequency and quantity of drug and alcohol use during four time periods: three months prior to pregnancy and during the first, second and third trimester of pregnancy (Richardson et al. 1999; Shankaran et al. 2004; Della Grotta et al. 2010). Interviewers were trained and certified in the administration of maternal interviews and utilized scripted introductions to ensure consistency between sites.
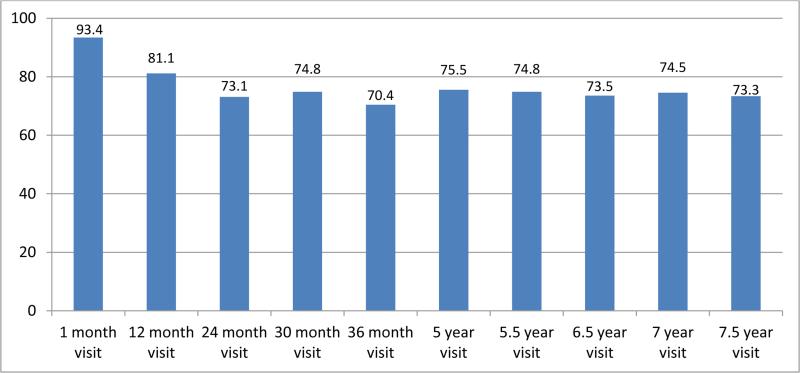
The longitudinal phase of this study included visits when the child was 1, 12, 24, 30, 36, 60, 66, 78, 84, and 90 months of age with each visit maintaining a retention rate of over 70%. An analysis of retention at each time point was completed with covariates examined for differences. (Figure 2) The covariates included race, SES, insurance, partner status, maternal education, gender, prenatal exposures-alcohol, tobacco, marijuana and MA, maternal age, birth weight, birth length, birth head circumference and gestational age. At 1 and 12 months of age, the difference between included vs. not seen showed differences in alcohol use and low SES, respectively. No other differences were found.
Figure 2.
Retention Percentages at each visit age-US IDEAL.
The Brief Symptom Inventory (BSI), a 53-item questionnaire administered at ages 1, 12, and 36 months, yielded an overall score of caregiver psychological symptoms(Derogatis and Melisaratos 1983). Caregiver depression was assessed using the Beck Depression Inventory-II (BDI-II) at ages 1, 12, and 36 months. The inventory is a 21-item self-report instrument with a high reliability (Beck et al. 1996).
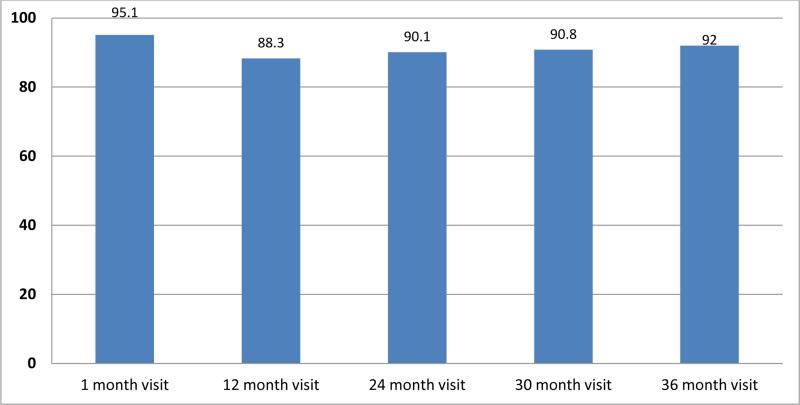
The IDEAL study was also conducted in New Zealand to increase the sample size of the IDEAL study, increase the observable effects of prenatal MA exposure in a group with a higher frequency of use and with less variability in the purity of MA, and increase understanding of the role of cultural, childrearing, and out of home placement issues on the outcomes of MA exposed children. The addition of New Zealand also gave us the opportunity to study a society that take a less punitive approach to drug dependent mothers and determine how child welfare and child development is affected by different policies. In New Zealand, recruitment was conducted through referrals from maternity services at participating hospitals and through independent midwife practices. These referrals were screened prior to birth to determine if the mother met the study criteria. If the mother agreed, the study staff met with her to explain the study in detail and obtain written consent to participate. New Zealand study staff met with the mother again post-partum prior to discharge to review the study protocol, affirm consent, collect meconium from all infants and obtain substance use and lifestyle data consistent with the US protocol. In New Zealand, data on total eligible participants and enrollment rates were not available given the recruitment procedure used, with midwives only referring expectant mothers who had expressed interest in participating. A total of 223 mother–infant dyads in New Zealand were enrolled, with 108 PME participants and 115 NPME comparisons. The New Zealand study included visits when the child was 1, 12, 24, 30, and 36 months of age with each visit maintaining a retention rate of 88% or higher (Table 3).
Table 3.
Frequency of self-reported tobacco use pre-pregnancy and by trimester of pregnancy- US IDEAL
| MA user (N=204) | Comparison (N=208) | MA User (N=204) | Comparison (N=208) | |||||
|---|---|---|---|---|---|---|---|---|
| Trimester | ||||||||
| Tobacco use | Pre-pregnancy | First | Second | Third | First | Second | Third | |
| N (%) | N(%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 126 (61.8%)** | 37 (17.8%) | 111 (54.4%)** | 94 (46.1%)** | 91 (44.6%)** | 28 (13.5%) | 25 (12.0%) | 20 (9.6%) |
| 3-6 days/wk. | 26 (12.7%)** | 8 (3.8%) | 33 (16.2%)* | 34 (16.7%)** | 17 (8.3%)* | 15 (7.2%) | 6 (2.9%) | 5 (2.4%) |
| 1-2 days/wk. | 4 (2.0%) | 4 (1.9%) | 11 (5.4%) | 5 (2.5%) | 10 (4.9%) | 4 (1.9%) | 2 (1.0%) | 4 (1.9%) |
| 1-3 days/mo. | 0 (0%) | 1 (0.5%) | 1 (0.5%) | 3 (1.5%) | 4 (2.0%) | 4 (1.9%) | 3 (1.4%) | 3 (1.4%) |
| 1-2 days/3 mos. | 0 (0%) | 0 (0%) | 2 (1.0%) | 3 (1.5%) | 3 (1.5%) | 3 (1.4%) | 4 (1.9%) | 1 (0.5%) |
| Not at all | 46 (22.5%)** | 155 (74.5%) | 46 (22.5%)** | 65 (31.9%)** | 79 (38.7%)** | 154 (74.0%) | 168 (80.8%) | 175(84.1%) |
| Cigarettes per day (mean, SD) | 10.7 (11.2)** | 3.2 (8.0) | 9.0 (10.7)** | 6.9 (8.9)** | 5.2 (7.6)** | 2.3 (6.2) | 1.4 (4.2) | 1.3 (4.4) |
Difference between MA users and comparisons, p<0.05
Difference between MA users and comparisons, p < 0.001
Findings
Results and Findings are based on the IDEAL-United States only cohort unless otherwise stated.
Maternal Sociodemographic Characteristics & Medical Complications
Mothers in the MA group were older, gained more weight, received less prenatal care and began prenatal care at a later gestational age and were more likely to have greater parity and gravida than comparison mothers. Further, the MA group had a lower SES, were less likely to have a partner and were diagnosed with a psychiatric disorder/emotional illness more frequently than mothers in the comparison group (Shah et al. 2012). Nearly 26% of MA exposed children had an adoptive parent as their primary caregiver (Table 1). The cumulative average Hollingshead social position index was significantly lower in the MA group. The cumulative average Brief Symptom Inventory and Beck Depression Inventory totals were also lower in the MA group (Table 1).
Table 1.
Maternal characteristics by MA exposure
| Number (Percent)/ Mean (SD) | |||
|---|---|---|---|
| Exposed (N = 204 ) | Comparison (N= 208) | P-Value | |
| Adoptive parent –cumulative birth through 7.5 years | 52 (25.5%) | 4 (1.9%) | <0.001 |
| ISP average – cumulative birth through 7.5 years | 29.8 (8.7) | 32.8 (9.3) | <0.001 |
| BSI average total score-cumulative 1m-36m | 0.50 (0.45) | 0.45 (0.41) | 0.202 |
| BDI average total score–cumulative 1m-36m | 9.9 (8.0) | 9.2 (6.1) | 0.357 |
ISP- Hollingshead Index of Social Position
BSI- Brief Symptom Inventory
BDI- Beck Depression Inventory
Mothers using MA were more likely to be diagnosed with gonorrhea. There were no other differences between the groups regarding the incidence of maternal medical complications previously associated with MA use including hepatitis, active genital herpes, syphilis, diabetes, receiving treatment for UTI, chronic hypertension, preeclampsia, abruptio placentae, placenta previa, delivery via cesarean section or the incidence of hospitalization during pregnancy (Shah et al. 2012).
Maternal Drug Use Patterns During Pregnancy
Preliminary findings after the first year of the two year enrollment period were based on 1,632 eligible mother-infant pairs who consented to participate in the study. Overall, 5.2% of enrollees used MA, 25% smoked tobacco, 22.8% drank alcohol, and 6% used marijuana prenatally (Arria et al. 2006). Further, of those enrolled in the longitudinal follow–up during the first year of enrollment (n=131; 50 MA group, 81 comparison subjects), prenatal MA use was associated with increased substance use among friends and family, increased risk for ongoing legal issues, increased likelihood of developing a substance abuse disorder, and decreased maternal perceptions of quality of life (Derauf et al. 2007).
An analysis of the full longitudinal sample (n=412) found that MA use decreased over the course of pregnancy, but 29.3% of the women maintained a high frequency of MA use throughout gestation (Della Grotta et al. 2010). There were no differences in sociodemographic characteristics among mothers who increased, decreased or used a constant low level of MA throughout pregnancy. Although both groups included individuals who had used alcohol, tobacco and marijuana during pregnancy, mothers in the MA group used all three drugs at a greater frequency.
The rates of use of MA, alcohol and tobacco during the pre-pregnancy period (3 months prior to pregnancy) and each trimester for the IDEAL-US and IDEAL-NZ cohorts are shown in Tables 2-7. Mothers who self-reported heavy MA use pre-pregnancy daily or 3-6 days/week in both IDEAL United States and IDEAL New Zealand were found to report continued use throughout the pregnancy at a mean of ~5 days a week, only decreasing to a mean of ~2 days a week in the third trimester (Tables 2 & 5). Mothers in the MA group in both the US and New Zealand cohort used alcohol and tobacco at significantly higher rates pre-pregnancy and continued the trend during each trimester of pregnancy (Table 3, 4, 6 & 7).
Table 2.
Frequency of self-reported methamphetamine use pre-pregnancy and by trimester of pregnancy- US IDEAL
| Heavy MA Use (N=35) | Some MA Use (N=161) | Heavy MA Use (N=35) | Some MA Use (N=161) | |||||
|---|---|---|---|---|---|---|---|---|
| Trimester | ||||||||
| MA use | Pre-pregnancy | First | Second | Third | First | Second | Third | |
| N (%) | N(%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 22 (62.9%) | 31 (19.3%) | 20 (57.1%) | 16 (45.7%) | 5 (14.3%) | 14 (8.7%) | 1 (0.6%) | 1 (0.6%) |
| 3-6 days/wk. | 8 (22.9%) | 38 (23.6%) | 13 (37.1%) | 17 (48.6%) | 12 (34.3%) | 40 (24.8%) | 9 (5.6%) | 2 (1.2%) |
| 1-2 days/wk. | 2 (5.7%) | 36 (22.4%) | 0 (0%) | 2 (5.7%) | 2 (5.7%) | 29 (18.0%) | 18 (11.2%) | 11 (6.8%) |
| 1-3 days/mo. | 1 (2.9%) | 16 (9.9%) | 0 (0%) | 0 (0%) | 1 (2.9%) | 17 (10.6%) | 21 (13.0%) | 14 (8.8%) |
| 1-2 days/3 mos. | 0 (0%) | 5 (3.1%) | 0 (0%) | 0 (0%) | 3 (8.6%) | 29 (18.0%) | 23 (14.4%) | 30 (18.8%) |
| Not at all | 2 (5.7%) | 33 (20.5%) | 2 (5.7%) | 0 (0%) | 11 (31.4%) | 30 (18.6%) | 88 (55.0%) | 102 (63.8%) |
| Days/week (mean, SD) | 5.47 (2.34) | 2.72 (2.61) | 5.70 (1.94) | 5.21 (1.86) | 2.70 (2.68) | 1.93 (2.19) | 0.48 (1.02) | 0.24 (0.76) |
a 8 of the 204 MA users in this study were identified as exposed by toxicology only
b 2 cases classified as heavy users who abstained in the 1st trimester: case 1: 2nd trimester-3.5 days/wk; 3rd trimester-5.5 days/wk; case 2: 2nd trimester-7 days/wk; 3rd trimester-3.5 days/wk.
Table 7.
Frequency of self-reported alcohol use pre-pregnancy and by trimester of pregnancy- IDEAL New Zealand
| MA user (N=108) | Comparison (N=115) | MA User (N=108) | Comparison (N=115) | |||||
|---|---|---|---|---|---|---|---|---|
| Trimester | ||||||||
| Alcohol use | Pre-pregnancy | First | Second | Third | First | Second | Third | |
| N (%) | N(%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 2 (1.9%) | 1 (0.9%) | 5 (4.6%)** | 6 (5.6%) | 1 (0.9%) | 0 (0%) | 1 (0.9%) | 0 (0%) |
| 3-6 days/wk. | 13 (12.1%) | 6 (5.2%) | 12 (11.1%) | 11 (10.2%) | 4 (3.7%) | 11 (9.6%) | 5 (4.4%) | 4 (3.5%) |
| 1-2 days/wk. | 9 (8.3%) | 13 (11.3%) | 10 (9.3%) | 13 (12.0%) | 9 (8.3%) | 17 (14.8%) | 21 (18.3%) | 6 (5.2%) |
| 1-3 days/mo. | 11 (10.2%) | 7 (6.1%) | 15 (13.9%) | 12 (11.1%) | 9 (8.4%) | 13 (11.3%) | 10 (8.7%) | 6 (5.2%) |
| 1-2 days/3 mos. | 18 (16.7%) | 11 (9.6%) | 15 (13.9%) | 11 (10.2%) | 9 (8.3%) | 15 (13.0%) | 8 (7.0%) | 14 (12.2%) |
| Not at all | 55 (50.9%)* | 77 (67.0%) | 51 (47.2%) | 55 (50.9%) | 76 (70.4%) | 59 (51.3%) | 70 (60.9%) | 85 (73.9%) |
| Absolute alcohol per day (mean, SD) | 1.32 (3.32) | 0.68 (1.43) | 0.71 (1.98) | 0.17 (0.66)* | 0.05 (0.23) | 0.33 (0.79) | 0.01 (0.06) | 0.02 (0.11) |
Difference between MA users and comparisons, p < 0.05
Difference between MA users comparisons, p < 0.001
Table 5.
Frequency of self-reported methamphetamine use pre-pregnancy and by trimester of pregnancy- IDEAL New Zealand
| Heavy MA Use (N=12) | Some MA Use (N=94) | Heavy MA Use (N=12) | Some MA Use (N=94) | |||||
|---|---|---|---|---|---|---|---|---|
| Trimester | ||||||||
| MA use | Pre-pregnancy | First | Second | Third | First | Second | Third | |
| N (%) | N(%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 5 (41.7%) | 19 (20.9%) | 5 (41.7%) | 3 (25.0%) | 1 (8.3%) | 7 (7.4%) | 2 (2.1%) | 0 (0%) |
| 3-6 days/wk. | 4 (33.3%) | 20 (22.0%) | 6 (50.0%) | 8 (66.7%) | 4 (33.3%) | 19 (20.3%) | 1 (1.1%) | 2 (2.1%) |
| 1-2 days/wk. | 1 (8.3%) | 27 (28.6%) | 1 (8.3%) | 1 (8.3%) | 1 (8.3%) | 24 (25.5%) | 17 (18.1%) | 7 (7.4%) |
| 1-3 days/mo. | 1 (8.3%) | 13 (14.3%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 14 (14.9%) | 13 (13.8%) | 10 (10.6%) |
| 1-2 days/3 mos. | 1 (8.3%) | 10 (11.0%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 24 (25.5%) | 11 (11.7%) | 16 (17.0%) |
| Not at all | 0 (0%) | 2 (2.2%) | 0 (0%) | 0 (0%) | 4 (33.3%) | 6 (6.4%) | 50 (53.2%) | 59 (62.8%) |
| Days/week (mean, SD) | 4.57 (2.67) | 2.91 (2.59) | 5.28 (1.85) | 5.10 (1.67) | 2.10 (2.44) | 1.84 (2.13) | 0.54 (1.16) | 0.24 (0.63) |
a 1 of the 108 MA users in this study were identified as exposed by toxicology only
Table 4.
Frequency of self-reported alcohol use pre-pregnancy and by trimester of pregnancy- US IDEAL
| MA user (N=204) | Comparison (N=208) | MA User (N=204) | Comparison (N=208) | |||||
|---|---|---|---|---|---|---|---|---|
| Trimester | ||||||||
| Alcohol use | Pre-pregnancy | First | Second | Third | First | Second | Third | |
| N (%) | N(%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 6 (2.9%)** | 0 (0%) | 1 (0.5%) | 1 (0.5%) | 4 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 3-6 days/wk. | 16 (7.8%)* | 2 (1.0%) | 8 (3.9%)** | 17 (8.3%)* | 12 (5.9%)** | 1 (0.5%) | 3 (1.4%) | 0 (0%) |
| 1-2 days/wk. | 20 (9.8%)** | 4 (1.9%) | 12 (5.9%) | 11 (5.4%)* | 10 (4.9%) | 5 (2.4%) | 3 (1.4%) | 3 (1.4%) |
| 1-3 days/mo. | 8 (3.9%) | 4 (1.9%) | 13 (6.4%)* | 8 (4.0%) | 13 (6.4%)** | 3 (1.5%) | 7 (3.4%) | 2 (1.0%) |
| 1-2 days/3 mos. | 11 (5.4%) | 5 (2.4%) | 11 (5.4%) | 14 (6.9%) | 19 (9.3%)** | 16 (7.7%) | 7 (3.4%) | 6 (2.9%) |
| Not at all | 143 (70.1%)** | 193 (92.8%) | 159 (77.9%)* | 153 (75.0%)** | 146 (71.6%)** | 183 (88.0%) | 188 (90.4%) | 197 (94.7%) |
| Absolute alcohol per day (mean, SD) | 0.36 (0.97)** | 0.05 (0.34) | 0.27 (1.12)** | 0.09 (0.45)* | 0.03 (0.20) | 0.01 (0.04) | 0.002 (0.01) | 0.001 (0.01) |
Difference between MA users and comparisons, p < 0.05
Difference between MA users comparisons, p < 0.001
Table 6.
Frequency of self-reported tobacco use pre-pregnancy and by trimester of pregnancy- IDEAL New Zealand
| MA user (N=108) | Comparison (N=115) | MA User (N=108) | Comparison (N=115) | |||||
|---|---|---|---|---|---|---|---|---|
| Trimester | ||||||||
| Tobacco use | Pre-pregnancy | First | Second | Third | First | Second | Third | |
| N (%) | N(%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 83 (76.9%)** | 47 (40.9%) | 74 (68.5%)** | 65 (60.2%)** | 62 (57.4%)** | 40 (34.8%) | 32 (27.8%) | 31 (27.0%) |
| 3-6 days/wk. | 11 (10.2%) | 7 (6.0%) | 16 (14.8%) | 12 (11.2%) | 13 (12.0%) | 10 (8.7%) | 10 (8.7%) | 8 (7.0%) |
| 1-2 days/wk. | 0 (0%) | 5 (4.3%) | 2 (1.9%) | 8 (7.4%) | 6 (5.6%) | 6 (5.2%) | 4 (3.5%) | 3 (2.6%) |
| 1-3 days/mo. | 0 (0%) | 0 (0%) | 0 (0 %) | 0 (0%) | 1 (0.9%) | 1 (0.9%) | 0 (0%) | 2 (1.8%) |
| 1-2 days/3 mos. | 0 (0%) | 1 (0.9%) | 0 (0%) | 0 (0%) | 2 (1.9%) | 2 (1.7%) | 1 (0.9%) | 0 (0%) |
| Not at all | 14 (13.0%)** | 55 (47.8%) | 16 (14.8%)** | 23 (21.3%)** | 24 (22.2%)** | 56 (48.7%) | 68 (59.1%) | 71 (61.7%) |
| Cigarettes per day (mean, SD) | 14.4 (10.2)** | 8.1 (8.5) | 10.6 (10.1)** | 7.7 (8.2)** | 6.8 (7.4)** | 4.3 (7.3) | 3.1 (5.8) | 2.7 (4.7) |
* Difference between MA users and comparisons, p < 0.05
Difference between MA users comparisons, p < 0.001
Mothers in the comparison group used more pain/sedation medication during pregnancy than those in the methamphetamine group. There was no difference in the administration of anesthetics, psychoactives, steroids, phenobarbital, tocolytics or antihypertensives between the groups during the pregnancy (Shah et al. 2012).
Meconium specimens were collected on all consented neonates (n=3,705). Of infants identified as MA exposed (n=210), 71% were identified by maternal self-report only, 25.2% by positive self-report and meconium screen, and 3.8% by meconium screen only. Maternal self-report was more sensitive than meconium analysis for detecting prenatal amphetamines exposure. The low number of positive meconium screens is not unexpected. Meconium begins to form in the twelfth week of gestation and collects endogenous and exogenous waste from the second trimester. If drug used stopped in the first trimester, as was the case for many women, meconium results would be negative. Cannabis and continine were also examined by meconium screening and self-report. Two participants had insufficient meconium remaining for cannabis testing and 634 had lack of adequate specimen to be evaluated for cotinine. Neonates who were cannabis exposed (n=261) were identified by maternal self-report (69.7%), positive self-report and meconium (16.5%), and by meconium alone (13.8%). Similar to amphetamines, few meconium specimens screened or confirmed positive if use was in the first and/or second trimester. Cotinine exposure was identified in 1008 neonates with meconium and self-report (47.2%), self-report alone (26.0%) and meconium alone (26.8%). This indicates that meconium testing and maternal self-report have equal sensitivity detection (Gray et al. 2009).
Newborn Medical Outcomes
There were no differences between the MA and comparison groups regarding the incidence of facial dysmorphism, skeletal or cardiac defects, or respiratory problems after birth (Shah et al. 2012). The incidence of admission to the NICU was higher in the exposed group and after adjusting for covariates, MA exposure remained significantly associated with poor suck and being less likely to be breastfed. There were no differences between the groups regarding central nervous system signs of drug withdrawal requiring pharmacological intervention, sweating or tachycardia. There was no difference in the incidence of abnormal head sonograms.
Maternal and Neonatal Outcomes comparison with New Zealand
New Zealand followed 107 children prenatally exposed to MA and 112 unexposed (Wu et al. 2013) with comorbidity of substance use disorders and other psychiatric disorders examined in a smaller subsample of participants who attended the one-month follow-up visit (New Zealand: 97 exposed, 110 unexposed; United States: 127 exposed, 193 unexposed)(Wouldes et al. 2013). Overall, MA-using mothers in both New Zealand and the United States were ten times more likely to have a substance use disorder and twice as likely to have a psychiatric disorder when compared to non-using mothers. Mothers in New Zealand were more likely to have comorbid disorders than the mothers in the United States.
MA-using mothers in both New Zealand and the United States were more likely to be single, waited longer for first prenatal care visit, had lower SES, had a CPS referral, and used alcohol and marijuana during pregnancy than the mothers who did not use MA. MA-using mothers in the United States had significantly less prenatal care than the mothers in the United States who did not use MA. Similarly, inadequate prenatal care was associated with increased CPS referral in the United States but not in New Zealand, and more CPS referrals were due to drug use in the United States than in New Zealand.
Childhood Growth Effects
Preliminary analysis of the of the IDEAL cohort after the first year of a two year recruitment period found the 84 MA-exposed infants were more likely to be small for gestational age (SGA) and have a lower birth weight than the 1534 eligible and consented unexposed infants(Smith et al. 2006) with an analysis of the full sample continuing to demonstrate an increased risk for being born SGA in the MA group (Nguyen et al. 2010). In this study, an initial set of 10 potential covariates [site, maternal age, mother's pre-pregnancy weight, SES, marital status, first born and use of drugs (yes vs. no) tobacco, alcohol, marijuana and cocaine] were used in multivariate models. Through a backward selection process using a p-value <=.10, extraneous variables were eliminated from the models. An analysis of the full longitudinal sample (n=412) found that despite matching by birth weight categories and adjusting for confounding variables, infants in the MA-exposed group had smaller head circumferences, and were shorter than the comparison group at birth (Shah et al. 2012). To assess growth effects over time, growth parameters were analyzed for participants who attended the birth, 12, 24, and 36 month visits. Throughout the first three years of life, the exposed children remained significantly shorter than the unexposed children but were growing at roughly the same rate as the unexposed children (Zabaneh et al. 2012). Because the MA exposed infants were more likely to be born ≤36 week gestation, the height trajectory for term-only infants was examined and the exposed remained lower through age 3.
The impact of prenatal MA exposure on growth in the USA vs. New Zealand cohorts found a stronger negative effect on infant and child length/height in the USA (Abar et al. 2014). Given the considerable differences in governmental and healthcare responses to maternal drug use across the countries, these findings suggest improved antenatal and postpartum provision for drug-using mothers in the USA is a potential way to prevent the decreased growth observed in the developing fetus and child.
Neurobehavioral Outcomes
Preliminary analysis of 166 infants, found the 74 MA-exposed infants who were administered the NICU Network Neurobehavioral Scale (NNNS) within the first five days of life were more likely to exhibit increased physiological stress than the unexposed infants (Cohen's d=0.20) (Smith et al. 2008). Increased CNS stress was also associated with higher amphetamine metabolites in meconium. Heavy MA use, defined as average use of MA >=3 days per week across pregnancy, was significantly associated with decreased arousal (Cohen's d=0.85) and increased physiological stress (Cohen's d=0.29) and lethargy (Cohen's d=0.52).
When the full sample was compared to a cohort of infants from New Zealand, all MA-exposed infants demonstrated poorer quality of movement, and increased physiological stress, total stress/abstinence, and CNS stress (LaGasse et al. 2011). Additionally, infants with heavy MA-exposure demonstrated lower arousal and less excitability when compared with unexposed infants. Similar to the partial United States sample, maternal MA use during the first trimester was associated with increased total stress/abstinence and also increased physiological stress. Third trimester use was associated with increased hypotonicity and increased lethargy. Only the MA-exposed infants in the New Zealand cohort had significantly increased hypotonicity, hypertonicity, nonoptimal reflexes and less total stress compared to the MA-exposed infants of the United States cohort (LaGasse et al. 2011). These findings from the United States and New Zealand increased the generalizability of the methamphetamine effects across various cultures.
Effects of Maternal Depression
Given the evidence of negative effects of prenatal depression on infants (Field et al. 2006; Alder et al. 2007; Hurley et al. 2008), the IDEAL study also examined the neurobehavioral effect of maternal depression on MA-exposed infants. Although prenatal MA-exposure combined with maternal depression was not significantly associated with any neurobehavioral outcomes, regardless of exposure status, maternal depression was associated with decreased handling and arousal scores, increased physiological stress, and increased hypotonicity (Paz et al. 2009). The full sample set was analyzed at the one-month visit and MA use was associated with increased depressive symptoms and higher scores on the Beck Depression Inventory-II compared to the mothers who did not use MA during pregnancy (Smith et al. 2012). At the one-month visit, regardless of exposure status, maternal depression was associated with increased autonomic stress and poorer quality of movement. Although the specific neurobehavioral scores differed from the first five days of life to the one-month visit, it is clear maternal depression impacts infant neurodevelopment.
Maternal MA use has also been associated with increased maternal depressive symptoms (Cohen's d=0.41) and parenting stress (Cohen's d=0.36) at 3 years of age when compared to mothers who did not use MA during pregnancy (Liles et al. 2012). Similar to the maternal depression findings regarding neurobehavioral outcomes, there were no significant differences between exposure and maternal depression in regards to perceived child behavior problems (Cohen's d=0.15). Additionally, depressive symptoms and perceived child behavior problems were significant predictors of parenting stress.
Childhood Behaviors
Internalizing and externalizing behaviors at age 3 years were analyzed in a sample of 290 (n=142 exposed) children who attended both the one-year and three-year appointments. Although no group differences were found based on MA exposure, decreased internalizing and externalizing behaviors were found in children with easy temperaments compared to children with difficult temperaments (Derauf et al. 2011). Easy and difficult temperaments were defined using the Infant Behavior Questionnaire at the one-year appointment. Additionally, children living in high environmental risk were more likely to display internalizing and externalizing behaviors than those children living in lower environmental risk.
Additional caregiver rated behavior problems were assessed at age 3 years and 5 years (n=330). Prenatal MA exposure (n=166) was associated with increased anxious/depressed problems and emotional reactivity at both age 3 and 5 years (LaGasse et al. 2012). Additionally, MA exposure was associated with increased externalizing behaviors and increased attention-deficit/hyperactivity disorder symptoms at age 5 years. When compared to caregiver ratings at age 3, exposed children at age 5 were rated to have significantly higher externalizing behaviors, and attention-deficit/hyperactivity disorder symptoms. Heavy MA exposure was also associated with increased attention problems and being withdrawn. No effects of MA exposure were found for internalizing behaviors.
At age 5.5 the Conners’ Kiddie Continuous Performance Test, a computerized visual examination that analyzes performance measures associate with ADHD was administered to the children (Kiblawi et al. 2013). Prenatal methamphetamine was associated with subtle differences in attention processing. The exposed children also had a higher ADHD confidence index score, suggesting a greater future risk for developing ADHD.
To further refine these clinically significant behavioral findings at age 5, the home environment and several primary caregiver risk factors were assessed (Twomey et al. 2013). We found that the more responsive the home environments were to children's developmental and emotional needs, the less risk of internalizing and externalizing behavior was observed. Further, psychological symptoms and parenting stress experienced by the primary caregivers were associated with increased child behavioral problems. These primary caregiver and home environment findings were independent of methamphetamine exposure highlighting the importance of interventions that address both the child and parental or primary caregiver needs in order to optimize child outcome.
Motor, Cognitive & Language Outcomes
At age three years, there were no effects of MA exposure on receptive and expressive language (Derauf et al. 2011), gross motor skills, or mental development (defined as memory, early number concepts, problem solving, generalization and vocalization) (Smith et al. 2011). Heavy prenatal MA exposure was associated with decreased grasping scores at ages one and three years, however there were no differences in any other fine motor skills (Smith et al. 2011).
There are numerous possible etiologies for the poorer motor performance in the MA exposed children. Administration of MA to laboratory animals results can lead to long lasting toxicity to the brain. MA administration to pregnant mice induces dopaminergic nerve terminal degeneration and long term motor deficits in the exposed offspring (Jeng et al. 2005). Prenatal MA exposure in 3 week old rats (equivalent to approximately age 5 years) demonstrate impaired postural motor movements (Šlamberová et al. 2006). These altered movements can be detected in the second generation of rats exposed to MA prenatally (Šlamberová et al. 2007).
Our cognitive findings are consistent with the preclinical findings that MA exposure during development leads to spatial learning and reference memory deficits in rats (Vorhees et al. 2007). Neuroimaging research has demonstrated volumetric reductions in the caudate nucleus in preschool children prenatally exposed to MA suggesting the caudate nucleus impacts cognitive control processes (Derauf et al. 2012b). Further, decreased putamen, globus pallidus and hippocampus volumes in these children correlated with decreased performance on sustained attention and delayed verbal memory indicating these decreased volumes may contribute to poorer learning(Chang et al. 2004b).
Executive Functioning
Children (n=137 exposed) who completed the 66-month visit (n=267), and had valid data on a directional Stroop-like task for school age children showed that heavy prenatal MA exposure was associated with reduced accuracy (incongruent, Cohen's d=0.73 and mixed, Cohen's d=0.55), signaling poorer inhibitory control (Derauf et al. 2012a). Additionally, caregiver psychological distress and child protective services involvement due to physical and/or sexual abuse were associated with reduced accuracy.
The relationship between prenatal methamphetamine exposure, early childhood adversity and subsequent childhood neurodevelopment was assessed using an adversity index score over the first three years of life. Prenatal methamphetamine exposure was associated with behavioral and emotional control findings at age 5 which was then associated with deficits in executive function at age 6.5. The findings also demonstrated that the effects of MA on neurodevelopment(Abar et al. 2013) functioned primarily through early adversity.
Limitations
There are potential limitations to the IDEAL study. A substantial proportion of women declined to participate at an early stage in the study and therefore, the findings may not be generalizable to all MA using pregnant women. It is possible that women who refused to participate would be likely to have more severe MA problems. Another potential limitation of the study is that the exposed group of subjects is primarily based on self-report and women may not have been able to recall the timing and amount of MA use during their pregnancy; however other research supports the use of the calendar method to overcome recall bias (Jacobson et al. 2002). Additionally, despite matching on birth weight and using type of insurance as a proxy of for SES, newborns in the exposed group were more likely to be ≤36 weeks gestation and have a lower SES. The exposure to alcohol and tobacco was increased in the methamphetamine using mothers relative to comparison subjects though this is mitigated in part by multivariate statistical techniques.
Summary
We have reviewed the published findings of the IDEAL study regarding prenatal MA exposure and subsequent child outcome. To our knowledge the IDEAL study is the first prospective, longitudinal controlled investigation of prenatal methamphetamine exposure utilizing maternal self-report and objective meconium confirmation. The strengths of the study design allows for the use of multivariate statistical techniques to account for findings in the context of the common co-exposures of alcohol, tobacco and marijuana. Further, the findings are also presented in the context of the home environment as well as the characteristics of the primary caregivers to allow for assessment of how the drug exposure and the quality of parenting and home environment interact on overall child outcome.
Our findings that there were no differences in maternal complications or newborn health outcomes are reassuring and counter many previous reports with smaller sample sizes. Further, there was no neonatal abstinence syndrome requiring pharmacologic intervention observed, indicating that clinicians should investigate for opioid co-exposures when working with a methamphetamine-exposed neonate demonstrating significant withdrawal symptoms.
Though there were no significant malformations in the exposed children, somatic growth was significantly decreased in the methamphetamine exposed children. In addition to having an increased risk for being born SGA, height velocity remained lower by age three. Given these growth difference were in the cohort in the United States relative to enrollees in New Zealand, these findings have significant policy implications. The differences in governmental and healthcare responses to maternal drug use in New Zealand suggest efforts targeted at antenatal prevention as well as postpartum support for drug-using mothers could diminish the impaired growth observed in the exposed children. In contrast to the MA-using mothers in the United States who are often reported for suspected child endangerment, there is no legal mandate in New Zealand to report a pregnant woman who reveals substance use during her pregnancy. In fact, there has never been a case of child removal due to drug use in New Zealand.
Despite the lack of a neonatal abstinence syndrome requiring pharmacologic intervention, numerous findings from the IDEAL study were associated with heavy drug exposure. Increased stress responses in the neonatal period were linked to higher drug metabolite concentrations in meconium. The poorer fine motor scores at one year were also linked to heavy use. Further, poorer inhibitory control observed in the heavy methamphetamine exposed children places them at high risk for impaired executive function. Collectively these findings demonstrate the importance of providing rapid, comprehensive drug counseling services to women actively using methamphetamine during pregnancy in order to optimize the long-term neurodevelopment of the exposed child.
In addition to the importance of drug dosing, the home environment and caregiver stress significantly influenced child outcomes. Independent of methamphetamine exposure, children with more responsive home environments to developmental and emotional needs demonstrated lower risks for internalizing and externalizing behavior. Further, increased psychological symptoms and parenting stress in the primary caregivers were associated with increased child behavioral problems. These findings again highlight the importance of interventions that address both the child and parental or primary caregiver needs in order to optimize child outcome.
Supplementary Material
Highlights.
A neonatal abstinence syndrome was not observed with methamphetamine exposure
Somatic growth was significantly decreased in methamphetamine exposed children
Methamphetamine exposure increased emotional reactivity in young children.
Heavy methamphetamine exposure was linked with poorer inhibitory control in children
Responsive home environments lowered the risk for behavior issues
Figure 3.
Retention Percentages at each Visit Age- NZ IDEAL
Acknowledgments
These findings were supported by NIDA Grant# 1RO1DA014948 and in part by the National Center on Research Resources Grant# 1UL1-TR000124 and 5P20 RR11091.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abar B, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, et al. Examining the relationships between prenatal methamphetamine exposure, early adversity, and child neurobehavioral disinhibition. Psychol Addict Behav. 2013 Sep;27(3):662–73. doi: 10.1037/a0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abar B, Lagasse LL, Wouldes T, Derauf C, Newman E, Shah R, et al. Cross-national Comparison of Prenatal Methamphetamine Exposure on Infant and Early Child Physical Growth: A Natural Experiment. Prev Sci. 2014 Aug 13;15(5):767–76. doi: 10.1007/s11121-013-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuff-Smith KD, George M, Lorens SA, Vorhees C V. Preliminary evidence for methamphetamine-induced behavioral and ocular effects in rat offspring following exposure during early organogenesis. Psychopharmacology (Berl) 1992;109:255–63. doi: 10.1007/BF02245871. [DOI] [PubMed] [Google Scholar]
- Acuff-Smith KD, Schilling M a., Fisher JE, Vorhees CV. Stage-specific effects of prenatal dmethamphetamine exposure on behavioral and eye development in rats. Neurotoxicol Teratol. 1996;18(2):199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern neonatal Med. 2007 Mar;20(3):189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- Arria AM, Derauf C, Lagasse LL, Grant P, Shah R, Smith L, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006 May;10(3):293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, et al. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002 Mar;186(3):487–95. doi: 10.1067/mob.2002.121073. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994 Jan;18(1):3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Larsson G, Zetterström R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatr Scand. 1980 Sep;69(5):675–80. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abus Negl. 1988;12(4):503–7. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- Brown J, Hohman M. The impact of methamphetamine use on parenting. J Soc Work Pract Addict. 2006;6(1-2):63–88. [Google Scholar]
- Cabrera TM, Levy AD, Li Q, Van de Kar LD, Battaglia G. Prenatal methamphetamine attenuates serotonin mediated renin secretion in male and female rat progeny: Evidence for selective long-term dysfunction of serotonin pathways in brain. Synapse. 1993;15:198–208. doi: 10.1002/syn.890150305. [DOI] [PubMed] [Google Scholar]
- Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996 Feb;85(2):204–8. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res - Neuroimaging. 2004a;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res - Neuroimaging. 2004b;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cho DH, Lyu HM, Lee HB, Kim PY, Chin K. Behavioral teratogenicity of methamphetamine. J Toxicol Sci. 1991;16(Suppl 1):37–49. doi: 10.2131/jts.16.supplementi_37. [DOI] [PubMed] [Google Scholar]
- Derauf C, LaGasse L, Smith L, Newman E, Shah R, Arria A, et al. Infant temperament and high-risk environment relate to behavior problems and language in toddlers. J Dev Behav Pediatr. 2011;32(2):125–35. doi: 10.1097/DBP.0b013e31820839d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, LaGasse LL, Smith LM, Grant P, Shah R, Arria A, et al. Demographic and psychosocial characteristics of mothers using methamphetamine during pregnancy: preliminary results of the infant development, environment, and lifestyle study (IDEAL). Am J Drug Alcohol Abuse. 2007 Jan;33(2):281–9. doi: 10.1080/00952990601175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Lagasse LL, Smith LM, Newman E, Shah R, Neal CR, et al. Prenatal methamphetamine exposure and inhibitory control among young school-age children. J Pediatr. Mosby, Inc. 2012a Sep;161(3):452–9. doi: 10.1016/j.jpeds.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Lester BM, Neyzi N, Kekatpure M, Gracia L, Davis J, et al. Subcortical and cortical structural central nervous system changes and attention processing deficits in preschool-aged children with prenatal methamphetamine and tobacco exposure. Dev Neurosci. 2012b Jan;34(4):327–41. doi: 10.1159/000341119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Eriksson M, Zetterström R. Amphetamine addiction during pregnancy: 10-year follow-up. Acta Paediatr. 1994 Nov;404:27–31. doi: 10.1111/j.1651-2227.1994.tb13380.x. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behav Dev. 2006 Jul;29(3):445–55. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Gray TR, LaGasse LL, Smith LM, Derauf C, Grant P, Shah R, et al. Identification of prenatal amphetamines exposure by maternal interview and meconium toxicology in the Infant Development, Environment and Lifestyle (IDEAL) study. Ther Drug Monit. 2009 Dec;31(6):769–75. doi: 10.1097/FTD.0b013e3181bb438e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Grotta S, LaGasse LL, Arria AM, Derauf C, Grant P, Smith LM, et al. Patterns of methamphetamine use during pregnancy: results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2010 Jul;14(4):519–27. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A de B. Four Factor Index of Social Status. New Haven, Conn. 1975 [Google Scholar]
- Hrebíčková I, Malinová-Ševčíková M, Macúchová E, Nohejlová K, Šlamberová R. Exposure to Methamphetamine During First and Second Half of Prenatal Period and Its Consequences on Cognition After Long-Term Application in Adulthood. Physiol Res. 2014;63(Suppl. 4):S535–45. doi: 10.33549/physiolres.932927. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Black MM, Papas MA, Caulfield LE, Caufield LE. Maternal symptoms of stress, depression, and anxiety are related to nonresponsive feeding styles in a statewide sample of WIC participants. J Nutr. 2008 Apr;138(4):799–805. doi: 10.1093/jn/138.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Ergui I, Young JI. Mol Psychiatry. January. Vol. 20. Nature Publishing Group; 2014. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. pp. 1–11. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–25. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jeng W, Wong AW, Ting-A-Kee R, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005:317–26. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kiblawi ZN, Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, et al. The effect of prenatal methamphetamine exposure on attention as assessed by continuous performance tests: results from the Infant Development, Environment, and Lifestyle study. J Dev Behav Pediatr. 2013 Jan;34(1):31–7. doi: 10.1097/DBP.0b013e318277a1c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C, et al. Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics. 2012 Apr;129(4):681–8. doi: 10.1542/peds.2011-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, et al. The Maternal Lifestyle Study (MLS): The Caretaking Environment of Infants Exposed to Cocaine/Opiates. Pediatr Res. 1999 Apr;45(4, Part 2 of 2):247A–247A. [Google Scholar]
- LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, et al. Neurotoxicol Teratol. 1. Vol. 33. Elsevier Inc.; 2011. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. pp. 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The Maternal Lifestyle Study: effects of substance exposure during pregnancy on Neurodevelopmental Outcome in 1-Month-Old Infants. Pediatrics. 2002 Dec 1;110(6):1182–92. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- Liles BD, Newman E, Lagasse LL, Derauf C, Shah R, Smith LM, et al. Perceived child behavior problems, parenting stress, and maternal depressive symptoms among prenatal methamphetamine users. Child Psychiatry Hum Dev. 2012 Dec;43(6):943–57. doi: 10.1007/s10578-012-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC. Methamphetamine Abuse During Pregnancy: Outcome and Fetal Effects. Obstet Gynecol. 1988 Oct;72(4):541–4. [PubMed] [Google Scholar]
- Lu LH, Johnson A, O'Hare ED, Bookheimer SY, Smith LM, O'Connor MJ, et al. Effects of prenatal methamphetamine exposure on verbal memory revealed with functional magnetic resonance imaging. J Dev Behav Pediatr. 2009 Jun;30(3):185–92. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell-Dowling K, Donlon M, Kelly JP. Methamphetamine exposure during pregnancy at pharmacological doses produces neurodevelopmental and behavioural effects in rat offspring. Int J Dev Neurosci. 2014;35:42–51. doi: 10.1016/j.ijdevneu.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, et al. J Pediatr. 2. Vol. 157. Mosby, Inc.; Aug, 2010. Intrauterine growth of infants exposed to prenatal methamphetamine: results from the infant development, environment, and lifestyle study. pp. 337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: Maternal and neonatal correlates. J Pediatr. 1987;111(4):571–7. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Paz MS, Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, et al. Neurotoxicol Teratol. 3. Vol. 31. Elsevier B.V.; 2009. Maternal depression and neurobehavior in newborns prenatally exposed to methamphetamine. pp. 177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Kolchugina GK, Butler RW, Corbett SM, Honeycutt EB, et al. Pharmacol Biochem Behav. 3. Vol. 98. Elsevier Inc.; May, 2011. Abnormalities in parentally rated executive function in methamphetamine/polysubstance exposed children. pp. 432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessinger MA. Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstet Gynecol Clin North Am. 1998 Mar;25(1):119–38. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- Pometlová M, Hrubá L, Šlamberová R, Rokyta R. Cross-fostering effect on postnatal development of rat pups exposed to methamphetamine during gestation and preweaning periods. Int J Dev Neurosci. 2009;27(2):149–55. doi: 10.1016/j.ijdevneu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL. Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999 Aug;104(2):e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- Shah R, Diaz SD, Arria A, LaGasse LL, Derauf C, Newman E, et al. Prenatal methamphetamine exposure and short-term maternal and infant medical outcomes. Am J Perinatol. 2012 May;29(5):391–400. doi: 10.1055/s-0032-1304818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Das A, Bauer CR, Bada HS, Lester B, Wright LL, et al. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004 Aug;114(2):e226–34. doi: 10.1542/peds.114.2.e226. [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Pometlová M, Charousová P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuro-Psychopharmacology Biol Psychiatry. 2006;30:82–8. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Pometlová M, Rokyta R. Effect of methamphetamine exposure during prenatal and preweaning periods lasts for generations in rats. Dev Psychobiol. 2007;49(3):312–22. doi: 10.1002/dev.20203. [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Vrajová M, Schutová B, Mertlová M, Macúchová E, Nohejlová K, et al. Prenatal Methamphetamine Exposure Induces Long-Lasting Alterations in Memory and Development of NMDA Receptors in the Hippocampus. Physiol Res. 2014;63(Suppl. 4):S547–58. doi: 10.33549/physiolres.932926. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006 Sep;118(3):1149–56. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30(1):20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Haning W, et al. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol. 2011;33(1):176–84. doi: 10.1016/j.ntt.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Paz MS, LaGasse LL, Derauf C, Newman E, Shah R, et al. Maternal depression and prenatal exposure to methamphetamine: neurodevelopmental findings from the infant development, environment, and lifestyle (ideal) study. Depress Anxiety. 2012 Jun;29(6):515–22. doi: 10.1002/da.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O'Connor MJ, Kan E, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci. 2010 Mar 17;30(11):3876–85. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . THe DASIS Report-- Primary Methamphetamine/Amphetamine Admissions to Substance Abuse Treatment. Rockville, MD: 2008. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Treatment Episode Data Set (TEDS) 2000-2010. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2012. DASIS Series S-61, HHS Publication No. (SMA 12-4701) [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Treatment Episode Data Set (TEDS):2001-2011. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2013. BHSIS Series S-65 HHS Publication No. (SMA-13-4772) [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009 Jun;113(6):1285–91. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Twomey J, LaGasse L, Derauf C, Newman E, Shah R, Smith L, et al. Prenatal methamphetamine exposure, home environment, and primary caregiver risk factors predict child behavioral problems at 5 years. Am J Orthopsychiatry. 2013 Jan;83(1):64–72. doi: 10.1111/ajop.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C V, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav Pharmacol. 2007;18(5-6):549–62. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AD, Caldecott-Hazard S. Developmental neurotoxicity to methamphetamines. Clin Exp Pharmacol Physiol. 1995;22:372–4. doi: 10.1111/j.1440-1681.1995.tb02022.x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown C a., Gudelsky G a., Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev Brain Res. 2003;147(1-2):163–75. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wouldes T a, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, et al. Drug Alcohol Depend. 1-3. Vol. 127. Elsevier Ireland Ltd; Jan 1, 2013. Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. pp. 101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Lagasse LL, Wouldes T, Arria AM, Wilcox T, Derauf C, et al. Predictors of inadequate prenatal care in methamphetamine-using mothers in New Zealand and the United States. Matern Child Health J. 2013 Apr;17(3):566–75. doi: 10.1007/s10995-012-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Yamamoto K, Fukui Y, Kurishita A. Teratogenic effects of methamphetamine in mice. Japanese J Leg Med. 1992;46:126–31. [PubMed] [Google Scholar]
- Zabaneh R, Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, et al. The effects of prenatal methamphetamine exposure on childhood growth patterns from birth to 3 years of age. Am J Perinatol. 2012 Mar;29(3):203–10. doi: 10.1055/s-0031-1285094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.