Abstract
During tumor progression, alterations within the systemic tumor environment, or macroenvironment, result in the promotion of tumor growth, tumor invasion to distal organs, and eventual metastatic disease. Distally produced hormones, commensal microbiota residing within mucosal surfaces, and myeloid cells and even the bone marrow impact the systemic immune system, tumor growth, and metastatic spread. Understanding the reciprocal interactions between the cells and soluble factors within the macroenvironment and the primary tumor will enable the design of specific therapies that have the potential to prevent dissemination and metastatic spread. This chapter will summarize recent findings detailing how the primary tumor and systemic tumor macroenvironment coordinate malignant progression.
Keywords: tumor-promoting inflammation, MDSC, emergency myelopoiesis, microbiota, pre-metastatic niche, tumor microenvironment, hormones, commensal microbiota
1. INTRODUCTION
Cancer is a systemic disease that affects multiple organs and systems in tumor-bearing hosts. In recent years, seminal mechanistic insight has been generated to understand the peculiarities of the tumor microenvironment (TME), which have resulted in novel therapeutic interventions. While much more work needs to address how multiple non-tumor cell types and structures at tumor beds influence malignant progression, it is becoming increasingly clear that tumors release factors that drive the orchestration of an environment in the host that involves the crosstalk between multiple distal compartments, at places beyond tumor beds. Systemic alterations include changes in the functioning of the bone marrow, where especially myelopoiesis is heavily altered in the presence of a tumor. Distal signals also involve the formation of pre-metastatic niches where disseminated tumor cells home, lay dormant, and eventually develop into growing metastatic masses, as well as hormonal signals and inflammatory mediators generated through interactions with commensal microorganisms. Together, these inflammatory, tumor-promoting pro-metastatic networks form a systemic “macroenvironment” in tumor-bearing hosts that influences both the function of distant tissues and the tumor itself. This chapter will focus on the role of immune cells and their products in the orchestration of this systemic “macroenvironment”, which is critical for the progression of aggressive tumors and eventually for fatal outcomes.
2. INTERACTIONS BETWEEN THE TUMOR MICROENVIRONMENT AND THE BONE MARROW
2.1. Pathological myelopoiesis promotes malignant progression
The hematopoietic system has evolved to adjust the production of leukocytes to the presence of viral or bacterial infections. By increasing the production of leukocytes derived from the myeloid lineage, the host quickly generates myeloid effectors that are mobilized to the periphery from the bone marrow to fight pathogens (Takizawa et al., 2012). These mechanisms of “emergency myelopoiesis” are co-opted by tumors for their own growth and dissemination. A common finding in advanced solid-tumor bearing hosts is that severe alterations of myelopoietic differentiation promote the expansion and accumulation of immature myeloid progenitors into the blood, lymph nodes, spleen, bone marrow and tumor sites (Gabrilovich et al., 2012; Ostrand-Rosenberg and Sinha, 2009). In cancer, unlike emergency myelopoiesis induced by acute infections, immature myeloid cells are also retained at early stages of differentiation by signals derived from the TME, which block the differentiation of immature precursors into lineage-committed leukocytes, further contributing to the accumulation of myeloid precursors. This impairs DC-mediated antigen presentation and macrophage-dependent cytotoxic (protective) activity.
Initially, immature myeloid cells mobilized in the presence of a tumor are not necessarily immunosuppressive, although they could play a role in tumor-promoting inflammation and neovascularization. As the tumor progress, however, immature myeloid cells constantly produced through expanded myelopoiesis are influenced by multiple tumor-derived factors that turn them into powerful suppressors of protective immune responses. These heterogeneous immature myeloid cells that are able to suppress anti-tumor T cell responses through a variety of mechanisms are generically termed myeloid-derived suppressor cells (MDSCs), and in mice, are characterized by the co-expression of high levels of Gr1, along with CD11b. Under steady-state conditions, Gr-1+CD11b+ cells comprise ~35% of bone marrow cells but only ~3% of splenocytes (Nagaraj and Gabrilovich, 2012). In certain advanced tumor models, however, they can represent more than 50% of total splenocytes, and ~12% of total cells in single-cell suspensions from dissociated human melanoma samples (Gros et al., 2012). They also accumulate in variable proportions at tumor beds where, besides promoting the suppression of tumor-specific immune responses, MDSCs drive angiogenesis (Yang et al., 2004) and IL-6-dependent tumor-promoting inflammation (Fukuda et al., 2011; Lesina et al., 2011). In addition, MDSCs mobilized from the bone marrow generate pre-metastatic niches that provide a favorable location for disseminated tumor cells to survive, expand and, by recruiting new myeloid cells, generate metastases that are eventually responsible for fatal outcomes (Kaplan et al., 2006; Kaplan et al., 2005; Peinado et al., 2012; Psaila and Lyden, 2009).
MDSCs are therefore immature myeloid cells that acquire immunosuppressive activity through pathological activation in tumor-bearing hosts at places distal to the TME. As we will describe below, MDSCs play a critical role as systemic drivers of malignant progression.
2.2. Tumor-derived secreted factors promote the expansion of immunosuppressive MDSCs
MDSC are induced by factors primarily produced in the TME, and in particular by inflammatory cytokines. Inflammatory mediators such as IL-1β, IL-6, and PGE2 have been demonstrated to play a role in the mobilization of MDSCs in tumor-bearing mice (Bunt et al., 2007; Eruslanov et al., 2010; Sinha et al., 2007). In addition, immunosuppressive CD33+ leukocytes can be generated from human peripheral blood cells by incubation with GM-CSF and IL-6 (Lechner et al., 2010). Correspondingly, an important transcriptional pathway associated with both the expansion and the differentiation blockade of myeloid progenitors is mediated by STAT3. STAT3 activation by tumor-induced cytokines promotes the expansion of myeloid cells by up-regulating both drivers of the cell cycle and anti-apoptotic factors (Gabrilovich et al., 2012; Sander et al., 2010; Yu et al., 2009). Activated STAT3 also promotes the up-regulation of C/EBPβ, which contributes to the acquisition of immunosuppressive activity (Marigo et al., 2010). In addition, STAT3 signaling in myeloid cells impairs dendritic cell and macrophage differentiation, at least in part by decreasing PKCβII (Farren et al., 2014; Nefedova et al., 2004). The combination of increased proliferation, decreased apoptosis and impaired differentiation all contribute to the accumulation of immature myeloid leukocytes at bone marrow and lymphatic locations. STAT3 activation is not only caused by secreted inflammatory cytokines, as heat shock proteins contained within tumor-derived exosomes have been also shown to contribute to the mobilization of MDSCs in cancer through this mechanism (Chalmin et al., 2010).
STAT3 activation in myeloid cells also elicits an autocrine loop whereby S100A9 protein secretion is increased. S100A8/9 dimers bind to the receptor for advanced glycation end-products (RAGE receptors) activating the NF-κB pathway (Sinha et al., 2008), further blocking the differentiation of these myeloid progenitors into macrophages and DCs (Cheng et al., 2008). In addition, S100A8/9 protein production in the TME promotes the recruitment of MDSCs to tumor beds and enhances their suppressive activity (Gabrilovich et al., 2012; Sinha et al., 2008).
3. SUBSETS OF MYELOID PRECURSORS PATHOLOGICALLY MOBILIZED IN TUMOR-BEARING HOSTS
While all MDSCs represent immature and pathologically activated myeloid cells, they include at least two main categories of precursors; granulocytes and macrophages/DCs. Thus, murine MDSCs were originally defined based on co-expression of CD11b and Gr-1 surface markers (Gabrilovich et al., 2007; Kusmartsev and Gabrilovich, 2002; Sinha et al., 2008). However, it became clear that this heterogeneous population could be sub-divided into Ly-6ClowLy-6G+ granulocytic/polymorphonuclear (PMN) MDSCs and Ly-6ChiLy-6G− monocytic MDSCs (Dolcetti et al., 2010; Youn et al., 2008).
The categorization of MDSCs in cancer patients is more complicated due to higher heterogeneity and differentiation from other myeloid subsets (e.g., bona fide neutrophils). Nevertheless, MDSC are typically lineage−CD11b+CD33+HLA-DR−/low leukocytes that include a “monocytic” population with the capacity to differentiate into CD14+ cells; and a CD15+ granulocytic type (Condamine et al., 2014; Gros et al., 2012; Ostrand-Rosenberg and Sinha, 2009; Ramachandran et al., 2013). Immature myeloid cells in cancer patients are, however, quite heterogeneous in every tumor, and much remains to be learned about the phenotypic diversity of myeloid leukocytes in freshly processed human tumors, before granulocytic populations die in the freeze/thawing process. In addition, because immature myeloid cells may not be intrinsically immunosuppressive at initial stages of tumor progression, the role of MDSCs in human tumors - resected as soon as they are detected – may not mirror the dramatic phenotypes identified in certain terminal transplantable tumors in mice.
Independently of additional subsets, granulocytic MDSCs outnumber monocytic MDSCs by a ratio of 3:1 (Youn et al., 2008). This reflects differences in the proportions of neutrophilic vs. monocyte/macrophage precursors during myelopoiesis, but also the fact that typical myelopiesis in cancer-bearing individuals is so corrupted that myelomonocytic precursors acquire the pathological capacity turning into granulocytes through epigenetic silencing of the retinoblastoma gene (Youn et al., 2013).
The two main subsets of MDSCs inhibit the protective activity of anti-tumor T cells through different mechanisms. Thus, the production of reactive oxygen species (ROS) is the main mechanism whereby granulocytic MDSCs suppress CD8 T cell responses, which requires cell-cell contact (Corzo et al., 2009; Youn et al., 2012). In addition, granulocytic MDSCs are more active at generating immunosuppressive adenosine, a process that involves the sequential activity of the CD39 and CD73 ectoenzymes (Ryzhov et al., 2011). In contrast, monocytic MDSCs primarily suppress through the generation of reactive nitrogen species (RNS) and the enzymatic activity of arginase (ARG1), which depletes the Amino Acid Arginine, thus promoting T cell unresponsiveness (Corzo et al., 2010; Gabrilovich et al., 2012).
4. LIENAGE-COMMITTED MYELOID POPULATIONS IN THE TME
Although tumor-derived factors hamper the differentiation of pathologically expanded immature myeloid progenitors into their lineage-committed cell types in tumor-bearing hosts, MDSCs contain precursors with the capacity to differentiate into macrophages, DCs and granulocytes. These myeloid cells are in fact the most abundant cell types identified in the microenvironment of most solid tumors. Tumor-derived MDSCs transferred into tumor-free syngeneic mice turn into immunocompetent macrophages and DCs (Gabrilovich et al., 2012; Narita et al., 2009). In contrast, MDSCs transferred into tumor-bearing hosts home to the TME where, under hypoxic conditions, turn primarily into immunosuppressive macrophages, but also into DCs (~5% of them), with phenotypes that remain to be characterized in terms of immunosuppressive vs. immunostimulatory potential (Corzo et al., 2010).
Monitoring the capacity of tumor-derived MDSCs for granulocytic differentiation is more challenging due to the short life and sensitive nature of neutrophils, but polymorphonuclear/granulocytic MDSCs turn into bona fide neutrophils in vitro (Youn et al., 2012). The need to process fresh samples as soon as they are resected for the analysis of tumor-infiltrating neutrophils has limited our understanding of their activities in the TME. However, recent evidence indicates that neutrophils with an activated phenotype comprised 5-25% of cells isolated from freshly digested human lung tumors. Rather than impairing anti-tumor immunity, these cells were able to stimulate T cell responses in vitro (Eruslanov et al., 2014). Whether this only reflects the nature of the myeloid cells that accumulate at tumor beds at relatively early stages of malignant progression (those that are resectable) remains unknown.
As aforementioned, macrophages are the most abundant leukocyte subset in the microenvironment of most tumors, at virtually any stage of malignant progression. Macrophages are known to promote angiogenesis through the production of VEGF-A and by promoting tumor cell intravasation (Noy and Pollard, 2014). They are also, in general, driving immune privilege at tumor locations. Among the immunosuppressive mechanisms that macrophages promote in the TME, the secretion of IL-10 and TGF-β1, the expression of PD-L1 on their surface and the production of ARG1 have all been shown to be significant (Noy and Pollard, 2014). From the therapeutic point of view, however, macrophages offer great potential because their tumor-promoting phenotype can be transformed into cytotoxic activities that are relevant for tumor shrinking. The potential of CD40 agonists, for instance, has been recently underscored in patients and preclinical models of pancreatic cancer, where macrophages (but not T cells) were responsible for objective clinical responses by altering the stroma (Beatty et al., 2011).
Besides macrophages, another cell type frequently identified in the microenvironment of epithelial malignancies includes DCs, both conventional and plasmacytoid (Chiba et al., 2012; Huarte et al., 2008; Scarlett et al., 2009; Scarlett et al., 2012; Wei et al., 2005). Because a continuum of differentiation and conflicting differentiation signals complicates the categorization of myeloid populations at tumor beds, the identification of bona fide DCs vs. macrophage populations has been frequently challenged. In our hands, the most frequent leukocyte subset infiltrating ovarian carcinoma masses in mice and humans (but not in human tumor ascites or other tumor types) shows phenotypic attributes and functional activities of canonical DCs. In human solid ovarian tumors, for instance CD1c+MHC-II+CD19−CD11c+ leukocytes outnumber CD11c+CD1c− cells. These myeloid cells are able to suppress allogeneic T cell responses in the absence of treatment (unpublished observations), and are therefore highly immunosuppressive. Corresponding surface determinants and functional activities are identified in different ovarian cancer models (Huarte et al., 2008; Scarlett et al., 2012). However, synergistic activation of CD40 and TLRs, or restoration of immunostimulatory miRNAs using synthetic reagents transforms them into an immunostimulatory cell type that is able to up-regulate co-stimulatory molecules and effectively process and present antigens to T cells (Conejo-Garcia et al., 2004; Cubillos-Ruiz et al., 2012; Cubillos-Ruiz et al., 2010; Huarte et al., 2008; Rutkowski et al., 2012; Scarlett et al., 2009; Scarlett et al., 2012).
Further supporting the true dendritic nature of this abundant population, in both mouse and human tumors we confirmed that they co-express markers associated with the dendritic lineage. For instance, they show Zbtb46 mRNA levels significantly higher than splenic macrophages and significantly augmented compared to bone marrow-derived DCs in mice, where they also showed high expression of DNGR1/CLEC9A (Meredith et al., 2012; Schraml et al., 2013).
Independently of nomenclature, the identification of cells with the capacity to present antigens taken up in the TME has great potential for the design of therapeutic interventions that can both reverse immunosuppression, and boost anti-tumor immunity in vivo and in situ.
5. METASTATIC SPREADING AND THE METASTATIC NICHE
Dissemination and metastatic growth of tumor cells accounts for the majority of cancer-related deaths, events that often occur before the primary mass is detected. Metastasis is a multi-stage process involving intrinsic changes to the tumor cells and exogenous soluble and cell-mediated factors that aid in extravasation of the primary tumor into the peripheral circulation, seeding and survival at distal sites, and eventual metastatic growth (Fig. 1). Long-term survival of disseminated tumor cells within the metastatic niche is facilitated by tumor-derived cytokines and immune cells that have been mobilized from the primary tumor microenvironment, or from the expression of tissue-specific cytokines and growth factors.
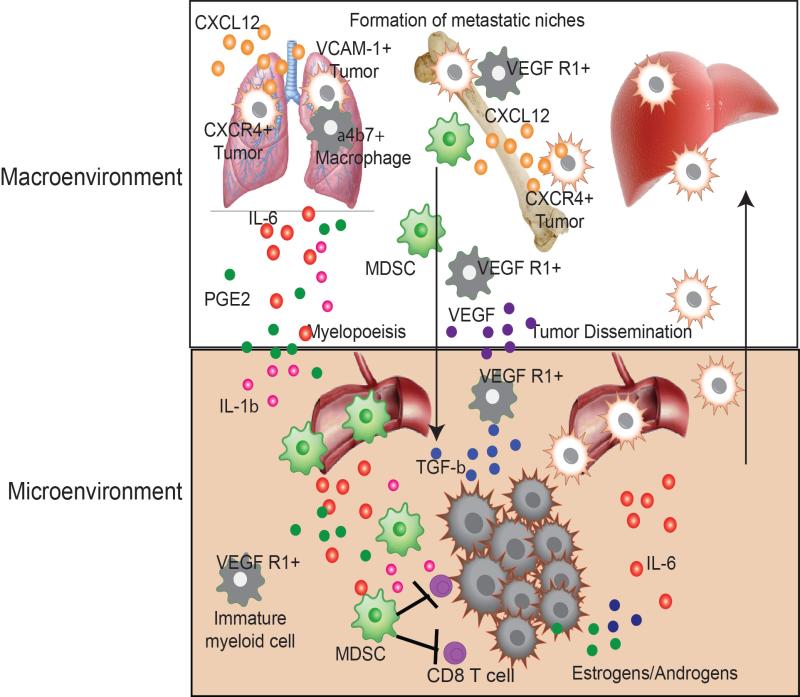
Figure 1. Crosstalk between the tumor macroenvironment and microenvironment result in dissemination and metastatic spread.
CXCR4 and VCAM-1 expressing tumor cells are recruited out of the tumor microenvironment and into the macroenvironment due high levels of CXCL12 expression in certain tissues. VCAM-1 helps tether tumor cells to tissue-associated macrophages which aide in the survival of tumor cells. VEGF R1 expressing immature myeloid cells are recruited out of the tumor environment by VEGF, TGFβ and IL-6, where they home to distal organs to set up a pre-metastatic niche for disseminated tumor cells. MDSC and immature myeloid cells are recruited from the bone marrow into the tumor microenvironment to impair effective anti-tumor immunity and aide tumor growth and dissemination.
5.1. Tumor-mediated influence on the pre-metastatic niche: Preparation for tumor seeding at distal sites
The Lynden and Rafii laboratories helped to establish the concept of a pre-metastatic niche orchestrated by hematopoietic cells prior to the formation and seeding of metastases, facilitating the survival and outgrowth of disseminated tumor cells (Kaplan et al., 2006; Kaplan et al., 2005; Psaila and Lyden, 2009; Sceneay et al., 2013). The original studies identified a population of VEGFR1+ immature myeloid cells and endothelial progenitors that were mobilized from the bone marrow in response to inflammatory signals that originated in the TME, such as VEGF-A, TGFβ and PlGF. Bone marrow-derived cells favorably homed to secondary organs such as the lung where proteins such as S100A8/9 and serum amyloid A3 (SAA3) are preferentially up-regulated, resulting in the establishment of an environment favoring growth and survival of metastatic tumor cells.
In patients with advanced colorectal cancer (CRC), high systemic levels of tissue inhibitor of metalloproteinases (TIMP)-1 are predictive of poor outcome (Holten-Andersen et al., 2000) and more metastatic disease (Seubert et al., 2015). Using mouse models, Seubert and colleagues found that in tumor-bearing mice with high systemic levels of TIMP-1, CXCL12 was increased in the liver, resulting in the recruitment of CXCR4-expressing neutrophils that establish a pre-metastatic niche for disseminated CRC cells (Seubert et al., 2015).
More recently, the secretion of exosomes by tumor cells has also been shown to play an important role in the formation of pre-metastatic niches. Tumor-derived exosomes enhance vascular leakiness by inducing a vasculogenic phenotype in bone marrow progenitor cells, essentially “educating” the bone marrow to support tumor dissemination (Peinado et al., 2012). The role of immunosuppressive mediators (in addition to activators of myelopoiesis) potentially contained in these exosomes deserves further investigation. Additionally, understanding the significance of exosome release and metastasis for other cancer types could potentially uncover novel therapies that either inhibit the release of exosomes from primary tumors or target the proteins that enable metastatic growth.
Together these studies have provided compelling data detailing mechanisms of tumor-induced metastatic spread and generation of the pre-metastatic niche in distal organs. However, the limitation to these studies is a lack of relevant pre-clinical animals that recapitulate the entire process of tumor dormancy and metastasis. Therefore, more clinical data is required to verify these events occur during the progression of malignant human disease (Keskinov and Shurin, 2015).
5.2. Tissue specific properties in the formation of metastatic niches
Metastatic cancers generally have a specific pattern of metastatic spread, with tropism for different tissues, indicating that tissue-specific factors may also be involved in the recruitment of disseminated tumor cells. Studies have shown that chemokine receptors CXCR4 and CCR7 are overexpressed in metastatic breast cancer cells while the respective ligands, CXCL12 and CCL21, are most abundantly expressed in the sites most commonly associated with breast cancer dissemination, such as the lymph nodes, lung, bone-marrow, and liver (Müller et al., 2001). CXCL12 is also involved with inducing neovascular growth (Jin et al., 2006), which results in the outgrowth of dormant tumor cells residing within the perivascular niche (Ghajar et al., 2013). These studies demonstrate that cytokines and chemokines present within the local tissue milieu also facilitate tumor dissemination and metastatic growth, uncovering a mechanism that explains the tropism of certain tumors for specific organ sites.
Pro-survival and growth signals on tumor cells have also been shown to be mediated by adhesion between tissue resident myeloid cells and disseminated tumor cells within each metastatic niche. For example, disseminated breast cancer cells in both the lungs and bone marrow utilize VCAM-1-mediated binding to intergrins on tissue-resident myeloid cells to survive in distal sites. Aberrant VCAM-1 overexpression on breast tumor cells in the bone marrow promotes the recruitment and activation of osteoclast progenitors, accelerating growth of breast tumor cells within the bone marrow (Lu et al., 2011). In the lungs, another niche involved in breast tumor metastasis, metastasized VCAM-1 overexpressing breast tumor cells tether themselves to lung macrophages through binding of α4β1 intergrins, resulting in the activation of pro-survival PI3K/AKT signaling (Chen et al., 2011). VCAM-1 mediated tumor-induced adhesion with myeloid cells in environments associated with metastatic spread may explain the high VCAM-1 expression in invasive breast tumor cell lines (Lu et al., 2011) and resected metastatic human tumor specimens (Minn et al., 2007).
Together, the primary tumor microenvironment and local tissue environment coordinate enrichment of pro-survival signals, allowing for survival of disseminated tumor cells. When disseminated tumor cells encounter these locations enriched in inflammatory and pro-angiogenic cells types, a niche is formed where tumor cells initiate the orchestration of a more complex tissue microenvironment where they will eventually form macroscopic masses. Targeted therapies inhibiting the soluble factors or cells that aide in supporting these events will provide novel treatment strategies for the control of highly metastatic cancers. For example, targeting the CXCL12/CXCR4 pathway has already been shown to reduce metastatic spread of prostate cancers (Wong et al., 2014), the recurrence of glioblastomas (Tseng et al., 2011), dissemination of tumor cells and immunosuppression in ovarian cancer (Gil et al., 2014), and breast tumor metastasis (Gil et al., 2013).
6. ROLE OF THE MICROBIOTA IN TUMOR PROGRESSION
The skin, respiratory tract, genitourinary tract and the gastrointestional tract are colonized by billions of microorganisms, comprised of viruses, fungi, protozoan, and predominantly bacteria. Commensal microbiota provide a first line of defense against invading pathogens, maintain homeostasis at mucosal surfaces, and aid in digestion. However, the microbiota also have an important role in the development and function of the immune system (Arpaia et al., 2013; Chappert et al., 2013; Duan et al., 2010; Furusawa et al., 2013; Ivanov et al., 2008; Wei et al., 2010) as well as influencing the activation of innate immune cells within the periphery during pathogenic infection (Abt et al., 2012; Clarke et al., 2010; Ichinohe et al., 2011).
Dysbiosis is an altered balance in the composition of the microbiota, resulting in dysregulated immune function and excess inflammation. When this occurs within the intestines, a cascade of events is initiated that may eventually lead to malignant growth. Although the link between dysregulation of commensal microbiota and colonic inflammation and cancer has been well established (Arthur et al., 2012; Hu et al., 2013), until recently, very few studies had addressed the role of the microbiota for tumors occurring at distal extra-intestinal locations.
6.1. Commensal microbiota are required for effective anti-tumor immune responses for extra-intestinal tumors
Two seminal studies demonstrated that the commensal microbiota were required for immune-mediated control of extra-intestinal tumors (Iida et al., 2013; Viaud et al., 2013). In mice bearing MC38 carcinoma, EL4 lymphoma, and B16 melanoma the commensal microbiota were required for myeloid cell production of TNFα during intratumoral immunotherapy with CpG-oligodeoxynucleotides (CpG-ODN) and inhibition of IL-10 signaling (Iida et al., 2013), an immunotherapeutic combination that has been shown to reverse dendritic cell immune suppression within the tumor microenvironment (Vicari et al., 2002). Direct or indirect activation of dendritic cells through TLR4-mediated interactions with the commensal microbiota primed the induction of TNFα production in response to TLR9 immunotherapy. The anti-tumor and immunogenic effects of oxaliplatin treatment were also dependent upon the commensal microbiota to induce the generation of ROS from myeloid cells (Iida et al., 2013).
Treatment using cyclophosphamide, an anticancer agent that elicits a therapeutic response by inducing an immunogenic cell death in tumor cells (Kroemer et al., 2012) was also shown to require the commensal microbiota (Viaud et al., 2013). Mechanistically, cyclophosphamide decreased the epithelial integrity of the intestinal mucosa, resulting in translocation of gram-positive microbiota to secondary lymph organs and the conversion of IL-17 producing pathogenic Th1 CD4 T cells and enhancement of memory Th1 responses (Viaud et al., 2013).
These studies were among the first to demonstrate that the commensal microbiota were indispensable for the establishment of effective immune responses during immunotherapy and immunogenic chemotherapy of cancers occurring outside of the intestines. Commensal-mediated differences in immune responses to each therapy was not “one size fits all”, indicating both treatment and context-dependent differences influenced priming and activation of the innate and adaptive immune system. These two studies also highlighted the role of specific microbial genera in controlling immune function. The Lactobacillus genus negatively correlated with TLR4-induced TNFα production while Ruminococcus genera positively correlated with the production of TNFα and the priming of dendritic cells (Iida et al., 2013). In cyclophosphamide treated mice, Lactobacilli and segmented filamentous bacteria (SFB), or Candidatus Savagella (Thompson et al., 2012), were shown to be positively associated with inducing pathogenic Th17 cells in peripheral lymph organs (Viaud et al., 2013).
6.2. The role of microbe-induced inflammation during malignant progression
Certain chemotherapies and cancer treatments suppress the immune system, creating a risk for individuals undergoing treatment to develop infections with opportunistic pathogens. Acute inflammation driven by infection with opportunistic pathogens has been suggested to promote metastasis to distal organ sites. LPS-delivery or infection in the lungs enhances chemotaxis of CXCR4 expressing tumor cells due to the induction of ubiquitins in the pulmonary epithelium (Yan et al., 2013), suggesting that treatment of cancer-bearing patients with antibiotics to prevent infection with opportunistic bacteria may result in a lower rate of metastasis. The caveat of this study is that certain antibiotics may eliminate commensal microbial species that facilitate anti-tumoral immune responses. The notion that acute inflammation, driven by pathogenic microorganisms, can drive metastasis or induce the formation of pre-metastatic niches warrants further studies and careful consideration of how treatment with antibiotics will alter the composition of the commensal microbiota in tumor-bearing hosts. Additionally, separate studies have demonstrated that inflammation induced by intratumoral injection of avirulent microorganisms, such as Toxoplasma gondii, induces a therapeutic benefit for the treatment of ovarian cancer and melanoma (Baird et al., 2013a; Baird et al., 2013b), demonstrating that for certain cancers acute inflammation can be beneficial to the host.
The composition of the commensal microbiota is influenced by common genetic polymoprhisms in pattern recognition receptors, resulting in systemic inflammation that affects the initiation and progression of tumors occurring outside of the intestinal tract. Homozygous and heterozygous carriers of a deleterious polymorphism in TLR5 (TLR5R392X) (Hawn et al., 2003) have increased long-term survival after an initial ovarian cancer diagnosis, but a reduced survival when diagnosed with luminal breast cancer (Rutkowski et al., 2015). Rutkowski et al. demonstrated that TLR5-mediated interactions with commensal microbiota increased systemic levels of IL-6, resulting in the recruitment of MDSCs into the tumor environment, and the induction of galectin-1 expression in tumor-associated γδ T cells, abrogating effective anti-tumor immunity. IL-6 driven immune suppression was only observed in TLR5+ animals bearing IL-6 responsive tumors (e.g. ovarian cancer, sarcoma), whereas IL-6 non-responsive tumors (e.g. breast tumors) grew significantly slower compared to TLR5−/− mice (Rutkowski et al., 2015). Importantly, differences observed in tumor progression required the commensal microbiota, as antibiotic depletion eliminated all TLR5-mediated differences in tumor progression.
In the absence of TLR5 signaling, TLR5−/− mice maintained significant differences in the composition of major species of commensal microbiota, despite prolonged co-housing, commonly used to equilibrate differences in the microbiota (Ivanov et al., 2008). In the presence of a tumor, a significant increase in the systemic levels of IL-17 was observed in TLR5-deficient individuals, and in the absence of IL-6, tumor progression was driven by IL-17 (Rutkowski et al., 2015). This study helped to clarify the controversial role of IL-17 in tumor progression, suggesting that IL-17 has a tumor-promoting effect only in the absence of IL-6. Human studies in the literature support this notion. In ovarian cancer, high IL-17 levels have been reported to associate with a positive prognosis (Kryczek et al., 2009) whereas in luminal breast cancer, tumors that have low levels of IL-6 (Hartman et al., 2013), IL-17 is associated with a poor prognosis (Chen et al., 2013). The production of IL-17 depends upon the commensal microbiota (Ivanov et al., 2008), demonstrating that these differences in tumor progression are driven by TLR5-mediated recognition of commensal microbes. This study implicated that common genetic polymorphisms in pattern recognition receptors, that are present at a high frequency within the general population (Casanova et al., 2011), have an important influence on tumor progression and survival outcome.
It is becoming increasingly clear that the microbiota influence systemic immunity during the progression of cancer (Fig. 2). What is now being appreciated is that differences in microbial composition result in systemic differences in the production of certain cytokines, such as TNFα, IL-6, and IL-17, which ultimately influence the immune environment and tumor growth. Genetic variation and the composition of the commensal microbiota will be important to consider for personalized treatment of cancer. Additionally, future studies that explore interventions that modify or reshape the microbiota with certain genera associated with the production of specific cytokines would enable the development of approaches that modify the systemic immune system to treat cancer.
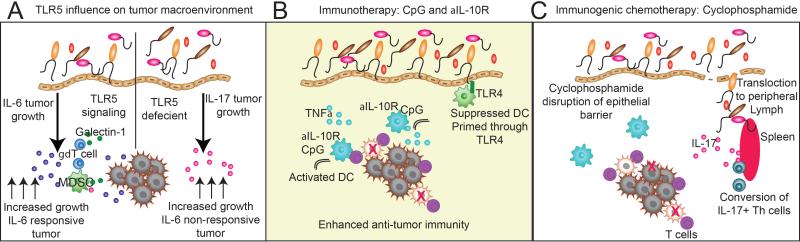
Figure 2. Commensal microbiota differentially influence the tumor environment and immunotherapy.
A) The influence of TLR5 expression on tumor progression. TLR5 responsive hosts have accelerated tumor progression in the presence of an IL-6 responsive tumor, resulting in increased levels of IL-6, increased recruitment of MDSCs into the tumor environment, and the induction of galectin-1 expression in tumor-associated γδ T cells. TLR5-deficient hosts have elevated levels of IL-17, which in the absence of IL-6, drives tumor progression. B) Commensal microbiota are required for effective immunotherapy with CpG and anti-IL-10R antibody treatment to reverse the immune suppression of tumor-associated DCs. The commensal microbiota prime DCs through TLR4 interaction, resulting in production of TNFα in response to CpG treatment. This results in conversion of suppressed DCs into immunostimulatory DCs, which elicit anti-tumor immunity. C) The effects of cyclophosphamide are mediated through the microbiota. Cyclophosphamide disrupts the mucosal surface, allowing for bacterial translocation to distal lymph organs, such as the spleen. This results in the conversion of IL-17 producing Th cells, which enhance anti-tumor immune response.
7. DISTALLY PRODUCED HORMONES INFLUENCE TUMOR PROGRESSION
The endocrine system contributes significantly to the host macroenvironment by affecting a wide variety of biological functions, such as homeostasis, development, and inflammation. Hormonal changes, either from exogenous or endogenous sources, are associated with several diseases including certain cancers. In addition to having a direct impact on tumor cell growth for hormone-dependent cancers, hormones have been hypothesized to indirectly impact tumors by influencing host stromal responses. Currently, anti-hormonal therapies are used as adjuvant therapy only in hormone-dependent cancers, such as estrogen receptor (ER) positive breast cancer (Burstein et al., 2014) and androgen receptor (AR) positive prostate cancer (Loblaw et al., 2004); however, due to their pleiotropic effects on the host macroenvironment, endocrine therapies represent potential novel avenues for therapy in non-hormone dependent cancers.
7.1. Estrogens and the tumor macroenvironment (Estrone, Estradiol, and Estriol)
Estrogens, produced mainly by the ovaries and adipose tissue in non-gravid women, play a major role in female sexual development and health. Consequently, they have been implicated most strongly in the gynecological malignancies: breast, ovarian, and endometrial cancer. The Women's Health Initiative and Million Women Study found that women placed on combined estrogen-progesterone (EPT) replacement therapy are at an increased risk of breast cancer which is sustained approximately three years following termination of EPT (Beral, 2003; Rossouw et al., 2002). Additionally, elevated serum levels of estradiol in women without hormone therapy is associated with increased risk of ER-positive, but not ER-negative, breast cancer (Farhat et al., 2011). The Million Women Study found that women who were placed on post-menopausal estrogen replacement therapy were found to be at higher risk of developing ovarian cancer (Beral et al., 2007), although this association is less clear when estrogen therapy is combined with progesterone (Anderson et al., 2003). Estrogen therapy alone is also known to increase the risk of endometrial cancer; however, this risk is mitigated when combined with progesterone (Weiderpass et al., 1999).
Canonically, estrogens directly regulate cell transcription via the steroid hormone receptors ERα and β (Nilsson et al., 2001). Upon ligand binding, ER's dimerize and translocate into the cell nucleus where they recognize estrogen response elements in gene promoters and enhancers. Depending on specific coregulators present within the nucleus, ER binding can activate or repress gene expression. ER can also form complexes with other transcription factors, such as AP-1 (Jakacka et al., 2001) and NF-κB (Galien and Garcia, 1997), thus directly affecting their activity in multiple cell types, including immune cells.
Estrogen signaling plays an important role in cancer by directly affecting ER-positive tumor cells. In vitro and in vivo studies using ER-positive breast tumor cells have shown that estradiol drives proliferation by inducing cell cycle progression via cyclin D1 (Hamelers et al., 2003). Treating breast cancer cells with the selective estrogen receptor modulator (SERM) tamoxifen results in cell cycle arrest and delayed tumor progression in vivo (Nunez et al., 2004). Clinically, SERMs used in ER-positive breast cancer patients have been shown to increase disease free survival and decrease mortality (Swaby et al., 2007). In ovarian cancer, the effect of estrogens on cell proliferation is less clear and seems to depend on the expression of the different estrogen receptors. ERα drives proliferation (Bossard et al., 2012) while ERβ and GPER are inhibitory (Bossard et al., 2012; Ignatov et al., 2013). Consequently, ovarian clinical trials using anti-estrogen therapy have failed to deliver the same benefits demonstrated in ER-positive breast cancer (Argenta et al., 2009). Endometrial tissue growth is driven by estrogen signaling (Groothuis et al., 2007), which may be a major contributing factor in type 1 endometrial tumorigenesis. Because tamoxifen acts as an agonist in endometrial tissue (Diel, 2002) other anti-estrogen therapies, such as aromatase inhibitors, have been used to treat pre-metastatic endometrial tumors; however, this anti-estrogen therapy results in relatively low response rates (Lindemann et al., 2014).
Due to the wide expression of ER's on cells comprising the tumor stroma, it has been hypothesized that estrogens can impact tumor growth independently of tumor cells. In a mouse xenograft model using ER-negative patient-derived tumor cells, it was shown that ERα-positive bone marrow cells were required for estrogen-triggered enhancement of tumor implantation and growth (Iyer et al., 2012). These cells appeared to contribute to the formation of tumor vasculature. Other studies have also implicated estrogen signaling in tumor vasculogenesis (George et al., 2012). In a model of liver cancer, estradiol was shown to suppress tumor growth by reducing IL-6 production by macrophages in response to chemical carcinogen induced liver damage (Naugler et al., 2007).
7.2. Androgens in the tumor macroenvironment (Testosterone and Dihydrotestosterone)
Exposure to androgens has long been associated with prostate cancer (Herger and Sauer, 1947). Testosterone upon recognition by androgen receptor (AR) induces initiation and progression of prostate cancer by stimulating tumor cell proliferation and inhibiting apoptosis (Green et al., 2012). Androgen deprivation therapy has been established as an effective treatment for advanced prostate cancer by inducing prostate cancer cell death (Feldman and Feldman, 2001). However, prostate cancer eventually recurs as castration resistant disease that relies upon alternative mechanisms for AR activation (Sharifi, 2013).
Besides direct effect on tumor cells, androgens have been shown to promote angiogenesis. Exposure to testosterone increased angiogenesis by selectively stimulating the AR in male endothelial cells in vitro and in vivo (Sieveking et al., 2010). This could further support progression and dissemination of cancer cells. High androgen levels may also help tumor promotion and progression by impairing the function of the antitumor immune response. Testosterone impairs Th1 differentiation by up-regulation of Ptpn1 (Kissick et al., 2014). Furthermore, orchiectomy has been shown to increase intratumoral infiltration of M1 macrophages and CD8α T cells using a mouse model of thyroid cancer (Zhang et al., 2015).
7.3. Insulin and Insulin-like Growth Factor-I (IGF-I) in the tumor macroenvironment
Other hormones that impact tumor initiation and progression are insulin and IGF-I. Epidemiological studies found a higher overall risk of cancer in patients who were on insulin (Hemkens et al., 2009). Besides increasing IGF-I secretion, insulin has been implicated in tumorigenesis by accelerating tumor growth both in vitro and in mouse models of type 2 diabetes (Fierz et al., 2010; Novosyadlyy et al., 2010; Zhang et al., 2010).
The role of IGF-I is clearly established in different types of cancer, such as breast, prostate and colorectal (Chan et al., 1998; Hankinson et al., 1998; Ma et al., 1999). Patients with acromegaly have an increase in IGF-I secondary to a Growth Hormone hypersecretion, which is associated with an increased risk of colorectal cancer (Melmed, 2009). Decrease of IGF-I by caloric restriction or using a liver-specific IGF-I-deficient mice model results in protection against tumors, which can be reversed by the administration of IGF-I (Dunn et al., 1997; Wu et al., 2003; Wu et al., 2002).
8. CONCLUSIONS
Understanding of the intrinsic mutational or immunosuppressive events that result in malignant progression have aided in the development of targeted therapies that have enhanced the survival and quality of life for many patients with metastatic disease. Although many studies have uncovered mechanisms utilized by tumor cells to escape certain chemotherapies or immunotherapies, the systemic burden of cancer remains an indelible consequence for the outcome of current therapies. There is a trend towards utilizing large-scale screening techniques to choose drug combinations that exploit genetic differences in individual patient tumors to identify optimal drug sensitivity (Barretina et al., 2012; Garnett et al., 2012). However, utilizing large-scale genomic screening approaches have drawn criticism (Haibe-Kains et al., 2013) as there is no account for somatic genetic differences or systemic differences in immune or microbial composition that influence the bioactivity of the drug in humans.
Personalized cancer therapy will thus have to enter into a new era, where not only genetic and immune profiles of tumor cells will be considered, in addition to considering the factors that influence the tumor macroenvironment to effectively develop therapies that have maximal effectiveness with minimal toxicity. For example, inhibitors targeting the PI3K and MEK signaling pathways, pathways utilized by many cells within the body, can have unintended effects on malignant progression, due to their influence on the immune system or epithelial integrity of the mucosal surfaces. Cancer is an inflammatory disease, indicating that therapeutic combinations should be devised that not only target the tumor cell, but also account for the influence of the macroenvironment in malignant progression and metastatic disease.
ACKNOWLEDGMENTS
This study was supported by R01CA157664, R01CA124515, R01CA178687, U54CA151662, P30CA10815, and Ovarian Cancer Research Fund (OCRF) Program Project Development awards. NS was supported by T32CA009171. APP was supported by the Ann Schreiber Mentored Investigator Award (OCRF).
Abbreviations
- MDSC
Myeloid-derived Suppressor Cell
- DC
Dendritic Cell
- TME
Tumor Microenvironment
- PMN
Polymorphonuclear
- RAGE
Receptor for advanced glycation end-products
- VEGF-A
Vascular Endothelial Growth Factor
- TGFβ
Transforming Growth Factor beta
- PlGF
Placental Growth Factor
- PMN
Polymorphonuclear cell
- ROS
Reactive Oxygen Species
- RNS
Reactive Nitrogen Species
- ARG1
Arginase
- CRC
Colorectal Cancer
- TIMP-1
Tissue inhibitor of metalloproteinases 1
- SAA3
Serum Amyloid A3
- CpG-ODN
CpG-oligodeoxynucleotides
- TLR
Toll-like receptor
- LPS
Lipopolysaccharide
- ER
Estrogen Receptor
- AR
Androgen Receptor
- EPT
Estrogen-progesterone replacement therapy
- SERM
Selective estrogen receptor modulator
REFERENCES
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA. 2003;290:1739–1748. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- Argenta PA, Thomas SG, Judson PL, Downs LS, Jr., Geller MA, Carson LF, Jonson AL, Ghebre R. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol. 2009;113:205–209. doi: 10.1016/j.ygyno.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013 doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JR, Byrne KT, Lizotte PH, Toraya-Brown S, Scarlett UK, Alexander MP, Sheen MR, Fox BA, Bzik DJ, Bosenberg M, et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013a;190:469–478. doi: 10.4049/jimmunol.1201209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JR, Fox BA, Sanders KL, Lizotte PH, Cubillos-Ruiz JR, Scarlett UK, Rutkowski MR, Conejo-Garcia JR, Fiering S, Bzik DJ. Avirulent Toxoplasma gondii generates therapeutic antitumor immunity by reversing immunosuppression in the ovarian cancer microenvironment. Cancer Res. 2013b;73:3842–3851. doi: 10.1158/0008-5472.CAN-12-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Beral V, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- Bossard C, Busson M, Vindrieux D, Gaudin F, Machelon V, Brigitte M, Jacquard C, Pillon A, Balaguer P, Balabanian K, Lazennec G. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS One. 2012;7:e44787. doi: 10.1371/journal.pone.0044787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky AJ, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- Chappert P, Bouladoux N, Naik S, Schwartz RH. Specific gut commensal flora locally alters T cell tuning to endogenous ligands. Immunity. 2013;38:1198–1210. doi: 10.1016/j.immuni.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature medicine. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide RH, English NR, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–2639. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, et al. Reprogramming tumor-associated dendritic cells in vivo using microRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Martinez D, Scarlett UK, Rutkowski MR, Nesbeth YC, Camposeco-Jacobs AL, Conejo-Garcia JR. CD277 is a Negative Co-stimulatory Molecule Universally Expressed by Ovarian Cancer Microenvironmental Cells. Oncotarget. 2010;1:329–328. doi: 10.18632/oncotarget.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol Lett. 2002;127:217–224. doi: 10.1016/s0378-4274(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell host & microbe. 2010;7:140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer research. 1997;57:4667–4672. [PubMed] [Google Scholar]
- Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. J Leukoc Biol. 2010;88:839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat GN, Cummings SR, Chlebowski RT, Parimi N, Cauley JA, Rohan TE, Huang AJ, Vitolins M, Hubbell FA, Manson JE, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103:562–570. doi: 10.1093/jnci/djr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren MR, Carlson LM, Netherby CS, Lindner I, Li PK, Gabrilovich DI, Abrams SI, Lee KP. Tumor-induced STAT3 signaling in myeloid cells impairs dendritic cell generation by decreasing PKCbetaII abundance. Sci Signal. 2014;7:ra16. doi: 10.1126/scisignal.2004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature reviews Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes. 2010;59:686–693. doi: 10.2337/db09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Wang SC, Morris J. P. t., Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013 doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res. 1997;25:2424–2429. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, Greninger P, Thompson IR, Luo X, Soares J, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AL, Rajoria S, Suriano R, Mittleman A, Tiwari RK. Hypoxia and estrogen are functionally equivalent in breast cancer-endothelial cell interdependence. Mol Cancer. 2012;11:80. doi: 10.1186/1476-4598-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H. c., Mori H, Matei IR, Evason KJ, Brazier H. l. n., Almeida D, Koller A, Hajjar KA, Stainier DY, et al. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, Komorowski MP, Seshadri M, Rokita H, McGray AJ, Opyrchal M, Odunsi KO, Kozbor D. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J Immunol. 2014;193:5327–5337. doi: 10.4049/jimmunol.1400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110:E1291–1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SM, Mostaghel EA, Nelson PS. Androgen action and metabolism in prostate cancer. Molecular and cellular endocrinology. 2012;360:3–13. doi: 10.1016/j.mce.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis PG, Dassen HH, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13:405–417. doi: 10.1093/humupd/dmm009. [DOI] [PubMed] [Google Scholar]
- Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18:5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibe-Kains B, El-Hachem N, Birkbak NJ, Jin AC, Beck AH, Aerts HJ, Quackenbush J. Inconsistency in large pharmacogenomic studies. Nature. 2013;504:389–393. doi: 10.1038/nature12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelers IH, Van Schaik RF, Sussenbach JS, Steenbergh PH. 17beta-Estradiol responsiveness of MCF-7 laboratory strains is dependent on an autocrine signal activating the IGF type I receptor. Cancer Cell Int. 2003;3:10. doi: 10.1186/1475-2867-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck SG, Mills GB, Brown PH. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer research. 2013;73:3470–3480. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. The Journal of experimental medicine. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herger CC, Sauer HR. A consideration of the effect of androgen control treatment of carcinoma of the prostate. New York state journal of medicine. 1947;47:494–501. [PubMed] [Google Scholar]
- Holten-Andersen MN, Stephens RW, Nielsen HJ, Murphy G, Christensen IJ, Stetler-Stevenson W, Brunner N. High preoperative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin Cancer Res. 2000;6:4292–4299. [PubMed] [Google Scholar]
- Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F, Stan RV, Keler T, Sarobe P, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki AC. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatov T, Modl S, Thulig M, Weissenborn C, Treeck O, Ortmann O, Zenclussen A, Costa SD, Kalinski T, Ignatov A. GPER-1 acts as a tumor suppressor in ovarian cancer. J Ovarian Res. 2013;6:51. doi: 10.1186/1757-2215-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (New York, NY) 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Frutos R. d. L., Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DRC. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, Zhang X, Lewis MT, Kuperwasser C. Estrogen promotes ER-negative tumor growth and angiogenesis through mobilization of bone marrow-derived monocytes. Cancer Res. 2012;72:2705–2713. doi: 10.1158/0008-5472.CAN-11-3287. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. 2015;220:236–242. doi: 10.1016/j.imbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2012;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Lindemann K, Malander S, Christensen RD, Mirza MR, Kristensen GB, Aavall-Lundqvist E, Vergote I, Rosenberg P, Boman K, Nordstrom B. Examestane in advanced or recurrent endometrial carcinoma: a prospective phase II study by the Nordic Society of Gynecologic Oncology (NSGO). BMC Cancer. 2014;14:68. doi: 10.1186/1471-2407-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben-Josef E, Middleton R, Porterfield H, Sharp SA, Smith TJ, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22:2927–2941. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Haffty BG, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. Journal of the National Cancer Institute. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Melmed S. Acromegaly pathogenesis and treatment. The Journal of clinical investigation. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Padua D, Bos P. Lung metastasis genes couple breast tumor size and metastatic spread. Proceedings of the, Ķ. 2007 doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich DI. Regulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cells. Semin Cancer Biol. 2012;22:282–288. doi: 10.1016/j.semcancer.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res. 2009;30:7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi PA, Molinolo AA, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer research. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez NP, Jelovac D, Macedo L, Berrigan D, Perkins SN, Hursting SD, Barrett JC, Brodie A. Effects of the antiestrogen tamoxifen and the aromatase inhibitor letrozole on serum hormones and bone characteristics in a preclinical tumor model for breast cancer. Clin Cancer Res. 2004;10:5375–5380. doi: 10.1158/1078-0432.CCR-04-0261. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran IR, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J, Gabrilovich D, Nefedova Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol. 2013;190:3815–3823. doi: 10.4049/jimmunol.1203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rutkowski MR, Stephen TL, Conejo-Garcia JR. Anti-tumor immunity: myeloid leukocytes control the immune landscape. Cell Immunol. 2012;278:21–26. doi: 10.1016/j.cellimm.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, Rogers NC, Moncaut N, Carvajal JJ, Reis e Sousa C. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell. 2013;154:843–858. doi: 10.1016/j.cell.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Seubert B, Grunwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N, et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61:238–248. doi: 10.1002/hep.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N. Mechanisms of androgen receptor activation in castration-resistant prostate cancer. Endocrinology. 2013;154:4010–4017. doi: 10.1210/en.2013-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieveking DP, Lim P, Chow RW, Dunn LL, Bao S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS, Ng MK. A sex-specific role for androgens in angiogenesis. The Journal of experimental medicine. 2010;207:345–352. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaby RF, Sharma CG, Jordan VC. SERMs for the treatment and prevention of breast cancer. Rev Endocr Metab Disord. 2007;8:229–239. doi: 10.1007/s11154-007-9034-4. [DOI] [PubMed] [Google Scholar]
- Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Vier R, Mikaelyan A, Wienemann T, Brune A. ‘Candidatus Arthromitus’ revised: segmented filamentous bacteria in arthropod guts are members of Lachnospiraceae. Environ Microbiol. 2012;14:1454–1465. doi: 10.1111/j.1462-2920.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (New York, NY) 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, Borneman J, Kronenberg M, Braun JC. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. Journal of immunology (Baltimore, Md : 1950) 2010;184:1218–1226. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- Weiderpass E, Adami HO, Baron JA, Magnusson C, Bergstrom R, Lindgren A, Correia N, Persson I. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91:1131–1137. doi: 10.1093/jnci/91.13.1131. [DOI] [PubMed] [Google Scholar]
- Wong D, Kandagatla P, Korz W, Chinni SR. Targeting CXCR4 with CTCE-9908 inhibits prostate tumor metastasis. BMC Urol. 2014;14:12. doi: 10.1186/1471-2490-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer research. 2003;63:4384–4388. [PubMed] [Google Scholar]
- Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer research. 2002;62:1030–1035. [PubMed] [Google Scholar]
- Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res. 2013;19:4706–4716. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fagan DH, Zeng X, Freeman KT, Sachdev D, Yee D. Inhibition of cancer cell proliferation and metastasis by insulin receptor downregulation. Oncogene. 2010;29:2517–2527. doi: 10.1038/onc.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LJ, Xiong Y, Nilubol N, He M, Bommareddi S, Zhu X, Jia L, Xiao Z, Park JW, Xu X, et al. Testosterone regulates thyroid cancer progression by modifying tumor suppressor-genes and tumor immunity. Carcinogenesis. 2015 doi: 10.1093/carcin/bgv001. [DOI] [PMC free article] [PubMed] [Google Scholar]