Detection of de novo complex chromosomal rearrangements (CCR) in prenatal testing is extremely rare. CCRs are defined as constitutional structural rearrangements involving three or more chromosomes or more than three breakpoints. A survey of 269,371 prenatal studies1 detected only 0.03% complex rearrangements out of 246 that were determined to be de novo. Recent whole-genome sequencing studies using large-insert jumping libraries have found that cryptic complexity, particularly cryptic inversions, often occurs at the breakpoints and in some cases can introduce a degree of complexity as significant as ‘chromothripsis’ to events that appear to be canonical rearrangements at karyotypic resolution2. The term chromothripsis was initially coined by Stephens et al.3 to explain the mechanism involved in massive chromosomal rearrangements in cancers. Once defined, the concept was widely adopted to help explain complex rearrangements in the germline 4,2. All reported chromothripsis rearrangements share several features in common, including: a) The occurrence of a single catastrophic genomic event resulting in chromosome shattering. The shattered pieces contain double stranded breaks that are reassembled into mosaic chromosomes. b) The reassembly of the majority of fragments of DNA in what appears to be random fashion with little sequence homology at the breakpoints. c) Unique to congenital chromothripsis, the lack of major duplications and deletions during reassembly. Most genomic changes detected in the germline are copy neutral or span only a few base pairs, lacking the frequent larger deletions and duplications observed in cancer chromothripsis. This is likely due to selection in these cases for fetal viability. There is also growing evidence that chromothripsis occurs mainly in spermatogenesis. For reviews, see Kloosterman et. al. 20114, 20125, 20136 and Pellestor, 20147.
We report a prenatal case initially diagnosed by karyotyping as a CCR with 6 breakpoints, 5 chromosomes involved in a four-way translocation and a separate two-way translocation. The p and q arms of the same chromosome 18 were involved in distinct translocations. Further analysis by whole-genome sequencing showed that two of the breakpoints were more complex than seen by karyotype, giving a total of 9 breakpoints in 5 chromosomes. Small < 1000 bp deletions or duplications were detected at these breakpoints, which interrupted 7 genes. We believe this case fits the criteria for chromothripsis.
A 28 year old woman in her first pregnancy presented for amniocentesis sampling at 21 weeks gestation. Ultrasound and MRI revealed bilateral ventriculomegaly (13mm and 15mm) and colpocephaly, with partial agenesis of the corpus callosum. The prior family history was unremarkable with no unusual environmental exposures known to the mother or father upon questioning.
The initial FISH analysis with AneuVysion (Abbott), suggested a normal female. However, the pregnancy was terminated at 22 weeks due to the ultrasound findings. Cytogenetic and FISH analysis with telomere probes on amniocytes harvested post termination revealed a 46,XX,t(3;18;5;7)(p25;p11.2;q13.3;q32),t(9;18)(p22;q21) karyotype in all cells examined. SNP oligonucleotide microarray analysis (Affymetrix Cytoscan HD) on fetal DNA showed no loss or gain of chromosomal material at any of the breakpoints. This unusual complex karyotype was confirmed in fetal kidney cells. Chromosomes from both parents were normal. Fetal genomic DNA was accessioned in the Developmental Genome Anatomy Project as case DGAP259. Next generation sequencing of fetal genomic DNA using large-insert jumping libraries at ~3 kb resolution, followed by PCR and Sanger validation, resolved 5 of the putative breaks. In addition to the 6 visible breakpoints, a 184.5kb cryptic inversion at the chr3/chr18 junction on the p arm of the derivative 18 was identified and the sixth break point on the derivative 5 was found to be more complex, involving the insertion of small portions of chromosomes 3 and 7 at the chr5/chr18 junction (Figure 1). The breakpoints were refined to 3p24.3, 3p26.3; 5q14.3; 7q35, 7q36.3 and inv(18p11.31p11.31). The formula for the 4 chromosome translocation was thus revised as: t(3;18;5;7)(7qtel→7q36.3::3p24.3→3qter;3pter→3p26.3::18p11.31p11.31::18p11.31→18q2 1.31::9p23→9qter;5pter→5q14.3::7q35→7q36.3::3p24.3→3p26.3::18p11.31→18pter;7pter →7q35::5q14.3-5qter). The two-chromosome translocation was rewritten as t(9;18)(p23;q21.31).
Figure 1.
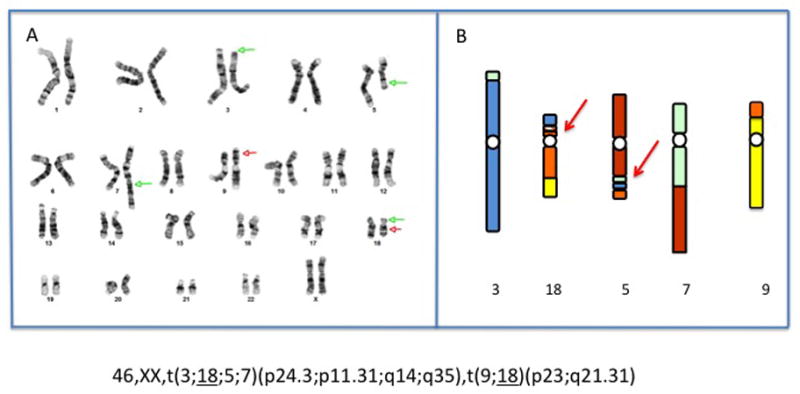
A. G-banded karyotype from kidney culture of the terminated fetus. Arrows indicate the 6 visible breakpoints. B. Diagram of the complex rearrangement after information from whole genome sequencing. The arrows indicate areas of complexity in 19p and 5q not seen by karyotype. The inversion at 18p11.31 is represented by slanted lines.
Using the new nomenclature for sequenced breakpoints proposed by Ordulu et al.8, this would be written as:
“46,XX,t(3;18;5;7)(p25;p11.2;q13.3;q32),t(9;18)(p22;q21)dn.seq[GRCh37/hg19](3,5,7,9,18)cx,der(3)(7qter->7q36.3(155,701,797)::3p24.3(17,392,144)->3qter) dn,der(5)(5pter->5q14.3(88,756,2{48-56})::7q35q36.3(147,718,91{1-9}-155,700,873)::AGAAC::3p24.3p26.3(17,392,136-1,408,99{6})::18p11.31(6,375,05{1})->1 8pter)dn,der(7)(7pter->7q35(147,718,90{7-8})::5q14.3(88,756,2{39-40})->5qte r)dn,der(9)(18qter->18q21.31(54,660,13{8})::9p23(9,646,47{5})->9qter)dn,der (18)(3pter->3p26.3(1,408,984)::18p11.31(6,559,611-6,375,0{52-48})::18p11.31 q21.31(6,559,{598-602}-54,660,136)::9p23(9,646,471)->9pter)dn”
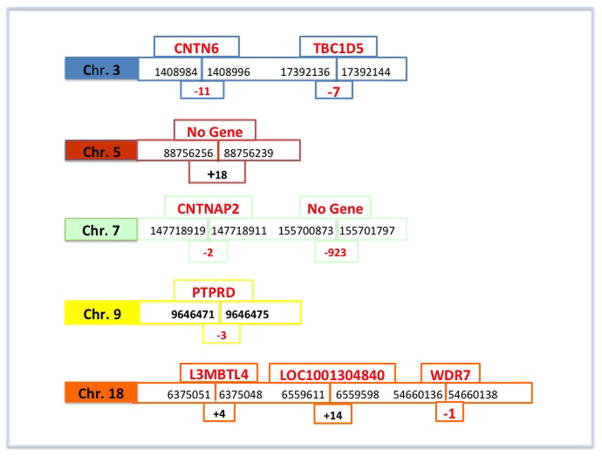
Seven OMIM annotated genes were disrupted at the breakpoints, with small base pair losses or gains in all 7 genes (Figure 2). On chromosome 3, CNTN6 (a neuronal membrane protein that functions as a cell adhesion molecule, believed to play a role in the development of the nervous system) and TBC1D5 (acts as a GTPase-activating protein for Rab family protein(s)) are interrupted. On chromosome 7, CNTNAP2 (a member of the neurexin family that acts as a cell adhesion molecule in the vertebrate nervous system and is implicated in numerous neurodevelopmental disorders) is disrupted. On chromosome 9, PTPRD (a signaling molecule regulating cell growth and development is disrupted. On chromosome 18, L3MBTL4 (a conserved gene down regulated or mutated in tumors, LOC100130480 (uncharacterized) and WDR7 (a gene possibly involved in cell cycle progression and gene regulation) are disrupted. The chromosome 5 breakpoint does not involve any gene disruption but does contain a gain of 18 bp. The 7q36.3 breakpoint does not involve a gene but resulted in a loss of 923 bp. This brings the total number of breaks in this chromosome complement to 9.
Figure 2.
Reassembly of all chromosomal regions that were involved in the translocations, according to HG19 (www.genome.ucsc.edu). At each breakpoint interrupted genes are shown above and bps of gain or deletion are shown below. The gains and losses were all well under the detection level of the microarray.
In our case the pregnancy was terminated because of the ultrasound abnormalities: a complete fetal autopsy was performed, which showed a very small brain for gestation (40 gm. vs. normal 75 gm.), the ventriculomegaly seen on fetal MRI, and an absent left kidney and small right kidney. The corpus callosum could not be visualized. All other structures were unremarkable. In the absence of USG detected anomalies, it will be very difficult to provide a risk for developmental abnormalities when chromothripsis is detected prenatally. Most reported cases with clinical data have been detected postnatally as apparently balanced rearrangements in patients with developmental delay. The spectrum of phenotype in individuals with chromothripsis “balanced” at the array level is yet to be determined, but will presumably reflect the nature of the disrupted genes. Chromothripsis seen prenatally is unlikely to contain major imbalances because of in utero selection for survival to the time of diagnosis.
The characterization of this extremely complex abnormality illustrates the necessity of both cytogenetic and molecular testing. Chiang et al.2 sequenced 141 breakpoints from what were originally classified as cytogenetically balanced rearrangements and found that 19.2% of these fit the criteria for CCRs, a much higher percentage than previously believed1. The number of congenital cases showing chromothripsis suggests that all de novo balanced rearrangements detected prenatally by karyotyping in cases with ultrasound abnormalities should ideally be further analyzed by sequencing to determine possible undetected genetic changes. It is ironic that as molecular testing is becoming extremely sophisticated, chromosomal analysis is at present the only reliable method to initially detect rearrangements that would fit the criteria for chromothripsis.
Chromothripsis is a recently described phenomenon whereby a single catastrophic event leads to multiple chromosome breaks and subsequent repair through non-homologous end joining. The result can present karyotypically as a complex rearrangement and occurs both congenitally and in cancer cells.
We report, to our knowledge, the first case of congenital chromothripsis uncovered prenatally through a combination of G-banded karyotype analysis and whole-genome sequencing by jumping libraries. The G-banded karyotype initially suggested the involvement of 5 chromosomes and 6 breakpoints. Whole-genome sequencing further resolved this event to include 9 total breakpoints that disrupt seven independent genes, all in the presence of a normal microarray result. This emphasizes the complementarity that whole-genome sequencing can provide to the initial karyotype analysis as a reflex test when a rearrangement is detected. We also discuss the dilemma of prognosis with this finding.
Acknowledgments
Funding source: NIH (R00MH095867, P01GM061354), the March of Dimes and the Charles Hood Foundation
Footnotes
Conflict of Interest None
References
- 1.Giardino D, Cort C, Ballarati L, et al. De novo balanced chromosome rearrangements in prenatal diagnosis. Prenat Diagn. 2009;29:257–265. doi: 10.1002/pd.2215. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C, Jacobsen JC, Ernst C, et al. Complex reorganization and predominant non-homologous repair following chromosomal breakage in karyotypically balanced germline rearrangements and transgenic integration. Nat Genet. 2012;44:390–7. doi: 10.1038/ng.2202:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens PJ, Greenman CD, Fu B, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloosterman WP, Guryev V, van Roosmalen M, et al. Chromothripsis as a mechanism driving complex de novo structural rearrangements in the germline. Hum Mol Genet. 2011;20:1916–1924. doi: 10.1093/hmg/ddr073. [DOI] [PubMed] [Google Scholar]
- 5.Kloosterman WP, Tavakoli-Yaraki M, van Roosmalen MJ, et al. Constitutional chromothripsis rearrangements involve clustered double-Stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 2012;1:648–655. doi: 10.1016/j.celrep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Kloosterman WP, Cuppen E. Chromothripsis in congenital disorders and cancer: similarities and differences. Curr Op Cell Biol. 2012;25:341–348. doi: 10.1016/j.ceb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Pellestor F. Chromothripsis: how does such a catastrophic event impact human reproduction? Hum Reprod. 2014;29:388–393. doi: 10.1093/humrep/deu003. [DOI] [PubMed] [Google Scholar]
- 8.Ordulu Z, Wong KE, Currall BB, et al. Describing sequencing results of structural chromosome rearrangements with a suggested next-generation cytogenetic nomenclature. Am J Hum Genet. 2014;94:695–709. doi: 10.1016/j.ajhg.2014.03. [DOI] [PMC free article] [PubMed] [Google Scholar]