Abstract
Human inborn errors of immunity mediated by the cytokines interleukin (IL)-17A/F underlie mucocutaneous candidiasis, whereas inborn errors of interferon (IFN)-γ immunity underlie mycobacterial disease. We report the discovery of bi-allelic RORC loss-of-function mutations in seven individuals from three kindreds of different ethnic origins with both candidiasis and mycobacteriosis. The lack of functional RORγ and RORγT isoforms resulted in the absence of IL-17A/F-producing T cells in these individuals, probably accounting for their chronic candidiasis. Unexpectedly, leukocytes from RORγ- and RORγT-deficient individuals also displayed an impaired IFN-γ response to Mycobacterium. This principally reflected profoundly defective IFN-γ production by circulating γδ T cells and CD4+CCR6+ CXCR3+ αβ T cells. In humans, both mucocutaneous immunity to Candida and systemic immunity to Mycobacterium require RORγ, or RORγT, or both.
Introduction
Inborn errors of human IL-17A/F or IFN-γ immunity are each associated with a specific set of infections. Inborn errors of IL-17A/F underlie chronic mucocutaneous candidiasis (CMC), which is characterized by infections of the skin, nails, oral and genital mucosae with Candida albicans, typically in the absence of other infections. Five genetic etiologies of CMC have been reported, with mutations in five genes (1, 2). Inborn errors of IFN-γ underlie Mendelian susceptibility to mycobacterial disease (MSMD), which is characterized by selective susceptibility to weakly pathogenic mycobacteria, such as Mycobacterium bovis Bacille Calmette-Guérin vaccines (BCG) and environmental mycobacteria. Eighteen genetic etiologies of MSMD have been reported, involving mutations of nine genes (3, 4). Only a few patients display both candidiasis and mycobacteriosis, including some patients with IL-12p40 and IL-12Rβ1 deficiencies, which impair IFN-γ immunity in all patients and IL-17A/F immunity in some patients (4). We studied seven patients from three unrelated consanguineous families with this unusual combination of infectious diseases but with no known genetic disorder. A Palestinian child (Fig. 1A, Kindred A, patient P1, SOM Case Reports) died at the age of six years from disseminated BCG disease. Two other children (P2 and P3) in Kindred A had similar clinical presentations but survived and are now 7 and 4 years old, respectively. A 6-year-old Chilean child (Kindred B, P4, SOM Case Reports) had disseminated BCG infection at age 16 months. Finally, three siblings from Saudi Arabia (Kindred C, P5, P6 and P7, SOM Case Reports), aged 9, 6 and 3 years, had mycobacterial diseases, caused by BCG in two children and by M. tuberculosis in the third. Six of the seven patients also had mucocutaneous candidiasis, of various severities (Table S1).
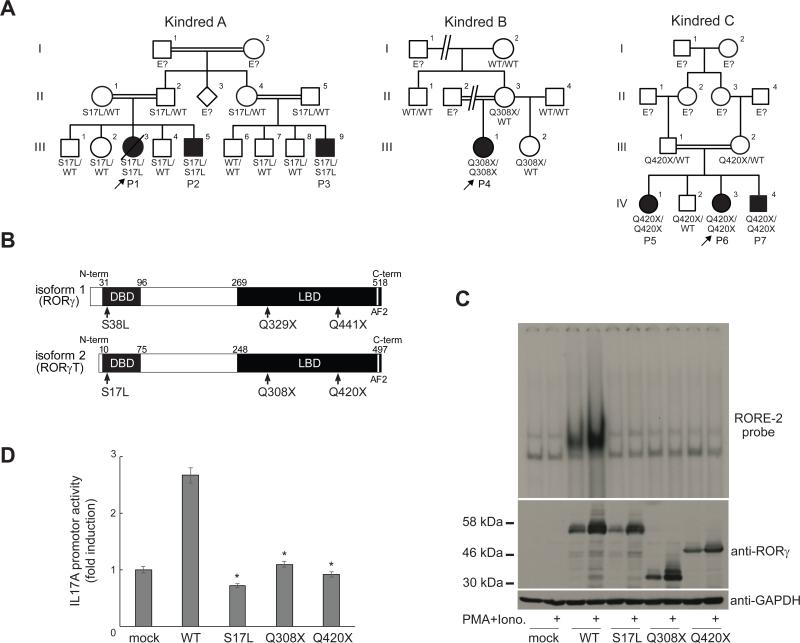
Fig. 1.
Identification of homozygous loss-of-function mutations affecting the human RORγT protein. (A) Sanger sequencing results and familial segregation of previously unidentified homozygous RORC mutations in three unrelated consanguineous families, indicating an autosomal recessive pattern of inheritance, with complete clinical penetrance. (B) Graphical representation of the RORγ and RORγT proteins, encoded by RORC isoforms 1 and 2, respectively. AF2, activation function 2 domain. Red arrows indicate the location of the sites affected by the RORC mutations found in the families. (C) HEK293T cells were either mock-transfected or transfected with the indicated plasmids. After 24 hours, cells were either left untreated or were stimulated with PMA and ionomycin. Whole-cell lysates were obtained and subjected to western blotting (lower panel), and nuclear lysates were subjected to EMSA with a 32P-labeled RORE-2 probe derived from the IL17A promoter sequence (upper panel). (D) IL17A reporter plasmids, the pRL-SV40 vector and WT or mutant RORC plasmid were used to transfect HEK293T cells. After 24 hours, cells were stimulated with PMA and ionomycin as in (C), then subjected to luciferase assays. Experiments were performed in triplicate, and IL17A promoter activity is expressed as fold-induction relative to mock-transfected cells.*p<0.05 versus WT controls, in two-tailed Mann-Whitney tests.
Bi-allelic RORC mutations
We combined whole-exome sequencing (WES) and genome-wide linkage (GWL) analysis to search for homozygous genetic lesions in the three probands (P1, P4, and P6) (Fig. S1). We identified a homozygous C/T mutation in the RORC gene in P1, P2, and P3, resulting in a missense S38L substitution in the retinoic acid-related orphan receptors γ (RORγ) isoform, or a S17L substitution in the RORγT isoform (Fig. 1A,B, Fig. S2). In P4, we identified a homozygous RORC C/T mutation converting the Q329 residue of RORγ (or Q308 in RORγT) into a stop codon (Fig. 1A,B, Fig. S2). In P5, P6 and P7, we identified a homozygous C/T mutation converting the Q441 residue of RORγ (or Q420 in RORγT) into a stop codon (Fig. 1A,B, Fig. S2). In each kindred, all unaffected family members were either heterozygous or homozygous for the WT allele (Fig. 1A, Fig. S2). The familial segregation of these mutant RORC alleles was therefore consistent with an autosomal recessive (AR) pattern of inheritance. There were no other genes mutated in the three kindreds among the 173 genes on the 6.87 Mb interval linked with disease (maximum LOD score 6.35). The S17L mutation affects a strictly conserved residue of the DNA-binding domain (DBD) of RORγT (Fig. 1B) and is predicted to be damaging by multiple software algorithms (5). The Q308X and Q420X nonsense mutations are predicted to result in truncated proteins lacking part of the ligand-binding domain (LBD, Fig. 1B). The Q308X and Q420X alleles were not found in the NCBI, Ensembl, ExAC, and dbSNP databases, our own in-house database of over 3,000 exomes, or in 1,052 controls from 52 ethnic groups in the CEPH-HGD panel, indicating that they were very rare variants, possibly private to these two kindreds. There were no nonsense or frameshift mutants affecting isoform 2 (RORγT) in these databases. The S17L allele was found in one heterozygous individual of the ExAC database, indicating that its frequency is less than 10−5. We therefore hypothesized that the biallelic RORC mutations found in these three kindreds were disease-causing.
Complete RORγ and RORγT deficiency
In mice and humans, the RORγ and RORγT isoforms are generated by transcription from different start sites (6-10) (Fig. 1B). Both molecules are transcription factors, but they have different expression patterns in inbred mice: RORγ is ubiquitous, whereas RORγT is restricted to leukocytes (10). RORγT plays an important role in T-cell development and function in mice (11, 12). Animals lacking only RORγT apparently have the same immunological phenotype as those lacking both isoforms (10). We first assessed the impact of RORC mutations, by transiently expressing wild-type (WT) and mutant RORγT and RORγ in HEK293T cells in the presence and absence of stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. We detected both the WT and S17L RORγT proteins, at the expected molecular weight (MW) of 56 kDa (Fig. 1C). The Q308X and Q420X RORγT mutant proteins had MW consistent with truncation at residues 308 and 420, respectively (Fig. 1C). Similar results were obtained upon expression of RORγ (Fig. S3). We then performed EMSA, to assess the ability of the mutant RORγT and RORγ isoforms to respectively bind to RORE-2 and RORE-1, the consensus binding sites in the promoter of IL17A (Fig. S3). The three mutations abolished DNA binding by RORγT to RORE-2 (Fig. 1C) and by RORγ to RORE-1 (Fig. S3), but not by disrupting the nuclear localization of the protein (Fig. S3). Each mutation resulted in the loss of IL17A promoter activation by RORγT (Fig. 1D) or RORγ (Fig. S4). Thus, each mutant allele was associated with a complete loss of function of the two encoded protein isoforms, identifying these patients as cases of human AR complete RORγ/RORγT deficiency (hereafter referred to as RORγT deficiency).
Broad immunological phenotype
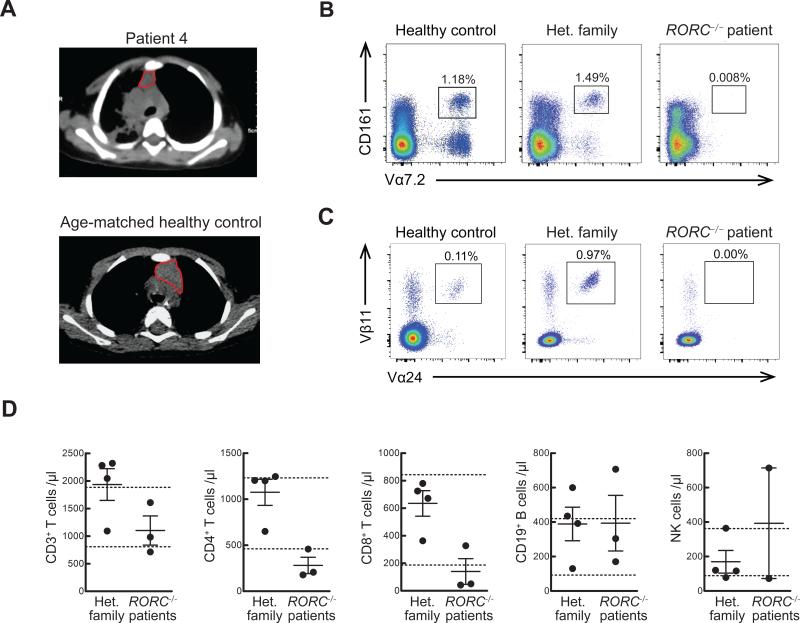
Mouse RORγT is expressed in lymphoid tissue inducer (LTi) cells, innate lymphoid cells type 3 (ILC3), type 1 natural killer T cells (NKT, also designated iNKT), some γδ T cells, immature CD4+CD8+ αβ thymocytes, and IL-17A/F-producing CD4+ αβ T cells (T helper (Th)17 cells) (7, 11, 13-16). LTi, ILC3, type 1 NKT and Th17 cells fail to develop in Rorc−/− mice, and CD4+CD8+ αβ thymocytes have a reduced life span (11, 14, 17). RORC−/− patients displayed clinical signs consistent with LTi deficiency, including absence of palpable axillary and cervical lymph nodes (despite visible tonsils), and had reduced thymus size (Fig. 2A). Like in Rorc−/− mice, ILC3 were barely detectable in the patients’ blood (Fig. S5). In Rorc−/− mice, the short lifespan of CD4+CD8+ αβ thymocytes results in an inability to use the most 5’ segments of the T cell receptor Vα array (12), including those encoding the Vα chains of mucosal associated invariant T (MAIT) (12) and type I NKT cells (18). High throughput sequencing of the TRA/TRD and TRG loci revealed that 5’ Vα gene segment use was decreased, while Vδ and Vγ usage was normal in RORC−/− T cell clonotypes (Fig. S6). Further, these patients lacked TRA clonotypes utilizing 5’ Vα and distal 3’ Jα pairings (Fig. S6). In total RORC−/− T cell clonotypes, usage of Vγ9 was elevated (Fig. S6), consistent with antigen-driven peripheral expansion of this subset, perhaps driven by mycobacteria (19). Abolished use of the Vα segments TRAV10 (encoding Vα24) and TRAV1.2 (encoding Vα7.2) was confirmed by qPCR (Fig. S7) and resulted in a lack of both CD161+Vα7.2+ MAIT cells and Vα24+Vβ11+ type I NKT cells (Fig. 2B,C and Fig. S7). Some Vα7.2+ cells other than MAIT cells have been recently shown to recognize Mycobacterium-derived mycolyl lipids (20); they were also missing in RORC−/− patients. Nevertheless, RORC−/− patients displayed only mild CD4+ and CD8+ αβ T-cell lymphopenia, with normal B- and NK-cell counts (Fig. 2D, Table S2). These patients did not, therefore, have T-cell, or “combined” immunodeficiency (CID), consistent with their lack of broad infectious and autoimmune phenotypes (21). Finally, the frequencies of circulating γδ T cells were normal (Table S2). Overall, these RORC−/− patients displayed the general immunological features characteristic of Rorc−/− mice (11, 12, 14, 22, 23). These studies also revealed that the development of MAIT and other Vα7.2+ T cells is critically dependent on RORγT, which had been predicted but not shown in mice. No infectious phenotype can be unambiguously assigned to any of these individual immunological anomalies.
Fig. 2.
RORC−/− patients display abnormal thymus size and TCRα rearrangement, in line with their mild T-cell lymphopenia with a complete absence of MAIT and type 1 NKT cells. (A) Chest CT scan of P4 at the age of 16 months, revealing right lung infiltrate and thymic hypoplasia. (B, C) PBMCs from WT, heterozygous family members or from RORC−/− patients were analyzed for MAIT (B) and type 1 NKT (C) cell frequencies by flow cytometry. Each plot is representative of n=3 experiments. (D) Cell counts were performed on fresh blood samples from heterozygous family members (n=4) and RORC−/− patients (n=3). Dotted lines indicate the normal ranges for each lymphocyte population per μL of blood, based on the results for healthy individuals tested at the Necker Hospital for Sick Children, Paris, France.
Abolished production of IL-17A/F
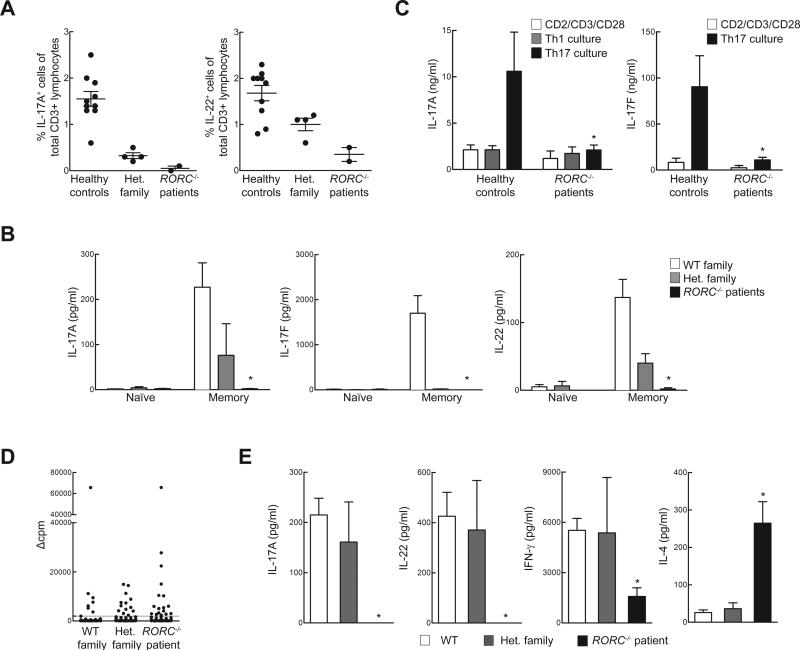
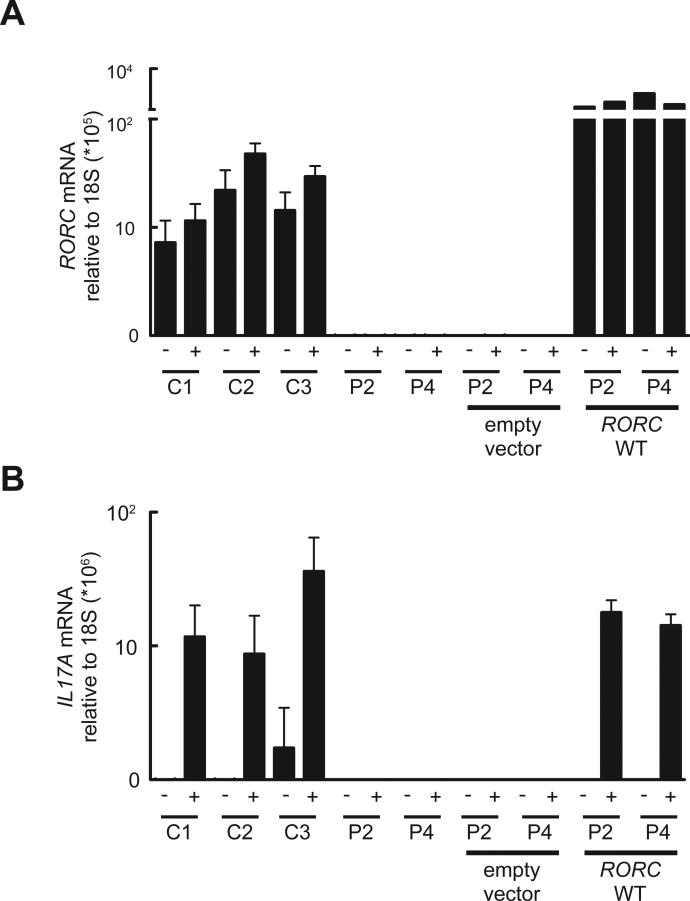
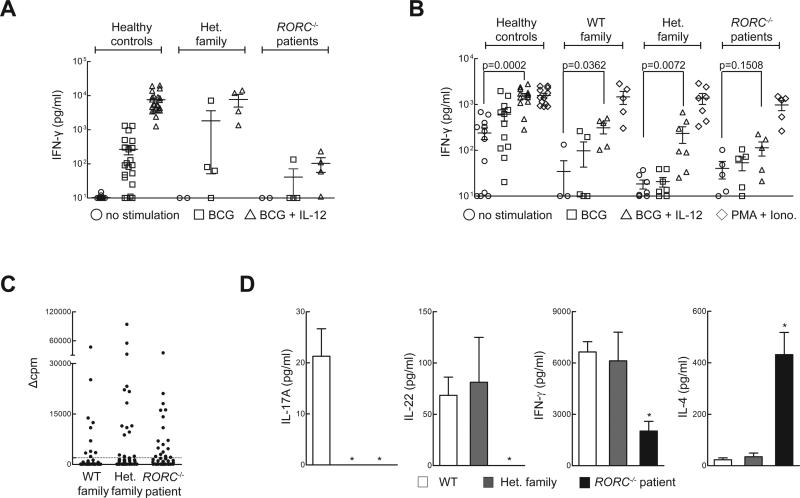
Given the critical role of murine RORγT in generating IL-17A/F- and IL-22-producing lymphocytes, including ILC3, γδ T cells, and Th17 cells (11, 13, 24), and the finding that patients with compromised IL-17A/F immunity are susceptible to mucocutaneous candidiasis (1) we assessed the development and function of IL-17A/F-producing lymphocytes in the patients. Circulating ILC3 cells were too few to assess their production of IL-17. CD3+ T cells from RORC−/− patients displayed a severe impairment in the production of IL-17A, IL-17F, and IL-22 both at the mRNA (Fig. S8) and at the protein level (Fig. 3A) after polyclonal stimulation. CD4+ αβ T cells are a major source of IL-17A/F (9). Memory (CD45RA−) CD4+ T cells from RORC−/−patients produced much less IL-17A, IL-17F, and IL-22 than WT and heterozygous controls (Fig. 3B). In contrast, the memory CD4+ T cells from these patients produced large amounts of IL-4, IL-5, and IL-13 (Fig. S8). In separate experiments, naïve (CD45A+ CCR7+) CD4+ T cells from RORC−/− patients cultured under IL-17-polarizing conditions secreted less IL-17A and IL-17F than cells from healthy donors or heterozygous relatives (Fig. 3C). We next assessed the proliferation and cytokine secretion of highly purified WT, heterozygous, and RORC−/− CCR6+CD4+ memory αβ T cells (Fig. S9), a population enriched in IL-17A/F-secreting cells (Th17, which express CCR4), as well as cells secreting IL-17A/F and IFN-γ (herein designated as Th1*, which express CXCR3) (25), following their stimulation with C. albicans lysate. By monitoring the incorporation of a radioactive label, we found that CD4+CCR6+ T cells from RORC−/− patients had normal frequencies of antigen-specific cells recognizing C. albicans (Fig. 3D). However, these cells (including both Th17 and Th1*, whose proportions were normal, Fig. S9) secreted much less IL-17A and IL-22 than control cells (Fig. 3E). IFN-γ was also reduced, but large amounts of IL-4 were secreted, serving as control (Fig. 3E). Finally, Herpesvirus saimiri-transformed CD4+ αβ T cells from RORC−/− patients showed abolished induction of RORC (Fig. 4A) and IL17A (Fig. 4B), but not IFNG serving as a control (Fig. S10). The defect in IL17A induction could be rescued by retroviral transduction with WT RORC (Fig. 4B). Collectively, these data demonstrate a profound diminution of IL-17A/F and IL-22 production by all leukocytes tested in RORC−/− patients. As CMC-causing germline mutations have previously been identified in IL17F, IL17RA, IL17RC, and ACT1 (1, 26, 27) we conclude that impaired IL-17A/F immunity in RORC−/− patients accounts for their development of CMC. Human IL-17A/F-producing ILC3, γδ T cells, and αβ T cells, or any of their subsets, may individually or collectively confer protection against Candida.
Fig. 3.
Cellular mechanisms of compromised IL-17 immunity and CMC in RORC−/− patients. (A) Whole blood from healthy WT donors, heterozygous family members, or RORC−/− patients was activated by PMA and ionomycin in the presence of brefeldin A, then assessed by intracellular flow cytometry for the production of IL-17A and IL-22. (B) Naïve and memory CD4+ T cells from WT controls (n=7), heterozygous family members (n=2) and RORC−/− patients (n=3) were cultured with T-cell activation and expansion (TAE) beads, and the culture supernatants were then assessed for secretion of the cytokine indicated (38). (C) Cytokine production by in vitro-differentiated CD4+ T cells from control donors and RORC−/− patients. Naïve (CD45RA+CCR7+) CD4+ T cells were purified from the PBMCs of WT controls (n=6) or RORC−/− patients (n=3), then cultured in the presence of TAE beads alone or TAE beads together with polarizing stimuli to induce the differentiation of Th1- or Th17-type cells (38). After 5 days, culture supernatants were assessed for the secretion of the cytokine indicated. (D) Sorted CCR6+ memory CD4+ T cells from WT controls, heterozygous family members and RORC−/− patients were initially polyclonally stimulated to generate T-cell libraries, then cultured with autologous irradiated B cells, with or without a 3 h pulse with C. albicans lysate (CA, 5 μg/mL)(38). Proliferation was assessed by evaluating radiolabel incorporation on day 4, and is expressed as Δcpm values (38). Dotted lines represent the cutoff values. The frequencies of specific T cells using the Poisson distribution were 315/106, 631/106, 874/106 in WT, Het. family and RORC−/− patient, respectively. (E) Concentration of the indicated cytokines were measured in the supernatants from positive cultures (Δcpm values above the cut-off value) from experiments performed as in (D) with cells from WT controls, heterozygous family members, and RORC−/− patients (n=2 each) The number of wells included was n=45-64 for WT controls, n=4-10 for heterozygous family members, and n=14-23 for RORC−/− patients. *p<0.05 versus WT controls, in two-tailed Mann-Whitney tests.
Fig 4.
T cell lines from RORC−/− patients fail to induce IL17A after mitogen stimulation. (A) Herpesvirus saimiri-transformed T cells from healthy donors (C1, C2, C3) or RORC−/− patients (P2, P4) were cultured in the presence (+) or absence (−) of PMA and ionomycin and then total RNA was extracted and used for qRT-PCR for total RORC. T cell lines from RORC−/− patients were transduced with retrovirus encoding either a tag only (empty vector), or tagged WT RORC isoform 2. (B) IL17A expression was assessed in the same RNA samples presented in (A). n=3, error bars represent SEM.
Selective defect in IFN-γ production
We then investigated the cellular mechanism underlying the patients’ surprising susceptibility to mycobacteria. The patients did not display chronic granulomatous disease or severe combined immunodeficiency, which can underlie BCG disease (4). The CD3+ T cells (including both γδ and αβ T cells) from RORC−/− patients produced IFN-γ normally, following the stimulation of whole blood or PBMC with PMA and ionomycin (Fig. S10 and data not shown). Likewise, total CD4+ αβ T cells, memory (CD45RA−) CD4+ T cells, naïve CD4+ T cultured under Th1-polarizing conditions, and herpesvirus saimiri-transformed T cells from the patients produced IFN-γ normally (Fig. S10). Overall, and in contrast to the IL-17A/F defect, RORγT deficiency does not impair IFN-γ secretion in conditions of polyclonal stimulation. We next assessed mycobacterium-specific IFN-γ responses from whole blood (Fig. 5A) or PBMC (Fig. 5B) of RORC−/− patients, heterozygous family members, and healthy controls. The patients’ cells produced very little IFN-γ in response to BCG plus IL-12 treatment (Fig. 5A,B). This defect was as profound as that seen in patients with IL-12Rβ1 deficiency (28). The production of IL-12p40 by RORC−/− cells was normal (Fig. S11). Impaired IFN-γ production may account for mycobacterial diseases in RORC−/− patients. This IFN-γ defect was not secondary to excessive IL-4, IL-5, or IL-13 production (Fig. S11) or to the IL-17A/F defect (Fig. S12). Many single-gene immunodeficiencies do not predispose to BCG disease despite impaired or abolished development or function of various αβ T cell subsets, including CD4+ T cells (29), CD8+ T cells (30), type 1 NKT cells (31, 32), and MAIT cells (32). Even rare patients deficient in total αβ T cell function (ZAP70−/− (33), TRAC−/− (34)) have not been reported to develop BCG disease. Whole blood or PBMC from such patients responded to BCG plus IL-12 normally, except for patients lacking all functional αβ T cells (Fig. S12). As MAIT cells were shown to respond to mycobacteria (35), we purified these cells from WT donor PBMC and added them to PBMC from RORC−/− patients before BCG stimulation. The lack of MAIT cells in RORC−/− patients did not account for their impaired IFN-γ production (Fig. S13). Overall, the absence of type 1 NKT and MAIT cells, the mild T-cell lymphopenia, and the poor development of IL-17A/F T cells may contribute marginally to mycobacterial susceptibility, but do not account for the low level of IFN-γ production by RORC−/− leukocytes stimulated with BCG and IL-12, and probably not for the patients’ mycobacterial disease.
Fig. 5.
Cellular mechanisms of impaired IFN-γ immunity to Mycobacterium in RORC−/− patients. (A) Whole-blood samples from healthy controls (n=23), heterozygous family members (n=4), or RORC−/− patients (n=4) were incubated for 48 h under three different sets of activation conditions: medium alone, live Mycobacterium bovis-BCG (BCG) at a MOI of 20 BCG cells/leukocyte, and with BCG plus 20 ng/ml IL-12. The IFN-γ levels of culture supernatants were determined by ELISA. (B) Equal numbers of live PBMCs from healthy controls, WT family members, heterozygous family members, or RORC−/− patients were cultured in the presence of live BCG, BCG and IL-12, or PMA/ionomycin for 48 hours. IFN-γ concentration in the culture supernatant was assessed by ELISA. (C) Sorted CCR6+ memory CD4+ T cells were polyclonally stimulated with PHA in the presence of irradiated allogeneic feeder cells and IL-2, to generate T-cell libraries, as in Fig. 3D. Library screening was performed 14-21 days after initial stimulation, by culturing thoroughly washed T cells with autologous irradiated B cells, with or without a three-hour pulse with Mycobacterium bovis-BCG peptide pools. Proliferation was measured by radiolabel incorporation on day 4, and is expressed as Δcpm values. Each symbol illustrates one culture. Dotted lines represent the cutoff value. The frequencies of specific T cells calculated using the Poisson distribution were 467/106, 749/106, 875/106 in WT, Het. family and RORC−/− patient, respectively. (D) The cytokines indicated were determined in the culture supernatants from (C), for wells with Δcpm values above the cutoff value. The number of wells included was n=45-64 for WT controls, n=4-10 for heterozygous family members, and n=14-23 for RORC−/− patients. *p<0.05 versus WT controls, in two-tailed Mann-Whitney tests.
Impaired IFN-γ production by γδ T cells
We thus systematically characterized the consequences of leukocyte population depletions on BCG-dependent IFN-γ production by PBMC in healthy controls. We found no overt IFN-γ defect as a consequence of depleting NK cells, CD14+ cells, CD4+ or CD8+ T cells. Depletion of αβ T cells, γδ T cells, or both resulted in diminished IFN-γ production (Fig. S14). To probe the kinetics of this phenotype, a similar experiment was repeated and supernatant was assessed at 6, 12, 18, 24 and 48h post-stimulation (Fig. S14). The effect of γδ T cell depletion was most apparent at 24h (Fig. S14). We observed high expression of RORC isoform 2 mRNA in both αβ and γδ T cells of healthy donors (Fig. S15), prompting further analyses of γδ T cell function. Flow cytometry analyses revealed that the TCRhigh γδ T cells from RORC−/− patients could not secrete IFN-γ in response to PMA-ionomycin, unlike TCRlow γδ T cells (Fig. S15). TCR Vδ2+ cells have been reported as the predominant cells responding to human BCG vaccination (19). RORC−/− patients had normal frequencies of TCR Vδ2+ cells, but these cells were unable to secrete IFN-γ when stimulated with PMA and ionomycin (Fig. S15), suggesting a possible contribution of this γδ T cell subset defect to mycobacterial susceptibility in RORC−/− patients. Overall, RORγT deficiency diminishes the IFN-γ-producing capacity of γδ T cells, which normally produce this cytokine in response to Mycobacterium stimulation.
The patients’ CCR6+ CD4+ αβ T cells produce little IFN-γ in response to BCG
Previous studies have demonstrated that the T-bet- and RORγT-expressing, IFN-γ and IL-17A/F-producing Th1* CCR6+CXCR3+ subset is strongly enriched for Mycobacterium-responsive CD4+ αβ T cells, unlike CCR6+CCR4+ Th17 cells that only express RORγT and IL-17A/F and are enriched for Candida-responsive T cells (25). We therefore purified CCR6+ cells (Fig. S9), and assessed their proliferation and cytokine production in response to a pool of BCG peptides. CD4+CCR6+ αβ T cells from RORC−/− patients had a normal or high frequency of antigen-specific cells recognizing BCG peptides, as demonstrated by the induction of proliferation (Figs. 5C and S16). However, although CD4+CCR6+ T cells from RORC−/− patients responded to mycobacterial antigens, they secreted much less IFN-γ than CD4+CCR6+ αβ T cells from normal donors (Fig. 5D). The normal proliferation and cytokine production of other CD4+ memory subsets in response to Candida and Mycobacterium (Fig. S17), and to irrelevant viral stimuli (Fig. S18), indicate a selective RORγT-dependent functional defect in Mycobacterium-specific CD4+CCR6+ αβ T cells. Although we did not purify and test Th1* cells, they were present in normal proportions in the patients (Fig. S9), implying that they are functionally defective for IFN-γ production. Collectively, these data suggest that mycobacterial diseases in RORC−/− patients may result from the poor production of IFN-γ by γδ T cells, CD4+CCR6+CXCR3+ αβ Th1* cells, or both, in response to mycobacteria. IFN-γ treatment may therefore be beneficial for RORC−/− patients. This combined defect probably also accounts for mycobacterial disease in SCID patients, as patients with various forms of CID are normally resistant to BCG (28, 34). Finally, the lack of MAIT and type 1 NKT cells, reduction in ILC3, and possibly of other lymphocytes not analyzed using blood samples (e.g. LTi), may aggravate the mycobacterial phenotype of RORC−/− patients.
Conclusion
Collectively, these data demonstrate that human RORC play a surprising dual role in host defence. These findings are clinically, immunologically, and genetically robust, as they were consistent in seven patients, from three ethnic groups, homozygous for three different RORC mutations that are loss-of-function for both isoforms. Interestingly, although the two infectious phenotypes are purely recessive, some immunological phenotypes showed co-dominant or dominant inheritance. The mild T-cell lymphopenia, small thymus, lack of palpable axillary and cervical lymph nodes, and absence of MAIT and type 1 NKT cells in RORC−/− patients were consistent with the phenotype of Rorc−/− mice (Table S3). Likewise, impaired IL-17A/F immunity was predicted to account for impaired protection against Candida albicans (36), as Rorc is the master gene controlling Th17 differentiation in inbred mice (11) and mutations affecting human IL-17A/F immunity underlie isolated CMC (1, 27, 37). The IL-17A/F defect therefore underlies CMC in RORγT-deficient patients, probably but not necessarily because of T cells as other cells can normally produce these cytokines. We expected these patients to be susceptible to candidiasis, but their susceptibility to mycobacterial disease, and its severity, were unanticipated. This phenotype does not seem to be human-specific, as we also found that mice deficient for Rorc (14) are susceptible to mycobacterial infection (Fig. S19). Our data conclusively demonstrate that human RORC plays an indispensable role in the induction of IFN-γ-dependent anti-mycobacterial systemic immunity. The mechanism underlying disease in these patients probably involves an impairment of the induction of IFN-γ production by γδ T cells, or CD4+CCR6+CXCR3+ αβ Th1* cells, or both, in response to mycobacteria. Other mechanisms may also be at work. Human RORC is essential not only for the development of IL-17A/F-producing lymphocytes protecting the mucocutaneous barriers against Candida, but also for the activation of IFN-γ-producing T cells, and for systemic protection against Mycobacterium.
Supplementary Material
One-Sentence Summary.
Human RORγ and RORγT deficiency impairs both IL-17A/F-dependent mucocutaneous immunity to Candida and IFN-γ-dependent systemic immunity to Mycobacterium.
Acknowledgments
We thank the patients and their families for their collaboration; both branches of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions and support; M. Hindiyeh for expert clinical care of the patients from Kindred A; G. C. Tsokos for providing the IL17A reporter luciferase plasmid; E. Van de Vosse for providing the pLZRS-IRES-ΔNGFR vector; D. Littman for helpful discussions; B. Fleckenstein and M. Schmidt for the generation of patient-derived T cell lines; and Y. Nemirovskaya, L. Amar, E. Anderson, M. Courat, and T. Nivare for administrative support. The data presented in the manuscript are tabulated in the main paper and in the supplementary materials. The sequence data are available in the Sequence Read Archive (www.ncbi.nlm.nih.gov/sra) with accession numbers SRS964935, SRS965039, SRS965040, and SRS965042. J.Mc. and The University of Melbourne filed Australian provisional patent application numbers 2014901185 and 2014901 that relate to ligands that bind MR1 and stimulate MAIT cells. The Laboratory of Human Genetics of Infectious Diseases is supported by grants from the National Center for Research Resources and the National Center for Advancing Sciences, NIH (8UL1TR000043); the French National Research Agency (ANR) under the “Investments for the Future” program (grant ANR-10-IAHU-01), grant IFNGPHOX (13-ISV3-0001-01 to J.B.), and grant GENCMCD (11-BSV3-005-01 to A.P); Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID); the National Health and Medical Research Council (NHMRC) (to E.K.D., C.S.M., S.G.T, and J.Mc.); the Rockefeller University; INSERM; Université Paris Descartes; the St. Giles Foundation; the National Institute of Allergy and Infectious Diseases (R37AI095983 to J.-L.C.); and the NIH (contract HHSN272200900044C to A.S.). S.O. was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (25713039 and 25670477), J.G.M. by the Canadian Institutes of Health Research, R.M.-B. by the European Molecular Biology Organization, Y.I. by the AXA Research Fund, L.A.H. by the Rheumatology Research Foundation's Scientist Development Award, and F.S. by grants from the European Research Council (323183 PREDICT) and the Swiss National Science Foundation (149475). The Institute for Research in Biomedicine and the Center of Medical Immunology are supported by the Helmut Horten Foundation. S.A.-M. is the bronchial asthma research chair of the Prince Naif Center for Immunology Research.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling Y, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–631. doi: 10.1084/jem.20141065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogunovic D, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26:454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medvedev A, Chistokhina A, Hirose T, Jetten AM. Genomic structure and chromosomal mapping of the nuclear orphan receptor ROR gamma (RORC) gene. Genomics. 1997;46:93–102. doi: 10.1006/geno.1997.4980. [DOI] [PubMed] [Google Scholar]
- 7.Villey I, de Chasseval R, de Villartay JP. RORgammaT, a thymus-specific isoform of the orphan nuclear receptor RORgamma / TOR, is up-regulated by signaling through the pre-T cell receptor and binds to the TEA promoter. Eur J Immunol. 1999;29:4072–4080. doi: 10.1002/(SICI)1521-4141(199912)29:12<4072::AID-IMMU4072>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Q, et al. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Guo J, et al. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 13.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 15.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Robinette ML, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michel ML, et al. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 20.Van Rhijn I, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14:706–713. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notarangelo LD. Functional T cell immunodeficiencies (with T cells present). Annu Rev Immunol. 2013;31:195–225. doi: 10.1146/annurev-immunol-032712-095927. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 23.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 24.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 26.Boisson B, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinberg J, et al. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol. 2004;34:3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- 28.Ouederni M, et al. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood. 2011;118:5108–5118. doi: 10.1182/blood-2011-05-352716. [DOI] [PubMed] [Google Scholar]
- 29.de la Calle-Martin O, et al. Familial CD8 deficiency due to a mutation in the CD8 alpha gene. J Clin Invest. 2001;108:117–123. doi: 10.1172/JCI10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin E, et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature. 2014;510:288–292. doi: 10.1038/nature13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AC, et al. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 33.Morgan NV, et al. Mutation in the TCRalpha subunit constant gene (TRAC) leads to a human immunodeficiency disorder characterized by a lack of TCRalphabeta+ T cells. J Clin Invest. 2011;121:695–702. doi: 10.1172/JCI41931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bourhis L, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 35.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Material and methods are available as supplementary materials on Science online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.