Abstract
One of the methods of controlling biofilms that has widely been discussed in the literature is to apply a potential or electrical current to a metal surface on which the biofilm is growing. Although electrochemical biofilm control has been studied for decades, the literature is often conflicting, as is detailed in this review. The goals of this review are to (1) present the current status of knowledge regarding electrochemical biofilm control, (2) establish a basis for a fundamental definition of electrochemical biofilm control and requirements for studying it, (3) discuss current proposed mechanisms, and (4) introduce future directions in the field. It is expected that the review will provide researchers with guidelines on comparing data sets across the literature and generating comparable data sets. The authors believe that, with the correct design, electrochemical biofilm control has great potential for industrial use.
Keywords: biofilm, biofilm control, electrochemical, current, potential
Introduction
Most microorganisms can colonize and form biofilms on metal surfaces, which are widely used for example in the food processing industry, oil refineries, paper mills, heat exchangers, and on ships’ hulls, and medical implants. The formation of biofilms adversely affects the processes for which these surfaces are used (Lewandowski and Beyenal 2014). Biofilms are also involved in nosocomial infections on wound surfaces (Dijkshoorn et al. 2007). Therefore, bacterial attachment and growth on such surfaces should be prevented, or should be delayed when prevention is not possible. Conventional biofilm control uses chemical agents or biocides to disinfect metal surfaces. Antibiotics or biocides are used to control biofilm-related infections. However, these strategies involving externally-added agents have several drawbacks: (1) Antibiotics or biocides cannot be delivered continuously. (2) The concentrations of antibiotic or biocides at the surface cannot be controlled because of diffusion limitations in biofilms. The concentration at the surface may be very low and ineffective even though the concentration in the bulk is high (Davison et al. 2010, Donlan and Costerton 2002, Stewart et al. 2000, Tkachenko and Karas 2012). (3) Cells in biofilms can develop resistance against biocides and antibiotics (Russell 2003). (4) In some cases biocides react with biofouling deposits and prevent their diffusion (Stewart et al. 2001). Therefore, alternative strategies, which allow the delivery of biofilm inhibitors for biofilm control, are needed. One solution is to coat a surface with biocide to delay biofilm growth or prevent cell attachment (Lv et al. 2014, Sun and Chen 2007). However, this process has limited use because of the limited supply of biocides in the coating. An alternative technology for long-life biocidal coatings could bring many advantages.
Electrochemical biofilm control is a technology where surface properties or reactions are controlled to delay or prevent cell attachment or to remove existing cells from that surface. Generally, electrochemical biofilm control is applied to targeted surfaces that are electrically conductive and inert (Hong et al. 2008). These conductive surfaces act as electrodes where electrochemical phenomena occur. Thus, electrochemical biofilm control prevents or delays biofilm formation through the application of a constant (electric) potential or constant (electric) current that manipulates electrochemical phenomena on the target surface. In this review, electrochemical biofilm control is discussed in electrochemical terms based on electrochemical reactions. The surface where biofilm growth is expected and to which electrochemical reactions are targeted is chosen as the biofilm electrode. For example, 316L stainless steel (SS) can be considered a good example of a material which mimics the surfaces used in the food industry (Kumar and Anand 1998). By controlling the current or potential of the 316L SS surface, cell attachment and growth can be delayed (Istanbullu et al. 2012). Information on both the current and the potential is required to characterize electrochemical phenomena and therefore to investigate the mechanism of action of electrochemical biofilm control (Istanbullu et al. 2012).
Several pertinent reviews summarize the broader field of study on the “bioelectric effect”, which focus on the combined application of antibiotics and either electric current or an electric field to enhance biofilm removal (Del Pozo et al. 2008, Freebairn et al. 2013). High voltages and current not typically used in aqueous electrochemical systems are included (see Table 1), which unnecessarily complicate the system under study. Although electrochemical biofilm control can be considered a subset of the bioelectric effect, the two are differentiated because the goal is targeted at understanding electrochemical phenomena within a specified range where complicating effects (see Figure 2) are minimized. Doing so allows an understanding to be gained of the mechanism of actions involved and subsequently allows electrochemical biofilm control to become a viable technology. It is recognized that electrochemical biofilm control has been studied for decades; however, there are many conflicting findings in the literature, as is summarized in Table 1 and discussed in detail in the following sections. Interestingly, it was often found that the outcome of biofilm control was emphasized over an understanding of the fundamental mechanisms involved. This observation, in addition to the authors desire to study the electrochemical phenomena governing electrochemical biofilm control, prompted the writing of this review.
Table 1.
Selected potential-controlled and current-controlled studies on electrochemical biofilm control of various microorganisms.
| Method | Bacterial strain | Current density/ Potential | Material type | Experimental system | Log reduction/ Percentage removal |
Imaging/Bacterial enumeration |
Exposure time | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|
|
Potential control |
P. fluorescens | −500 mVAg/AgCl −200 mVAg/AgCl |
WE: Gold-covered glass CE: Pt wire RE: Ag/AgCl (sat. KCl) |
Thin film flow cell No membrane |
Cell adhesion prevented: ~90% at −500 mVAg/AgCl ~80% at −200 mVAg/AgCl |
Microscopy, gray scale images |
Polarization and then 15 min attachment |
Electrostatic repulsive force |
1.(Busalmen and de Sanchez 2001) |
| P. fluorescens | −200 mVAg/AgCl −500 mVAg/AgCl 500 mVAg/AgCl 800 mVAg/AgCl |
WE: Gold-covered glass CE: Pt wire RE: Ag/AgCl (3 M NaCl) |
Flow cell No membrane |
No effect on cell growth at −200 mVAg/AgCl. Most bacteria did not adhere on surface at −500 mVAg/AgCl; microcolonies formed at 500 mVAg/AgCl; no cell growth at 800 mVAg/AgCl |
Phase contrast microscopy |
2 min initial attachment then polarization for 3 or 8 h |
Electrostatic repulsion, oxygen reduction at −0.5 VAg/AgCl, pH increase |
2.(Busalmen and de Sanchez 2005) | |
| V. alginolyticus | 0 mVAg/AgCl −200 mVAg/AgCl −600 mVAg/AgCl |
WE: Conductive paint and TiN film electrode CE: Iron bar RE: Ag/AgCl |
Nylon yarn fishnet in water disinfection reactor |
0 desorption ~14% desorption ~48% desorption |
Live/dead staining and fluorescence microscopy |
30 min | No toxic substance generation or pH change, electrochemical sterilization |
3.(Matsunaga and Lim 2000) | |
|
Current control |
P. aeruginosa PAO1 |
±0.015 mA cm−2 | WE: ITO CE: ITO No RE |
Flow cell reactor No membrane |
~80% detachment | Live/dead staining and fluorescence microscopy |
90 min initial attachment then 40 min polarization |
Electrostatic and electrophoretic repulsive forces |
4.(Hong et al. 2008) |
| P. aeruginosa | ±12 V/cm (DC) ±2.1 mA cm−2 |
Pt wire and stainless steel sample studs |
Electrified modified Robbins device |
1 log reduction (individual application of biocide and electric field) Eradication of biofilm (combined application) |
Viable colony forming unit (CFU) count |
24 h | Bactericidal action No specific mechanism confirmed |
5.(Blenkinsopp et al. 1992) | |
|
P. aeruginosa EX226 |
9 mA cm−2> with ciprofloxacin with polymyxin B with piperacillin |
Silastic rubber | Electrical colonization cell (ECC), biofilm on dialysis membrane between two parallel electrode plates |
Population reduced to <1% after 12 h <0.1% after 24 h Bacteriostatic |
Fluorescence microscopy, scanning electron microscopy (SEM) |
1–2 h initial attachment then 22 h treatment with medium and then 12 h exposure to electricity |
Low electrical current enhancing the activity of some antibiotics defined as bioelectric effect. |
6. (Jass and LappinScott 1996) | |
|
P. aeruginosa UR-21 |
0.1 mA cm−2(alternating) | WE: Stainless steel CE: Stainless steel |
Flow cell. Antibiotic alone Electricity alone Electricity + Antibiotic |
In CFU cm−2: 1.51×107 2.25×108 8×102 |
Viable CFU count, SEM |
24 h 48 h |
Electrophoresis effect |
7.(Costerton et al. 1994) | |
|
S. epidermidis HBH276 |
0.00286 mA cm−2 (DC) 0.00476 mA cm−2 (DC) 0.00286 mA cm−2 (alternative polarity, 50% cycle, 1 Hz) 0.00476 mA cm−2 (alternative polarity, 50% cycle, 1 Hz) |
WE: Stainless steel CE: ITO No RE |
Parallel plate flow chamber No membrane |
37% detachment 78% detachment 24% detachment 31% detachment |
Live/dead staining and confocal laser scanning microscopy (CSLM) |
Initial attachment for 200 min then polarization for 360 min |
Electro-osmotic fluid flow directed to and from the surface |
8.(van der Borden et al. 2004) | |
|
S. epidermidis HBH276 |
0.0007 mA cm−2DC 0.00286 mA cm−2 DC 0.00476 mA cm−2 DC and alternative polarity, 5–50% cycle, 0.1–2 Hz |
WE: Stainless steel CE: ITO No RE |
Parallel plate flow chamber No membrane |
Detachment was independent of cycle. Average 76% detachment for current applied with alternate polarity (0.1 Hz). |
Live/dead staining and CSLM, metallurgical microscope with CCD-MXR camera |
Initial attachment for 90 min then polarization for 150 min |
Electro-osmotic fluid flow; bactericidal effect but no H2O2 production tested |
9. (van der Borden et al. 2005) | |
|
S. aeurus Xen 30 or S. epidermidis Xen 43 or P. aeruginosa Xen 5 |
0.015 mA cm−2 DC 0.15 mA cm−2DC 1.5 mA cm−2DC |
WE and CE: stainless steel or graphite cylinders |
Biofilms grown on Teflon disks (CDC biofilm reactor) for 48 h then placed in biofilm treatment device |
4 to 5 log CFU cm−2 6 log CFU cm−2 3.5 to 5 log CFU cm−2 |
Viable CFU count | At least 2 days At least 2 days 7 days |
No H2O2 detected; pH changes may be involved |
10. (Del Pozo et al. 2009a) | |
|
S. epidermidis or S. aureus |
−0.0106 mA cm−2 DC −0.106 mA cm−2 DC |
WE and CE: catheters made of 15% carbon- impregnated polyurethane |
WE and CE inserted into an agar plate inoculated with bacteria |
10.2–11.5 mm average zone of inhibition ~40% decrease in CFU cm -1 of catheter |
Zone of inhibition test Viable CFU count |
16 h 14 h |
A salt bridge used; H2O2 produced at the cathode |
11.(Liu et al. 1997) | |
|
S. epidermidis or P. aeruginosa |
Four levels DC 0.7–1.8 mA cm−2with Chloride 1.8 mA cm−2 with Sulfate Nitrate Phosphate |
WE and CE: Pt electrodes |
Biofilms grown on polycarbonate disks (CDC biofilm reactor) and transferred to a customized well |
Up to 6.7 log10 CFU cm−2 3 log CFU/cm2 2 log CFU/cm2 0 log CFU/cm2 |
Live/dead staining, epifluorescence microscopy, viable CFU count |
24 h | Electrolysis reaction forming hypochlorous acid from NaCl |
12.(Sandvik et al. 2013) | |
| V. anguillarum | 0.005 mA cm−2 Cell potential: 1950 mV Cathode potential: −560 mVAg/AgCl |
WE and CE: SnO2 RE: saturated calomel electrode |
Cell constructed of transparent conductive SnO2 glass with nafion cationic membrane separating WE and CE compartments |
~ 33 times decrease in bacterial concentration |
Dark field and phase contrast microscopy, SEM, chemical spot test for H2O2 and Cl2 |
30 min | Generation of H2O2 from dissolved O2 present in the electrolyte |
13. (Dhar et al. 1981) | |
| Mixed-species water biofilm |
0.37 mA cm−2 | WE and CE: stainless steel |
Annular reactor | No bactericidal action | Viable CFU count, free chlorine determined by spectrophotometer and modified DPD colorimetric method |
7 days | No bactericidal action |
14. (Shirtliff et al. 2005) |
Figure 2.
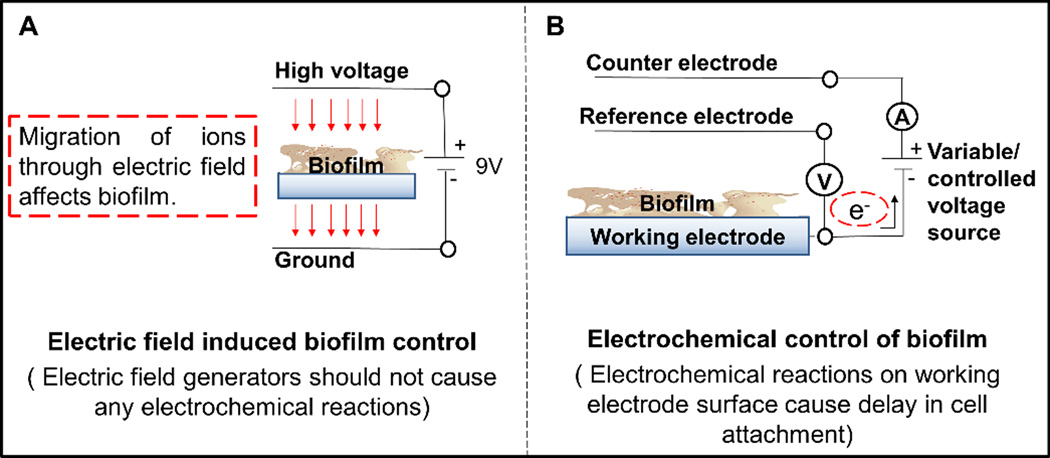
Systems used for biofilm control: (A) electric field induced biofilm control and (B) electrochemical biofilm control.
The goals of this review are to (1) present the current status of knowledge regarding electrochemical biofilm control, (2) establish a fundamental definition of electrochemical biofilm control and the requirements for studying it, (3) discuss current proposed mechanisms, and (4) introduce future directions in the field. The authors expect to establish a basis for future work at a fundamental level and help researchers to generate comparable data sets. They also concisely present their understanding of the “bioelectric effect” as it relates to electrochemical biofilm control. For more detailed discussion on the bioelectric effect, readers are referred elsewhere (del Pozo et al. 2008).
Current status of electrochemical biofilm control
As summarized in Table 1, various approaches to controlling biofilm growth based on electrochemical methods are reported in the literature. Most of these approaches are focused on either the delay/prevention of cell attachment or the removal/eradication of biofilm, as illustrated in Figure 1. Bacterial cells usually have a net negative charge. When a surface is negatively polarized such that a net negative surface charge exists, (1) a repulsive electrostatic interaction with bacterial cells occurs that reduces cell attachment and/or (2) electrochemical reactions occur at the surface, generating biocides such as reactive oxygen species (ROSs). If the surface is positively polarized such that a net positive surface charge exists, (1) an attractive electrostatic interaction with bacterial cells occurs that enhances cell attachment. At sufficiently large positive polarizations in the presence of chloride, reactive chlorine species (RCSs) may be generated and remove/eradicate bacteria. Choosing a wrong polarization potential or allowing too high a current to pass may cause unexpected and uncontrolled results such as a change in the solution pH near the surface, causing modification of the bacterial cell charge and thus migration to the surface as opposed to repulsion (Burke and Gibson 1933).
Figure 1.
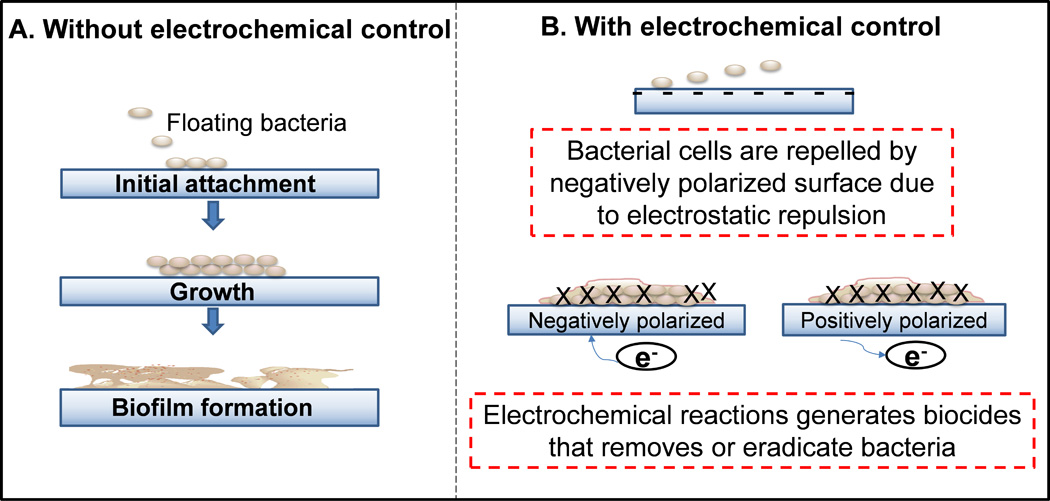
Schematic illustration of biofilm systems with and without electrochemical biofilm control. (A) Bacterial attachment leading to biofilm formation on a metal surface without electrochemical control. (B) Delay in bacterial attachment when the surface is negatively polarized or has a net negative surface charge. Bacterial cells having a negative charge are repelled by the surface. Electrochemical reactions can generate biocides such as ROSs (eg H2O2 and hydroxyl free radicals). When the surface is positively polarized RCSs (eg NaOCl, HOCl) can be generated which eradicate cells.
Delaying cell attachment
For a clean surface, the first goal is to prevent cell attachment, as shown in Figure 1B. Currently, no technology exists that completely prevents cell attachment to surfaces. Most biofilm prevention technologies modify surfaces to delay attachment. One of the uses of the electrochemical method is to delay cell attachment to surfaces (Figure 1B). In the examples in Table 1, researchers applied either alternative potential or constant potential to surfaces, which resulted in findings that are difficult to compare.
Matsunaga and Lim (2000) applied alternating potentials of 1200 mVAg/AgCl and −600 mVAg/AgCl to TiN film electrodes in a water disinfection system that detached ~50% of the cells in 30 min. In a thin film electrochemical flow-cell system, Pseudomonas aeruginosa cell attachment on a gold-coated glass surface was reduced by ~90% with a constant applied potential of −500 mVAg/AgCl for 15 min. Attachment was reduced by ~80% with a potential of −200 mVAg/AgCl compared to a control with a nonpolarized surface (Busalmen and de Sanchez 2001). This decrease in cell attachment can be explained by the mechanisms illustrated in Figure 1B and indicates that in addition to electrochemical reactions, surface charge plays a critical role in cell attachment. For instance, in the thin film electrochemical flow cell system, a gold electrode was negatively polarized at an applied potential of −200 mVAg/AgCl, causing a repulsive electrostatic interaction and thus delaying or preventing bacterial attachment. At less negative potentials close to the potential of zero charge of gold (0 mVAg/AgCl), reversible adhesion was observed (Busalmen and de Sanchez 2001, 2005). At a more negative potential of −500 mVAg/AgCl, where oxygen reduction was activated, cell clusters loosened from the electrode surface. On the other hand, the application of a more positive potential was reported to have a major effect on the growth or cell attachment of Pseudomonas fluorescens: at 500 mVAg/AgCl the formation of compact microcolonies was observed, while at 800 mVAg/AgCl there was no cell growth at all (Busalmen and de Sanchez 2005). Ueshima et al. (2002) used reactive hydroxyapatite as an electrode surface that caused the migration of charged Ca2+ under a negatively charged condition. It bound more bacterial cells (Ueshima et al. 2002) rather than repulsing them, as explained in Figure 1B. This phenomenon is similar to that illustrated in Figure 2A and different from the electrochemical biofilm control that requires an inert material as the electrode.
Iontophoresis is another method of delaying cell attachment. Iontophoresis is defined as the introduction of an ionized substance into intact cells or tissues using direct current (DC). The application of a steady current of 1.9 mA cm−2 from a 9 V DC source to a silver-wired electrified catheter released toxic silver ions into urine. This achieved a 5-log reduction in the viable cells in urine and a 156-h delay in cell attachment vs 22 h in the control catheters (Chakravarti et al. 2005). The application of such high current between conductive surfaces causes an electric field that drives a silver ion flow (Figure 2A). However, this mechanism is different from that of the electrochemical methods in which current initiates specific reactions at the electrode surface (Figure 2B).
Removing or eradicating biofilm
One method for electrochemical biofilm control is applying a constant potential to the biofilm electrode to generate biocides at the electrode surface in contact with the biofilm as shown in Figure 2B. Success in the removal or further eradication of biofilm using electrochemically generated biocides will vary depending on a number of factors. These include the biocide concentration, exposure time, biofilm thickness and/or growth stage, and bacterial strain, as observed in Table 1. For example, reactive oxygen species (ROSs) can be generated near the electrode surface through oxygen reduction in the presence of oxygen (Babauta et al. 2013, Istanbullu et al. 2012). Such ROSs can delay bacterial attachment/growth on the biofilm electrode (Istanbullu et al. 2012, Schmidt-Malan et al. 2015, Sun et al. 2012). Continuous application of a constant potential and thus delivery of ROSs can also remove preexisting biofilms from an electrode surface (Dhar et al. 1981). Dhar et al. (1981) observed generation of H2O2 (5×10−6 M) near a SnO2 cathode surface at an applied potential of −560 mVAg/AgCl with a current density of 0.005 mA/cm2. This reduced the cell density of Vibrio anguillarum ~33 times compared to a control. Researchers have further integrated electric fields with exogenous biocides to increase biocide efficacy and the eradication of biofilms (Blenkinsopp et al. 1992; del Pozo et al. 2009b). The mechanism behind the increasing efficacy of biocides when a weak electric field is applied as stated in the literaure is still unknown (Del Pozo et al. 2008). For example, SS electrode polarity was altered every 64 s within a 24 h period by 3 V using a DC power source. This was reported to produce a low-strength electric field of ±12 V cm−1 and a current density of ±2.1 mA cm−2 that enhanced the efficacy of low-concentration industrial biocides. The combined application of an electric field with biocides achieved the complete eradication of biofilm, whereas just a 1-log reduction in the number of viable cells was achieved with the application of biocides alone (Blenkinsopp et al. 1992). No mechanism was confirmed by these authors. Possibly an altering polarity affecting the surface charge (Figure 1B) is the factor controlling cell attachment and influencing cell detachment. The continuous alteration of polarity at a constant period has been reported to be an effective approach to bacterial removal and eradication (Borole et al. 2011). For instance, Borden et al. (2011) reported >76% detachment of initially adhered Staphylococcus epidermidis cells from surgical SS for both the application of constant cathodic current and the application of alternating cathodic and anodic currents of magnitudes <0.005 mA cm−2. They reported alternating cathodic and anodic current achieved faster detachment. However, this comparison was done for two separate studies with initial attachment times of 200 min and 90 min, respectively (van der Borden et al. 2004, 2005). This shows that current exposure time also may affect biofilm removal. For example, variation in S. epidermidis biofilm removal from a teflon coupon was observed when it was exposed to a current density of 0.935 mA cm−2 delivered by SS for 4 h and 24 h (Del Pozo et al. 2014). Other factors, such as current density and the ionic strength of the medium, can also affect biofilm removal. Bacterial displacement 1.2 µm from an ITO electrode was reported when an anodic current density ranging from 0.0075 to 0.03 mA cm−2 was applied for 10 s, and a decrease in bacterial motility with increased ionic strength of the medium was also observed (Kang et al. 2011). As shown in Figure 2A, this may be the result of the electric field causing migration (Kang et al. 2011). Electric field induced biofilm control (Figure 2A) is different from electrochemical control (Figure 2B), since their mechanisms are different.
Electrochemical systems for biofilm control
The first prerequisite for electrochemical control is to know the dominant electrochemical reaction in the system being studied. Alternatively, a dominant electrochemical reaction could be proposed and a hypothesis designed which is testable in a well-defined electrochemical system. The second prerequisite is to understand the impacts of current, potential, and chemical flux (mass transfer) on the dominant electrochemical reaction operating at the electrode. Electrochemical control requires knowledge of these three basic concepts, which are interrelated through various governing equations including: (1) the Butler-Volmer equation (Equation 1), (2) the Nernst-Planck equation (Equation 2), (3) the Cottrell equation (Equation 3), and (4) double-layer theory (which encompasses a set of equations) (Bard and Faulkner 1980). These equations describe the electrochemical reactions and mass transfer occurring at an electrode surface. Once the two prerequisites are fulfilled, it is possible to propose a mechanism by which cells will interact with electrode surface processes and the products that will be generated through electrochemical reactions. The sections below specifically expand upon electrochemical reactor design and how it directly affects current, potential, and mass transfer rates.
| (1) |
| (2) |
| (3) |
Electrochemical reactor design is centered on the biofilm electrode and its control. The first step in electrochemical biofilm control research is to decide on the biofilm electrode material, size, and geometry based upon the needs of the experimental analysis to be done before, during, or after electrochemical control. The materials for biofilm electrochemistry have been covered in a separate review (Babauta et al. 2012). The second step is to decide whether to control electrode potential or current and measure the other so that both current and potential are known. Potential control directly determines the overpotential (Butler-Volmer equation, Equation 1), by which specific electrochemical reactions are activated, deactivated, or reversed. In all cases, a positive overpotential, ie anodic polarization, causes anodic oxidation to occur. A negative overpotential, ie cathodic polarization, causes a cathodic reduction to occur. Experimentally, the overpotential can be based upon the difference between the applied potential and the open circuit potential, which is often referred to as the resting potential. As an example, Figure 3 shows the measured current density as the potential was varied from high to low and from low to high. The voltammogram in Figure 3 is split into three regions based upon current, denoted as region A, region B, and region C. Region A is the cathodic current region that resulted from reduction reactions when the potential was brought from the open circuit potential to a lower potential, ie a negative overpotential. Region B is a region of minimal current (essentially zero compared to regions A and C) and therefore no detectable electrochemical reactions. Region C is the anodic current region that resulted from an oxidation reaction when the potential was brought to a higher potential than the open circuit potential, ie a positive overpotential. Figure 3 demonstrates that using potential as the independent variable allows for precise control over the electrochemical reactions activated at an electrode surface. For example, in region A, oxygen reduction and proton reduction generate cathodic current. On the other hand, in region C, water oxidation generates anodic current (Vetter 1967). The transitions between these regions are discussed in detail in the following sections.
Figure 3.
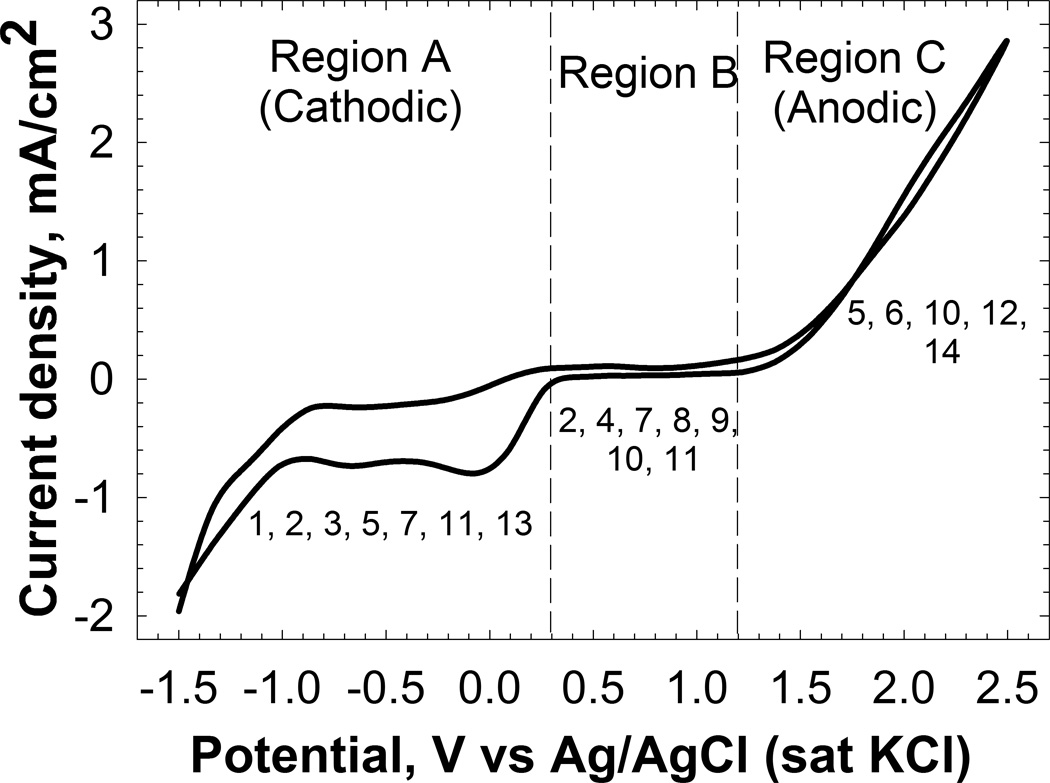
Current density dependence on applied potential on a Pt electrode recorded at a scan rate of 10 mVs−1. The regions indicate the activation of cathodic and anodic electrochemical reactions in tryptic soy broth (TSB), the medium that is typically used to grow biofilms. (The numbers below the curve in each region represent the region of potential or current density used in the references listed in Table 1.)
Figure 3 also shows that if an anodic current of 1 mA cm−2 is applied to the same electrode, the resulting potential will fall within region C. Similarly, an applied cathodic current of −1 mA cm−2 will bring the potential within region A. Current control, which is unlike potential control, controls the rate of a particular reaction but not the energy required to activate it. Figure 4 shows a simplified chronopotentiometric plot of an experiment in which the electrode potential was measured over 48 h at a variety of constant cathodic current densities. In this type of experiment, the current is the independent variable and the potential is measured. As the cathodic current density was increased from −0.1 mA cm−2 to −4 mA cm−2, the potential decreased from −1200 mVAg/AgCl to −2150 mVAg/AgCl. Under current control, the measured potential is determined by the electrochemical reaction that has: (1) the lowest cathodic activation energy (the most positive formal potential) and (2) facile kinetics that supply the requested current. If one reaction cannot supply 100% of the requested current, then the potential proceeds to the reaction that has the next lowest cathodic activation energy. Thus, the potential measured in a current-controlled system is the potential required to maintain the current value.
Figure 4.
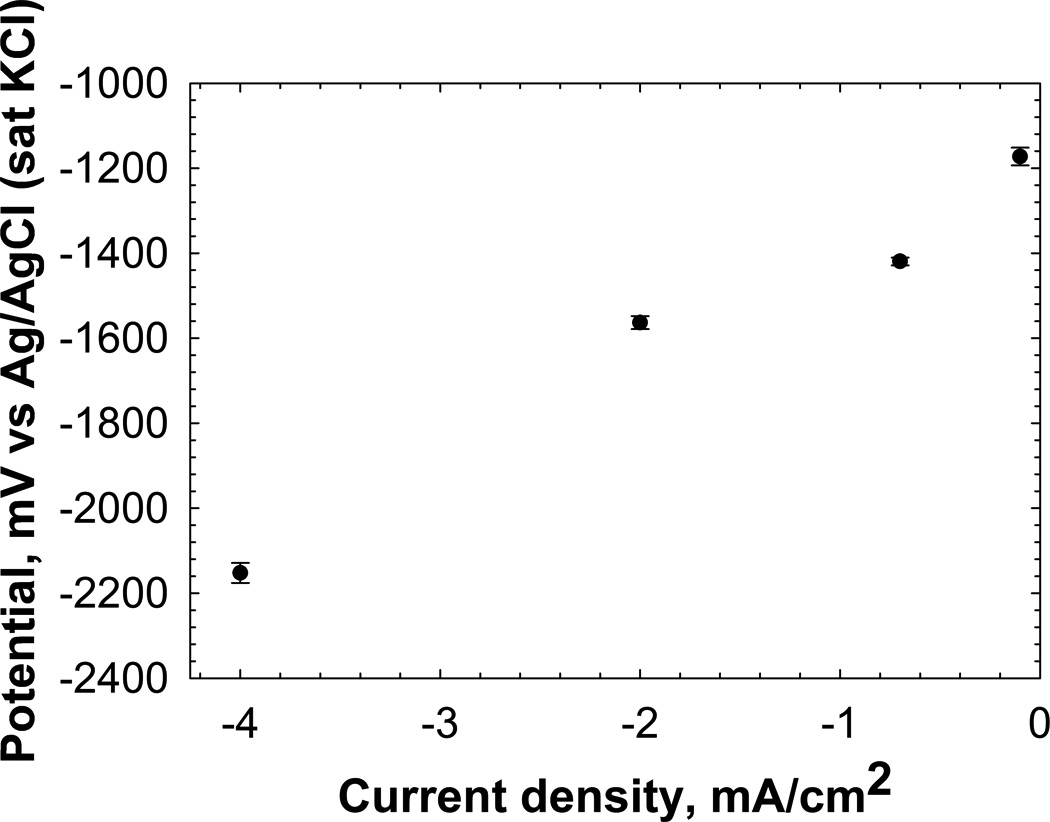
Example of current density dependence of the potential of a 316 L SS working electrode. The potentials are averages of data collected over 48 h for applied current density, and the error bar represents the SD. The potential shifts toward the negative with increasing cathodic current density.
Both potential-controlled and current-controlled systems offer advantages for electrochemical biofilm control when properly designed. The design and practical implementation of electrochemical reactors which can be used to control biofilms are detailed in Kissinger and Heineman (1996). The implications of solution resistance, electrode geometry/size, inter-electrode distances, and the electronics behind potential/current control are also described therein. Two-and three-electrode systems and separators (eg cation-exchange membranes) are critical components of these systems. The simplest setup is a two-electrode cell in which current or potential is applied between two electrodes (Figure 5A). If bacterial cells are added to the system in Figure 5A, both the anode and the cathode are exposed to cells. Therefore, the cells can attach to both electrodes and grow. A separator can be used to isolate one of the electrodes from the cells. In Figure 5B, the cathode is separated from the cells and the anode becomes the biofilm electrode since the cells only attach to the anode. Note that it is also possible to add cells to the cathode only, in which case the cathode becomes the biofilm electrode. The systems shown in Figure 5A and Figure 5B are “relative reference”-based systems, in which the biofilm electrode is controlled relative to the counter electrode, which is used as an auxiliary electrode. For example, the anode in Figure 5A will always be a set value more negative than the cathode potential in order for electrons to flow from low potential to high potential (ie anode to cathode). If the cathode potential is allowed to drift, then the anode potential will drift accordingly, making the system behave in an unpredictable way. Thus, in this type of system, the applied potential can change both the anode and cathode potentials. This relative change will depend on the electrode size, materials and any electrochemical reactions at the electrodes (Babauta et al. 2012, Bard and Faulkner 2001). If the cathode is not designed to account for drift, the potential of the biofilm electrode will also be uncontrolled. This fact renders potential control ineffective. “Fixed reference”-based systems (Figure 5C and Figure 5D) are designed to prevent potential drift by separating the auxiliary electrode into a counter electrode and a reference electrode. The counter electrode “carries the current” while the reference electrode “fixes the electrode potential.” The working, counter, and reference electrodes make up the three-electrode system and allow both accurate and precise control of potential. The difference between Figure 5C and Figure 5D is similar to that between Figure 5A and Figure 5B except that the reference electrode should be placed in the biofilm electrode compartment. Three-electrode systems allw the biofilm electrode potential to be controlled against a well-defined reference electrode. There are other benefits to using three-electrode systems. These include compensating for the majority of the resistance of the solution by placing the reference and working electrodes close to each other and the ability to select appropriate references based on compatibility with the system.
Figure 5.
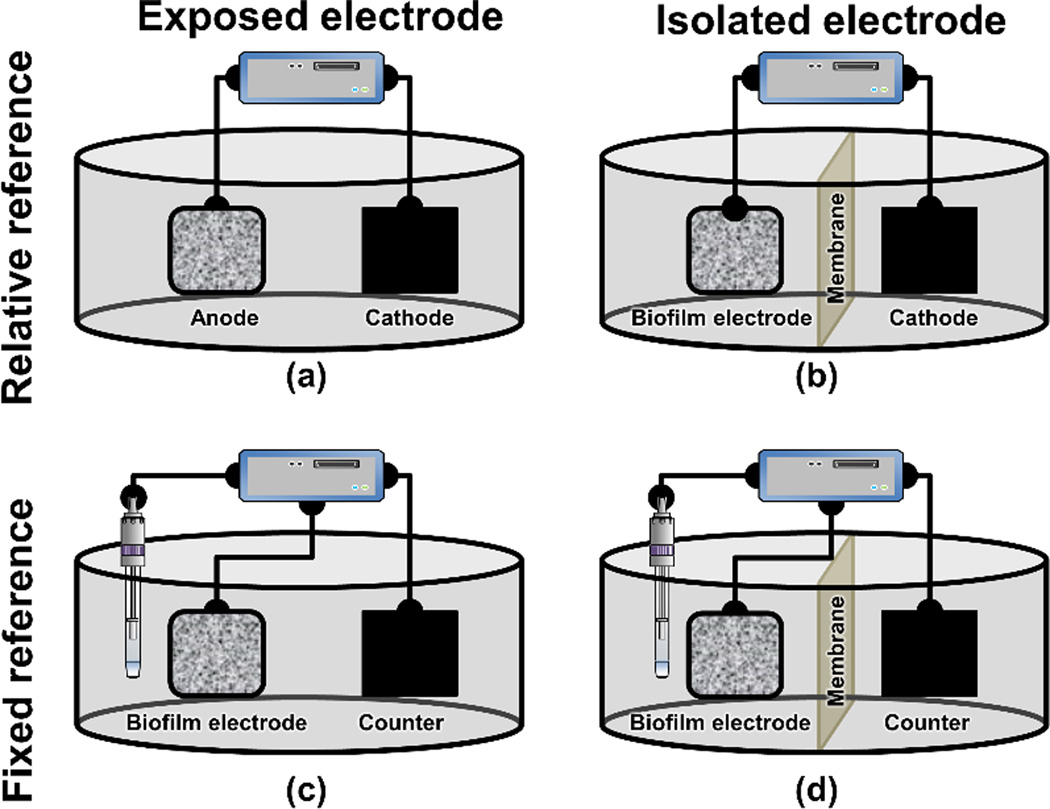
Electrochemical system that can be used to control biofilms. (A) Two electrodes connected to a potentiostat/ galvanostat or a power source; (B) the same as (A) except that the electrodes are separated by a membrane to prevent by-products generated by the cathode from diffusing to the biofilm electrode; (C) a three-electrode electrochemical system with a reference electrode controlled using a potentiostat; (D) the same as (C) except that the biofilm and counter electrodes are separated by a membrane to prevent by-products generated by the counter electrode from diffusing to the biofilm electrode. In the figure the biofilm is arbitrarily shown to be grown on the anode. Biofilms can also grow on cathodes.
Mechanisms of action
Generation of strong oxidants by electrochemical reaction
One of the mechanisms which have been suggested for the electrochemical control of biofilm is the formation of H2O2, which is a result of the partial reduction of oxygen on metal surfaces (Bard and Faulkner 2001).
| (4) |
| (5) |
Since the standard reduction potential of H2O2 is +85 mVAg/AgCl, polarizing the electrode below this potential generates negative overpotentials and starts H2O2 production (Vetter 1967). Thus, in a bioelectrochemical system with oxygen as the only cathodic reactant, polarizing the biofilm electrode at potentials below +85 mVAg/AgCl will result in H2O2 generation by oxygen reduction. It should be noted that this reaction requires the presence of oxygen at the electrode surface within the biofilm. Therefore, an attempt to generate H2O2 in an anaerobic environment within the biofilm will not be successful and it is always critical to test for the presence of oxygen.
As explained in the previous section, overpotential activates certain reaction mechanisms. According to the Butler-Volmer equation (Equation 1), the current density, which is dependent on the exchange current density and the electrode material, controls the overpotential, so the potential range for a reaction mechanism will also depend on the electrode material (Dhar et al. 1981). For example, at a Pt electrode, oxygen is reduced at low current densities, virtually entirely by the four-electron path (Equation 4). At higher current densities, as it reaches the overpotential for complete reduction to water, the two-electron path (Equation 5) contributes a greater share to the total cathodic current (Hoare 1968). In most cases, the oxygen reduction at the metal surface initially starts with Equation 5. At more negative potentials, peroxide is further reduced to water (Equation 6). The direction is towards the complete reduction of oxygen as per Equation 4 (Hoare 1968, Vetter 1967); this is also observed in region A in Figure 3. This transition from two- to four-electron transfer is highly dependent on the electrode materials (Vetter 1967).
| (6) |
On iron electrodes, the transition from Equation 5 to Equation 4 occurs between −400 mVAg/AgCl and −800 mVAg/AgCl; beyond −800 mVAg/AgCl, peroxide production is negligible (Vetter 1967). The oxygen reduction mechanism varies widely depending upon the type of electrode and whether a carbon electrode is used. On a glassy carbon disk electrode, Equation 5 occurs at two different potentials, −295 mVAg/AgCl and −740 mVAg/AgCl, producing H2O2. In contrast, for carbon nanotubes, H2O2 is produced in a potential range −400 mVAg/AgCl to −800 mVAg/AgCl and further reduced to OH− at potentials more negative than −900 mVAg/AgCl.
Thus, to identify the production of such oxidants as H2O2, Cl2 or chlorine compounds in the electrochemical system for electrochemical biofilm control, it is very important to understand the dominant reactions at the electrode surface and choose the correct operating potential range. For instance, Busalmen and de Sanchez (2005) did not observe any growth inhibition for P. aeruginosa at −200 mVAg/AgCl applied to a gold surface, whereas there was no bacterial attachment at −500 mVAg/AgCl because of H2O2 generation at this potential. These potentials are in region A in Figure 3; however, likely because of a different activation overpotential for oxygen reduction to H2O2 on gold, the reduction started below −200 mVAg/AgCl. Similarly, in a current-controlled system it is important to monitor and report the potential of the biofilm electrode, since the applied potential determines the overpotential for possible reactions. For example, recently it was reported that the application of direct current (0.7 mA cm−2 to 1.8 mA cm−2) caused the oxidation of chloride to chlorine, removing biofilms from a Pt-based biofilm electrode in the presence of NaCl (Sandvik et al. 2013). Here the suggested reactions at the anode surface are as follows (Bard and Faulkner 2001):
| (7) |
| (8) |
As per the reported applied current, oxidation reactions will happen in region C as in Figure 3. However, the chloride oxidation reaction (Equation 7) has a higher activation overpotential (ie a lower cathodic activation energy) than that for water oxidation (Equation 8) (Oldham and Myland 1994). Thus, if chloride oxidation cannot supply all the required current, the measured potential will move to water oxidation, which has a lower activation overpotential, ie a higher cathodic activation energy (Patil et al. 2011). Thus, without knowing the potential of the working electrode in a current-controlled system, it is difficult to form a conclusion about chlorine generation or further formation to hypochlorous acid (Sandvik et al. 2013). On the other hand,Shirtliff et al. (2005) applied 0.37 mA cm−2 through SS electrodes with mixed species biofilms and reported no bactericidal action. This applied current is in region C for the anode and in region A for the cathode in Figure 3. Since the anode is the biofilm electrode, the biofilm electrode potential will fall in region C, where water electrolysis happens. Water electrolysis produces O2, which might increase bacterial growth as observed in their work (Shirtliff et al. 2005).
Summarizing the mechanisms proposed in the literature
A schematic of the mechanisms proposed in previous literature is shown in Figure 6. Simply, the proposed mechanisms can be categorized into the application of electric potential and the application of current. The mechanisms proposed in most of the literature are: (1) electrochemical generation of oxidants on the surface (Costerton et al. 1994, Liu et al. 1997, Sandvik et al. 2013), (2) migration of ions and generation of oxygen through the electrolysis of water (Stewart et al. 1999), and (3) electrostatic or electrophoretic repulsive forces (Hong et al. 2008, van der Borden et al. 2005). Most of these results in the literature are conflicting, as the electrochemical reactions near surfaces have not been investigated, mostly because of the unavailability of appropriate tools. Further, it is important to note that it is only possible to detect the electrochemical generation of an oxidant when the applied potential or current reaches the required overpotential to activate that reaction on the electrode surface. In certain circumstances, oxidants may be present in very small quantities compared to the substances present in the electrolyte of the redox system. Moreover, the redox potentials of certain reactions may vary with pH and the detection of any oxidant generation may be delayed because of reaction kinetics and mass transfer limitations (Lewandowski and Beyenal 2014, Wang 2006).
Figure 6.
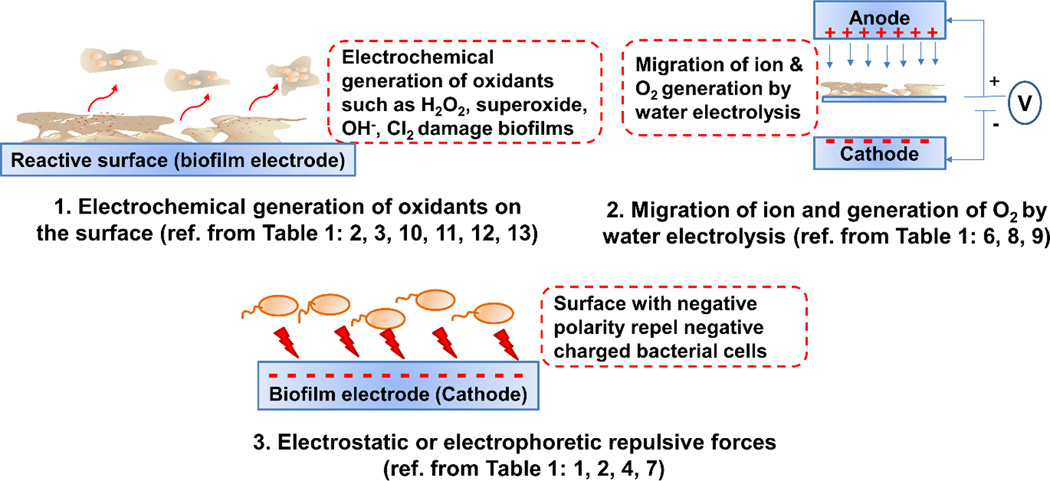
Schematic of the proposed mechanisms based on the literature review in Table 1.
In order to compare the results obtained from different electrochemical biofilm control systems studied in the literature, it is crucial that these systems have geometric and electrochemical similarity. Moreover, two important parameters to be considered and specified are current and potential, which help to understand the reaction rate and dominant reaction mechanism, respectively. As observed in Table 1, the studies report either current (current-controlled system) or potential (potential-controlled system), whereas experimental data for both potential and current are important for reproducibility and the comparison of data (Istanbullu et al. 2012). Most importantly, the potential of the biofilm electrode must be controlled against a reference electrode for reproducibility of the results. In addition, both the current and the potential data should be known in order to understand the mechanism and avoid any unwanted reactions on the electrode. For instance, in a study by del Pozo et al. (2009a) a current density of 0.935 mA cm−2 was applied to a SS working electrode (cathode) and it was found that the electrode corroded. This is likely because of the change in electrode potential promoting iron oxidation (del Pozo et al. 2009a).
Table 1 shows that most of these systems are difficult to compare since they lack both current or potential data and geometric description. For example,Liu et al. (1997) proposed mechanism 1 for a controlled-current biofilm control system where H2O2 was produced on a carbon cathode following Equation 5. A zone of inhibition was identified as due to the formation of H2O2 on an inoculated agar plate with an anode and a cathode. Sandvik et al. (2013) proposed the same mechanism for a two-electrode system. They reported the formation of hypochlorous acid on a Pt electrode with the application of four levels of direct current in a saline system as given in Table 1. In contrast, the application of current between 0.0076 and 0.76 mA cm−2 to a SS or graphite electrode in a similar reactor did not produce detectable H2O2; they concluded that pH was the cause of decreased biofilm growth. For an applied current density of 0.76 mA cm−2 over 7 days, they reported the bulk pH inside their system increased from 7 to 12 for a SS electrode while for a graphite electrode it decreased from 7 to 4 (del Pozo et al. 2009c). This variation in pH might only be because of the different electrode potential.
Several reports in Table 1 suggest a mechanism in which electrostatic or electrophoretic repulsive forces inhibit biofilm formation or adhesion to a surface, but the results are contradictory. For instance, the individual application of either cathodic or anodic current with alternating polarity with a magnitude of 0.015 mA cm−2 to an ITO electrode in a two-electrode flow cell reactor achieved ~80% P. aeruginosa cell detachment (Hong et al. 2008). These results contradict similar work done previously (van der Borden et al. 2004). Van derBorden et al. (2004) applied cathodic and alternating current densities with magnitudes of 0.00286 and 0.00476 mA cm−2 respectively, to SS electrodes in a similar flow cell system. The authors observed 37% and 24% S. epidermidis cell detachment for 0.00286 mA cm−2 and 78% and 31% detachment for 0.00476 mA cm−2 current (van der Borden et al. 2004). Differences in the applied cathodic current, electrode material and bacterial strains were reported as the reason behind this contradiction (Hong et al. 2008). Overall, studies on electrochemical biofilm control have used a range of AC or DC voltages, current settings, polarities of the biofilm electrode, configurations of the reactor, application times and other variables (Table 1). Furthermore, many researchers have confused electric field induced biofilm control systems with electrochemically controlled systems when analyzing their experimental outcomes. As a consequence, it is difficult to compare the systems and draw conclusions about the general effectiveness of electrochemical biofilm control (Isseroff and Dahle 2012).
In the authors laboratory, a well-controlled experimental setup for studying electrochemical biofilm control has been established which allows them to obtain both current and potential data and to explain the mechanism. This three-electrode system similar to the one in Figure 5C was used to explain the mechanism of the electrochemical biofilm control on 316 SS surfaces (Istanbullu et al. 2012). Identical P. aeruginosa biofilms grown on polarized and non-polarized surfaces were studied. On the non-polarized surfaces there was significant biofilm growth, whereas on surfaces polarized negatively at −600 mVAg/AgCl the growth was negligible. The generation of H2O2 with a continuous flux of dissolved oxygen to the polarized surface was identified as the mechanism for controlling biofilm growth. Microelectrodes were used to measure the H2O2 concentration near the metal surface. An increase in H2O2 concentration was observed when the surface was polarized to −600 mVAg/AgCl whilst at the same time the dissolved oxygen concentration decreased. The generation of H2O2 on the surface occurs because of the partial reduction of oxygen that occurs at potentials between −400 mVAg/AgCl and −800 mVAg/AgCl (Istanbullu et al. 2012). Beyond this potential, the reduction of oxygen to water dominates, which may be a reason for conflicting reports in the literature on H2O2 detection. This explanation also supports the importance of considering both current and potential data to understand electrochemical biofilm control. The use of a catalase-positive bacterium that causes H2O2 decomposition is another possible source of undetectable H2O2 in the bulk solution, as stated in the literature (Istanbullu et al. 2012). Once the generated concentration of H2O2 and the current are known, biofilms can be administered a similar concentration of exogenous H2O2 and a similar electric current, both alone and in combination, as control experiments. These can also give a better understanding of the possible combined effect of electric current and ROSs generated by electrochemical reactions in the biofilm.
Can an electrical current increase the effectiveness of antibiotics and biocides against biofilms?
Recent approaches claim electrical current increases the efficacy of antibiotics and biocides (Blenkinsopp et al. 1992, Del Pozo et al. 2008, Stewart et al. 1999, Zhang et al. 2014). Antimicrobial agents such as antibiotics are widely used to eradicate or inhibit the growth of microorganisms, and chemical agents, or biocides, are used as disinfectants. Although these are very effective for planktonic cells, they do not work well for biofilm control. Biofilms are resistant to most antimicrobial agents used in clinical practice, which delays recovery from biofilm-associated infections in humans. Because of this failure of antimicrobial agents in biofilm-associated infection treatment, several researchers investigated novel and innovative therapeutic approaches (Costerton et al. 1994, del Pozo et al. 2009b, van der Borden et al. 2004). It has been demonstrated by many researchers that direct electric current substantially enhances the activity of antimicrobial agents in in vitro experiments: this has been defined as the bioelectric effect (Del Pozo et al. 2008). Costerton et al. (1994) applied a direct current electric field with a current density of 0.015 to 2.1 mA cm−2 with tobramycin. The concentration of tobramycin required to eradicate the biofilm in the system effectively was ar ~1.5 to 4.0 times that needed for planktonic cells. Other researchers have used an electric current along with a wide variety of antimicrobial agents such as aminoglycosides, quinolones, tetracycline, erythromycin, daptomycin, and moxifloxacin against biofilm-associated bacteria such as P. aeruginosa, E. coli, K. pneumoniae, S. epidermidis, MRSA, and S. gordonii (Del Pozo et al. 2008). However, for this technology to be applied in clinical settings, it is critical to understand the mechanism of this approach and to generalize this effect to a variety of antimicrobial classes. Many mechanisms have been suggested so far, but there is still no satisfactory explanation, as is discussed in the literature (Del Pozo et al. 2008). Moreover, because of limited information in the literature, it is difficult to generalize this approach to all antimicrobial agents or to all bacterial species. The bioelectric effect is discussed in detail in a review by del Pozo et al. (2008). This review details some hypothetical mechanisms proposed in the literature for the bioelectric effect. These include an electric current disrupting the capacity of a biofilm for binding the antimicrobial agent and thus allowing penetration (Blenkinsopp et al. 1992), electroporation increasing cell permeability (Blenkinsopp et al. 1992, Costerton et al. 1994), and electrolytically generated O2 enhancing biofilm metabolic activity (Stewart et al. 1999). However, most of these mechanisms are not confirmed and a satisfactory explanation is yet to be developed, as is detailed in the review by Del Pozo et al. (2008). Efforts have been focused on optimizing operating parameters (e g electric field strength, current density, and time of application) to achieve maximum biocidal effect (Blenkinsopp et al. 1992, del Pozo et al. 2009c, Niepa et al. 2012, Sandvik et al. 2013, Wellman et al. 1996). However, without a known mechanism, it is difficult both to decide which parameter is more important and to optimize the antimicrobial concentration for maximum effect (Del Pozo et al. 2008).
Furthermore, although the application of electric current with antibiotics has been identified as a promising method for controlling infections, longer exposures to current may cause other issues for skin and tissue (Butterwick et al. 2007; Sandvik et al. 2013). Moreover, some of these works also report that the application of a high current density can cause a joule effect and heat up the reactor, which may promote biofilm growth rather than inhibiting it. Further research is required to understand these mechanisms and to optimize the effectiveness of the bioelectric effect.
Future directions
From the literature discussed in this review, it is clear that electrochemical biofilm control methods using applied potential or current have the potential to increase biofilm removal. This is a potential alternative and a more environment-friendly approach than the conventional chemical biofilm control approaches. In this method, electrochemical reactions generate chemical agents such as biocides near biofilm surfaces. Unlike chemical biofilm control, this allows direct exposure to biocides at the base of biofilms and may be more effective in biofilm eradication. One of the limitations of this approach may be a limited availability of reactants in the system to generate certain biocides: in practice, this may result in low concentrations of biocide and ineffective biofilm removal. However, to understand the underlying mechanism and for this method to achieve reproducibility and efficacy in future research, it is critical that the working electrode potential be reported against a standard reference electrode along with current data and that the geometry of the system be defined. Furthermore, the application of proper noninvasive tools for the detection of any toxic substances is important. With defined electrochemical parameters and a well-defined system geometry, electrochemical biofilm control can be a promising technology generating reproducible results. Electrochemical biofilm control could allow the development of an antibiotic-free biofilm treatment strategy.
Acknowledgements
This research was funded by NSF-CAREER award #0954186 and in part by a grant from the National Institute of Environmental Health Sciences (5R25ES023632). This contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring agencies.
Nomenclature
- A
area of the electrode
- Ci*
concentration of the specie
- Ci*
initial concentration of the specie
- Di
diffusivity of the chemical specie i
- F
Faraday constant
- i
net current
- i0
exchange current
- Ji
current flux
- n
number of electrons involved in the electrode reaction
- R
universal gas constant
- T
absolute temperature
- t
time
- 𝑣
solution velocity
- zi
valence of ionic species
- α
charge transfer co-efficient, dimensionless
- ɳ
activation overpotential
References
- Babauta J, Renslow R, Lewandowski Z, Beyenal H. Electrochemically active biofilms: facts and fiction. A review. Biofouling. 2012;28:789–812. doi: 10.1080/08927014.2012.710324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babauta JT, Nguyen HD, Istanbullu O, Beyenal H. Microscale gradients of oxygen, hydrogen peroxide, and pH in freshwater cathodic biofilms. Chemsuschem. 2013;6:1252–1261. doi: 10.1002/cssc.201300019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. New York: Wiley; 1980. [Google Scholar]
- Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. 2nd ed. New York (NY): John Wiley & Sons, Inc.; 2001. [Google Scholar]
- Blenkinsopp SA, Khoury AE, Costerton JW. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology. 1992;58:3770–3773. doi: 10.1128/aem.58.11.3770-3773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borole AP, Reguera G, Ringeisen B, Wang Z-W, Feng Y, Kim BH. Electroactive biofilms: current status and future research needs. Energy & Environmental Science. 2011;4:4813–4834. [Google Scholar]
- Burke V, Gibson FO. The Gram reaction and the electric charge of bacteria. Journal of Bacteriology. 1933;26:211–214. doi: 10.1128/jb.26.2.211-214.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busalmen JP, de Sanchez SR. Adhesion of Pseudomonas fluorescens (ATCC 17552) to nonpolarized and polarized thin films of gold. Applied and Environmental Microbiology. 2001;67:3188–3194. doi: 10.1128/AEM.67.7.3188-3194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busalmen JP, de Sanchez SR. Electrochemical polarization-induced changes in the growth of individual cells and biofilms of Pseudomonas fluorescens (ATCC 17552) Applied and Environmental Microbiology. 2005;71:6235–6240. doi: 10.1128/AEM.71.10.6235-6240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterwick A, Vankov A, Huie P, Freyvert Y, Palanker D. Tissue damage by pulsed electrical stimulation. Biomedical Engineering, IEEE Transactions on. 2007;54:2261–2267. doi: 10.1109/tbme.2007.908310. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Gangodawila S, Long MJ, Morris NS, Blacklock ARE, Stickler DJ. An electrified catheter to resist encrustation by Proteus mirabilis biofilm. Journal of Urology. 2005;174:1129–1132. doi: 10.1097/01.ju.0000168618.79096.cb. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrobial Agents and Chemotherapy. 1994;38:2803–2809. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison WM, Pitts B, Stewart PS. Spatial and temporal patterns of biocide action against Staphylococcus epidermidis biofilms. Antimicrobial Agents and Chemotherapy. 2010;54:2920–2927. doi: 10.1128/AAC.01734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JL, Rouse MS, Patel R. Bioelectric effect and bacterial biofilms. A systematic review. International Journal of Artificial Organs. 2008;31:786–795. doi: 10.1177/039139880803100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JL, Rouse MS, Euba G, Kang C-I, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrobial Agents and Chemotherapy. 2009a;53:4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrobial Agents and Chemotherapy. 2009b;53:35–40. doi: 10.1128/AAC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrobial Agents and Chemotherapy. 2009c;53:41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JL, Rouse MS, Euba G, Greenwood-Quaintance KE, Mandrekar JN, Steckelberg JM, Patel R. Prevention of Staphylococcus epidermidis biofilm formation using electrical current. Journal of Applied Biomaterials & Functional Materials. 2014;12:81–83. doi: 10.5301/jabfm.5000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar HP, Bockris JO, Lewis DH. Electrochemical inactivation of marine-bacteria. Journal of the Electrochemical Society. 1981;128:229–231. [Google Scholar]
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nature Reviews Microbiology. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15 doi: 10.1128/CMR.15.2.167-193.2002. 167-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freebairn D, Linton D, Harkin-Jones E, Jones DS, Gilmore BF, Gorman SP. Electrical methods of controlling bacterial adhesion and biofilm on device surfaces. Expert Review of Medical Devices. 2013;10:85–103. doi: 10.1586/erd.12.70. [DOI] [PubMed] [Google Scholar]
- Hoare JP. Electrochemistry of oxygen. 1968 [Google Scholar]
- Hong SH, Jeong J, Shim S, Kang H, Kwon S, Ahn KH, Yoon J. Effect of electric currents on bacterial detachment and inactivation. Biotechnology and Bioengineering. 2008;100:379–386. doi: 10.1002/bit.21760. [DOI] [PubMed] [Google Scholar]
- Isseroff RR, Dahle SE. Electrical stimulation therapy and wound healing: where are we now? Adv Wound Care (New Rochelle) 2012:238–243. doi: 10.1089/wound.2011.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istanbullu O, Babauta J, Nguyen HD, Beyenal H. Electrochemical biofilm control: mechanism of action. Biofouling. 2012;28:769–778. doi: 10.1080/08927014.2012.707651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J, LappinScott HM. The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms. Journal of Antimicrobial Chemotherapy. 1996;38:987–1000. doi: 10.1093/jac/38.6.987. [DOI] [PubMed] [Google Scholar]
- Kang H, Shim S, Lee SJ, Yoon J, Ahn KH. Bacterial translational motion on the electrode surface under anodic electric field. Environ Sci Technol. 2011;45:5769–5774. doi: 10.1021/es200752h. [DOI] [PubMed] [Google Scholar]
- Kissinger P, Heineman WR. Laboratory techniques in electroanalytical chemistry, revised and expanded. CRC press; 1996. [Google Scholar]
- Kumar CG, Anand SK. International Journal of Food Microbiology. Second Edition. Vol. 42. Taylor & Francis; 1998. Significance of microbial biofilms in food industry: a review; pp. 9–27. r esearch, [DOI] [PubMed] [Google Scholar]
- Liu WK, Brown MRW, Elliott TSJ. Mechanisms of the bactericidal activity of low amperage electric current (DC) Journal of Antimicrobial Chemotherapy. 1997;39:687–695. doi: 10.1093/jac/39.6.687. [DOI] [PubMed] [Google Scholar]
- Lv L, Yuan SJ, Zheng Y, Liang B, Pehkonen SO. Surface modification of mild steel with thermally cured antibacterial poly(vinylbenzyl chloride)-polyaniline bilayers for effective protection against sulfate reducing bacteria induced corrosion. Industrial & Engineering Chemistry Research. 2014;53:12363–12378. [Google Scholar]
- Matsunaga T, Lim TK. Electrochemical prevention of biofouling. Electrochemistry. 2000;68:847–852. [Google Scholar]
- Niepa THR, Gilbert JL, Ren D. Controlling Pseudomonas aeruginosa persister cells by weak electrochemical currents and synergistic effects with tobramycin. Biomaterials. 2012;33:7356–7365. doi: 10.1016/j.biomaterials.2012.06.092. [DOI] [PubMed] [Google Scholar]
- Oldham KB, Myland JC. Fundamentals of electrochemical science. Academic Press; 1994. [Google Scholar]
- Patil RS, Juvekar VA, Naik VM. Oxidation of chloride ion on platinum electrode: dynamics of electrode passivation and its effect on oxidation kinetics. Industrial & Engineering Chemistry Research. 2011;50:12946–12959. [Google Scholar]
- Russell AD. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infectious Diseases. 2003;3:794–803. doi: 10.1016/s1473-3099(03)00833-8. [DOI] [PubMed] [Google Scholar]
- Sandvik EL, McLeod BR, Parker AE, Stewart PS. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PloS one. 2013;8:e55118. doi: 10.1371/journal.pone.0055118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Malan SM, Karau MJ, Cede J, Greenwood-Quaintance KE, Brinkman CL, Mandrekar JN, Patel R. Anti-biofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrobial Agents and Chemotherapy. 2015 doi: 10.1128/AAC.00483-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtliff ME, Bargmeyer A, Camper AK. Assessment of the ability of the bioelectric effect to eliminate mixed-species biofilms. Applied and Environmental Microbiology. 2005;71:6379–6382. doi: 10.1128/AEM.71.10.6379-6382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Wattanakaroon W, Goodrum L, Fortun SM, McLeod BR. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrobial Agents and Chemotherapy. 1999;43:292–296. doi: 10.1128/aac.43.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Roe F, Rayner J, Elkins JG, Lewandowski Z, Ochsner UA, Hassett DJ. Effect of catalase on hydrogen peroxide penetration into Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology. 2000;66:836–838. doi: 10.1128/aem.66.2.836-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Rayner J, Roe F, Rees WM. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. Journal of Applied Microbiology. 2001;91:525–532. doi: 10.1046/j.1365-2672.2001.01413.x. [DOI] [PubMed] [Google Scholar]
- Sun Y-S, Peng S-W, Cheng J-Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics. 2012;6:034117. doi: 10.1063/1.4750486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chen Z. Making of rechargeable antimicrobial anti-biofilm product used in e.g. medical devices for preventing growth of e.g. bacteria, involves adding N-halamine biocidal compounds comprising rings and nitrogen heteroatom(s) to target material. 2007 [Google Scholar]
- Tkachenko O, Karas JA. Standardizing an in vitro procedure for the evaluation of the antimicrobial activity of wound dressings and the assessment of three wound dressings. Journal of Antimicrobial Chemotherapy. 2012;67:1697–1700. doi: 10.1093/jac/dks110. [DOI] [PubMed] [Google Scholar]
- Ueshima M, Tanaka S, Nakamura S, Yamashita K. Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. Journal of Biomedical Materials Research. 2002;60:578–584. doi: 10.1002/jbm.10113. [DOI] [PubMed] [Google Scholar]
- van der Borden AJ, van der Werf H, van der Mei HC, Busscher HJ. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Applied and Environmental Microbiology. 2004;70:6871–6874. doi: 10.1128/AEM.70.11.6871-6874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Borden AJ, van der Mei HC, Busscher H. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials. 2005;26:6731–6735. doi: 10.1016/j.biomaterials.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Vetter KJ. Electrochemical kinetics: theoretical and experimental aspects. New York: Academic Press; 1967. [Google Scholar]
- Wang J. Analytical electrochemistry. John Wiley & Sons; 2006. [Google Scholar]
- Wellman N, Fortun SM, McLeod BR. Bacterial biofilms and the bioelectric effect. Antimicrobial Agents and Chemotherapy. 1996;40:2012–2014. doi: 10.1128/aac.40.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Neoh KG, Hu X, Kang E-T. Mechanistic insights into response of Staphylococcus aureus to bioelectric effect on polypyrrole/chitosan film. Biomaterials. 2014;35:7690–7698. doi: 10.1016/j.biomaterials.2014.05.069. [DOI] [PubMed] [Google Scholar]


