Abstract
Transcriptome studies have revealed that protein-coding loci within the human genome are overlapped at their 3′-termini by noncoding RNA (ncRNA) transcripts. Small duplex RNAs designed to be fully complementary to these 3′ ncRNAs can modulate transcription of the upstream gene. Robust regulation by designed RNAs suggests that endogenous small RNAs might also recognize 3′ ncRNAs and regulate gene expression. A genome-wide evaluation revealed that sequences immediately downstream of protein-coding genes are enriched with miRNA target sites. We experimentally tested miRNA mimics complementary to the well-characterized 3′-terminus of the human progesterone receptor (PR) gene and observed inhibition of PR transcription. These results suggest that recognition of ncRNA transcripts that overlap gene termini may be a natural function of endogenous small RNAs.
Introduction
Recent studies have revealed unexpectedly complex transcription within the human genome. These investigations have shown that many protein-coding genes are flanked at both their 5′ and 3′ termini by noncoding (ncRNA) transcripts.1,2 These transcripts are produced in both the sense and antisense direction with respect to the adjacent gene. The natural biological function of these ncRNAs has not been determined, however their proximity to protein-coding regions suggest they might be involved in regulating gene expression.
Consistent with reports of complex transcription at gene loci, we have identified ncRNA transcripts that overlap either the promoter or terminus of the human progesterone receptor (PR) gene.3,4 We have shown that small duplex RNAs that are fully complementary to these transcripts can modulate transcription of the PR gene in a potent and robust fashion.3–9 Our experiments have demonstrated that these small RNAs recruit argonaute 2 (AGO2) to their respective target ncRNAs and alter levels of RNA Pol II at the PR gene transcription start site.3–5,7
Initially, modulation of transcription by small RNAs complementary to sequences beyond the 3′ terminus of the PR gene was puzzling because approximately 1 00 000 bases separate the target site from the gene's transcription start site. Experiments have revealed, however, that the PR locus loops and juxtaposes the transcription start site with the gene terminus.4 This looping occurs in multiple cell lines independent of whether the PR gene is expressed at high or low levels, a modulatory small RNA is present, or biological stimuli are applied to cells. The proximity of the 5′ and 3′ gene termini provides a direct path allowing transcriptional modulation by small RNAs targeted to distal genomic regions.
MicroRNAs (miRNAs) are endogenous small RNAs that can recognize sequences within 3′-UTRs of mRNA transcripts.10,11 In addition, recent reports have shown that miRNAs can target ncRNAs that overlap gene promoters and subsequently regulate transcription.12–16 Our observation of efficient gene regulation by duplex RNAs that are fully complementary to sequences downstream of gene termini suggests that miRNAs might also target these sequences, although recognition could occur through partial complementarity to their target sequences. To test this hypothesis we investigated the genome-wide occurrence of miRNA target sites within sequences immediately downstream of protein-coding loci and tested whether miRNAs complementary to the well-characterized ncRNA overlapping the 3′ terminus of the PR gene could modulate gene expression.
Results
Detecting miRNA target sites within sequences downstream of gene termini
To evaluate the potential for miRNAs to target the 3′-termini of protein-coding genes we developed a miRNA target prediction algorithm. A major determinant of miRNA targeting is perfect base complementarity between the miRNA seed sequence (bases 2–8 of the mature miRNA) and its target sequence. Our algorithm searches for miRNA seed sequence matches and, if found, calculates additional metrics for comparison (Fig. 1A). First, we determine the minimum free energy of hybridization (MFE) between the miRNA and its target sequences. Second, we calculate a complementarity score using the Needleman-Wunsch algorithm with a scoring matrix optimized for small RNA interactions.17 While these values are not mutually exclusive, they provide two independent criteria for comparison.
Fig. 1.
Computational approach for identifying miRNAs that target ncRNAs produced from sequences downstream of gene termini. (A) Schematic of algorithm used to identify potential miRNA target sites. (B) Diagram of sequences analyzed for miRNA target sites.
Our algorithm incorporates several additional layers of sequence analysis for predicted miRNA targets. First, we discriminate unique target sequences from those that occur within repeat elements. Next, we determine cross-species conservation values for target sequences using the PhastCons analysis of the multiz alignment of 44 vertebrate species.18 Finally, we analyze the predicted RNA secondary structure of target sequences calculated using EvoFold.19
miRNA sequences were obtained from miRBase, the public repository for miRNAs.20 Gene termini sequences were obtained from the UCSC genome browser and consisted of the 1000 bases immediately downstream of the annotated transcription termination site for each gene in the human genome. We used the genomic sequences of gene termini to construct sequence sets corresponding to potential ncRNA transcripts in both the sense and antisense orientations relative to their upstream gene (Fig. 1B). We also obtained sequences corresponding to the 3′-UTR of all annotated genes to compare the frequencies of matches within these datasets.
Sequences downstream of gene termini are enriched with putative miRNA target sites
We used our algorithm to perform a genome-wide evaluation of potential miRNA target sites within putative ncRNAs produced from sequences downstream of gene termini. In addition, we compared these results to predicted target sites within 3′-UTRs. We calculated the total number of seed sequence matches within each dataset and normalized the values to the number of bases within each respective dataset. We found that the frequencies of predicted miRNA target sites within downstream ncRNAs, in both the sense and anti-sense orientations, were indistinguishable from those within 3′-UTRs (Fig. 2A).
Fig. 2.
Regions beyond gene termini are enriched with putative miRNA target sites. (A) Relative frequencies of seed sequence matches within 3′-UTRs and sequences downstream of gene termini. (B) Enrichment of seed sequence matches within 3′-UTRs and sequences downstream of gene termini with respect to randomized sequences. (C) Examples of miRNAs that are highly complementary to regions downstream of gene termini (seed sequences show in red). Alignments allow for G–U base pairing. **P < 0.01.
To determine if ncRNAs overlapping gene termini are enriched with predicted miRNA target sites we compared the frequency of seed matches in our initial analysis to the frequency with which they occur in randomized sequences. Each sequence in the dataset was subjected to 100 iterations of randomization followed by screening for the number of seed sequence matches. We observed a significant enrichment of potential miRNA target sites within both sense and antisense oriented ncRNAs with respect to randomized sequences (Fig. 2B). This enrichment was comparable to that of predicted miRNA target sites within 3′-UTRs (Fig. 2B).
We identified many miRNAs with a striking degree of complementarity to their predicted target sites. A selected subset of predicted miRNA target sites within both sense and antisense oriented ncRNAs are shown in Fig. 2C. Each alignment is accompanied by the location of the target site with respect to the transcription termination site for the adjacent gene along with the MFE value for the alignment. Taken together, these results suggest that ncRNAs produced from sequences downstream of gene termini are promising candidates for miRNA targets.
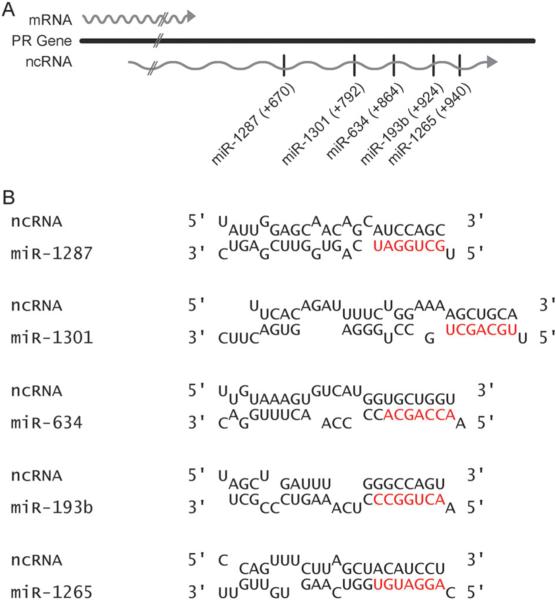
miRNA target sites within the ncRNA overlapping the PR gene terminus
Our computational results prompted us to experimentally evaluate the possibility that miRNAs can target ncRNAs produced from sequences downstream of gene termini. We have previously used the human PR gene as a model system for studying transcriptional regulation by small RNAs. While characterizing the transcriptional landscape of the PR locus in our prior studies we identified a 3.2 kb ncRNA transcript that overlaps the 3′-terminus of the gene and is expressed at ~4% relative to PR mRNA.4 The ncRNA is transcribed in the same direction as the PR gene and shares 1.7 kb of sequence content with PR mRNA. The remaining 1.5 kb of the transcript is unique to the ncRNA. Several assays including RT-PCR, rapid amplification of cDNA ends (RACE), and branched DNA (bDNA) verified that the ncRNA is not an extension of PR mRNA.
Our algorithm identified 84 putative miRNA target sites within the unique region of the ncRNA. To test the hypothesis that miRNAs can regulate gene expression by recognizing sequences downstream of gene termini, we selected a subset of 5 miRNAs that displayed a high degree of complementarity to the target ncRNA (Fig. 3A and B). We designed miRNA mimics corresponding to each miRNA that consisted of the miRNA sequence and a fully complementary RNA carrier strand. The miRNA mimics were transfected into T47D breast cancer cells and PR protein expression was monitored. An siRNA targeting PR mRNA (PRC1) and a duplex RNA previously shown to target the terminus-overlapping transcript and inhibit PR expression (PR13580) were used as positive controls for modulation of PR expression.4 A mismatched duplex RNA (MM) that does not affect PR expression was used as a negative control to demonstrate the specificity for recognition of the target ncRNA.
Fig. 3.
miRNA target sites within the ncRNA that overlaps the PR gene terminus. (A) Schematic of selected miRNA target sites within the target ncRNA. (B) Alignments of selected miRNAs with the target ncRNA overlapping the PR gene terminus (seed sequences shown in red). Alignments allow for G–U base pairing.
Of the 5 miRNA mimics tested, we observed that the miR-193b mimic was the most promising inhibitor of PR protein expression (Fig. 4A). miR-193b has a single predicted target site within the target ncRNA roughly 1000 bases downstream of the transcription termination site of the PR gene and no target sites within PR mRNA. Inhibition of PR by the miR-193b mimic was potent and dose dependent, with an IC50 near 15 nM (Fig. 4B and C), a value similar to the previously-determined IC50 for PRC1 (11.5 nM) or PR13580 (10.7 nM).
Fig. 4.
miR-193b inhibits PR protein expression. (A) Western analysis showing inhibition of PR protein expression by miRNA mimics. miRNA mimics and siRNAs were added to cells at 25 nM. (B) Dose dependent inhibition of PR protein expression by miR-193b. (C) Quantification of multiple dose response experiments.
Inhibition of transcription by miRNA mimics that target the ncRNA overlapping the PR terminus
To determine if inhibition of PR was occurring at the level of RNA we used quantitative RT-PCR (RT-qPCR) to evaluate expression of PR mRNA. We observed a 70% reduction in PR mRNA expression after treatment with the miR-193b mimic which was similar to the level achieved by fully complementary RNA PR13580 (Fig. 5A). Designed inhibitory small RNAs that are fully complementary to the PR terminus-overlapping ncRNA also decrease expression of the target ncRNA. Using RT-qPCR we found that the miR-193b mimic decreased expression of the ncRNA by 70%, which again was similar to the levels observed following treatment with PR13580 (Fig. 5B).
Fig. 5.
miR-193b silences transcription of the PR gene. (A) RT-qPCR measuring PR mRNA expression. (B). RT-qPCR measuring expression of the ncRNA overlapping the PR gene terminus. (C) RT-qPCR measuring PR hnRNA expression. (D) ChIP-qPCR measuring Pol II occupancy on the PR promoter. miRNA mimics and siRNAs were added to cells at 25 nM. Error bars indicate s.d. (n = 3). *P < 0.05, **P < 0.01. P-values were calculated using the two-tailed unpaired Student's T-test with equal variances.
To investigate whether inhibition of PR was occurring at the level of transcription we used two independent assays. First, we used RT-qPCR to monitor expression of pre-spliced PR mRNA, also termed heteronuclear RNA (hnRNA). We observed a 50% decrease in PR hnRNA expression following treatment with the miR-193b mimic (Fig. 5C). Second, we used chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR) to measure RNA Polymerase II (Pol II) occupancy on the PR promoter. Treatment with the miR-193b mimic decreased Pol II occupancy by greater than 80% (Fig. 5D). Taken together, these results indicate that miR-193b is capable of targeting the ncRNA overlapping the PR gene terminus and regulating transcription of the PR gene.
Discussion
miRNAs have typically been associated with recognition of mRNA transcripts, usually at sequences within 3′-UTRs. miRNA binding results in destabilization of the target mRNA and ultimately reduction in target gene expression.21 It has become clear that miRNAs are powerful regulators of gene expression at the post-transcriptional level.
Transcriptome profiling studies, however, have transformed our current understanding of cellular RNAs. These studies have found that many regions of the genome that do not encode protein still produce RNA transcripts which are termed noncoding RNAs (ncRNAs). While some of these ncRNAs are directly involved in transcriptional regulation, the molecular function of most ncRNAs remains unclear.22–29 In addition, it has been proposed that certain classes of ncRNAs may be promising targets for the development of therapeutics.30 Regardless of ncRNA function, it does not appear that miRNAs can discriminate their targets based on protein coding potential and it is reasonable to hypothesize that many ncRNAs may be targeted by miRNAs.31
These transcriptome profiling studies have revealed that promoter regions of protein-coding loci are overlapped by ncRNA transcripts.1,2 These ncRNAs could serve as miRNA targets and the fact that they overlap key regulatory regions of the genome suggests that recognition by miRNAs may affect gene expression. Indeed, several reports have characterized miRNAs that target promoter-overlapping ncRNAs and subsequently regulate transcription of the downstream gene.12–14 The ability of miRNAs to target gene promoters not only increases the sequence space available for targeting, it also complements the well-known role of miRNAs in post-transcriptional gene regulation.
Robust transcriptional regulation by miRNAs that target ncRNAs overlapping gene promoters prompted us to search for additional classes of ncRNAs that might serve as targets for small RNAs. Specifically, we focused on ncRNAs that overlap gene termini. Terminus-overlapping ncRNAs, similar to promoter-overlapping RNAs, are a common feature of protein-coding loci and their proximity to protein-coding genes also suggests that they may play a role in gene regulation. Furthermore, using the PR gene as a model system we have been able to identify small duplex RNAs that are fully complementary to a ncRNA that overlaps the PR gene terminus and are capable of regulating PR transcription.
In this study we have found that putative ncRNA transcripts that overlap gene termini are significantly enriched with predicted miRNA target sites. In addition, we have experimentally tested a subset of miRNAs that display a high degree of complementarity to a ncRNA overlapping the PR gene terminus and found that synthetic miR-193b is a potent inhibitor of PR expression. In contrast to our previous studies with designed small RNAs where introduction of mismatched bases disrupted silencing activity, miR-193b is extensively mismatched relative to its target sequence. In addition, our designed small RNAs targeted just over 500 bases downstream of the PR gene while miR-193b targets almost 1000 bases downstream. While we did not investigate involvement of proteins in this study, we had previously observed that AGO2 is required for recognition of promoter-overlapping ncRNAs by miRNA mimics.16
It is important to note that we have used exogenously added miRNA mimics as a proof-of-principle for the ability of miRNAs to act as transcriptional regulators. This approach has allowed us to take advantage of our previous detailed characterization of transcription at the PR locus. Identifying endogenous examples of miRNAs that target gene termini will be the focus of future experiments.
Our findings extend miRNA function to the recognition of sequences downstream of gene termini and also highlight a largely unappreciated role for gene termini in transcriptional regulation. However, miRNAs that target gene termini must overcome some mechanistic hurdles before they can regulate transcription of the upstream gene. For example, at the PR locus more than 100 kb separates the promoter from the gene's terminus. Experiments have revealed, however, that the PR promoter and terminus are in close physical proximity. Such gene looping is relatively common and may provide a scaffold for communication between miRNAs that target gene termini and the transcription machinery at the promoter of the upstream gene (Fig. 6).32–34
Fig. 6.
Model for mechanism. miRNAs can target ncRNA transcripts that overlap gene termini. Gene looping allows this interaction to occur in close physical proximity to gene promoters and affects transcription of the upstream gene.
As more comprehensive 3-dimensional maps of the human genome are compiled, it will be interesting to evaluate the ability of miRNAs to regulate transcription of a given gene when targeted to more distal or even inter-chromosomal regions of the genome.35 The ability to integrate signals over large genomic distances raises the possibility that miRNAs may play a role in long-range chromatin interactions and overall genome structure.
Materials and methods
Sequence analysis
miRNA sequences were obtained from miRBase (Release 13.0) and gene sequences were obtained from Genome Browser (Mar. 2006 genome assembly). Sequences overlapping gene termini were defined as the 1000 bases downstream of the annotated 3′ end of the gene. All gene sequences were searched for perfect complementarity to miRNA seed sequences (bases 2–8 of the mature miRNA sequence). The total number of seed matches was calculated and compared to total seed matches within 100 iterations of randomized sequences (randomized from actual 1000 bases downstream of gene termini). Minimum free energy (MFE) of hybridization values were calculated for miRNA sequences and sequences overlapping gene termini that contained seed matches. MFE values were calculated using RNAhybrid. Cross-species conservation values were obtained from the PhastCons analysis of 44 vertebrate genomes. RNA secondary structure was predicted using EvoFold.
Cell culture
T47D breast cancer cells (American Type Culture Collection, ATCC) were maintained in RPMI-1640 medium (ATCC) supplemented with 10% (v/v) FBS (Atlanta Biologicals), 0.5% (v/v) nonessential amino acids (Sigma), 10 mM HEPES (Sigma), 1 mM Sodium Pyruvate (Sigma), 0.4 units ml−1 bovine insulin (Sigma). Cells were cultured at 37 °C and 5% CO2.
Cellular delivery of miRNA mimics and siRNAs
RNAiMAX (Invitrogen) was used to deliver small duplex RNAs into T47D cells as per the manufacturer's instructions. For RNA and protein isolation, cells were plated in 6-well dishes at densities ranging between 150 K–200 K cells/well. For chromatin immunoprecipitation, cells were plated in 10 cm2 dishes at a density of 4.5 × 106 cells/dish. Cells were transfected 48 h after plating. Sequences for miRNA mimics and siRNAs are listed in Supplementary Table 1A.†
Western blotting
Cells were harvested 5 days post-transfection for protein isolation. Cell pellets were lysed and protein concentrations were quantified by BCA assay (Pierce). Western blots were performed on protein lysates (30 mg per well). Primary antibodies used were α-PR (Cell Signaling Technology) and α-β-actin (Sigma). Protein was visualized with horseradish peroxidase-conjugated α-mouse secondary antibody (Jackson Immunolabs) and Supersignal developing solution (Pierce).
Quantitative PCR
Cells were harvested 72 h post-transfection for RNA isolation. RNA was isolated using TRI Reagent (Sigma) as per the manufacturer's instructions. For each sample, 2 μg of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RNA was treated with DNase I (Worthington) prior to reverse transcription. qPCR was performed on an ABI7900 real-time PCR (Applied Biosystems) using iTaq SYBR Green Supermix (Bio-Rad). Primers for PR and GAPDH mRNA were supplied by Applied Biosystems. All additional primers were designed using Primer3. Only those primer sets that showed linear amplification over several orders of magnitude were used for quantification. Primers and PCR conditions are listed in Supplementary Table 1B.†
Chromatin immunoprecipitation
ChIP was performed essentially as described.36 Cells were harvested 72 h post-transfection. α-RNA Polymerase II and normal mouse IgG antibodies were supplied by Millipore. Primers for the PR promoter and GAPDH promoter were designed using Primer3. Only those primer sets that showed linear amplification over several orders of magnitude were used for quantification. Primers and PCR conditions are listed in Supplementary Table 1B.†
Statistical analysis
Data are presented as means ± standard deviation of three or more independent results. Statistical significance was assessed using a two-tailed unpaired Student's t-test.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (NIGMS 77253 to DRC), The Robert A. Welch Foundation (I-1244), Alnylam Pharmaceuticals, and an NIH Pharmacological Sciences Training Grant (GM07062 to STY).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c1mb05090g
References
- 1.Gingeras TR. Genome Res. 2007;17:682–690. doi: 10.1101/gr.6525007. [DOI] [PubMed] [Google Scholar]
- 2.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz JC, Younger ST, Nguyen NB, Hardy DB, Monia BP, Corey DR, Janowski BA. Nat. Struct. Mol. Biol. 2008;15:842–848. doi: 10.1038/nsmb.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yue X, Schwartz JC, Chu Y, Younger ST, Gagnon KT, Elbashir S, Janowski BA, Corey DR. Nat. Chem. Biol. 2010;6:621–629. doi: 10.1038/nchembio.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu Y, Yue X, Younger ST, Janowski BA, Corey DR. Nucleic Acids Res. 2010;38:7736–7748. doi: 10.1093/nar/gkq648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janowski BA, Huffman KE, Schwartz JC, Ram R, Hardy D, Shames DS, Minna JD, Corey DR. Nat. Chem. Biol. 2005;1:216–222. doi: 10.1038/nchembio725. [DOI] [PubMed] [Google Scholar]
- 7.Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, Minna JD, Corey DR. Nat. Struct. Mol. Biol. 2006;13:787–792. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- 8.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Nat. Chem. Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 9.Younger ST, Corey DR. ChemBioChem. 2009;10:1135–1139. doi: 10.1002/cbic.200900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambros V. Nat. Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, Saetrom P, Snove O, Jr., Rossi JJ. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16230–16235. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majid S, Dar AA, Saini S, Yamamura S, Hirata H, Tanaka Y, Deng G, Dahiya R. Cancer. 2010;116:5637–5649. doi: 10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younger ST, Pertsemlidis A, Corey DR. Bioorg. Med. Chem. Lett. 2009;19:3791–3794. doi: 10.1016/j.bmcl.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younger ST, Corey DR. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr155. DOI: 10.1093/nar/gkr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Needleman SB, Wunsch CD. J. Mol. Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 18.Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, Weinstock GM, Wilson RK, Gibbs RA, Kent WJ, Miller W, Haussler D. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen JS, Bejerano G, Siepel A, Rosenbloom K, Lindblad-Toh K, Lander ES, Kent J, Miller W, Haussler D. PLoS Comput. Biol. 2006;2:e33. doi: 10.1371/journal.pcbi.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, Ingolia NT, Weissman JS, Bartel DP. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 23.Hirota K, Miyoshi T, Kugou K, Hoffman CS, Shibata T, Ohta K. Nature. 2008;456:130–134. doi: 10.1038/nature07348. [DOI] [PubMed] [Google Scholar]
- 24.Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- 25.Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 26.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wutz A, Smrzka OW, Schweifer N, Schellander K, Wagner EF, Barlow DP. Nature. 1997;389:745–749. doi: 10.1038/39631. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahlestedt C. Drug Discovery Today. 2006;11:503–508. doi: 10.1016/j.drudis.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C, Fabbri M, Cimmino A, Lee EJ, Wojcik SE, Shimizu M, Tili E, Rossi S, Taccioli C, Pichiorri F, Liu X, Zupo S, Herlea V, Gramantieri L, Lanza G, Alder H, Rassenti L, Volinia S, Schmittgen TD, Kipps TJ, Negrini M, Croce CM. Cancer Cell. 2007;12:215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan JM, Tan-Wong SM, Morillon A, Lee B, Coles J, Mellor J, Proudfoot NJ. Nat. Genet. 2004;36:1014–1018. doi: 10.1038/ng1411. [DOI] [PubMed] [Google Scholar]
- 33.Tan-Wong SM, French JD, Proudfoot NJ, Brown MA. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5160–5165. doi: 10.1073/pnas.0801048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiwari VK, McGarvey KM, Licchesi JD, Ohm JE, Herman JG, Schubeler D, Baylin SB. PLoS Biol. 2008;6:2911–2927. doi: 10.1371/journal.pbio.0060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts JK, Yu D, Charisse K, Montaillier C, Potier P, Manoharan M, Corey DR. Nucleic Acids Res. 2010;38:5242–5259. doi: 10.1093/nar/gkq258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.