Abstract:
Background:
The effects of different modes of mechanical ventilation in the same ventilatory support level on ventilator-induced diaphragm dysfunction onset were assessed in healthy rabbits.
Methods:
Twenty New Zealand rabbits were randomly assigned to 4 groups (n = 5 in each group). Group 1: no mechanical ventilation; group 2: controlled mechanical ventilation (CMV) for 24 hours; group 3: assist/control ventilation (A/C) mode for 24 hours; group 4: high-level pressure support ventilation (PSV) mode for 24 hours. Heart rate, mean arterial blood pressure, PH, partial pressure of arterial oxygen/fraction of inspired oxygen and partial pressure of arterial carbon dioxide were monitored and diaphragm electrical activity was analyzed in the 4 groups. Caspase-3 was evaluated by protein analysis and diaphragm ultra structure was assessed by electron microscopy.
Results:
The centroid frequency and the ratio of high frequency to low frequency were significantly reduced in the CMV, A/C and PSV groups (P < 0.001). The percent change in centroid frequency was significantly lower in the PSV group than in the CMV and A/C groups (P = 0.001 and P = 0.028, respectively). Electromyography of diaphragm integral amplitude decreased by 90% ± 1.48%, 67.8% ± 3.13% and 70.2% ± 4.72% in the CMV, A/C and PSV groups, respectively (P < 0.001). Caspase-3 protein activation was attenuated in the PSV group compared with the CMV and A/C groups (P = 0.035 and P = 0.033, respectively). Irregular swelling of mitochondria along with fractured and fuzzy cristae was observed in the CMV group, whereas mitochondrial cristae were dense and rich in the PSV group. The mitochondrial injury scores (Flameng scores) in the PSV group were the lowest among the 3 ventilatory groups (0.93 ± 0.09 in PSV versus 2.69 ± 0.05 in the CMV [P < 0.01] and PSV versus A/C groups [2.02 ± 0.08, P < 0.01]).
Conclusions:
The diaphragm myoelectric activity was reduced in the PSV group, although excessive oxidative stress and ultra-structural changes of diaphragm were found. However, partial diaphragm electrical activity was retained and diaphragm injury was minimized using the PSV mode.
Key Indexing Terms: Diaphram electric activity, Caspase-3, Injury, Mechanical ventilation, Pressure support ventilation, Modes, Diaphragm, Mitochondrial injury
Overwhelming evidence has shown that controlled mechanical ventilation (CMV) induces inactivity of the diaphragm, which activates pathophysiological cascades leading to a loss of contractile force and muscle mass. This phenomenon is referred to as ventilator-induced diaphragm dysfunction (VIDD). VIDD has been observed not only to occur in animals but also in humans.1–3 Some studies have shown that pressure support ventilation (PSV) reduces the onset of VIDD due to retention of diaphragm motion.4,5 However, diaphragm electrical activity (Edi) characteristics under different modes of ventilation, set at the same level of ventilator support, are still unknown. Although various modes of ventilation at the identical levels of ventilatory support may all cause VIDD during PSV, the patient triggers each breath and the ventilator delivers a flow up to a preset pressure limit. Thus, it is possible that Edi characteristics, and impact on the diaphragm, are different with PSV compared with CMV and assist/control ventilation (A/C). To date, there has not been sufficient evidence to confirm this phenomenon.
The objective of this study was to explore the effects of PSV on the diaphragm in an animal model and to compare PSV with other modes of mechanical ventilation with all modes providing the same level of support. The data presented herein may provide guidance when selecting a mode of mechanical ventilation.
MATERIALS AND METHODS
Ethics Statement
The study was conducted with the approval of the Animal Care Committee of Zhejiang University (China). All animal procedures were performed in accordance with National Research Council's Guide for the Care and Use of Laboratory Animals.6
Animals
Twenty New Zealand White (NZW) rabbits, weighing 2.0 to 2.3 kg, were used in this study. All animals were randomized to 4 groups, according to a random numbers table. Rabbits were anesthetized with 4 mL/kg of 8% chloral hydrate (Shanghai Jinjinle Industry Co, Shanghai, China) administered through intraperitoneal injection. After reaching a surgical plane of anesthesia, each animal was weighed and intubated (inner diameter of 4 mm) through a tracheostomy. A flow sensor was connected to the endotracheal tube and ventilator circuit. Two 20-gauge catheters were placed in 2 marginal ear veins for fluid and drug administration. One catheter was for saline (0.9% saline) and the other was for the sodium midazolam (1 mg·kg−1·hr−1) also used, which provided mild sedation that preserved the drinking reflex. This ensured consistency with the clinical situation and reduced the effect of the sedatives on respiration. Another catheter was inserted into the left common carotid artery to monitor blood pressure and heart rate and for sampling of arterial blood gases, including PH, partial pressure of arterial carbon dioxide (PaCO2) and partial pressure of arterial oxygen. A laparotomy was performed during the experiment and a pair of wire electrodes was inserted into the 2 flanks of the central tendon portion of the diaphragm for measurement of diaphragmatic electrical activity (RM6240 multi-channel physiological signal acquisition and processing systems, Chengdu Medical Instrument Company, Chengdu, China). All surgical procedures were performed using aseptic techniques. Blood samples were taken from all animals (Cobas B 221 Blood Gas System; Roche Ltd.) at baseline (0 hours) and after 12 and 24 hours of mechanical ventilation. The settings for the ventilation rate were adjusted according to the ABG results. Body temperature was monitored and maintained at 37 ± 1°C with a heat lamp. During the experimental period, animals were kept in a humidity controlled, adequately insulated, quiet environment. Prevention of lung atelectasis was also ensured.
Experimental Groups
Animals were randomly assigned to 4 groups (n = 5 per group) and the experiment time was 24 hours.7 Group 1: nonventilated animals (Control); group 2: CMV; group 3: A/C; and group 4: PSV. Animals were mechanically ventilated using a 840 Puritan Bennett Ventilator with a neonate circuit (Neonatal type: Tyco Healthcare Group LP Nellcor Puritan Bennett Division, Ireland, England). The inspired air was humidified.
Ventilator Settings
Control Group: animals were sedated for 24 hours without mechanical ventilation.
CMV Group (volume control ventilation–control mechanical ventilation, VCV–CMV): animals were mechanically ventilated for 24 hours using time-triggered and volume-target ventilation. The tidal volume was 10 mL/kg of body weight and the respiratory rate (RR) was 35 to 40 breaths per minute with a fraction of inspired oxygen (FiO2) of 21% and 1 cmH2O positive end-expiratory pressure (PEEP). RR was adjusted to maintain PaCO2 within 35 to 45 mm Hg.
A/C Group (pressure control ventilation-assist/control, PCV-A/C): animals were mechanically ventilated using time triggered and volume target ventilation (10 mL/kg) and assessed plateau pressure (Pplat). The ventilator was then changed to A/C mode and was time/flow triggered. The pressure target was set according to Pplat in the CMV mode (range from 10 to 12 cmH2O). The trigger was 0.1 L/min; the RR was 35 to 40 breaths per minute, with a FiO2 of 21% and 1 cmH2O PEEP. Inspiration time (Ti) 0.5 seconds and the RR was adjusted to maintain PaCO2 within 35 to 45 mm Hg.
PSV Group: animals were mechanically ventilated using time triggered and volume target ventilation (10 mL/kg) and Pplat was assessed. The ventilator was then changed to PSV mode. The pressure target was set according to Pplat in the CMV mode (range from 10 to 12 cmH2O). The flow trigger was 0.1 L/min, with a FiO2 of 21% and 1 cmH2O PEEP. The expiratory trigger was fixed at 25% of expiration peak flow. Back-up settings were the same as in the A/C group.
Main Outcome Measures
All animals were euthenized after 24 hours. The diaphragm of each animal was then quickly removed and split into 3 portions. One section was frozen in liquid nitrogen and stored at −80°C for caspase-3 activity detection by Western blot assay and the 2nd section was immersed in 10% formalin for qualitative observation of caspase-3 protein expression elevation through immunohistochemistry since caspase-3 has been extensively characterized as a regulator of cellular DNA fragmentation in numerous models in skeletal muscle atrophy.8 A third section was dipped in 2.5% glutaraldehyde solution at 4°C for observation of diaphragm cell ultra-microstructure through electron microscopy.
Electromyography of Diaphragm Analysis
Diaphragmatic EMG included measurements of low frequency (L) electrical activity (20 ∼ 40 Hz), high frequency (H) electrical activity (150 ∼ 350 Hz) and centroid frequency (Fc, Hz). The ratio of high frequency to low frequency power (H/L) of the diaphragmatic EMG was calculated. The electromyography of diaphragm integral amplitude was analyzed.
Caspase-3 Protein Expression Detection by Western Blot
Activation of caspase-3 protein expression was quantified using a Western blot. The procedure was as follows: extraction of total protein in the diaphragm, determination of protein content, sodium dodecyl sulfate polyacrylamide gel electrophoresis, transfer film, immune response, chemiluminescence and imaging and analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference antibody. Quantity 1 software, using Western Blot protein bands, was used to calculate the gray value of a relative expression level of gray scale values. In other words, the target protein expression level = the target band gray value/reference material strip gray value. Anticaspase 3 activated polyclonal antibody was obtained from Abcam Company (USA). The blot contained evidence of AB binding.
Immunohistochemistry (IHC) Analysis
Caspase-3 expression was observed by IHC in the diaphragm cells. Active caspase-3 protein expression was assessed in the diaphragm muscle. Ten high-power fields were randomly observed and at least 1,000 cells were counted. Scoring was done according to high-power, field-positive cell percentage as follows: ≤10% positive cells = 0, 10% to 50% positive cells = 1, 51%–75% positive cells = 2 and ≥75% positive cells = 3 points. Cells were also scored according to color intensity: no coloring = 0 points, weakly stained, pale yellow = 1 point, medium yellow = 2 points; intensely stained, brownish yellow = 3 points. The product of the 2 scores (defined as Apoptotic index, AI) determined the level of protein expression: <4 points was considered low expression and ≥6 points was considered high expression.
Analysis of Diaphragm Ultra-Structure Injury Through Electron Microscopy (EM)
Diaphragm samples were fixed in a 2.5% glutaraldehyde solution for subsequent assay. The fixative was discarded and tissues were then dehydrated, embedded and sliced. Tissues were imaged using an electron microscope (JEM-1200EX; JEOL Company, Japan). Ultra-structural changes of the diaphragm tissue were observed and documented.
Mitochondrial injury scores (Flameng scores) were evaluated to assess the extent of mitochondrial damage. Five fields were obtained from every sample (20 mitochondria were selected in every field) for quantitative analysis. Scoring was done as follows: Grade 0 (0 points): mitochondrial structures are normal and their particles are intact. Grade I (1 point): mitochondrial structures are normal, but the particles are lost. There is also mild swelling, reduced density matrix and ridge separation; grade II (2 points): mitochondrial swelling (slight swelling), but the matrices are transparent and clear. Grade III (3 points): breaks in mitochondrial ridges and the matrix solidification. Grade IV (4 points): extensive destruction of mitochondrial cristae with the inside and outside of the membrane ruptured and incomplete.
Statistical Analysis
SPSS software (Version 19.0; IBM Corp, Armonk, NY) was used for analysis. Descriptive data are reported as either mean ± SD or number and percentage. Normal distribution of the data was assessed using the Kolmogorov–Smirnov test. Differences between various modes were evaluated using one-way analysis of variance. In the case of significant results, a post hoc multiple comparison analysis was performed using the Bonferroni correction. P < 0.05 was considered statistically significant.
RESULTS
Systemic and Physiological Responses to Mechanical Ventilation
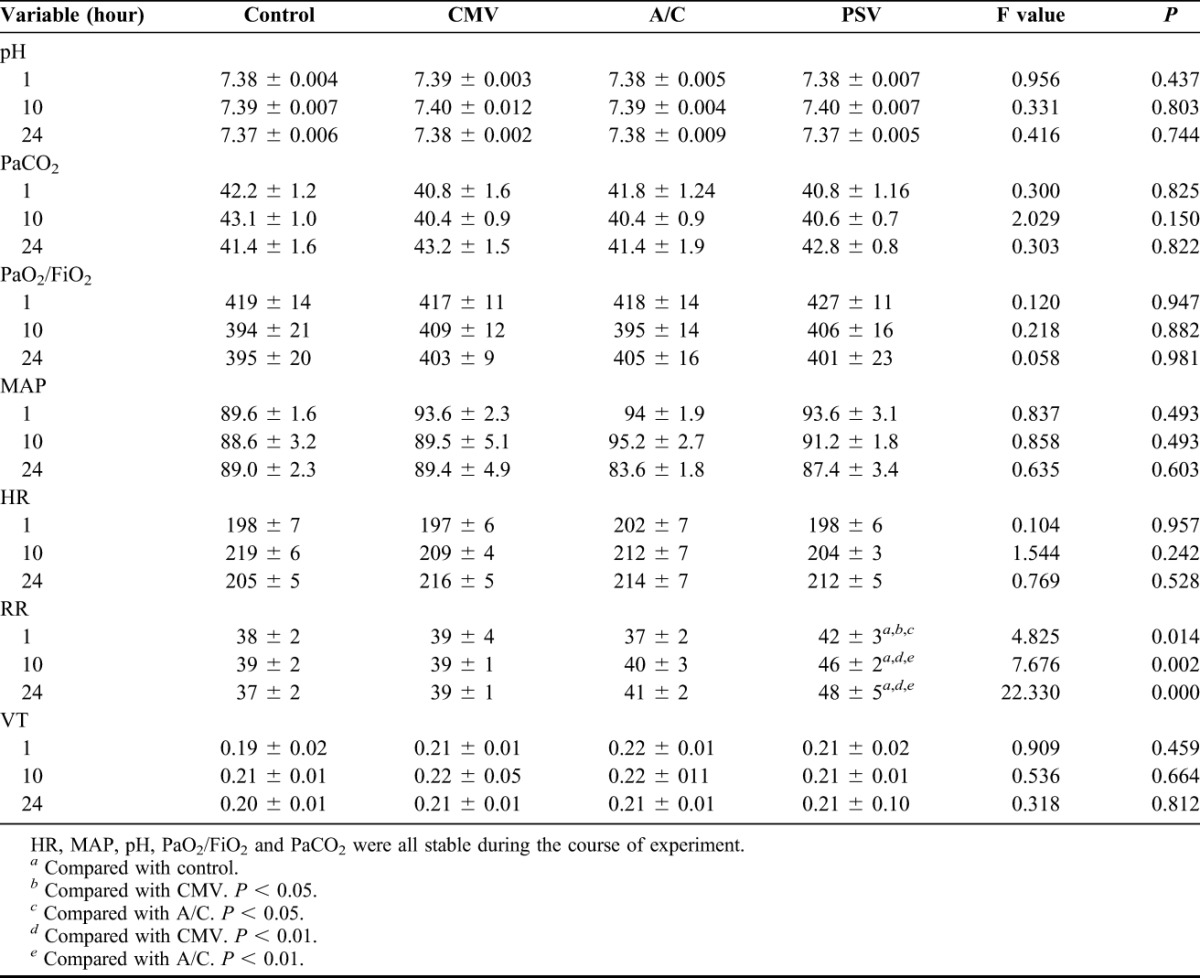
The initial body weights were not significantly different between the Control, CMV, A/C and PSV groups (2.1 ± 0.2, 2.3 ± 0.1, 2.2 ± 0.2 and 2.3 ± 0.2 kg, respectively). The physiological parameters are described in Table 1. Heart rate, mean arterial blood pressure, PH, partial pressure of arterial oxygen/FiO2 and PaCO2 were stably maintained in all animals. All groups received equal amounts of fluid boluses. There were differences in the Edi among the 4 groups during the 24-hour experimental time frame. There was a decrease in amplitude and the duty cycles of the diaphragm (Ti/Ttotal) were 30% ± 3.5%, 25% ± 1.8%, 20% ± 2.2% and 40% ± 3.9%, for Control, CMV, A/C and PSV, respectively. RR in the PSV group was varied compared with the other 3 groups (P < 0.05).
TABLE 1.
The vital signs and physiologic items during experiment
Diaphragm Electrical Activity
Edi in the 3 mechanically ventilated groups was significantly reduced compared with the Control group. Centroid frequency (Fc) and H/L were 139.41 ± 0.60 Hz and 5.61 ± 0.19 in the Control group, 79.31 ± 0.62 Hz and 1.02 ± 0.43 in the CMV group, 87.22 ± 0.65 Hz and 1.04 ± 0.19 in the A/C group and 90.72 ± 1.14 Hz and 1.09 ± 0.20 in the PSV group. The percent change in Fc was significantly lower in the PSV group (decreased by 34.9%), compared with the CMV (decreased by 43%) and A/C groups (decreased by 37.1%) (P = 0.001 and P = 0.028, respectively). The high frequency components (H) decreased and the low frequency components (L) increased in the mechanical ventilation (MV) groups, compared with the Control group. The CMV, A/C and PSV H/L ratios decreased by 82.3%, 81.8% and 80.6%, respectively (Figure 1). Edi integral amplitude decreased 90% ± 1.48%, 70.2% ± 4.72% and 67.8% ± 3.13% in CMV, A/C and PSV, respectively (P < 0.001).
FIGURE 1.
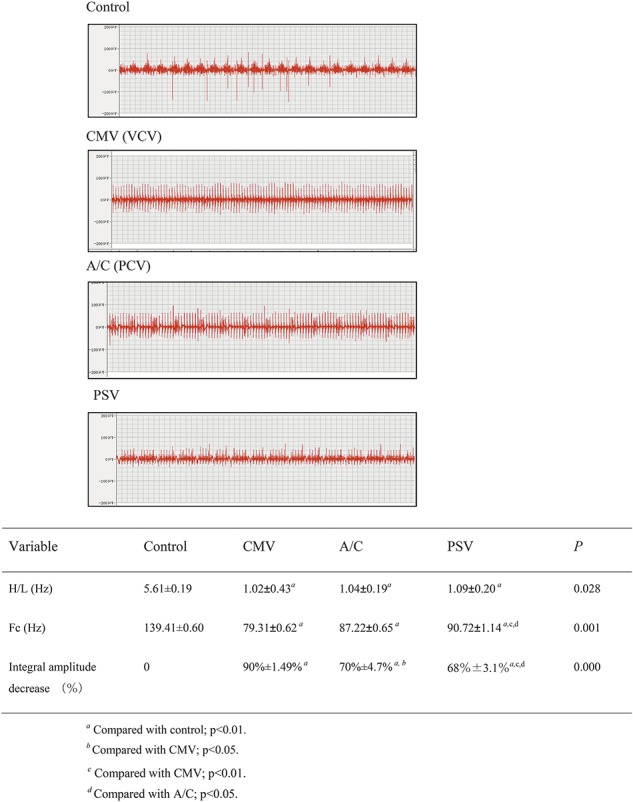
Frequency and integral amplitude of Edi Fc: Centroid frequency: ratio of high frequency to low frequency. Values are mean ± SD. Fc and H/L were 139.41 ± 0.60 Hz and 5.61 ± 0.19 in Control, 79.31 ± 0.62 Hz and 1.02 ± 0.43 in CMV, 87.22 ± 0.65 Hz and 1.04 ± 0.19 in A/C, 90.72 ± 1.14 Hz and 1.09 ± 0.20 in PSV. The percentage in Fc was significantly lower in the PSV group (decreased by 34.9%), compared to the CMV (decreased by 43%) and A/C groups (decreased by 37.1%) p = 0.001, p = 0.028 respectively. Compared with Control group, CMV, A/C, and PSV H/L decreased by 82.3%, 81.8%, and 80.6%, respectively. Electromyography of diaphragm integral amplitude decreased 90% ± 1.48%, 70.2% ± 4.72% in A/C and 67.8% ± 3.13% in PSV (p < 0.01).
Expression and Activation of Caspase-3
Caspase-3 is expressed mainly in the nucleus and also by the cell membrane to a lesser extent. Thus, IHC analysis of caspase-3 expression is shown here as brown or tan in muscle cell nuclei and cell membranes. The positive rate of caspase-3 expression in the PSV group was 25% to 70% (AI 4.47 ± 0.67 points) indicating moderate expression. The positive expression rate was 45% to 85% (AI 6.40 ± 0.57 points) in the CMV group and 32% to 70% (AI 5.98 ± 0.67 point) in the A/C group, both of which indicate relatively high expression (P < 0.01). Caspase-3 expression in the PSV group was significantly lower compared with the CMV and A/C groups (P < 0.01) (Figure 2).
FIGURE 2.
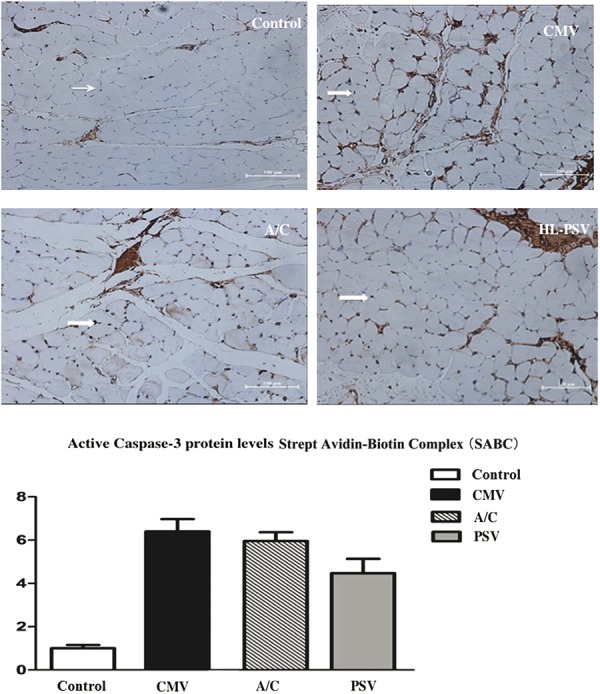
Caspase-3 protein expression through immunohistochemistry (SABC method). The positive rate in the PSV group was 25% to 70% (AI 4.47 ± 0.67 points) indicating moderate expression. Expression of caspase-3 in the CMV and A/C groups was 45% to 85% (AI 6.40 ± 0.57 points) and 32% to 70% (AI 5.98 ± 0.67 point), respectively, indicating relatively high expression (P < 0.01). Expression of caspase-3 in PSV was significantly lower than in the CMV and A/C groups (P < 0.01). Caspase-3 expression in the muscle cell membrane and nucleus is brown or tan and shown by block arrows. Scale bar = 100 μm.
A Western blot was also performed to analyze the level of caspase-3 activation with GAPDH used as an internal reference (Figure 2). The caspase-3/GAPDH ratio indicated the relative expression level of the caspase-3 protein and the corresponding control group after the caspase-3 expression levels had been normalized to 1-fold. Caspase-3 activity was significantly elevated in the CMV (∼2.5-fold), A/C (∼2.3-fold) and PSV (∼1.8-fold) groups compared with the Control group. PSV attenuated the increase in caspase-3 activation, compared with the CMV and A/C groups (P = 0.035 and 0.033) (Figure 3).
FIGURE 3.
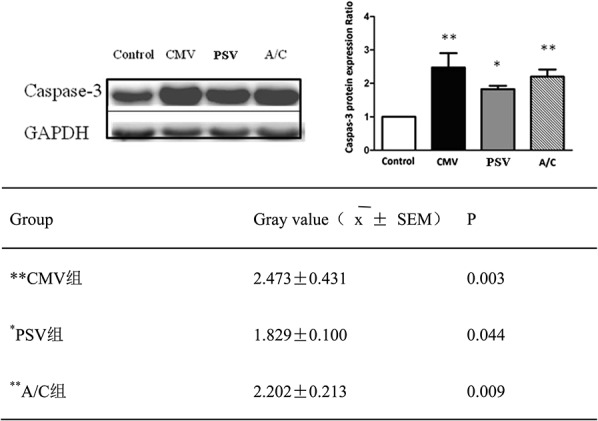
Western blotting for active caspase-3 protein levels in diaphragm muscle expressed as fold changes of control. Values are mean ± SD. CMV and A/C versus control, P < 0.01. PSV significantly attenuated the elevation of caspase-3 activation, compared with CMV group and A/C group (P = 0.035 and P = 0.033, respectively).
Ultra-Structure Analysis of Diaphragmatic Muscle Tissue
Observations of diaphragmatic muscle fiber ultra-structure showed different degrees of damage in the MV groups, mainly in the mitochondria (Figure 4). Of the 3 MV groups, the PSV group had the least mitochondrial injury. Mitochondrial swelling was observed in the PSV group, along with dense and rich cristae, the muscle fibrils were neatly arranged, sarcomere integrity was maintained, there were clear and neat Z lines, and a large number of vacuoles and lipid vacuoles had formed within the muscle fibers. In the CMV group, there was myofibrillar mitochondrial swelling and low density of cell matrix, along with crest deformation and dissolution. In addition, some mitochondria were flocculent, there was myeloid and vacuolar degeneration, some parts of myofibrils were dissolved and cracked, the arrangement was loose and there were irregular myofilament cracks. The Z lines were generally neat, however, some appear blurred, and there was structural disorder. In the A/C group, there was mitochondrial swelling and cristae deformation and dissolution and the myofibrils were arranged in neat rows, with some loosely arranged. Some of the Z lines were fuzzy, there was structural disorder and some myofibril vacuolization (Figure 3). There were significant differences in mitochondrial Flameng scores between each group (P < 0.01) (scores shown in Figure 3).
FIGURE 4.
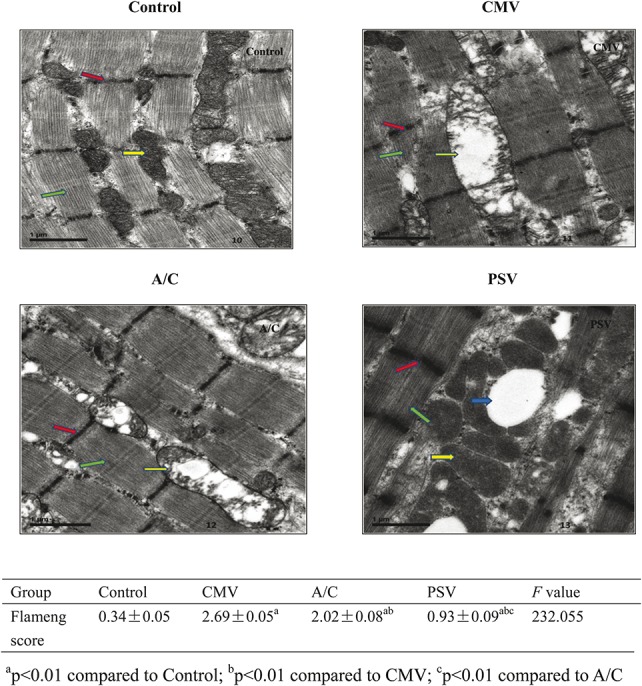
Diaphragm ultra-structure injury through electron microscopy. Green arrows indicate muscle fiber, the red arrow indicates the Z line, the yellow arrow indicates the mitochondria and the blue arrow indicates the vacuoles. In the CMV group, there was myofibrillar mitochondrial swelling and low density of cell matrix; and crest deformation and dissolution. Some mitochondria were flocculent; there was myeloid and vacuolar degeneration; some parts of myofibrils were dissolved and cracked; with a loose arrangement and irregular myofilament cracks. The Z lines were generally neat but some had a blurred appearance along with structural disorder. In the A/C group, there was mitochondrial swelling and cristae deformation and dissolution; the myofibrils were arranged in neat rows with some loosely arranged; part of the Z lines were fuzzy and there was structural disorder and some myofibril vacuolization. In contrast to the CMV group, which had myofibrillar mitochondrial swelling and crest deformation and dissolution (see yellow arrow), the PSV group, had mitochondrial swelling, but the cristae were dense and rich (see yellow arrow); the muscle myofibrils were neatly arranged, sarcomere integrity was maintained (green arrow), the Z lines neat and clear (red arrow); and there was formation of a large number of vacuoles and lipid vacuoles among the muscle fibers (blue arrow) (Bar = 1 μm). The Flameng scores in PSV were significantly lower compared with the CMV and A/C modes (P < 0.01).
DISCUSSION
The current study found that full ventilation support resulted in a significant reduction in diaphragm electrical activity in the PSV, CMV and A/C ventilation groups. PSV preserved some diaphragm electrical activity. In the PSV mode, the rabbits triggered the ventilator and determined the cycle, and the respiratory rate was variable. Ventilator-induced diaphragmatic atrophy, which is accompanied by an increase in both oxidative stress and protease activity, occurred in the CMV, A/C and PSV groups, however, PSV attenuated ventilation-induced caspase-3 activity and inhibited the diaphragm structural injury. These results support the hypothesis that diaphragmatic damage is lower with high level PSV compared with the same level of support when provided by CMV and A/C.
In contrast to other skeletal muscles, the diaphragm is normally activated rhythmically on a 24-hour basis. MV imposes a unique form of muscle disuse on the diaphragm, because the latter is simultaneously mechanically unloaded, intermittently shortened and electrically suppressed by the ventilator breaths.9 During continuous mandatory ventilation, the diaphragm remains inactive, which activates pathophysiological cascades leading to a loss of contractile force and muscle mass. Uncoupling between diaphragm activation and pressure generation is simply caused by the delivery of mechanical assistance. Diaphragm disuse in CMV reduces the Ca2+ uptake capacity of the sarcoplasmic reticulum and leads to VIDD.10 Based on evidence from human and animal research, ventilatory-induced diaphragmatic dysfunction is relieved when diaphragmatic activity is retained; even nerve stimulation of the diaphragm is reduced in CMV while synchronous diaphragm activity is decreased.
More electrical activity was maintained with PSV, even at the same level of support and with a variable respiratory rate, during the experiment, likely due to trigger and cycling itself. Edi responded directly to the rabbits' ventilatory demand and the strength of the central respiratory drive.11 When the rabbits were set on volume control mode, the amplitude and frequency of Edi decreased rapidly. This was reflected in the CMV animals, which had full support ventilation and depressed diaphragm activity. In the A/C mode with pressure control ventilation, the amplitude of Edi was higher than in the CMV mode likely due to the retention of trigger. In contrast, the amplitude and frequency of Edi was highest in the PSV group, among the 3 ventilatory groups. However, amplitude and frequency of Edi was decreased, with increased pressure support, in the PSV compared with the nonventilated control group, meaning that PSV decreased the work of breathing and therefore, some of the diaphragm activity. The Edi waveform is a reliable signal for monitoring a patient's neural respiratory drive and patient–ventilator interaction.12 A better design is to assess the value of neural index so that patient–ventilator interaction can be analyzed.
The anesthetic agent, sodium midazolam, can affect respiratory depression.13 However, in the current study, both MV and control animals were anesthetized with sodium midazolam, thus comparisons between the groups are valid. Furthermore, the drinking reflex was preserved, indicating that there was no or minimal depression of diaphragm activity due to the drug.
Diaphragm inactivity with controlled mechanical ventilation (CMV) decreases diaphragm force and produces myofibril damage. This damage contributes to the reduced force and is time-dependent. A previous study reported a decrease in in vitro maximal specific force in the rat diaphragm after 12 hours of controlled mechanical ventilation.14 Likewise, Sassoon et al examined the time course of in vivo and in vitro diaphragmatic force alterations after 1 or 3 days of controlled mechanical ventilation and reported a time-dependent decline in rabbit diaphragmatic function. In particular, Pdimax was reduced by 27% and 51% after 1 and 3 days of controlled mechanical ventilation, respectively.7 In the current study, rabbits were ventilated for 24 hours, resulting in attenuating diaphragm injury due to PSV. Robert et al15 found that diaphragm blood flow and microvascular partial pressure of oxygen diminished in Sprague–Dawley rats during 6 hours of controlled mechanical ventilation. Over time, diaphragm injury due to disuse and diminished partial pressure of oxygen may become more obvious. Extension of the time of CMV should be considered to further confirm the benefits of the PSV.
Muscle atrophy, oxidative stress and diaphragm structural injury have been documented after CMV.1–3 Regulation of myonuclear loss in skeletal muscle could occur through extrinsic death receptor and intrinsic mediated pathways. Caspase activation results in protein cleavage and functions in the intrinsic and extrinsic pathways of apoptosis. Since forced passive shortening of diaphragm pressure ventilation damages the diaphragm, the diaphragm muscle fibers appear deconstructed, leading to fatigue and eventually rendering the patient, ventilator dependent.16 In diaphragms of human MV patients, mitochondrial biogenesis and content were downregulated and there was a specific defect in respiratory chain cytochrome-c oxidase.17 Mitochondria with deficient respiratory chain function can permit excessive electron leakage with consequent increases in reactive oxygen species production.18–20 Adequate mitochondrial function is a prerequisite for normal muscle oxidative capacity. Bernard and colleagues conducted experiments in which rabbits underwent CMV ventilation for approximately 49 hours. They found myofibrillar breaks, increased sarcoplasmic lipid vacuoles, mitochondria that appeared smaller, along with and membrane ruptures, and a significant decrease in the volume and density of the mitochondria. Respiratory muscle structural damage directly leads to a decline in respiratory function, which causes VIDD and/or difficulty in ventilation weaning due to a reduction in mitochondrial oxidative phosphorylation coupling efficiency.21 In the current study, the PSV mode inhibited the mitochondrial injury.
It is important to note that both partial and full support MV results in diaphragmatic atrophy, albeit the MV-induced diaphragmatic atrophy that occurs during partial support ventilation occurs at a slower rate compared with the atrophy induced by full support ventilation.22 A study by Futier et al demonstrated that high-levels of prolonged PSV resulted in significant diaphragmatic atrophy and contractile dysfunction with no changes in plasma cytokines. In that study, the PSV setting was 5 to 7 cmH2O (partial pressure support) and was chosen according to the animals' minute volume.5 Jennifer et al23 compared the Edi of crural with transdiaphragmatic pressure (Pdi) during varying levels of PSV in 13 intubated patients. They found no changes in neuro-mechanical coupling of the diaphragm with increasing pressure support levels. A portion of diaphragm electrical activity was maintained and the complete neuromuscular coupling. This seems to be the main factor in the reduction of diaphragmatic breathing muscles injury with PSV. In this study, airway pressure was also evaluated during CMV and the pressure target for PSV was set according to the Pplat to examine the effect on the diaphragm with different modes at the same level of ventilation support. It was found that full ventilation support resulted in significantly reduced diaphragm electrical activity in PSV and in CMV and A/C. However, the Edi characteristics were different. The highest Fc occurred in the Hi-PSV mode and high-level PSV and A/C decreased Edi activity, but to a lesser extent than did CMV. Diaphragmatic levels of caspase-3 were higher in all of the MV groups compared with the Control group. However, PSV mode reduced the occurrence of diaphragm cell apoptosis. These findings are consistent with the notion that Edi reduction is the reason for MV-induced protease activation in the diaphragm.24,25
There were several limitations in this study. First, transdiaphragmatic pressure/Edi and the phrenic motor nerve conduction should be considered for evaluation. In addition, extending the time of PSV should be considered to verify PSV's attenuation of VIDD.
CONCLUSIONS
Although PSV caused some reduction in diaphragm myoelectric activity and induced oxidative stress and ultra-structure changes of diaphragm, partial diaphragm electrical activity was retained and VIDD was attenuated. Further research is warranted to help optimize the level of ventilation support for VIDD prevention in critically ill animals and clinical patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. They would also like to thank Yunbin Yao, who provided technical help.
Footnotes
The authors have no conflicts of interest to disclose.
Source of funding: Education Department of Scientific Research Foundation of Zhejiang Province (Y201017208). Natural Science Foundation of Zhejiang Province (LY14H030002).
REFERENCES
- 1.Sanford L, Taitan NB, Nyali T, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327–35. [DOI] [PubMed] [Google Scholar]
- 2.Jaber S, Jung B, Matecki S, et al. Clinical review: ventilator-induced diaphragmatic dysfunction—human studies confirm animal model findings! Crit Care 2011;15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang H, Lee M, Khuong A, et al. Diaphragm muscle atrophy in the mouse after long-term mechanical ventilation. Muscle Nerve 2013;48:272–8. [DOI] [PubMed] [Google Scholar]
- 4.Hering R, Bolten JC, Kreyer S, et al. Spontaneous breathing during airway pressure release ventilation in experimental lung injury: effects on hepatic blood flow. Intensive Care Med 2008;34:523–7. [DOI] [PubMed] [Google Scholar]
- 5.Futier E, Constantin JM, Combaret L, et al. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care, National Research Council of China; 2008;12:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regulation for the administration of affairs concerning experimental animals. State Science and Technology Commission; 2009. [Google Scholar]
- 7.Sassoon CS, Caiozzo VJ, Manka A, et al. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Phyiol (1985) 2002;92:2585–95. [DOI] [PubMed] [Google Scholar]
- 8.Nelson WB, Smuder AJ, Hudson MB. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit Care Med 2012;40:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 2010;16:19–25. [DOI] [PubMed] [Google Scholar]
- 10.Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364–371. [DOI] [PubMed] [Google Scholar]
- 11.Luo YM, Moxham J. Measurement of neural respiratory drive in patients with COPD. Respir Physiol Neurobiol 2005;146:165–74. [DOI] [PubMed] [Google Scholar]
- 12.Sinderby C, Liu SQ, Colombo D, et al. An automated and standardized neural index to quantify patient–ventilator interaction. Crit Care 2013;17:R239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozé H, Germain A, Perrier V, et al. Effect of flumazenil on diaphragm electrical activation during weaning from mechanical ventilation after acute respiratory distress syndrome. Br J Anaesth 2015;114:269–75. [DOI] [PubMed] [Google Scholar]
- 14.Powers SK, Shaneley RA, Coombes JS, et al. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol (1985) 2002;92:1851–8. [DOI] [PubMed] [Google Scholar]
- 15.Robert TD, Christian SB, John NS, et al. Mechanical ventilation reduces rat diaphragm blood flow and impairs O2 delivery and uptake. Crit Care Med 2012;40:2858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zergeroglu MA, McKenzie MJ, Shanely RA, et al. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol (1985) 2003;95:1116–24. [DOI] [PubMed] [Google Scholar]
- 17.Oicard M, Jung B, Liang F, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med 2012;186:1140–9. [DOI] [PubMed] [Google Scholar]
- 18.Powers SK, Hudson MB, Nelson WB, et al. Mitochondrial-targeted antioxidants protect against mechanical-ventilation-induced diaphragm weakness. Crit Care Med 2011;39:1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang H, Lee M, Bundak MT, et al. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel fos/foxol/stat3-bim axis. FASEB J 2011;25:2921–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung B, Constantin JM, Rossel N, et al. Adaptive support ventilation prevents ventilator-induced diaphragmatic dysfunction in piglet: an in vivo and in vitro study. Anesthesiology 2010;112:1435–43. [DOI] [PubMed] [Google Scholar]
- 21.Bernard N, Matecki S, Py G, et al. Effects of prolonged mechanical ventilation on respiratory muscle ultrastructure and mitochondrial respiration in rabbits. Intensive Care Med 2003;29:111–18. [DOI] [PubMed] [Google Scholar]
- 22.Hudson MB, Smuder AJ, Nelson WB, et al. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 2012;40:1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennifer B, Gottfried SB, Paolo N, et al. Electrical activity of the diaphragm during pressure support ventilation in acute respiratory Failure. Am J Respir Crit Care Med 2001;164:419–24. [DOI] [PubMed] [Google Scholar]
- 24.Uchlyama A, lmanaka H, Taenaka N, et al. Comparative evaluation of diaphragmatic activity during pressure support ventilation and intermittent mandatory ventilation in animal model. Am J Respir Crit Care Med 1994;150:1564–8. [DOI] [PubMed] [Google Scholar]
- 25.Parthasarathy S, Jubran A, Tobin MJ. Assessment of neural inspiratory time in ventilator-supported patients. Am J Respir Crit Care Med 2000;162:546–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


