ABSTRACT
In this case report, we examine the impact of a simplified two-drug highly active antiretroviral therapy (HAART) regimen of raltegravir and lamivudine in a patient co-infected with human immunodeficiency virus (HIV) and hepatitis C, D and B viruses (HCV/HDV/HBV) under immunosuppressive therapy after liver transplantation. Pharmacokinetic interactions between integrase inhibitors and immunosuppressant drugs are described. Raltegravir, the first integrase inhibitor, associated with lamivudine, was introduced because its metabolism does not interfere with immunosuppressant therapy. During post-orthotopic liver transplantation follow-up, the patient's transaminases level increased and his antiretroviral therapy (HAART) of tenofovir/emtricitabine and fosamprenavir was changed, due to suspected drug toxicity. After seven months of follow-up, the patient showed good tolerance, good viro-immunological control with undetectable HIV viraemia and stable concentrations of immunosuppressive drugs. This case indicates that the combination of raltegravir and lamivudine is an optimal and effective strategy because it resulted in an important reduction of hepatic transaminases in a patient with very critical clinical conditions.
Keywords: HIV-1, lamivudine, liver transplantation, raltegravir, viral hepatitis
RESUMEN
En este reporte de caso, examinamos el impacto de un régimen de terapia antirretroviral de gran actividad (TARGA) simplificada con dos drogas – raltegravir y lamivudina – en un paciente coinfectado con el virus de inmunodeficiencia humana (VIH) y los virus de la hepatitis C, D y B (HCV/HDV/HBV) bajo terapia inmunosupresiva después del trasplante del hígado. Se describen interacciones farmacocinéticas entre los inhibidores de la integrasa y los medicamentos inmunosupresores. El raltegravir – el primer inhibidor de la integrasa, asociado a la lamivudina – se introdujo porque su metabolismo no interfiere con la terapia inmunosupresora. Durante el seguimiento del trasplante hepático post-ortotópico del hígado, se produjo un aumento del nivel de transaminasas del paciente, y fue necesario cambiar la terapia antirretroviral de gran actividad (TARGA) con tenofovir-emtricitabina y fosamprenavir, debido a sospecha de toxicidad de los fármacos. Tras siete meses de seguimiento, el paciente mostró buena tolerancia, buen control viro-inmunológico con viremia del VIH indetectable y concentraciones estables de las medicamentos inmunosupresivos. Este caso indica que la combinación de raltegravir y lamivudina es una estrategia óptima y eficaz, ya que trajo consigo una reducción importante de las transaminasas hepáticas en un paciente con condiciones clínicas muy críticas.
INTRODUCTION
The history of transplantation and the first human renal transplant dates to December 23, 1954, when Joseph Murray performed a kidney transplant between identical twin brothers in Boston. In 1963, the introduction of azathioprine and steroid combination therapy produced important results and became the mainstay of immunosuppression until the use of cyclosporine in 1983. Human immunodeficiency virus (HIV)-1 infection in patients with advanced hepatic disease raises questions about the choice of post-transplantation anti-retroviral therapy, because of potential interaction between immunosuppressant and antiretroviral drugs via cytochrome P450 inhibition, resulting in an increased incidence of adverse events.
Raltegravir, the first integrase inhibitor approved for experienced (October 2007) as well as naïve patients [July 2009] (1), represents an evolutionary step and fundamental change in the therapy of transplanted HIV/hepatitis C virus (HCV) co-infected patients (2). This new class of drugs has been shown to be highly effective in leading to a profound and sustained viral load reduction, in both experienced and naive patients.
The good tolerance and optimal safety of lamivudine makes it an ideal drug to be associated with raltegravir in hepatitis B, C and D viruses (HBV/HCV/HDV)/HIV-experienced people with several clinical conditions after liver transplantation (3).
Here, we examine the impact of a simplified two-drug highly active antiretroviral therapy (HAART) regimen including raltegravir and lamivudine, in an HIV/HCV/HDV/HBV co-infected patient on immunosuppressive therapy after liver transplantation. Pharmacokinetic interactions between integrase inhibitors and immunosuppressant drugs will be described.
CASE REPORT
The patient, DB, aged 42 years, Italian, HIV-1 infected since 1987, HCV/HBV/HDV since 1992 with a history of decompensated hepatic cirrhosis, was admitted in our hospital “Ospedali Riuniti” of Ancona, for inclusion on the liver transplant list. His virological profile was: HCV-RNA with high replication, genotype 1b-c; Hbs-Ag positive; anti-Hbs negative; anti-Hbc positive; anti-Hbe positive; HBV-DNA negative; HDV-DNA negative.
At time of admission to our department (July 2008), the patient's HAART combination had been represented by zidovudine 300 mg twice a day, lamivudine 300 mg singledose, lopinavir/ritonavir 800 mg and 200 mg, respectively, with pre-transplantation CD4 T-cell count of 329 cell/mm3 and undetectable plasma HIV RNA concentration (detection limit 50 copies/mL), aspartate transaminase (AST) 97 IU/mL, alanine transaminase (ALT) 76 IU/mL, total bilirubin 2.8 mg/dL and HCV viraemia of 132 471 IU/mL. Clinical histopatological studies and ultrasound imaging of the liver identified decompensated liver cirrhosis HIV/HCV/HBV/HDV-related, complicated by complete portal vein thrombosis. Sonographic studies showed liver hypertrophy of the left lobe, marked splenomegaly (bipolar diameter 21 cm) with portal hypertension, fibroscan 25.1 KPa with stage F4 fibrosis according to Metavir, oesophageal varices (grade F3) at gastroscopy and ascites.
In July 2008, the patient received liver transplant and on 16th day, he started a new HAART combination based on tenofovir, emtricitabine and fosamprenavir/ritonavir. Tenofovir is one of the drugs of choice in HIV/HBV co-infected patients in whom antiretroviral therapy is advised. Its co-formulation with emtricitabine (Truvada) is particularly convenient for treating both HIV and HBV in co-infected individuals and it is an optimal drug for post-transplantation HBV prophylaxis. Contemporaneously, the patient was treated with immunosuppressant drugs according to the following pattern: in the early post-transplant period, corticosteroids and cyclosporine were given; on the 5th day, everolimus was added and on the 16th day, ciclosporine was changed to tacrolimus. These drug doses were adjusted on the basis of standard protocols or plasmatic concentrations (tacrolimus and everolimus), by microparticle enzyme immunoassay (MEIA) method based on the Abbott IMX®(Abbott Laboratories, Abbott Park, IL, USA). Plasma concentration of tacrolimus and everolimus were performed twice a week in the first two months post-transplant, then once per week for the other two months and then monthly.
To prevent hepatitis B recurrence post orthotopic liver transplantation (OLT), the patient was treated with doses of 2160 IU intramuscular hepatitis B immunoglobulin (HBIG) monthly and according to anti-Hbs surface antigen levels. Low-dose intramuscular HBIG, in combination with lamivudine, has been regarded as the most cost-effective regimen for the prevention of post-transplant HBV recurrence in recipients without pre-transplant lamivudine resistance. This therapy reduces the risk of recurrence to less than 5% at five years. At the time of writing, the patient's HBV-DNA was still negative, Hbs Ag was negative and anti-Hbs surface antigen levels were always above protective values. Viro-immunological examinations made after OLT showed HCV-RNA 132 471 UI/mL and CD4 cells 148/mm3. During subsequent follow-up and after eight months of therapy post-transplant, the patient had maintained good clinical conditions and a discrete adherence to HAART and immunosuppressive therapy. Post-OLT sonographic studies have demonstrated a regular hepatic morphovolumetry and echostructure, without focal lesions, and complementary Doppler studies showed no vascular obstruction.
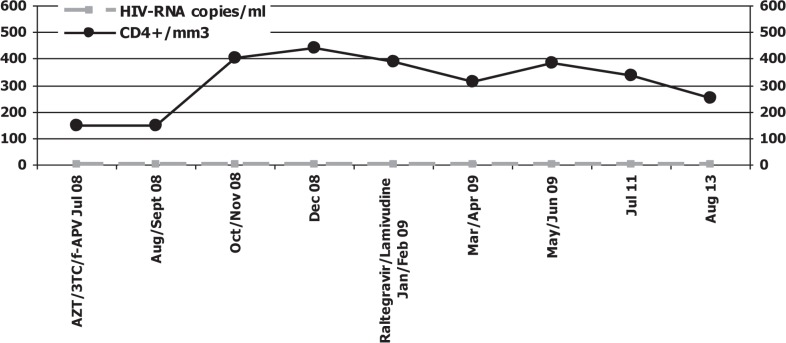
In February 2009, biochemical and haematological parameters demonstrated an elevation of the serum ALT concentration to 255 IU/L and AST to 228 IU/L (reference range [rr] 1–36 IU/L); bilirubin 0.8 mg/dL, alkaline phosphatase 737 IU/L (rr 0–270 IU/L) and gamma-glutamyl transferase (γGT) 452 IU/L (rr 4–45 IU/L). A liver biopsy showed signs of acute mild rejection (rejection activity index (RAI) grade 5 according to Banff) with peacemeal necrosis. This histological evidence could be related to: i) the reinfection of the graft liver by HCV (soon after the transplant), ii) drug toxicities, or iii) inadequate immunosuppressant plasma concentrations because of interactions with antiretroviral drugs. For these reasons, the patient interrupted the antiretroviral therapy (at date of interruption, the patient had CD4 T-cell count of 388 cell/mm3 and complete suppression of HIV-1 viral load) [Fig. 1].
Fig. 1. HIV-RNA viral load, CD4+ cell count and antiretroviral treatment.
AZT: zidovudine; 3TC: lamivudine; f-APV: fosamprenavir
Note: undetectability of HIV-RNA levels and stable CD4 cell count
The patient started the new regimen based on raltegravir, 400 mg twice a day and lamivudine 300 mg single dose. This simplified HAART regimen was chosen because raltegravir has fewer interactions with immunosuppressive drugs due to its metabolism.
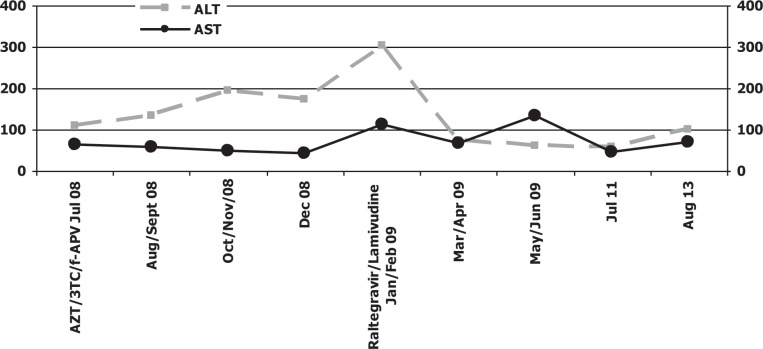
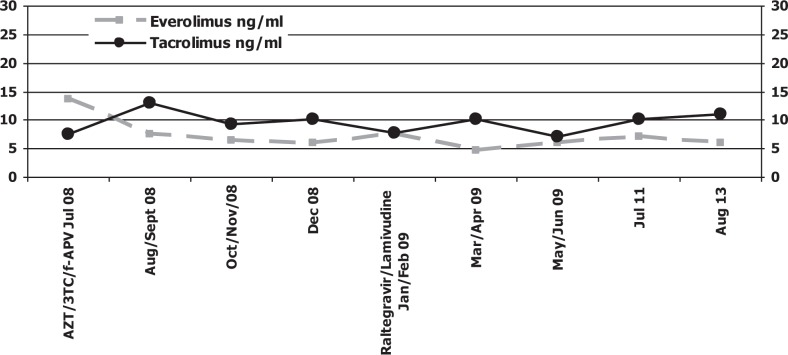
After seven months of therapy with raltegravir and lamivudine, laboratory examination revealed a very important reduction of serum transaminases (at baseline: ALT 181 UI/mL, AST 120 UI/mL and at date, 28 August 2013: ALT 50 UI/mL, AST 62 UI/mL), a sustained viro-immunological response and optimal tolerance [Figs. 2–3]. During the follow-up period, the patient did not experience any side effects related to raltegravir. Everolimus and tacrolimus showed a relatively flat concentration in this patient during therapy with integrase inhibitor [Fig. 4].
Fig. 2. Serum transaminases levels.
ALT: alanine transaminase; AST: aspartate transaminase; AZT: zidovudine; 3TC: lamivudine; f-APV: fosamprenavir
Note: data refer to mean values. At date of introduction of the last therapy with raltegravir and lamivudine, transaminases (especially ALT) had a high peak in serum; then they decreased until the last control, in September 2009, where they demonstrated an increase probably caused by HCV reinfection or raltegravir collateral effects.
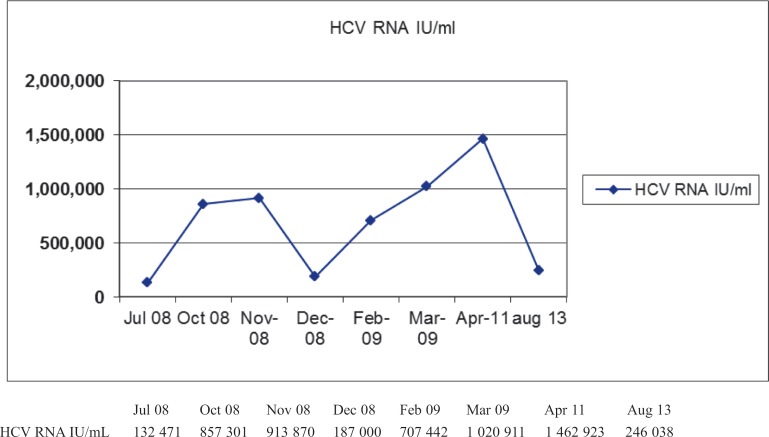
Fig. 3. Hepatitis C virus (HCV) RNA viral load.
Fig. 4. Everolimus and tacrolimus plasma concentrations.
AZT: zidovudine; 3TC: lamivudine; f-APV: fosamprenavir
Note: data refer to mean values. The drugs are maintaining stable concentrations in plasma.
DISCUSSION
Everolimus and tacrolimus are macrolide lactones and are metabolized in the liver by the CYP3A system and some of the HAART regimen medications require elimination or metabolism viathe P-glycoprotein and multidrug-resistant protein transporter or viathe cytochrome P450 enzyme system. Since these transporters and enzymes are also responsible for the clearance of immunosuppressant drugs, interactions are likely to occur, especially between protease inhibitors or non-nucleoside reverse transcriptase inhibitors (NNRTIs; nevirapin and efavirenz are both inducers of CYP 3A4; delavirdine acts as an inhibitor of these enzymes) and immunosuppressive drugs that require a reduction in drug dosage. On the other hand, most nucleoside reverse transcriptase inhibitors (NRTIs) are predominantly excreted by the renal system and interactions based upon CYP are not encountered (4).
A main challenge to improving transplantation outcome in HIV co-infected patients is to manage drug interaction between antiretroviral drugs and immunosuppressive treatments. It is crucial in the early post-transplant period that therapeutic trough levels of immunosuppressant drugs are achieved promptly without toxicity. Therefore, the risk of an acute rejection would be reduced. For this reason, raltegravir, a new integrase inhibitor with in vitroand in vivoactivity against HIV-1 and HIV-2, has a good safety profile because it is not a substrate of CYP 450 enzymes and it is metabolized viathe UGT1A1-mediated glucuronidation pathway (5). Immunosuppressive drug dosage was easy to manage in the patient after raltegravir introduction. Prompt and stable target levels would be obtained with standard dosages, because of the lack of significant interactions between raltegravir and calcineurin inhibitors [Fig. 4].
This case reports the use of raltegravir and lamivudine in a post transplanted patient with HIV/HCV/HDV/HBV infection in critical clinical conditions, a high grade of toxicity correlated to HAART therapy after his liver transplantation (tenofovir, emtricitabine and fosamprenavir/ritonavir), poor adherence and low tolerance. The patient had HIV/HCV/HDV/HBV with severe hepatic cirrhosis. Although both HIV-1 and HCV co-infection may be considered relatively common in the occidental world, the presence of hepatic cirrhosis raises the question whether the co-presence may be responsible for more severe hepatic and systemic damage in post-transplanted patients with HIV-1 infection. Therefore, serologic tests demonstrate that HIV-1 infected patients with HCV/HDV/HBV co-infection post-transplant have few limiting therapeutic options in second-line and salvage therapy.
In this severe condition, raltegravir maintains stable biochemical, haematological and clinical parameters. The main characteristic of this drug is represented by the optimal tolerance in a patient simultaneously treated with everolimus and tacrolimus because of its pharmacokinetic characteristics and the lack of interactions with immunosuppressant drugs.
Another important issue is represented by the definition of second-line regimens in HIV/HCV infected patients with failure or intolerance to HAART regimens. Zidovudine and lamivudine, to date, represent a common option for patients with very limited active options and hepatotoxicity related to HAART therapy.
In post-transplanted patients with several clinical conditions, few options are, however, available for the design of simplified second-line regimens, and the recently introduced integrase inhibitors represent a turning point in transplantation history and a promising advance in this scenario. In our case, a prompt response was observed, but it is still uncertain to what extent a durable response may be obtained with these compounds and which drugs represent the best partners for them. Thus, it is important to note that this simplified HAART regimen is motivated by the need to minimize the toxicity in the patient taking immunosuppressant therapy after liver transplantation. Pharmacokinetic studies showed stability of everolimus and tacrolimus concentrations, due to the good tolerance to raltegravir and lamivudine.
Our case indicates that the combination of raltegravir and lamivudine is an optimal and effective strategy since it reduces hepatic transaminases in a patient with very critical clinical conditions. However, these enzymes at present are increasing (last control in 28 August 2013: ALT 137 IU/L and AST 102 IU/L, rr 1–36 IU/L); this is most likely related to the reinfection of liver graft by HCV in an immunocompromised patient or side effects of raltegravir. A longer follow-up is necessary.
Further studies will have to be done to properly evaluate the best drug association to minimize toxicity and establish the best clinical scenario to use raltegravir in posttransplanted patients with HIV/HBV/HCV/HDV co-infection in critical conditions and who have limited therapeutic active options.
REFERENCES
- 1.Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection. Results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 2.Moreno A, Quereda C, Moreno L, Pérez-Elías MJ, Muriel A, Casado JL, et al. Safe use of raltegravir and serolimus in an HIV-1 infected patient with renal impairment after orthotopic transplantation. AIDS. 2008;22:547–548. doi: 10.1097/QAD.0b013e3282f37478. [DOI] [PubMed] [Google Scholar]
- 3.Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 4.Hassane I, Vacher VL, Baumelo A, Deray G. Antiretroviral and immunosuppressive drug-drug interactions: an update. Kidney Int. 2004;66:532–541. doi: 10.1111/j.1523-1755.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 5.Tricot L, Teicher E, Peytavin G, Zucman D, Conti F, Calmus Y, et al. Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant. 2009;9:1946–1952. doi: 10.1111/j.1600-6143.2009.02684.x. [DOI] [PubMed] [Google Scholar]