ABSTRACT
Objective:
To evaluate femoral cartilage thickness in patients with Behçet's disease (BD) by using ultrasonography.
Methods:
Thirty-one patients with BD (18 M, 13 F; mean age: 32.87 ± 8.5 years) and 31 age-, gender-and body mass index-matched healthy subjects were enrolled. Demographic features and medications of the patients were recorded. The femoral cartilage thicknesses of both knees were measured with a 7–12 MHz linear probe while subjects' knees were held in maximum flexion. Three mid-point measurements were taken from both knees: lateral femoral condyle (LFC), intercondylar area (ICA) and medial femoral condyle (MFC).
Results:
Cartilage measurements of BD patients were significantly thinner at the ICA (p = 0.009) and LFC (p = 0.007) on the left knee, and at the MFC on both sides (both p < 0.05). Left knee cartilage thickness value at MFC (p = 0.005) was decreased in BD patients with arthritis compared to the healthy control group.
Conclusion:
These preliminary findings of decreased femoral cartilage thickness in BD patients with arthritis should be complemented with future studies. However, the possibility of early knee joint degeneration and eventual osteoarthritis in BD should also be kept in mind.
Keywords: Behçet's disease, femoral cartilage, thickness, ultrasound
RESUMEN
Objetivo:
Evaluar el grosor del cartílago femoral en pacientes con enfermedad de Behçet (EB) mediante el uso de la ultrasonografía.
Métodos:
Se enrolaron treinta y un pacientes con EB (18 V, 13 H; edad promedio: 32.87 ± 8.5 años), y 31 pacientes saludables pareados por edad, género e índice de masa corporal. Se registraron las características demográficas y medicamentos de los pacientes fueron registrados. Los grosores del cartílago femoral de ambas rodillas se midieron con una sonda lineal MHz 7 – 12 mientras que las rodillas de los sujetos se mantenían en flexión máxima. Se hicieron tres mediciones de punto medio de ambas rodillas: cóndilo femoral lateral (CFL), área intercondílea (ICA), y el cóndilo femoral medial (CFM).
Resultados:
Las mediciones del cartílago de pacientes con EB fueron significativamente más delgadas en el ICA (p = 0.009) y el CFL (p = 0.007) de la rodilla izquierda, y en el CFM de ambos lados (ambos p < 0.05). El valor del grosor del cartílago de la rodilla izquierda en CFM (p = 0.005) disminuía en pacientes EB con artritis, en comparación con el grupo de control sano.
Conclusión:
Estos hallazgos preliminares del grosor disminuido del cartílago femoral en pacientes EB con artritis deben ser complementados con estudios futuros. Sin embargo, debe tenerse también en mente la posibilidad de degeneración temprana de la articulación de la rodilla así como la eventual osteoartritis en los casos de EB.
INTRODUCTION
Behçet's disease (BD) is a chronic multi-systemic vasculitis that usually presents with recurrent oral/genital ulcers and ocular manifestations. In addition to the characteristic triad, BD commonly manifests with cardiovascular, pulmonary, neurological, gastrointestinal and articular problems (1, 2).
The articular involvement is seen in 5% to 76% of BD patients and the clinical scenario is that of intermittent, self-limiting, non-erosive mono/oligoarticular episodes commonly attacking the knee and ankle joints (3). Further, it has been reported that interleukin-1 beta (IL-1β) and tumour necrosis factor-alpha (TNF-α), found in the synovial fluid of BD patients, exert destructive effects on the joint cartilage (4). However, to the best of our knowledge, no articular cartilage has been studied by imaging in the relevant literature. Accordingly, the aim of our study was to evaluate the femoral cartilage thickness of BD patients by using ultrasound, which has previously been shown to be a valid and reliable method in that regard (5–9).
SUBJECTS AND METHODS
Thirty-one patients (18M, 13F) with a diagnosis of BD – according to the International Study Group Classification Criteria (10) – were enrolled in this study. Patients were recruited from the physical and rehabilitation medicine departments of two centres between January and March 2013. Cartilage measurements pertaining to 31 age-, gender-and body mass index (BMI)-matched healthy subjects were acquired from the authors' previously recorded pool of data. The study procedure was explained to every patient and they gave written consent. The protocol was approved by one of the centres' local ethics committee.
Patients were excluded if they had collagen tissue disorders or other inflammatory articular diseases, previous knee surgery, malignancy, chronic kidney/liver/thyroid disease and pregnancy.
Demographic features (ie gender, age of symptom onset, age of diagnosis, smoking history and BMI) and medications of the patients were recorded. Patients were also questioned as regards to the presence of arthritis. Joint pain was evaluated by a 10 cm visual analogue scale (VAS). Laboratory tests included complete blood count, liver/renal function tests, erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP).
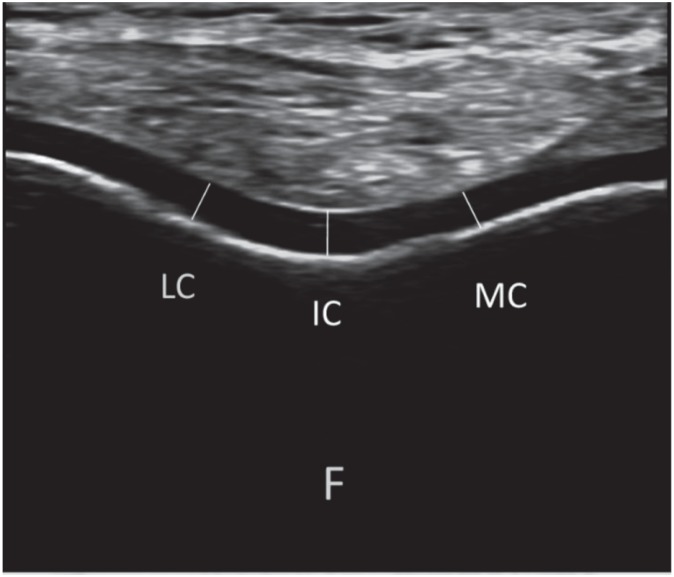
Cartilage measurements were performed bilaterally using a linear probe (7–12 MHz Logiq P5, GE Medical Systems, USA). While subjects were seated on the examination table with their knees in maximum flexion, the probe was placed (axially) on the suprapatellar area; the distal femoral cartilage was visualized as a strongly anechoic structure between the sharp bony (femur) cortex and the suprapatellar fat (Figure). Three mid-point measurements – lateral femoral condyle (LFC), intercondylar area (ICA) and medial femoral condyle (MFC) – were taken from each knee. All the ultrasounds were done by the same individual at the same centre.
Figure. Ultrasonographic image (suprapatellar axial view) demonstrating the femoral cartilage measurements. (LC: lateral condyle, IC: intercondylar area, MC: medial condyle, F: femur).
Statistical analysis
Statistical analysis was done using SPSS version 15.0. Data are expressed as mean ± standard deviation. Correlations between patients' characteristics and femoral cartilage thickness measurements were analysed using Pearson's correlation coefficients. Paired samples t-test and Wilcoxon signed-rank test were used to compare the mean knee cartilage thickness values between groups. Chi-squared test was used for frequency comparisons. Statistical significance was set at p < 0.05.
RESULTS
Measurements of 31 BD patients (62 knees) and 31 age-, gender- and BMI-matched healthy subjects (62 knees) were analysed. Mean age of the patients and controls was 32.8 ± 8.5 (range 17–51) years. Patients and the control group was similar as regards to BMI (23.9 ± 3.1 vs 23.9 ± 2.7, respectively) and number of smokers (eight vs 13) [both p > 0.05]. Mean disease duration of the patients was 53.9 ± 59.5 months. Current medications of the patients are given in Table 1. Twenty patients had eye involvement, 18 patients had mucocutaneous lesions and 13 patients had arthritis (eight knee, three ankle and two elbow involvement).
Table 1. Medications of the patients.
| Medication | (n = 31) |
|---|---|
| Colchicine + DMARD | 11 |
| Colchicine | 10 |
| CS + DMARD | 4 |
| DMARD | 3 |
| CS + IFN | 2 |
| Anti-TNF | 1 |
DMARD: disease-modifying antirheumatic drug, TNF: tumour necrosis factor, CS: corticosteroids, IFN: interferon
Mean femoral cartilage thickness values of the patients and controls are shown in Table 2. Cartilage measurements of BD patients were significantly thinner at the ICA (p = 0.009) and LFC (p = 0.007) on the left knee, and at the MFC on both sides (both p < 0.05).
Table 2. Comparison of femoral cartilage thickness measurements (mm).
| Patients (n = 31) | Controls (n = 31) | p | ||
|---|---|---|---|---|
| Right | LFC | 2.0 ± 0.3 | 2.2 ±0.4 | 0.143 |
| ICA | 2.0 ±0.4 | 2.2 ±0.5 | 0.142 | |
| MFC | 1.9 ±0.3 | 2.2 ±0.5 | 0.038 | |
| Left | LFC | 2.0 ±0.3 | 2.2 ±0.4 | 0.007 |
| ICA | 1.9 ±0.3 | 2.2 ±0.5 | 0.009 | |
| MFC | 1.9 ±0.3 | 2.2 ±0.4 | 0.013 |
LFC: lateral femoral condyle, ICA: intercondylar area, MFC: medial femoral condyle
When subgroups were compared (with their paired healthy controls) according to the presence of arthritis, left knee cartilage thickness values at MFC (p = 0.005) and ICA (p = 0.059) were decreased in BD patients with arthritis (n = 13).
Cartilage thickness measurements did not correlate with patients' characteristics or laboratory results (p > 0.05).
DISCUSSION
In this study, we have evaluated the femoral cartilage thicknesses of BD patients, to our best knowledge, for the first time in the literature. The results showed that BD patients had thinner femoral cartilage thicknesses when compared with those of healthy controls. Further, the presence of arthritis seemed to be associated with this decrease in thickness.
Joint involvement is one of the most frequent manifestations following oral and genital ulcers in BD (4). Although it usually tends to cause no deformities, joint involvement may occasionally result in synovial hypertrophy and contractures, and rarely, in the form of an erosive arthropathy (11). Similarly, established joint deformity was not present in any of our patients and only 42% reported a previous arthritis episode.
In the pertinent literature, joint imaging has been generally performed with scintigraphy (12, 13) and magnetic resonance imaging [MRI] (14, 15) in BD. There seems to be only a few papers that have used ultrasound for imaging the joints and nearby tendons (14, 16, 17). Further, to our best knowledge, there are no data regarding femoral cartilage thicknesses of BD patients. As such, and together with the fact that increased synovial fluid levels of IL-1β are believed to be responsible for cartilage destruction in BD (18), we reasoned that knee joint cartilage might have been affected in these patients. In this regard, our finding of decreased femoral cartilage thicknesses in patients with arthritis seems to be noteworthy. Although the type of joint involvement was not the knee in all of our arthritis patients (eight out of 13), it would not be unsound to speculate on the possibility of subclinical joint inflammation (13). On the other hand, we could not find any correlation between cartilage thickness values and patient characteristics, and we believe that this might be attributed to our small sample size. Another limitation of our study would be its cross-sectional design.
To conclude, in light of these preliminary results, we imply that BD patients with arthritis have decreased femoral cartilage thickness when compared with their healthy controls. As such, these patients may become prone to early knee joint degeneration and eventual osteoarthritis. Keeping in mind the other possible soft tissue changes of these patients (16), aside from medications, joint protection strategies also seem to be paramount in their treatment protocols. Future studies with larger samples (and perhaps with prospective follow-up) are definitely awaited to confirm our results and to uncover possible differences among various BD subgroups as well. Lastly, ultrasonography seems to be promising in that sense.
REFERENCES
- 1.Bodur H, Borman P, Özdemir Y, Atan Ç, Kural G. Quality of life and life satisfaction in patients with Behçet's disease: relationship with disease activity. Clin Rheumatol. 2006;25:329–333. doi: 10.1007/s10067-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 2.Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet's disease. Int J Dermatol. 2003;42:346–351. doi: 10.1046/j.1365-4362.2003.01741.x. [DOI] [PubMed] [Google Scholar]
- 3.Cho SB, Lee JH, Ahn KJ. Anti-cyclic citrullinated peptide antibodies and joint involvement in Behçet's disease. Yonsei Med J. 2012;53:759–764. doi: 10.3349/ymj.2012.53.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ertenli I, Kiraz S, Calgüneri M, Celik I, Erman M, Haznedaroglu IC. Synovial fluid cytokine levels in Behçet's disease. Clin Exp Rheumatol. 2001;19:37–41. [PubMed] [Google Scholar]
- 5.Lee CL, Huang MH, Chai CY, Chen CH, Su JY, Tien YC. The validity of in vivo ultrasonographic grading of osteoarthritic femoral condylar cartilage: a comparison with in vitro ultrasonographic and histologic gradings. Osteoarthritis Cartilage. 2008;16:352–358. doi: 10.1016/j.joca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Möller I, Bong D, Naredo E, Filippucci E, Carrasco I, Moragues C, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage. 2008;16:S4–S7. doi: 10.1016/j.joca.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Yoon CH, Kim HS, Ju JH, Jee WH, Park SH, Kim HY. Validity of the sonographic longitudinal sagittal image for assessment of the cartilage thickness in the knee osteoarthritis. Clin Rheumatol. 2008;27:1507–1516. doi: 10.1007/s10067-008-0956-3. [DOI] [PubMed] [Google Scholar]
- 8.Castriota-Scanderberg A, De Micheli V, Scarale MG, Bonetti MG, Cammisa M. Precision of sonographic measurement of articular cartilage: inter- and intraobserver analysis. Skeletal Radiol. 1996;25:545–549. doi: 10.1007/s002560050132. [DOI] [PubMed] [Google Scholar]
- 9.Mathiesen O, Konradsen L, Torp-Pedersen S, Jorgensen U. Ultrasonography and articular cartilage defects in the knee: an in vitro evaluation of the accuracy of cartilage thickness and defect size assessment. Knee Surg Sports Traumatol Arthrosc. 2004;12:440–443. doi: 10.1007/s00167-003-0489-x. [DOI] [PubMed] [Google Scholar]
- 10.International Study Group for Behcet's Disease Evaluation of diagnostic (‘classification’) criteria in Behçet's disease – towards internationally agreed criteria. Br J Rheumatol. 1992;31:299–308. [PubMed] [Google Scholar]
- 11.Gur A, Sarac AJ, Burkan YK, Nas K, Cevik R. Arthropathy, quality of life, depression, and anxiety in Behcet's disease: relationship between arthritis and these factors. Clin Rheumatol. 2006;25:524–531. doi: 10.1007/s10067-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 12.Yurtkuran M, Yurtkuran M, Alp A, Sivrioglu K, Dilek K, Tamgaç F, et al. Hand involvement in Behçet's disease. Joint Bone Spine. 2006;73:679–683. doi: 10.1016/j.jbspin.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Şahin M, Yildiz M, Tunç SE, Cerci S, Suslu H, Cure E, et al. The usefulness of Tc-99m-MDP bone scintigraphy in detection of articular involvement of Behçet's disease. Ann Nucl Med. 2006;20:649–653. doi: 10.1007/BF02984675. [DOI] [PubMed] [Google Scholar]
- 14.Choi JA, Kim JE, Koh SH, Chung HW, Kang HS. Arthropathy in Behçet disease: MR imaging findings in two cases. Radiology. 2003;226:387–389. doi: 10.1148/radiol.2262011982. [DOI] [PubMed] [Google Scholar]
- 15.Sugawara S, Ehara S, Hitachi S, Sugimoto H. Hand and wrist arthritis of Behçet disease: imaging features. Acta Radiologica. 2010;51:183–186. doi: 10.3109/02841850903401349. [DOI] [PubMed] [Google Scholar]
- 16.Özçakar L, Onat AM, Üreten K, Cetin A, Kiraz S, Ertenli I, et al. Sonographic evaluation of the tendons in familial Mediterranean fever and Behçet's disease. Joint Bone Spine. 2006;73:514–517. doi: 10.1016/j.jbspin.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Ceccarelli F, Priori R, Iagnocco A, Coari G, Accorinti M, Pivetti Pezzi P, et al. Knee joint synovitis in Behçet's disease: a sonographic study. Clin Exp Rheumatol. 2007;5:76–79. [PubMed] [Google Scholar]
- 18.Pay S, Erdem H, Pekel A, Simsek I, Musabak U, Sengul A, et al. Synovial proinflammatory cytokines and their correlation with matrix metalloproteinase-3 expression in Behçet's disease. Does interleukin-1b play a major role in Behçet's synovitis? Rheumatol Int. 2006;26:608–613. doi: 10.1007/s00296-005-0040-0. [DOI] [PubMed] [Google Scholar]