Abstract
Knowledge of feeding habits of small rodents is necessary for understanding food webs, trophic structure, and plant–animal interactions in Neotropical forests. Despite several studies that have investigated community structure and feeding behavior of rodents, large gaps remain in our understanding of their guild occupancy. Our objective was to investigate the diets of 7 species of small (< 100g) sympatric sigmodontine rodents in a high (3,500 m) Andean montane rainforest in Peru. We qualitatively and quantitatively assessed diet items in fecal samples from livetrapped rodents from 2009 to 2012. Frequency data for 4 diet categories indicated that all 7 species of rodents contained 4 diet categories in fecal samples: arthropods (88%), remains of leaves and fibers from plants (61%), intact seeds (with or without fruit pulp; 50%), and mycorrhizal spores (45%). Omnivory was found to be a strategy used by all species, although contingency table analysis revealed significant differences among and within species in diet categories. Cluster analysis showed 2 main groupings: that of the Thomasomys spp. plus Calomys sorellus group which included high amounts of intact seeds and plant parts in their fecal samples, and those of the genera Akodon, Microryzomys, Oligoryzomys, which included a greater proportion of arthropods in their fecal samples, but still consumed substantial amounts of fruit and plant parts. Intact seed remains from at least 17 plant species (9 families) were found in fecal samples. We concluded that this assemblage of sigmodontine rodents is omnivorous but that they likely play an important role as frugivores and in seed dispersal in tropical montane forests in Peru.
El conocimiento de los hábitos alimenticios de roedores pequeños es necesario para comprender cadenas alimenticias, estructura trófica, e interacciones planta-animal en los bosques neotropicales. A pesar de que numerosos estudios han investigado la estructura de comunidades y el comportamiento de forrajeo en roedores, aún existen grandes vacíos en nuestra comprensión de sus gremios tróficos. Nuestro objetivo fue investigar las dietas de siete especies de pequeños (< 100 g) roedores sigmodontinos simpátricos en un bosque montano andino a 3.500] m en Perú. Cualitativamente y cuantitativamente evaluamos la dieta en muestras fecales de roedores capturados entre el 2009 y el 2012. Datos de frecuencia para cuatro categorías de dieta indicaron que las siete especies de roedores consumieron cuatro categorías de dieta: artrópodos (88%), pedazos de hojas y fibras de plantas (61%), semillas intactas (con o sin pulpa de frutos; 50%), y esporas de micorrizas (45%). Omnivoría fue la estrategia utilizada por todas las especies, aunque el análisis con tablas de contingencia reveló diferencias significativas entre y dentro de especies en categorías de dieta. El análisis de agrupación presentó 2 grupos principales: el grupo Thomasomys spp. y Calomys sorellus, que incluye una gran proporción de semillas intactas, y partes de plantas en las muestras fecales y el grupo que incluye los géneros Akodon, Microryzomys y Oligoryzomys, el cual incluyó una proporción mayor de artrópodos en sus muestras fecales, pero con niveles altos de semillas intactas. Semillas intactas de al menos 17 especies de plantas (9 familias) fueron encontradas en las muestras fecales. Concluimos que este ensamble de roedores sigmodontinos es omnívoro y que probablemente las especies juegan un rol importante como frugívoros y en la dispersión de semillas en los bosques montanos tropicales en el Perú.
Key words: Akodon, Calomys, Microryzomys, Oligoryzomys, rodent frugivory, Sigmodontinae, Thomasomys, tropical forest
Knowledge of food habits and trophic structure of rodent assemblages are crucial for understanding ecosystem function and plant–animal interactions in Neotropical forest habitats. In the Neotropics, where rodents are diverse and abundant (Voss and Emmons 1996; Pacheco et al. 2009), in desert (Marquet 1994), Andean (Pizzimenti and De Salle 1981; Novillo and Ojeda 2014), and rainforest habitats (Fonseca 1989; Patterson et al. 1998; Solari et al. 2006; Pacheco et al. 2009, 2011), knowledge of rodent feeding habits can facilitate understanding not only the ecology of their habitats but also provide information necessary for management, conservation, and restoration of forest rodent populations (Pacheco et al. 2013; Salas et al. 2013). For North America, a rich literature exists regarding the ecology and dietary habits of small desert-dwelling rodents (Brown and Lieberman 1973; Reichman 1975; Meserve 1981; Wolff et al. 1985; Kotler and Brown 1988; Brown 1989; Kelt et al. 2004; Stevens and Tello 2009; Meserve et al. 2011; Lobo et al. 2013; Stevens and Anderson 2014). A general consensus from these studies is that rodents, through their seed consumption, seed predation, scatter-hoarding, and caching behavior, elicit profound influences on plant reproductive success and community structure (Ostfeld et al. 1997; Kelt 2011).
In the South American Neotropics, diet and seed dispersal in rodents have been primarily studied in larger rodents belonging to the genera Cuniculus, Dasyprocta, Myoprocta (Smythe 1978; Forget 1990; Henry 1999; Forget et al. 2002; Dubost and Henry 2006), and Proechimys (Adler 1995; Mangan and Adler 2002). These studies showed that impacts by rodents are wide and far ranging; for example, rodents of the genus Proechimys consume and disperse mycorrhizal spores (Janos et al. 1995; Mangan and Adler 2002), and act as seed predators and possibly seed dispersers via scatter-hoarding (Forget and Milleron 1991; Forget 1993; Adler 1995; Forget 1996; Jansen and Forget 2001). Rodents belonging to the genus Dasyprocta, Myoprocta, and Cuniculus include fruit in their diets (Smythe 1978; Henry 1999; Dubost and Henry 2006) and can consume scatter-hoarded seeds as well as germinating seedlings (Forget and Milleron 1991).
The subfamily Sigmodontinae (Cricetidae) includes approximately 400 species in some 86 genera and 9 tribes of small rodents (12–500 g—D’Elía et al. 2007; Salazar-Bravo et al. 2013; D’Elía and Pardiñas 2015). They range from North to South America, inhabiting deserts, highlands, and tropical forests. They are ubiquitous and diverse, with a high degree of endemism (D’Elía and Pardiñas 2015). The natural history, feeding behavior, and ecological roles of these small rodents in Neotropical forests are for the most part unknown, hampering our understanding of ecosystem structure and function in lowland and highland tropical forests.
Some recent studies of sigmodontines in the Neotropics indicate that they exhibit flexible diet strategies. Sigmodontine rodents were omnivorous, granivorous, or herbivorous in the Monte Desert in Argentina (Campos et al. 2001; Giannoni et al. 2005). Akodon spp. were found to be omnivorous in the temperate forests of Chile (Meserve et al. 1988). In the Brazilian Atlantic forest, sigmodontine rodents were recorded consuming fruits in captivity (Viera et al. 2003, 2006). In southern Brazil, rodent species were generalist in relation to diet composition (Caella and Caceres 2006). Stomach contents of 5 species of sigmodontine rodents from the montane forests of Huánuco, Peru, were recorded and arthropod consumption was found for Akodon orophilus, omnivory for Microryzomys altissimus and M. minutus, and herbivory (plant remains and seeds) for Thomasomys notatus and T. kalinowskii (Noblecilla and Pacheco 2012). Given the captive feeding experiments for Brazilian rodents and seeds found in stomach contents from Thomasomys spp. from montane forests in Peru, fruit consumption by sigmodontine rodents may be more common than previously thought. If this is true in Neotropical forests, then a reassessment of the guild occupancy of small-rodent communities, ecological roles, as well as niche occupancy relative to other, better studied taxa Neotropical forests such as birds, bats, and primates may be useful. However, for both lowland and montane rainforest, data on rodent diets are incomplete.
Herein, we document the diet of sigmodontine rodents found in a tropical montane forest in Ayacucho, Peru. Montane forests host many endemic species, including rodents (Gentry 1992; Leo 1995; Olson and Dinerstein 1998; Pacheco 2002; Pacheco et al. 2009) that are highly threatened by human activity such as road-building, agriculture, logging, and climate change (Young 1994; Foster 2001; Kintz et al. 2006). We addressed the questions: what is the diet composition of 7 species of sympatric sigmodontine rodents in a tropical montane forest? What are qualitative and quantitative differences in diet among species of rodents? and, is there evidence of frugivory within this assemblage of rodents?
Materials and Methods
Study site.
As part of a larger study to determine potential impacts of a natural gas pipeline on rodent populations, we collected fecal samples of rodents caught in Sherman live traps (Pacheco et al. 2013; Salas et al. 2013). Our study site was located near Chiquintirca, Department of Ayacucho, in the province of La Mar (13°03′34″S, 73°42′25″W). It is near the upper limit of montane forests of the Apurimac River valley ranging in elevation from 3,200 to 3,500 m. This area is cataloged as belonging to the Pluvial Montane Subtropical Forest (Instituto Nacional de Recursos Naturales 1995), upper montane pluvial forest of the yungas (Josse et al. 2007), and the Apurimac river valley montane forest ecotone (Langstroth et al. 2013).
Rodent capture and sample collection.
In 2009–2010, 8 sampling plots 20×150 m in size were established to evaluate the small mammal community and potential influence of construction of a pipeline (Pacheco et al. 2013; Salas et al. 2013). Each plot consisted of 2 parallel lines separated by 15–20 m, and each line had 16 sampling stations 10 m apart. We placed 2 Sherman traps at each sampling station. When possible, 1 of the traps at each station was placed on a branch of a shrub or tree, 1–2 m above ground. Plots were evaluated 3 times during the rainy season for 5 nights each (November 2009–March 2010) and 3 times in the dry season (August–November 2010), for a total of 14,400 trap nights (Pacheco et al. 2013; Salas et al. 2013). In 2011–2012, 9 sampling plots were sampled for 4 nights prior to the rainy season (October–November 2011) and after the rainy season (May 2012) for a total of 6,912 trap nights. Traps were baited late in the afternoon, left open at night and evaluated in the morning. All traps were washed with a small amount of powdered laundry detergent in between captures. Rodents captured were identified to species, aged (juvenile or adult), weighed, tagged, and released. Animal capture and handling followed ASM guidelines (Sikes et al. 2011) and was approved by The Smithsonian National Zoological Park-Institutional Animal Care and Use Committee (NZP-IACUC #09-39).
Plant species richness and fruit availability.
In 2011, plant species richness and abundance was recorded in the rodent sampling grids. In addition, 13 modified Whittaker plots of 0.1 ha were sampled. Seven of these were located in the pipeline right-of-way (RoW), 3 in long-term “control” plots, and 3 alongside the RoW that included forest and shrub habitat. For each sample plot, the number of plant species and individuals per plant species was recorded. Leaves, stems, fruits, and seeds in the plant study grids were collected and used as reference. These were deposited in the herbarium collection of the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos. These data were tabulated and a list of plant species found at our study site was compiled. This was used to complement the plant species list and fruit availability data compiled by Servat et al. (2013).
Fruit availability was recorded May 2012. A 90-m-long line-transect was established in each rodent sampling grid, with 10×2-m-long perpendicular transects every 10 m (n = 10). We recorded the number of plants with fruits on each transect, and estimated the number of fruits per shrub and tree by counting the total number of fruits in 3 infructescences (shrubs) or branches (trees), and by multiplying the average number of fruits by the number of branches or infructescences with fruits on each individual plant. We then calculated the number of fruits per species/m2. Rainfall data were obtained from a pluviometer at the study site.
Diet analysis of rodents.
Diet was determined via analysis of fecal remains of captured rodents (Meserve 1981; Cortes et al. 2002a, 2002b; Lopez-Cortes et al. 2007). After a captured rodent was measured, tagged, identified, and released, feces found in the trap were collected and placed in 2-ml-labeled plastic cryovial tubes with 70% ethanol. A total of 554 samples were collected from 2009 to 2012. We did not have recaptures in the same trapping night but did have recaptures within a trapping session (5 nights). We utilized these in our diet analyses. Twelve fecal samples from each tube were placed in a 140×20mm petri dish that was placed atop grid paper with 1×1mm squares. Ten observational fields of 10×10mm were chosen randomly, and frequency (presence/absence) of arthropods, intact, undamaged seeds (with or without fruit pulp), plant parts (leaves, stems, shoots), and mycorrhizal spores were recorded using a 20× Leica RX and a 7× zoom lens stereoscope. A 40–100× Labomed microscope was utilized to identify fragments and mycorrhizal spores. Four diet categories (2009–2012) and items (2009–2010; see below) were identified via a reference collection of insects, plants, fruits, and seeds from this study and a previous habitat analysis (Servat et al. 2013). Seeds were identified to family, genus, and species level when possible, using identification keys found in Gentry (1993), Ponte (1998), and Caceres (2004). Histological analysis of plant remains was not conducted.
For arthropods, parts of exoskeletons of adults were identified whenever possible to lowest taxonomic level (Tripplehorn and Johnson 2005). In 2009–2010, 7 diet categories were recorded: 1) arthropod larvae, 2) adult arthropods, 3) monocotyledon plant parts, 4) dicotyledon plant parts, 5) monocotyledon fruit remains, 6) dicotyledon fruit remains, and 7) mycorrhizal spores. Intact seeds in fecal samples with or without fruit pulp were considered as evidence of fruit consumption.
Analysis of data.
Using frequencies of occurrence for arthropods, intact seeds, plant remains, and mycorrhizal spores from 2009 to 2012, contingency table analysis was utilized to determine whether: 1) observed values of diet categories were different from expected; 2) species were associated with diet category; and 3) season was associated with diet category among and within species. Association between diet and year was not calculated due to different sampling dates and effort among years. A Bray–Curtis dissimilarity index was calculated for frequency of occurrence data from 2009 to 2012, and utilized in a cluster analysis to visualize grouping patterns according to diet.
Statistical analyses were conducted using SPSS version 21 and PAST version 7.
Results
Rodent community.
A total of 554 captures of 7 species of sigmodontine rodents were obtained during this study. Species captured (sample size of adult rodents, and average mass [g] ± SD in parenthesis) were the cloud forest grass mouse, Akodon torques (n = 44 adult females, = 25.93±7.39; n = 74 adult males, = 27.40±3.79); the Peruvian vesper mouse, Calomys sorellus (n = 9 females, = 23.04±3.70; n = 11 males, = 22.12±3.77); the forest small rice rat, Microryzomys minutus (n = 23 adults, both sexes, = 17.59±3.48); the Andean pygmy rice rat, Oligoryzomys andinus (n = 5 adults, both sexes, = 29.0±3.7); the golden Thomasomys, Thomasomys aureus (n = 9 adults, both sexes, = 91.2±11.5); Kalinowski’s Thomasomys, Thomasomys kalinowskii (n = 26 females, = 44.65±6.95; n = 13 males, = 52.50±4.23); and the montane fairy Thomasomys, Thomasomys oreas (n = 27 females, = 32.79±5.43; n = 34 males, = 37.97±6.80). Adult rodents ranged in average mass from 17.6g (M. minutus) to 91.2g (T. aureus).
Diet.
From 2009 to 2012, all of the 7 species of rodents captured contained 4 diet categories in fecal samples; arthropods, intact seeds, plant parts, and mycorrhizal spores. Frequency of occurrence of each diet category tabulated for each species indicated differences in feeding strategies (Table 1). Differences among species and within diet categories were significant (n = 554, d.f. = 6, χ2 = 35.59, P < 0.001, arthropods; n = 554, d.f. = 6, χ2 = 152.34, n = 554, d.f. = 6, P < 0.001, intact seeds; n = 554, d.f. = 6, χ2 = 91.86, P < 0.001, plant parts; n = 554, d.f. = 6, χ2 = 29.98, d.f. = 6, P < 0.001, mycorrhizal spores) indicative of diet differences or variable access to resources among rodent species.
Table 1.
Proportion frequency of occurrence of diet category for 7 species of sigmodontine rodents, 2009–2012, at Chiquintirca, Ayacucho, Peru. All 7 rodent species contain each diet category in fecal samples which is highly indicative of omnivory in this assemblage. Thomasomys aureus samples contain the highest frequency of intact seeds and plant parts and the lowest frequency of arthropods. Akodon torques samples contain the lowest frequency of intact seeds and plant parts and the highest frequency of arthropods.
| Species | N | Diet category | |||
|---|---|---|---|---|---|
| Arthropods | Intact seeds | Plant parts | Mycorrhizal spores | ||
| All species | 554 | 0.88 | 0.61 | 0.50 | 0.46 |
| Akodon torques | 283 | 0.93 | 0.39 | 0.33 | 0.40 |
| Calomys sorellus | 47 | 0.89 | 0.83 | 0.51 | 0.26 |
| Microryzomys minutus | 46 | 0.91 | 0.52 | 0.71 | 0.60 |
| Oligoryzomys andinus | 11 | 0.91 | 0.45 | 0.73 | 0.36 |
| Thomasomys aureus | 19 | 0.53 | 0.95 | 1.0 | 0.42 |
| Thomasomys kalinowskii | 57 | 0.81 | 0.94 | 0.56 | 0.67 |
| Thomasomys oreas | 91 | 0.80 | 0.96 | 0.77 | 0.55 |
Association between season (summer, autumn, winter, spring) and diet category for all species combined was significant for 4 categories: arthropods, intact seeds, plant parts, and mycorrhizal spores (n = 554, d.f. = 3, χ2 = 23.019, P < 0.001; χ2 = 23.861, P < 0.001; χ2 = 40.259, P < 0.001; χ2 = 56.994, d.f. = 3, P < 0.001). A contingency table analysis for season by species revealed that A. torques differed significantly among seasons for all diet categories (n = 283, d.f. = 3, χ2 = 19.2, P < 0.001, arthropods; χ2 = 21.94, P < 0.001, intact seeds; χ2 = 41.94, P < 0.001, intact seeds; χ2 = 71.29; P < 0.001, mycorrhizal spores) whereas C. sorellus did not differ among seasons in the presence/absence of arthropods, plant remains, or mycorrhizal spores (n = 47, d.f. = 3, χ2 = 1.35, P = 0.72, arthropods; χ2 = 2.54, P = 0.47, plant remains; χ2 = 3.3, P = 0.35, mycorrhizal spores) but differed significantly among seasons in intact seed occurrence (n = 47, d.f. = 3, χ2 = 10.82, P = 0.013). M. minutus did not show significant differences among seasons for diet items except for the presence of mycorrhizal spores (n = 46, d.f. = 3, χ2 = 4.34, P = 0.23, arthropods; χ2 = 6.03, P = 0.11, intact seeds; χ2 = 1.97, P = 0.58, plant parts; χ2 = 9.72, P = 0.021, mycorrhizal spores). T. kalinowskii showed significant differences among season only for intact seed consumption (n = 57, d.f. = 3, χ2 = 3.47, P = 0.33, arthropods; χ2 = 16.89, P = 0.001, intact seeds; χ2 = 0.61, P = 0.89, plant parts; χ2=6.45, P = 0.09, mycorrhizal spores) whereas T. oreas showed significant differences among seasons only for arthropods but not for fruits and seeds (n = 91, d.f. = 3, χ2 = 9.52, P = 0.02, arthropods; χ2 = 2.81, P = 0.42, intact seeds; χ2 = 3.08, P = 0.379, plant parts; χ2 = 9.216, P = 0.27, mycorrhizal spores). Due to small sample sizes, we were not able to reach conclusions regarding diet and seasonality for O. andinus and T. aureus.
Diet composition.
Diet composition, determined for samples collected in 2009–2010, indicated that all species (except for T. aureus [59%; Table 2]) had high frequency of occurrence of arthropods (between 90% and 100%). Arachnids were an infrequent diet item, although A. torques was found to consume spiders and scorpions. Within the class Insecta, coleopterans were the most common diet item, found most frequently in C. sorellus and A. torques, but absent from T. aureus. A. torques had the highest diversity of insects in its diet, with coleopterans, hemipterans, hymenopterans, isopterans, orthopterans, and other unidentified invertebrates present in fecal samples. C. sorellus was found to include at least 3 orders of insects (Coleoptera, Hemiptera, Hymenoptera); M. minutus 2 orders (Coleoptera, Hymenoptera); O. andinus 2 orders (Coleoptera, Hymenoptera); T. kalinowskii 2 orders (Coleoptera, Hemiptera); and T. oreas 3 orders (Coleoptera, Hymenoptera, Orthoptera).
Table 2.
Diet composition and proportion frequency of occurrence of diet items for 2009–2010 at Chiquintirca, Ayacucho, Peru. Starred items were found only in 2011–2012 samples, have the corresponding n next to frequency, and are included here to complete our diet composition list for plants.
| Species | Akodon torques | Calomys sorellus | Microryzomys minutus | Oligoryzomys andinus | Thomasomys aureus | Thomasomys kalinowskii | Thomasomys oreas |
|---|---|---|---|---|---|---|---|
| n = 162 | n = 21 | n = 34 | n = 7 | n = 17 | n = 23 | n = 64 | |
| Invertebrata | 1.00 | 1.00 | 1.00 | 1.00 | 0.59 | 1.00 | 0.91 |
| Arachnida | |||||||
| Araneae | 0.006 | 0.03 | |||||
| Scorpiones | 0.02 | ||||||
| Insecta | |||||||
| Coleoptera | 0.80 | 0.90 | 0.44 | 0.71 | 0.61 | 0.36 | |
| Hemiptera | 0.09 | 0.05 | 0.04 | ||||
| Hymenoptera | 0.20 | 0.10 | 0.03 | 0.14 | 0.05 | ||
| Isoptera | 0.01 | ||||||
| Orthoptera | 0.10 | 0.02 | |||||
| Unidentified larvae | 0.60 | 0.42 | 0.38 | 0.29 | 0.35 | 0.39 | 0.42 |
| Plantae | |||||||
| Plant parts | 0.85 | 0.95 | 0.97 | 1.00 | 1.00 | 1.00 | 0.97 |
| Monocotyledoneae | 0.10 | 0.06 | 0.18 | 0.04 | 0.06 | ||
| Dicotyledoneae | 0.29 | 0.61 | 0.59 | 0.57 | 0.94 | 0.65 | 0.73 |
| Seeds | |||||||
| Monocotyledoneae | |||||||
| Bromeliaceae | |||||||
| Greigia sp. | 0.39 | ||||||
| Dicotyledoneae | |||||||
| Annonaceae | |||||||
| Guatteria sp. | 0.59 | 0.04 | 0.02 | ||||
| Brassicaceae | |||||||
| Brassicaceae sp. 1 | 0.01 | 0.03 | 0.29 | 0.04 | 0.03 | ||
| Brassicaceae sp. 2 | 0.03 | ||||||
| Ericaceae | |||||||
| Gaultheria sp. 1 | 0.17 | 0.52 | 0.32 | 0.28 | 0.23 | 0.30 | 0.30 |
| Gaultheria sp. 2 | 0.03 | 0.05 | 0.06 | ||||
| Demosthenesia sp. | 0.01 | 0.13 | 0.03 | ||||
| Melastomataceae | |||||||
| Miconia sp. 1 | 0.14 | 0.06 | 0.59 | 0.43 | 0.26 | ||
| Miconia sp. 2 | 0.03 | 0.18 | 0.06 | ||||
| Myrtaceae | |||||||
| Myrteola sp. | 0.02 | ||||||
| *Rosaceae sp. 1 | 11 (0.09) | ||||||
| *Rubiaceae sp. 1 | 3 (0.33) | ||||||
| *Rubiaceae sp. 2 | 4 (0.25) | ||||||
| Solanaceae | |||||||
| Solanaceae sp. 1 | 0.59 | ||||||
| Solanaceae sp. 2 | 0.59 | 0.09 | |||||
| Solanaceae sp. 3 | 0.59 | 0.04 | |||||
| Solanaceae sp. 4 | 0.13 | ||||||
| Unidentified seeds | 0.02 | 0.05 | 0.12 | 0.22 | 0.06 | ||
| Fungi | |||||||
| Glomeromycetes | |||||||
| Glomerales | |||||||
| Glomus sp. | 0.58 | 0.57 | 0.71 | 0.57 | 0.47 | 0.74 | 0.59 |
Nine plant families and 17 plant morpho-species belonging to a minimum of 10 genera were identified by intact seeds occurring in rodent fecal remains. Based on identification of intact seeds, rodents consumed fruits that occur in families that are most commonly associated with small trees or shrubs that likely produce berries with small seeds. Positively identified families in the diets included Annonaceae (Guatteria sp.), Bromeliaceae (Greigia sp.), Brassicaceae (sp. 1, sp. 2), Ericaceae (Gaultheria sp. 1, Gaultheria sp. 2, Demosthenesia sp.); (Solanaceae sp. 1–4), Melastomataceae (Miconia sp. 1 and sp. 2), and Myrtaceae (Myrteola sp.). These represent approximately 13% of the plant families identified thus far at the study site but is likely a conservative estimate, as we were not able to identify all fruit pulp occurring in fecal samples.
Monocotyledon plant, fruit, and intact seed remains were found much less frequently than dicotyledon plant items in all species. Among the monocots, seeds belonging to the genus Greigia (Bromeliaceae) were found only in T. kalinowskii fecal samples. Seeds from the genus Miconia sp. 1 and sp. 2 (Melastomataceae) were found in 5 species and 3 genera of rodents (Akodon, Microryzomys, Thomasomys spp.). Seeds from the genus Gaultheria (sp. 1 and/or sp. 2; Ericaceae) were found in samples from all species of rodents. Seeds belonging to the Solanaceae family were found infrequently in T. kalinowskii and T. aureus feces. Demosthenesia sp. 1 (Ericaceae) seeds were found at low frequencies in T. kalinowskii and T. oreas fecal samples as were unidentified seeds belonging to the Rosaceae (sp. 1) and Rubiaceae (sp. 1 and sp. 2). Mycorrhizal spores belonging to the genus Glomus were frequent in samples from all species of rodents. T. kalinowskii had the highest percent occurrence of Glomus sp. spores, followed by O. andinus.
Bray–Curtis index of dissimilarity.
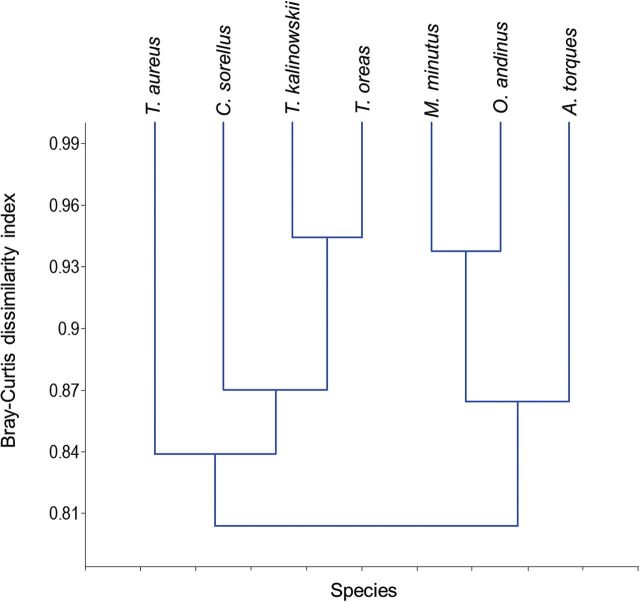
A cluster analysis utilizing a Bray–Curtis dissimilarity index showed 2 main clusters according to diet. The genus Thomasomys plus C. sorellus formed a group with T. aureus being the most dissimilar of this group. The 2nd cluster was formed by O. andinus, A. torques, and M. minutus, with A. torques being the most dissimilar of this group (Fig. 1).
Fig. 1.
Cluster analysis for rodent species based on Bray–Curtis dissimilarity index for frequency of occurrence of 4 diet categories at Chiquintirca, Ayacucho, Peru. Two clusters are apparent; one is comprised of Thomasomys spp. but also includes Calomys sorellus due to the high levels of intact seeds and plant parts in fecal samples. The 2nd cluster is comprised of Akodon torques, Microryzomys minutus, and Oligoryzomys andinus which are omnivorous, but have lower proportions of intact seeds and plant remains in samples as compared to the 1st group.
Plant species richness and fruit production.
A total of 172 species and morpho-species (54 families, 94 genera) were recorded at the study site. Rodents were found to pass whole, undamaged seeds from 17 species (10% of plant species registered). Plant families and genera in the diet of rodents primarily are associated with small trees and shrubs as well as the bromeliad Greigia, which commonly is found along the ground in montane cloud forest. We were not able to confirm the identity of all fruit pulp found in feces, so the number of fruit-bearing plants consumed may be larger than that reported for whole seed consumption and passage.
In May 2012, after the rainy season (Fig. 2), 57 species of plants were producing fruits (21 families and 33 genera) and 9 species of intact seeds were identified in feces (16% of fruiting species). Total fruit production at this time was 136 fruits/m2 in forest habitat and 142.35/m2 in shrub habitat.
Fig. 2.
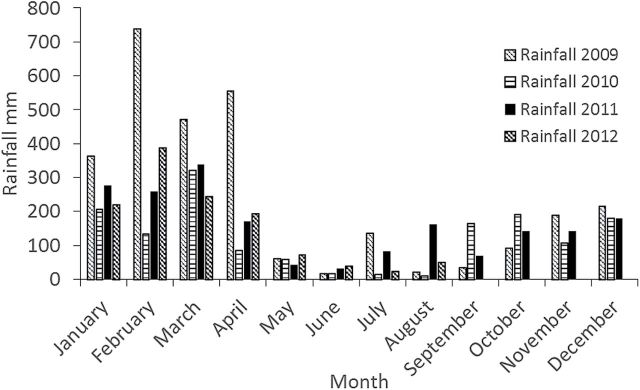
Rainfall for 2009–2012 from the study site at Chiquintirca, Ayacucho, Peru, illustrating variability in precipitation within and between years. For 2012, data from September through December are missing.
Discussion
The 7 species of sigmodontine rodents in our study area have flexible diet strategies with all species being omnivorous. Due to the high presence of intact seeds, we believe that fruit consumption is an important component of the diet for this rodent assemblage. All 7 species consumed arthropods, fruits, plant remains (stems, leaves, shoots), and mycorrhizal spores. Campos et al. (2001) and Giannoni et al. (2005) reported flexibility in the sigmodontine diet in montane and desert habitats, but did not report fruit as part of their diet. Viera et al. (2003, 2006) found that captive sigmodontine rodents ate fruit when it was offered. Omnivory was found in Akodon spp. in a temperate rainforest (Meserve et al. 1988). Noblecilla and Pacheco (2012) found intact seeds in stomach contents of T. kalinowskii and T. notatus, indicative of fruit consumption.
As expected, the frequency of occurrence of diet categories among genera and species was significantly different, suggestive of diet preferences among taxa and/or differential access to resources. Within rodent species, frequency of occurrence of diet categories also was significantly different, also suggestive of diet preference. Variation in diet categories among seasons is also significant, indicating variable access to resources over time. Although we do not have plant phenology recorded over the entire course of this study, rainfall data at our study site are indicative of pronounced rainy and dry seasons. Smythe (1978) found seasonal consumption of fruit pulp by Dasyprocta punctata and variable consumption of seeds and plant parts were found for D. leporinae (Henry 1999). Seasonal variation was found in fruit pulp consumption versus seed consumption in Dasyprocta spp. and Myoprocta spp. (Forget et al. 2002). Mangan and Adler (2002) found seasonal consumption of mycorrhizal spores by Proechimys semispinosus, whereas Janos et al. (1995) found seasonal occurrence of mycorrhizal spores in Proechimys and Oryzomys spp. fecal samples. Additional data on resource seasonality at our study site throughout the year would be useful for differentiating between resource availability and foraging strategies.
Akodon torques had the highest frequency of occurrence for arthropods and the lowest frequency of occurrence for fruits. Solari (2007) found that in the Andean highlands, A. torques and A. subfuscus were primarily insectivorous; in the montane forests of Huanuco, Peru, A. orophilus also exhibited a high degree of insectivory (Noblecilla and Pacheco 2012). Pizzimenti and de Salle (1980) found that Akodon was primarily insectivorous, with only one species A. jelskii, being principally herbivorous. Akodon spp. were found to be omnivorous in a Chilean temperate rainforest and consumed fruit (Meserve et al. 1988). In our study, A. torques fed principally on coleopterans, although spiders and scorpions also were included in its diet. Nonetheless, plant parts were a common item in its diet, as were fruits and mycorrhizal spores.
Microryzomys minutus and O. andinus showed a preference for arthropods but also consumed fruits, plants, and mycorrhizal spores, concurring with Noblecilla and Pacheco (2012) who noted that M. minutus and M. altissimus were also omnivorous in montane tropical forests. Solari (2007) noted that Oligoryzomys sp. B showed tendencies toward omnivory in the high grasslands of Manu National Park. M. minutus consumed primarily coleopterans, as did O. andinus, and both consumed fruits, although M. minutus had a higher percent volume of mycorrhizal spores in fecal samples.
Calomys sorellus also had a high frequency of occurrence of arthropods in samples, but this frequency of occurrence was almost equal to its consumption of plant remains and fruits, similar to T. kalinowskii. Pizzimenti and De Salle (1980) stated that C. sorellus was atypical for its genus by being small and insectivorous, but this study indicated that its diet is more flexible than previously noted and could be habitat dependent.
The genus Thomasomys was notable for its high frequency of occurrence of intact seed remains in fecal samples as well as plant remains, mycorrhizal spores, and arthropods. T. aureus, the largest rodent in our study, had the highest frequency of occurrence of intact seeds plus plant remains and the lowest frequency of occurrence of arthropod remains. Our study indicates that T. aureus appeared to specialize on fruits (as evidenced by consumption of intact seeds) and plant parts.
Thomasomys oreas had a very high frequency of occurrence of intact seeds and arthropods in its diet. T. kalinowskii was the most generalist species of the assemblage studied. Its diet included both a high frequency of intact seeds as well as arthropods and mycorrhizal spores. Noblecilla and Pacheco (2012) found that T. kalinowskii had the largest niche width of the rodent assemblage studied in Huanuco, Peru. Lopez-Arevalo et al. (1993) found that for T. laniger, diet was composed of fruits, seeds, and insects, with an increase of insects in the wet season. For T. niveipes, the diet was composed mainly of young leaves and other plant remains.
Thomasomys kalinowskii was the only species of rodent that was found to have Greigia sp. seeds in its feces. Gregia sp. is a bromeliad that often grows at ground level and exhibits a red, berry-like fruit. Troya et al. (2004) found that Andean bears, which inhabit montane rainforest, also include Greigia in their diet. Gaultheria spp. (Ericaceae) were consumed by all 7 species of rodents, ranging from 17% to 52% frequency of occurrence. Gaultheria species (of which there are at least 4 at our study site) are usually found as shrubs, producing fleshy berries with numerous small seeds.
Phenology data, taken shortly after the rainy season, indicated that during at least 1 portion of the year, fruits are plentiful and are not likely limiting. However, without year-round data on phenology and fruit consumption by potential competitors such as bats and birds, we cannot make conclusions as to whether fruit resources are limiting throughout the year.
Biological significance.
The rodent assemblage we studied is similar in diversity at the generic level and number of species to sigmodontine communities found in other montane tropical forests (Pacheco et al. 2013). We can hypothesize that similar diet patterns may be found for small rodents in tropical montane forests, with omnivory being common, and certain taxa specializing on either arthropods or fruit and plant parts. Two distinct clusters existed according to diet in our study; one that was comprised of the Thomasomys spp. + C. sorellus group and specializes more on plants (fruits, leaves, stems), and the 2nd one which consisted of genera that while omnivorous, consumed equal or higher quantities of arthropods in their diet.
Fruits consumed generally belong to taxa that are commonly associated with shrubs and produce berries with small-seeded fruits. All species of rodents consumed mycorrhizal spores belonging to the genus Glomus, in accord with several other studies that indicate rodents are dispersers of vesicular–arbuscular mycorrhizal fungi in tropical forests (Janos et al. 1995; Mangan and Adler 2002). Thus, the pattern shown in this study showed us a range of feeding strategies which varied from a high degree of arthropod specialization (A. torques) to arthropod-preferring omnivores, plant-preferring omnivores, and a tendency toward fruit specialization (T. aureus and T. oreus). Therefore, these sigmodontine rodents likely play important ecological roles in montane tropical forests, not only as arthropod predators but also as frugivores and seed dispersers.
Acknowledgments
We thank the government of Peru (Dirección General Forestal y de Fauna, Ministerio de Agricultura) for granting us permits to conduct the study (No. 440-2009-AG-DGFFS-DGEFFS, No. 344-2010-AG-DGFFS-DGEFFS, No. 144-2012-AG-DGFF-DGEFFS). We are indebted to E. Rengifo, C. Barriga, M. Peralta, O. Centty, D. Figueroa, J. Tito, and W. Calderon for assistance in the field, and to E. Arias and P. Nina for assistance in the laboratory. A. de la Cruz, A. Pace, O. Sissa, and K. Ledesma provided logistical support. We are also grateful to 2 anonymous reviewers whose comments greatly improved this manuscript. We thank the Smithsonian Institution and PERU LNG for financial support. This is publication # 31 from the Peru Biodiversity Program, Center for Conservation and Education, Smithsonian Conservation Biology Institute.
Literature Cited
- Adler G. H. 1995. Fruit and seed exploitation by Central American spiny rats, Proechimys semispinosus . Studies on Neotropical Fauna and Environment 30:237–244. [Google Scholar]
- Brown J. S. 1989. Desert rodent community structure: a test of 4 mechanisms of coexistence. Ecological Monographs 59:1–20. [Google Scholar]
- Brown J. H., Lieberman G. A. 1973. Resource utilization and coexistence of seed-eating desert rodents in sand dune habitats. Ecology 54:788–797. [Google Scholar]
- Caceres P. 2004. Caracterización dendrológica de las especies de los géneros Ficus y Cecropia (Moraceae) en el valle de Chanchamayo (Junín-Perú). Tesis para optar al título de Ingeniero Forestal. Universidad Nacional Agraria la Molina-UNALM, Lima, Peru. [Google Scholar]
- Caella J., Caceres N. C. 2006. Diet of 4 small mammal species from Atlantic forest patches in South Brazil. Neotropical Biology and Conservation 1:5–11. [Google Scholar]
- Campos C., Ojeda R., Monge S., Dacar M. 2001. Utilization of food resources by small and medium-sized mammals in the Monte Desert biome, Argentina. Austral Ecology 26:142–149. [Google Scholar]
- Cortes A., Miranda E., Jiménez J. E. 2002a. Seasonal food habits of the endangered long-tailed chinchilla (Chinchilla lanigera): the effect of precipitation. Mammalian Biology 67:167–175. [Google Scholar]
- Cortes A., Rau J. R., Miranda E., Jiménez J. E. 2002b. Hábitos alimenticios de Lagidium viscacia y Abrocoma cinerea: roedores sintópicos en ambientes altoandinos del norte de Chile. Revista Chilena de Historia Natural 75:583–593. [Google Scholar]
- D’Elía G., Pardiñas U. 2015. Subfamily Sigmodontinae Wagner, 1843. Pp. 63–70 in Mammals of South America. Volume 2, Rodents (J. L. Patton U. F. J. Pardiñas G. D’Elía , eds.). University of Chicago Press, Chicago, Illinois. [Google Scholar]
- D’Elía G., Pardiñas U. F. J., Teta P., Patton J. L. 2007. Definition and diagnosis of a new tribe of sigmodontine rodents (Cricetidae: Sigmodontinae), and a revised classification of the subfamily. Gayana 71:187–194. [Google Scholar]
- Dubost G., Henry O. 2006. Comparison of diets of the acouchy, agouti and paca, the 3 largest terrestrial rodents of French Guianan Forests. Journal of Tropical Biology 22:641–651. [Google Scholar]
- Fonseca G. A. B. 1989. Small mammal species diversity in Brazilian tropical primary and secondary forests of different sizes. Revista Brasileira de Zoologia 6:381–422. [Google Scholar]
- Forget P. M. 1990. Seed-dispersal of Vouacapoua americana (Caesalpinaceae) by rodents in French Guiana. Journal of Tropical Ecology 6:459–468. [Google Scholar]
- Forget P. M. 1993. Post-dispersal predation and scatterhoarding of Dipteryx panamensis (Papillionaeceae seeds) by rodents in Panama. Oecologia 94:255–261. [DOI] [PubMed] [Google Scholar]
- Forget P. M. 1996. Removal of seeds of Carapa procera (Meliaceae) by rodents and their fate in rainforest in French Guiana. Journal of Tropical Ecology 12:751–761. [Google Scholar]
- Forget P. M., Milleron T. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87:596–599. [DOI] [PubMed] [Google Scholar]
- Forget P. M, Hammond D. S, Milleron T., Thomas R. 2002. Seasonality of fruiting and food hoarding by rodents in Neotropical forests: consequences for seed dispersal and seedling recruitment. Pp. 241–256 in Seed dispersal and frugivory: ecology, evolution and conservation (D. J. Deey W. R. Silva M. Galetti , eds.). CAB International, Wallingford, United Kingdom. [Google Scholar]
- Foster P. 2001. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Science Reviews 55:73–106. [Google Scholar]
- Gentry A. H. 1992. Tropical forest biodiversity: distributional patterns and their conservational significance. Oikos 63:19–28. [Google Scholar]
- Gentry A. H. 1993. A field guide to the families and genera of woody plants of northwest South America (Colombia, Ecuador, Peru). Conservation International, Washington, D.C. [Google Scholar]
- Giannoni S. M., Borghi C. E., Dacar M., Campos C. M. 2005. Main food categories in diets of sigmodontine rodents in the monte (Argentina). Mastozoologia Neotropical 12:181–187. [Google Scholar]
- Henry O. 1999. Frugivory and the importance of seeds in the diet of the orange-rumped agouti (Dasyprocta leporina) in French Guiana. Journal of Tropical Ecology 15:291–300. [Google Scholar]
- Instituto Nacional De Recursos Naturales. 1995. Mapa ecológico del Perú. Mapa y guía explicativa. Instituto Nacional de Recursos Naturales, Ministerio de Agricultura, Lima, Perú. [Google Scholar]
- Janos D. P., Sahley C. T., Emmons L. H. 1995. Rodent dispersal of vesicular-arbuscular mycorrhizal fungi in Amazonian Peru. Ecology 76:1852–1858. [Google Scholar]
- Jansen P. A., Forget P. M. 2001. Scatterhoarding rodents and tree regeneration. Pp. 275–288 in Nouragues: dynamics and plant-animal interactions in a Neotropical rainforest (F. Bongers P. Charles-Dominique P. M. Forget M. Thery , eds.). Springer, Dordrecht, Netherlands. [Google Scholar]
- Josse C., et al. 2007. Ecological systems map of the Amazon Basin of Peru and Bolivia. Classification and Mapping. Scientific Report. NatureServe, Arlington, Virginia. [Google Scholar]
- Kelt D. A. 2011. Comparative ecology of desert small mammals: a selective review of the past 30 years. Journal of Mammalogy 92:1158–1178. [Google Scholar]
- Kelt D. A., Meserve P. L., Karina Nabors L., Forister M. L., Gutierrez J. R. 2004. Foraging ecology of small mammals in semiarid Chile: the interplay of biotic and abiotic effects. Ecology 85:33–397. [Google Scholar]
- Kintz D. B., Young K. R., Crews-Meyer K. S. 2006. Implications of land use/land cover change in the buffer zone of a national park in the tropical Andes. Environmental Management 38:238–252. [DOI] [PubMed] [Google Scholar]
- Kotler B. P., Brown J. S. 1988. Environmental heterogeneity and the coexistence of desert rodents. Annual Review of Ecology and Systematics 19:281–307. [Google Scholar]
- Langstroth R., Dallmeier F., Casaretto C., Servat G. P. 2013. Ecological landscape units across the Eastern Andean Valleys, High Andes, and Pacific Watershed Region of the Peru LNG Megaproject. Pp. 10–20 in Monitoring biodiversity: lessons from a trans-Andean Megaproject (A. Alonso F. Dallmeier G. P. Servat , eds.). Smithsonian Institution Scholarly Press, Washington, D.C. [Google Scholar]
- Leo M. 1995. The importance of tropical montane cloud forests for preserving vertebrate endemism in Peru: The Rio Abiseo National Park as a case study. Pp. 198–211 in Tropical montane cloud forests (L. S. Hamilton J. O. Juvik F. N. Scalena , eds.). Springer-Verlag, New York. [Google Scholar]
- Lobo N., Green D. J., Millar J. S. 2013. Effects of seed quality and abundance on the foraging behavior of deer mice. Journal of Mammalogy 94:1449–1459. [Google Scholar]
- Lopez-Arevalo H., Montenegro-Diaz O., Cadena A. 1993. Ecología de los pequeños mamíferos de la Reserva Biológica Carpanta, en la Cordillera Oriental Colombiana. Studies on Neotropical Fauna and Environment 28:193–210. [Google Scholar]
- Lopez-Cortés F., Cortés A., Miranda E., Rau J. 2007. Dietas de Abrothrix andinus, Phyllotis xanthopygus (Rodentia) y Lepus europaeus (Lagomorpha) en un ambiente altoandino de Chile. Revista Chilena de Historia Natural 80:3–12. [Google Scholar]
- Mangan S. A., Adler G. H. 2002. Seasonal dispersal of arbuscular mycorrhizal fungi by spiny rats in a Neotropical forest. Oecologia 131:587–597. [DOI] [PubMed] [Google Scholar]
- Marquet P. A. 1994. Diversity of small mammals in the Pacific coastal desert of Peru and Chile and in the adjacent Andean area-biogeography and community Structure. Australian Journal of Zoology 42:527–542. [Google Scholar]
- Meserve P. L. 1981. Trophic relationships among small mammals in a Chilean semiarid thorn scrub community. Journal of Mammalogy 62:304–314. [Google Scholar]
- Meserve P. L., Dickman C. R., Kelt D. A. 2011. Small mammal community structure and dynamics in aridlands: overall patterns and contrasts with Southern Hemispheric systems. Journal of Mammalogy 92:1155–1157. [Google Scholar]
- Meserve P. L., Lang B. K., Patterson B. D. 1988. Trophic relationships of small mammals in a Chilean temperate rainforest. Journal of Mammalogy 69:721–730. [Google Scholar]
- Noblecilla M. C., Pacheco V. 2012. Dieta de roedores sigmodontinos (Cricetidae) en los bosques montanos tropicales de Huanuco, Peru. Revista Peruana de Biologia 19:317–322. [Google Scholar]
- Novillo A., Ojeda R. A. 2014. Elevation patterns in rodent diversity in the dry Andes: disentangling the role of environmental factors. Journal of Mammalogy 95:99–107. [Google Scholar]
- Olson D. M., Dinerstein E. 1998. The Global 200: a representation approach to conserving the Earth’s most biologically valuable ecoregions. Conservation Biology 12:502–515. [Google Scholar]
- Ostfeld R. S., Manson R. H., Canham C. D. 1997. Effects of rodents on survival of tree seed and seedlings invading old fields. Ecology 78:1531–1542. [Google Scholar]
- Pacheco V. R. 2002. Mamiferos del Peru. Pp. 503–550 in Mamiferos tropicales (G. Ceballos and J. A. Simonetti , eds.). Conabio-UNAM, D.F., Mexico. [Google Scholar]
- Pacheco V. R., Cadenillas R., Salas E., Tello C., Zeballos H. 2009. Diversidad y endemismo de los mamíferos del Peru. Revista Peruana de Biología 16:5–32. [Google Scholar]
- Pacheco B., Marquez G., Salas E., Centty O. 2011. Diversidad de mamíferos en la cuenca media del rio Tambopata, Puno, Peru. Revista Peruana de Biología 18:231–244. [Google Scholar]
- Pacheco V., Salas E., Barriga C., Rengifo E. 2013. Small mammal diversity in disturbed and undisturbed montane forest in the area of influence of the Peru LNG Pipeline, Apurimac River Watershed, Ayacucho. Peru. Pp. 90–100 in Monitoring biodiversity: lessons from a Trans-Andean megaproject (A. Alonso F. Dallmeier G. Servat , eds.). Smithsonian Institution Scholarly Institute Press, Washington, D.C. [Google Scholar]
- Patterson B. D., Stotz D. F., Solari S., Fitzpatrick J. W., Pacheco V. 1998. Contrasting patterns of elevational zonation for birds and mammals in the Andes of southeastern Peru. Journal of Biogeography 25:593–607. [Google Scholar]
- Pizzimenti J. J., De Salle R. 1980. Dietary and morphometric variation in some Peruvian rodent communities: the effect of feeding strategy on evolution. Biological Journal of the Linnean Society 13:263–285. [Google Scholar]
- Pizzimenti J. J., De Salle R. 1981. Factors influencing the distributional abundance of 2 trophic guilds of Peruvian cricetid rodents. Biological Journal of the Linnean Society 15:339–354. [Google Scholar]
- Ponte M. 1998. Inventario y análisis florístico de la estructura del bosque. Pp. 43–65 in La Zona Reservada de Tumbes, biodiversidad y diagnóstico socioeconómico (W. Wust , ed.). The John D. and Catherine C. MacArthur Foundation/Fondo Nacional por Las Áreas Protegidas por El Estado (PROFONANPE), Lima, Peru. [Google Scholar]
- Reichman O. J. 1975. Relation of desert rodent diets to available resources. Journal of Mammalogy 56:731–751. [PubMed] [Google Scholar]
- Salas E., Barriga C., Rengifo E., Pacheco V. 2013. Assessment of the impact of the Peru LNG Pipeline on Sigmodontine rodent populations in a montane forest of Ayacucho, Peru. Pp. 101–109 in Monitoring biodiversity: lessons from a Trans-Andean megaproject. (A. Alonso F. Dallmeier G. Servat , eds.). Smithsonian Institution Scholarly Press, Washington, D.C. [Google Scholar]
- Salazar-Bravo J., Pardiñas U. F. J., D’elía G. 2013. A phylogenetic appraisal of Sigmodontinae (Rodentia, Cricetidae) with emphasis on phyllotine genera: systematics and biogeography. Zoologica Scripta 42:250–261. [Google Scholar]
- Servat G. P., Feria T. P., Hurtado N., Mendoza W., Alcocer F R. 2013. Potential distribution and habitat characterization of Atlapetes melanopsis (Aves: Emberizidae) in a montane forest ecotone of the Apurimac river valley. Pp. 141–153 in Monitoring biodiversity: lessons from a Trans-Andean megaproject (A. Alonso F. Dallmeier G. Servat , eds.). Smithsonian Institution Scholarly Press, Washington D.C. [Google Scholar]
- Sikes R. S., Gannon W. L. and The Animal Care and Use Committee of the American Society of Mammalogists. 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 92:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe N. 1978. The natural history of the Central American agouti (Dasyprocta punctata). Smithsonian Contributions to Zoology 257:1–60. [Google Scholar]
- Solari S. 2007. Trophic relationships within a highland rodent assemblage from Manu National Park, Cusco, Peru. Pp. 225–240 in The quintessential naturalist: honoring the life and legacy of Oliver P. Pearson (D. A. Kelt E. P. Lessa J. S. Bravo J. L. Patton , eds.). University of California Press, Berkeley. [Google Scholar]
- Solari S., Pacheco V., Luna L., Velasco P. M., Patterson B. D. 2006. Mammals of the Manu Biosphere Reserve. Pp. 13–23 in Mammals and birds of the Manu biosphere Reserve, Peru. Fieldiana: Zoology, New Series No. 110 (B. D. Patterson D. F. Stotz S. Solari , eds.). Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Stevens R. B., Anderson E. M. 2014. Habitat associations and assemblages of small mammals in natural plant communities of Wisconsin. Journal of Mammalogy 95:404–420. [Google Scholar]
- Stevens R. D., Tello J. S. 2009. Micro and macrohabitat associations in Mojave Desert rodent communities. Journal of Mammalogy 90:1431–1439. [Google Scholar]
- Tripplehorn C. A, Johnson N. F. 2005. Borror and DeLong’s introduction to the study of insects. Thomson Brooks/Cole, Belmont, California. [Google Scholar]
- Troya V., Cuesta F., Peralvo M. 2004. Food habits of Andean bears in the Oyacachi River Basin, Ecuador. Ursus 15:57–60. [Google Scholar]
- Viera E. M., Paise G., Machado H. D. 2006. Feeding of small rodents on seeds and fruits: a comparative analysis of 3 species of rodents of the Araucaria forests, southern Brazil. Acta Theriologica 51:311–318. [Google Scholar]
- Viera E. M., Pizo M. A., Izar P. 2003. Fruit and seed exploitation by small rodents of the Brazilian Atlantic forest. Mammalia 67:533–540. [Google Scholar]
- Voss R. S., Emmons L. H. 1996. Mammalian diversity in Neotropical lowland rainforests: a preliminary assessment. Bulletin of the American Museum of Natural History 230:1–115. [Google Scholar]
- Wolff J. O., Dueser R. D., Berry K. S. 1985. Food habits of Peromyscus leucopus and Peromyscus maniculatus . Journal of Mammalogy 66:795–798. [Google Scholar]
- Young K. R. 1994. Roads and the environmental degradation of tropical montane forests. Conservation Biology 8:972–976. [Google Scholar]