Abstract
At the time of implanting bone-related implants into human body, a variety of biological responses to the material surface occur with respect to surface chemistry and physical state. The commonly used biomaterials (e.g. titanium and its alloy, Co–Cr alloy, stainless steel, polyetheretherketone, ultra-high molecular weight polyethylene and various calcium phosphates) have many drawbacks such as lack of biocompatibility and improper mechanical properties. As surface modification is very promising technology to overcome such problems, a variety of surface modification techniques have been being investigated. This review paper covers recent advances in surface modification techniques of bone-related materials including physicochemical coating, radiation grafting, plasma surface engineering, ion beam processing and surface patterning techniques. The contents are organized with different types of techniques to applicable materials, and typical examples are also described.
Keywords: surface modification, physicochemical coating, radiation grafting, plasma surface engineering, ion beam processing, surface patterning, bone-related materials
Introduction
Biological responses to implants largely depend on the surface properties of biomaterials, such as surface chemistry and physical structure [1–5]. As implants are inserted in human body, a variety of acute or chronic responses occur at the biomaterial surface.
In most bone-related implants, sufficient mechanical strength is required [6, 7]. For example, an intervertebral fusion cage needs to possess high compressive strength and fine fatigue strength [8], and an artificial hip joint should resist wearing associated with friction between femur head and acetabular cup [6]. Therefore, metallic biomaterials, bioceramics and polymers with good mechanical properties were developed to meet such requirements. Besides having good mechanical properties, biocompatibilities of such materials are very important factors to be considered for the long-term success of implants, especially for metallic biomaterials as these materials are known as biotolerant [9–11].
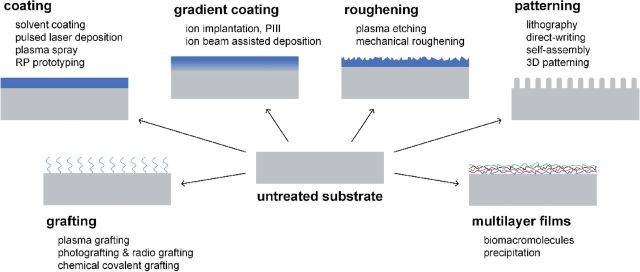
As for an example, Ti–6Al–4V and Co–Cr alloys have been commonly used for artificial joints, however, their wear debris produced by long-term friction would induce inflammatory responses and even result in aseptic loosening of the joint [12, 13]. Therefore, various surface modification techniques were developed to improve their tribological properties. Some commonly used surface modification techniques and their effects are shown in Fig. 1.
Figure 1.
Schematic diagrams of surface modification techniques.
Biomaterial surface can be modified by these illustrated methods to overcome or reduce their inherent shortages or disadvantages. As for the requirements of better biomaterials, the cutting edge techniques were introduced to improve surface physical, chemical and biological properties of bone grafts, so as to meet the clinical requirements of bone defect substitution and repair. This review covers recent advances in coating, non-coating and patterning techniques for the surface modification of bone graft materials.
In this review article, contents are arranged with the clue of different types of the surface modification techniques. The commonly used substrate materials and typical examples are involved in each section. It is important to note that laser is a widely used light beam source for many modification techniques, since it is able to rapidly and effectively induce physical and/or chemical changes (e.g. roughness [14], deformation [15], polymerization and grafting) on various biomaterial surfaces. Since many of surface modification techniques involve the use of laser, the applications of laser technique are described in relevant sections rather than a separate one.
Surface Modification with Coating Layer
Coating techniques are simple and intuitive approaches to obtain a modified surface. A variety of conventional physical and chemical coating methods (e.g. solvent evaporation, plasma spraying, and physical/chemical vapor deposition (CVD)) have been industrialized, while novel approaches involving recently developed and developing techniques are continuously coming forth. What will be introduced in this section are state-of-the-art physical and chemical methods for creating functional coatings on different biomaterial surfaces.
Rapid prototyping
Rapid prototyping (RP) comprises a series of techniques using three-dimensional computer-aided design (CAD) data to quickly fabricate a model or duplicate a same part. Some of RP techniques were used to construct coating for biomaterials, especially for metallic biomaterials.
Laser engineered net shaping (LENS) is an additive RP manufacturing technique that uses a focused, high-energy laser beam to melt metallic powders directly injected to the focused laser beam spot to form a new layer. Balla et al. [16] coated titanium with tantalum by using a LENS process to obtain better osseointegration property. Graded Co–Cr–Mo alloy coating was also successfully created on porous Ti6Al4V surface by LENS to obtain a high hardness interface [17]. Besides fabricating metallic coating, LENS is able to prepare ceramic coating. Roy et al. [18] successfully fabricated calcium phosphate coating on titanium without phase transition of the ceramic coating.
Pulsed laser deposition
Pulsed laser deposition (PLD), a physical vapor deposition (PVD) method, is popular for fabricating calcium phosphate coating on metallic substrate, since it is able to stoichiometrically transfer material from target to substrate and could obtain a ultra-thin coating layer (thickness of several atoms) [19]. Although PLD has been introduced to the surface modification of biomaterials for nearly 20 years, this technique is continuously developing in the field. For example, in its recent development, PLD was used to fabricate calcium phosphate coating on porous Ti6Al4V substrate produced by selective laser melting (SLM, one of RP techniques) [20]; water-assisted PLD was developed to improve coating-substrate binding strength [21]. PLD has also been introduced to the surface coating on polymers in recent years. Prosecka et al. [22] fabricated thin layer of hydroxyapatite (HA) on caprolactone/polyvinyl alcohol composite nanofibers. Besides calcium phosphate coating, bioceramic coating composed of akermanite (Ca2MgSi2O7) was successfully created on both non-biodegradable polysulfone and bioresorbable polylactic acid (PLA) surface by PLD [23].
Ion beam-assisted deposition
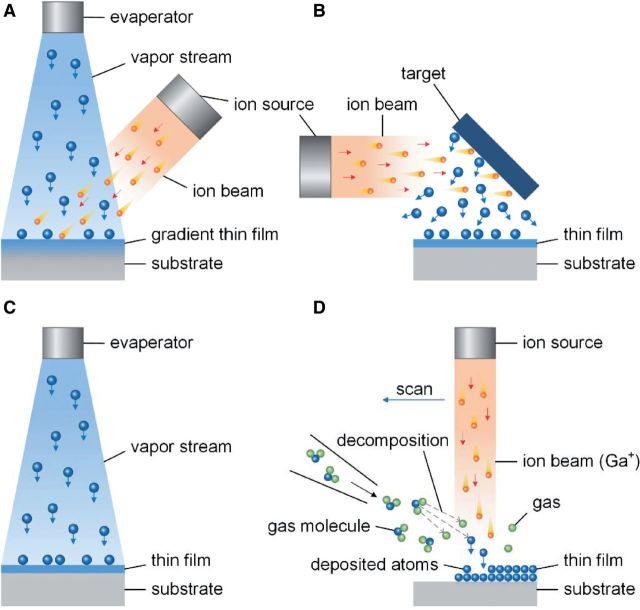
Ion beam-assisted deposition (IBAD), which is also called ‘ion beam enhanced deposition’ (IBED) is a vacuum deposition surface modification technique that combines PVD and ion implantation (described in the section ‘Ion implantation and plasma immersion ion implantation’). In the IBAD, an ion beam bombardment is continuous throughout the process to clean substrate surface prior to the deposition and control depositing film properties during the deposition. A significant advantage of IBAD is that such technique is able to create a gradual transition layer mixed with substrate material and depositing material between the substrate and the deposited film, thereby the coating adheres strongly to the substrate.
It is important to distinguish IBAD from some other surface modification techniques that also use ion beam and have similar names, including ion beam deposition (IBD), ion beam induced deposition (IBID) and ion beam sputtering deposition (IBSD). IBD is a direct beam deposition (DBD) process that directly applies an ionized particle beam onto substrate surface to fabricate thin film [24]. A significant difference between IBD and ion implantation is that the ionized particle beam in the IBD has low energy, and the particles arrive at substrate surface with a ‘soft landing’ [24]. IBID is a CVD technique that uses focused ion beam (usually Ga+ ion beam) to decompose gaseous molecules and deposit non-volatile component onto substrate surface [25]. IBSD is a PVD process that an ion beam bombards a target and ejects particles in atomic scale from the target to form thin film on nearby substrate surface [26]. Schematic diagrams of these ion beam surface modification methods are illustrated in Fig. 2. Each of IBD, IBID and IBSD can be utilized for creating thin film coating on material surface, however, they are hardly to create gradual transition between the substrate and the deposited film as IBAD, thus obtaining relatively lower adhesive strength.
Figure 2.
Schematic diagrams of ion beam surface modification methods (A: IBAD, B: IBSD, C: IBD and D: IBID).
IBAD has been used for the surface modification of biomaterials for decades and is still in development. As a typical application, Cui et al. [27] fabricated HA coating on Ti–6Al–4V substrate with an atomic intermixed coating/substrate interface by IBAD. The deposition was performed by a multifunctional IBAD system. Before the deposition, the substrate surfaces were cleaned by Ar+ ion beam bombardment. Then, a composite target containing HA and tricalcium phosphate was sputtered by Ar+ ion beam to form the coating on the substrate, which was simultaneously bombarded by another energetic Ar+ ion beam. In the deposition process, the bombardment energy of Ar+ ion beam was relatively higher at first to produce atomic intermixed layer of the coating and the substrate, and then the bombardment energy was reduced to increase the thickness of the coating and reinforce the compactness. During the deposition, the temperature of the substrate was below 100°C, which did not affect the substrate. The adhesive strength of the coating fabricated by IBAD was tested to be nearly twice to that prepared by IBSD with the same processing environment. Chen et al. [28, 29] prepared calcium phosphate thin film coating on pure titanium by using IBAD and further created biomimetic apatite precipitation layers by immersing the coating in Dulbecco’s phosphate buffered saline solutions containing calcium chloride, as well as biomolecules to modulate precipitation processes and enhance bioactivities.
IBAD was also applicable for the fabrication of metallic, bioceramic and composite thin film coating for many varieties of biomaterials for bone graft (e.g. titanium, stainless steel and ultra-high molecular weight polyethylene [UHMWPE]) [30–33]. A drawback for IBAD is that it is a line-of-sight modification technique, so it is difficult for IBAD to treat an irregular surface with non-line-of-sight regions.
Plasma coating
Plasma is a state of matter that is partially or fully ionized, and contains charged particles of free ions, electrons, radicals, as well as neutral particles of atoms and molecules. Plasma could be divided into thermal (high-temperature/hot/equilibrium) one and nonthermal (low-temperature/cold/nonequilibrium) one. The thermal plasma is nearly fully ionized, and electrons and heavy particles have the same temperature. The temperature required to generate thermal plasma is typically ranging from 4000 to 20 000 K [34]. Such high temperature is destructive for biomaterials, especially for those polymers. For nonthermal plasma, only a small fraction of the gas molecules are ionized, and ions and neutrals are at a much lower temperature (may as low as room temperature), although the temperature of electrons could reach several thousand degrees Celsius. The plasma used for the surface modification of biomaterials is the nonthermal one, which can be generated by different sources, including corona discharge, dielectric barrier discharges, radio frequency discharges and so on [35].
Plasma surface engineering is a series of economic and effective approaches for the surface modification of biomaterials and has been applied to commercialized products [36]. Plasma treatments can be used to modify material surfaces via different processes, including etching (or ablation), sputtering, polymerization, grafting and spray [35]. Wherein, plasma spray, plasma sputtering and plasma polymerization could be used to produce coating on a surface. Other plasma processes for the surface modification will be attributed to non-coating techniques.
Plasma spray
Plasma spray is a coating process that sprays melted or partially melted coating material onto substrate surface, and this technique has been applied to commercially available bone implants. The energy and temperature of the plasma environment of plasma spray are relatively higher than plasma surface engineering techniques descripted above. Due to its high operating temperature, plasma spray is usually applied to fabricate various coatings on metallic biomaterials, such as apatite and its derivatives coating [37–39], calcium silicate coating [40, 41], bioglass coating [42–44], zirconia coating [45], titanium coating [46] and composite coating [47, 48]. Moreover, bioceramic coatings have been successfully fabricated on polymer substrate by using plasma spraying, for example, HA coating on polyetheretherketone (PEEK) or carbon fiber-reinforced polyamide 12 [49, 50], and titanium coating on carbon fiber-reinforced PEEK [51]. However, the influences of the high-energy plasma on the polymer substrate were not discussed by these studies.
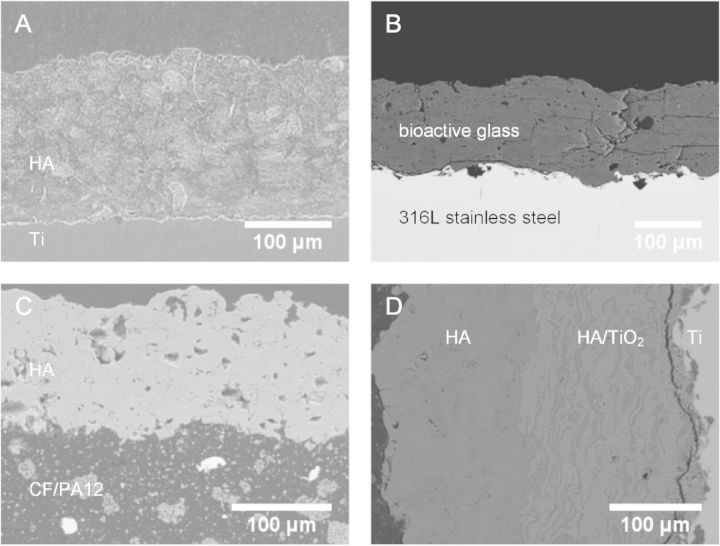
Figure 3 shows cross-sectional micrographs of coatings on various biomaterials that have been used for bone implants. The thickness of plasma sprayed coatings was usually more than 100 μm, and the interface could be clearly observed between the substrate and the coating. Therefore, the interface binding strength produced by plasma spray is relatively lower than those by IBAD with a gradient transition layer.
Figure 3.
Cross-sectional micrographs of coatings on various substrates prepared via plasma spray. (A: HA coating on titanium substrate (Reproduced with permission from Ref. [52], Copyright 2007 Elsevier Ltd.), B: 31SiO2-56CaO-2MgO-11P2O5 bioactive glass coating on 316L stainless steel substrate (Reproduced with permission from Ref. [53], Copyright 2013 Elsevier Ltd.), C: HA coating on carbon fibers/polyamide 12 (CF/PA12) composite substrate (Reproduced with permission from Ref. [49], Copyright 2005 John Wiley & Sons, Inc.), D: HA and HA/TiO2 coating on titanium substrate (Reproduced with permission from Ref. [54], Copyright 2004 Elsevier Ltd.)).
Plasma polymerization
Plasma polymerization is a process that ionizes monomer gas into plasma state and induces radical polymerization to create polymer coating on a substrate, so as to enhance corrosion resistance of metallic biomaterials or improve biocompatibility and bioactivity of relatively inert materials [55–57]. For example, Lewis et al. [55] fabricated fluorocarbon film on 316L stainless steel, and the corrosion rate was significantly decreased compared with those uncoated; Liu et al. [58] employed plasma polymerization to modify surfaces via generating different functional groups (amine, carboxyl, methyl and hydroxyl) and found that the plasma polymerization of allylamine on the surface promoted osteogenic differentiation of human adipose-derive stem cells [58].
Surface Modification with Grafting and Implantation
Coating approaches can effectively modify surface properties for bone implants. However, these coating techniques create isolation layer between the material and surrounding organisms, thus cutting off the interaction between them. Therefore, many advantageous properties of the substrates become useless for surrounding organisms after being coated. Moreover, most of those coatings are physically bound to the substrate, and the binding strength is limited.
Besides coating, there are many other techniques partially modify surface physical and/or chemical properties by, for example, grafting molecules on a surface, or injecting ions into superficial layer of a substrate. In this section, those surface modification techniques without forming coating are summarized as ‘non-coating’ methods, and several surface grafting methods and ion implantation techniques for the surface modification of bone implant materials will be introduced. Furthermore, these non-coating techniques do not modify topographic features in either micro- or nano-scale.
Chemical covalent bonding
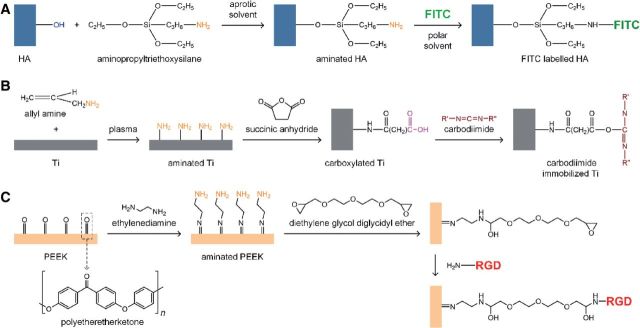
The use of functional groups on material surface to form covalent bond between the substrate and the coating is a classical approach for constructing chemical coating. The reaction is specific and binding effect is stable. Figure 4 shows some illustrative examples for chemical covalent bonding processes on biomaterials.
Figure 4.
Chemical covalent bonding processes on different biomaterials. (A: silanization on calcium phosphate bioceramic [59, 60], B: carbodiimide immobilization on titanium metal [61] and C: polypeptide grafting on biopolymer [62]).
Silanization is a low-cost and effective covalent coating method to modify material surface that are rich in hydroxyl groups, such as HA, bioglass, titania and many other metal oxide surfaces. There are many types of commercially available silane coupling agents, which are easy to react with hydroxylated surface and introduce active groups (e.g. amino group and carboxyl group) to the surface. Figure 4A takes HA as the example to illustrate chemical structure of the modified surface. Silanized surface can easily be modified by further grafting. Zhang et al. labeled nanometer HA with fluorescein isothiocyanate (FITC) by modifying HA with 3-aminopropyltriethoxysilane (AMPTES), and then grafting FITC via reaction with the amino group [59, 60]. Although the silanization is simple and effective, the reaction conditions such as concentration of the silane and reaction time must be carefully controlled to prevent from forming thick polymerized silane network on the surface. Otherwise, the bond between silane and the surface can also subject to hydrolysis in some conditions [63].
Photografting and radiation grafting
Chemical grafting has been widely used to obtain stable surface modification results for biomaterials. Active groups (e.g. –OH, –COOH and –NH2) exposed to the surface are necessary to acquire high chemical reactivity for the grafting. It is difficult to perform chemical grafting on the surface of those bioinert materials, since there are only a few or no active groups exposed to their molecular surface.
However, many relatively inert materials are being used as bone implants. In order to conduct grafting on the surface of these biomaterials, extra energy must be introduced to the grafting reaction. As the name suggests, photografting and radiation grafting make use of radiations, including UV radiation (photografting), gamma radiation and high-energy electron beam. The radiation breaks chemical bonds on material surface to be grafted, and form free radicals. The reactive surface will be then exposed to monomers to initiate surface graft polymerization [64].
The use of photografting and radiation grafting in the field of biomaterials is focused on surface modification of polymers, especially those hydrophobic and bioinert. Various materials commonly used in the preparation of bone substitutes, such as PEEK, UHMWPE and some biodegradable polymers, have been investigated to modify physical and chemical properties, as well as improve biocompatibility and osteointegration by radiation grafting and photografting [65, 66]. Photografting by UV radiation was used to improve the tribological performance of UHMWPE [66], enhance hydrophilicity of PEEK and biodegradable polymers [67, 68] and adjust biodegradation rate of PLA [69]. Gamma radiation grafting was reported to graft poly(N-isopropylacrylamide), a polymer had low critical solution temperature onto the surface of polystyrene Petri dish to control attachment and detachment of cells [70]; Cho et al. [71] used gamma radiation for the surface modification of UHMWPE by the graft polymerization of methyl methacrylate (MMA) monomer, so as to improve interfacial strength with poly(methyl methacrylate) (PMMA) bone cement. Electron beam grafting was used to enhance the hydrophilicity of PMMA [72], and improve biocompatibility of bioinert polymer [73].
Plasma etching and grafting
Plasma can not only be used to prepare coatings on biomaterials, but is also be able to conduct various non-coating surface modification processes, for example, plasma etching and plasma grafting.
Plasma etching modifies a surface by shooting a high-speed stream of plasma onto the substrate. Plasma etching is helpful to improve surface activity for bioinert polymers, with less influence on surface topography than chemical etching process [74].
Plasma grafting are used to modify surface chemical properties of biomaterials by grafting active groups on the surface, for example, plasma grafting of zinc oxide onto polypropylene to obtain an antibacterial surface [75].
Ion implantation and plasma immersion ion implantation
Ion implantation
Ion implantation is a physical surface modification process that injects accelerated high-energy ions into the surface of a material to modify its physicochemical and biological properties (Fig. 5). Almost every atom within the periodic table is available for ion implantation. For a biomaterial surface, ion implantation could be used to enhance corrosion resistance, reduce wear debris, regulate hardness and improve biocompatibility and bioactivities. For example, iridium was implanted into Ti–6Al–4V surface to enhance corrosion resistance [76]; nitrogen ion implantation into Ti–6Al–4V and UHMWPE can reduce surface wear [77]; ion implantation of silver into surfaces of 317L stainless steel, titanium, and Ti–Al–Nb alloy increased their anti-bacterial natures [78]; graphene could attain a good cytocompatibility by NH2 ion implantation [79]. Ion implantation has also been used to modify surface properties of polymers for biomedical applications [80, 81].
Figure 5.
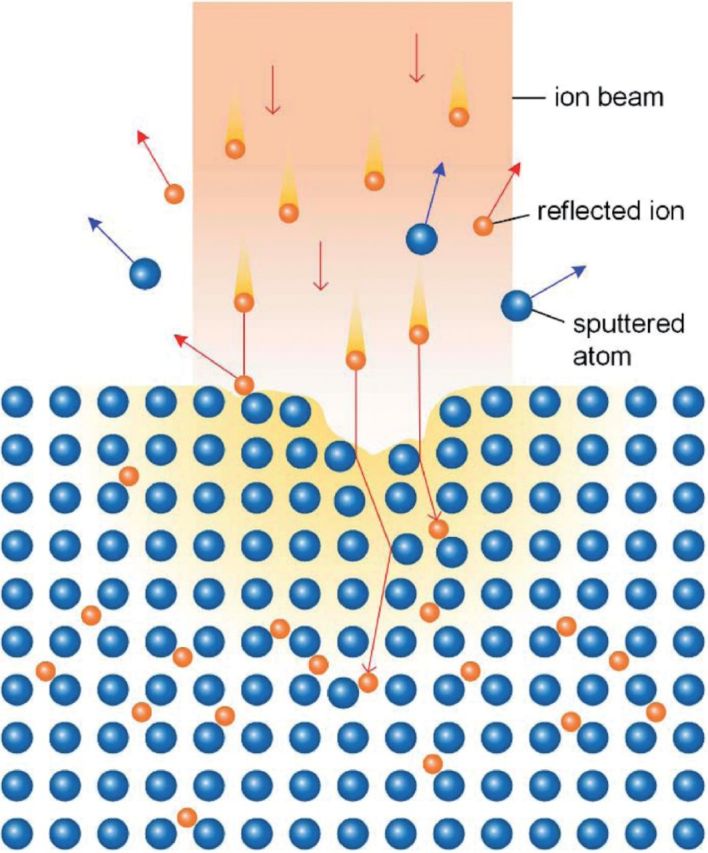
Schematic diagram of ion implantation process.
Ion implantation has many advantages: material of the substrate is unrestrictive, since the high-energy ions are forcibly injected into substrate surface; the implanted ions dispersed within a certain depth of substrate surface without forming a new layer, avoiding drawbacks (e.g. cracking and detachment) of traditional coatings; low operating temperature (sometimes at room temperature) did not affect the substrate material. However, traditional ion implantation is a line-of-sight processing technique, and is thus not suitable for the treatment of those bone implants with complex shape and internal structure, for example, a hip joint prosthesis with complex curved surface [82].
Plasma immersion ion implantation
Plasma immersion ion implantation (PIII) was invented by Prof. J. Conrad in 1980s [83], and overcame the limitation of line-of-sight processing of traditional ion implantation [84, 85]. In a PIII process, a workpiece to be modified on the surface is immersed in a plasma atmosphere and applied with a direct current (DC) or a high voltage pulsed DC, ions from the plasma are accelerated in the electric field of plasma sheath surrounding and perpendicular everywhere to workpiece surface, and finally implanted into the surface [86]. Therefore, PIII is able to modify complex surface or even inner surface of a material [85], which is of significance to those bone grafts with irregular shape and structure. In early 1990s, plasma immersion ion implantation and deposition (PIII&D) was then developed based on PIII by Brown et al. [87, 88] to build up a thin film with an atomically mixed interface with the substrate, so as to expand pure ion implantation technique to a hybrid coating technique.
PIII and PIII&D have now been applied to the surface modification of various biomaterials, including metals and polymers, in terms of surface mechanical properties, biocompatibility, bioactivity, antibacterial activity and so on [89, 90]. For example: nitrogen, oxygen and hydrogen implanted metallic materials (e.g. Ti–6Al–4V and stainless steel) and polymers (e.g. UHMWPE and PEEK) used for orthopedic implants showed better wear performance than those untreated [91–94]; corrosion resistance of alloys containing hazardous elements for the health (e.g. Ni–Ti alloy) could be enhanced by using oxygen, nitrogen, carbon or acetylene PIII and PIII&D [95–98]; for those bioinert materials for medical use (e.g. titanium, polytetrafluoroethylene and PEEK), it has been reported that biocompatibility and bioactivities (e.g. osteogenic) are able to be significantly improved by applying PIII and PIII&D using oxygen, hydrogen, water, calcium or zinc plasma on the surface modification [99–103]; antibacterial surface for biomaterials could be fabricated by introducing silver (Ag) or copper (Cu) element into the surface by using PIII and PIII&D [104–106].
Surface Patterning of Biomaterials in Micro- and Nano-Scale
Studies on cell biology demonstrated that the topography of the extracellular matrix (ECM) could regulate stem cell behaviors and fate, such as cell growth and differentiation, via physical interactions with the cells. Such physical interactions are affected by some geometric cues in different scales, including molecular conformation, surface topography or roughness, fiber diameter and so on [107]. The bone tissue was proposed to be divided into as many as nine levels from molecules (e.g. collagen and HA) to a bone organ, wherein many levels have their specific patterns in sub-nano- or micro-scale. For example, array patterns of mineralized collagen fibrils in nano-scale, and material patterns of woven bone, parallel fibered bone and lamellar bone in micro-scale [108]. Therefore, behaviors and fate of osteocytes would be regulated by topographies of bone tissue in various scales.
Based on the regulation effects of topography on cell behavior and fate in natural tissues, material could be functionalized by modifying surface topography, for example, creating patterns. As early as 1911, Harrison [109] found the influence of topography on cell behaviors based on the observation of the relationship between the movements of embryonic cells and the material shapes in contact with the cells. As the development of nano- and micro-processing techniques, some of them have been employed to fabricate nano- and micro-patterns on biomaterial surfaces, and interactions between patterned biomaterial surfaces and cells were investigated.
Surface patterning techniques are widely used and are developing rapidly in the field of microelectronics. Recently advanced methods for surface patterning on biomaterials were partially derived from those in microelectronics industry [110]. In this section, methodologies for the fabrication of nano- and micro-patterns on material surfaces are summarized.
Photolithography with mask
Photolithography was the first surface patterning technique introduced to make patterns for controlling cell behaviors [111]. Another well-known application of photolithography in the field of biology is the fabrication of DNA arrays [112, 113]. A photolithography process commonly comprises following steps (as shown in Fig. 6): (i) prepare a clean and flat substrate; (ii) coat a light sensitive polymer (called photoresist) onto the substrate; (iii) expose the photoresist under a mask (usually quartz or metal) to form a desired pattern; (iv) transfer the pattern to the substrate by an etching process (development process) and (v) remove the photoresist. Wherein, the photoresist could be either a positive one that areas exposed to the light beam can be dissolved in the development process (illustrated as ‘positive’ one in Fig. 6), or a negative one works conversely (illustrated as ‘negative’ one in Fig. 6). Due to the optical diffraction of the focused light beam, the resolution of the pattern created by photolithography is restricted to about half of the wavelength of the light source, typically several hundred nanometers [114]. Actually, 1 µ would be the finest resolution for photolithography in practice. Therefore, photolithography is suitable to create patterns with the size comparable to a cell.
Figure 6.
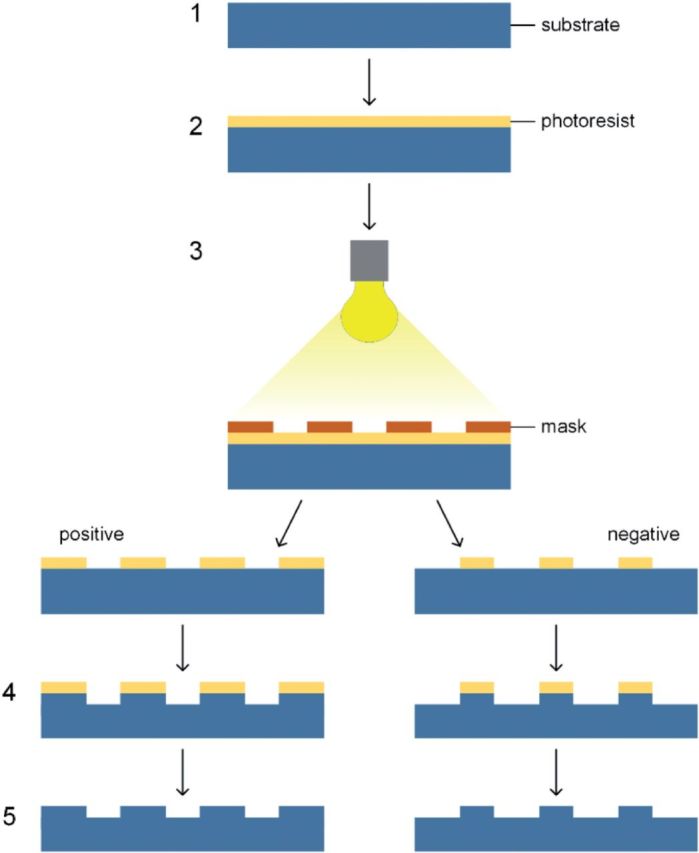
Schematic diagram of photolithography procedures.
Direct-write photolithography
Above classic photolithography with mask is an indirect approach for the fabrication of surface patterns. As the advance of photolithography, a direct-write mode without using mask has been developed via using a focused light beam to fabricate pattern directly on material surface [115–117]. In this maskless photolithography, laser is more commonly used as the light source to provide high-intensity light beam for the fabrication of surface patterns. In order to fabricate patterns, the light beam may induce two types of reactions on material surface: one is photochemical reaction for photoactive surfaces [118], the other is physical reactions involving ablation, melt or deformation of the substrate caused by the high energy of the laser [119, 120].
Some typical examples by using direct-write photolithography are: Pfleging et al. [121, 122] created micro-patterns on polystyrene surface by using laser photolithography combined with UV radiation to enhance L929 cells adhesion and protein adsorption; Rebollar et al. [123] fabricate submicro-patterns on polystyrene substrate to guide cell alignment and improve cell proliferation; Ahrem et al. [124] used pulsed laser to make 3D channels on bacterial cellulose hydrogels without chemical modifications or chemical strength lose, and observed inward migration of chondrocytes in the channels, as well as good matrix production and phenotypic stabilization.
Electron beam lithography
With the similar principle and fabrication process to photolithography, electron beam lithography (EBL) is able to create nano-sized patterns on material surfaces, since the electron beam is considered as a de Broglie wave, the wavelength of the electron is much shorter than that of light beams [125]. For example, a typical electron beam provided by electron microscope is accelerated by 100 kV electric field, and the wavelength is 0.003 nm. EBL is used to make patterns on electron sensitive material surfaces, which would be crosslinked [126, 127], chemically transformed [128, 129], polymerized [130] and so on under the electron beam. For example, Idota et al. [130] used EBL to polymerized and grafted N-isopropylacrylamide onto a hydrophilic polyacrylamide-grafted glass surfaces to form patterns, so as to regulate cell attachment directions and detachment. In spite of the high spatial resolution in nano-scale, EBL has its own limitations, such as high cost and low throughput. Therefore, EBL is now more commonly investigated in laboratory, rather than industrialization applications.
Moreover, EBL was used to fabricate special patterns that gave extraordinary functions to material surfaces. Wang et al. [131] constructed a patterned surface with sub-micron sized polyethylene glycol (PEG) microgels by EBL technique, since the PEG microgels were non-adhesive to both cells and bacteria, the patterned surface would be non-adhesive to bacteria with comparable size to the PEG microgels, as well as did not affect adhesion and behaviors of normal cells. Such interesting effects were further investigated and many similar multi-functional patterned surfaces were developed [132, 133].
Scanning probe lithography
Scanning probe lithography (SPL) is a direct-write method that moves a micro- or nano-stylus on material surface to mechanically ‘write’ patterns. SPL can be divided into two different types according to the patterning manners: the one is constructive that matters are transferred to the surface from the stylus (such as dip-pen nanolithography (DPN)); and the other one is destructive that the surface is deformed (such as nano-imprinting/engraving).
DPN uses a tip of atomic force microscope to create patterns by directly writing on the surface using a variety of molecular inks (solutions of molecules). In biomedical applications, DPN has been used to create patterns with polymers [134–136], biomolecules (including proteins [137], peptides [138], lipids [139, 140], enzymes [141] and DNA [142]), nano-particles [143–145], as well as living cells [146, 147], onto different substrates. Similar to other nano-patterning techniques mentioned above, the throughput of DPN is relatively low. To overcome such disadvantage, a high-throughput DPN was developed by parallelly operating a 2D probe arrays consisted of 55 000 tips [148].
Nano-imprinting and engraving uses a hard stylus to indent or scratch material surface to create patterns. Thus, the process is destructive to the surface. By using this process, nano-patterns were created on thin film (such as self-assembly monolayer) coated surface [149]. Moreover, nano-patterns of proteins were fabricated by grafting protein molecules onto the exposed substrate after the scratching [150, 151].
Patterning with master
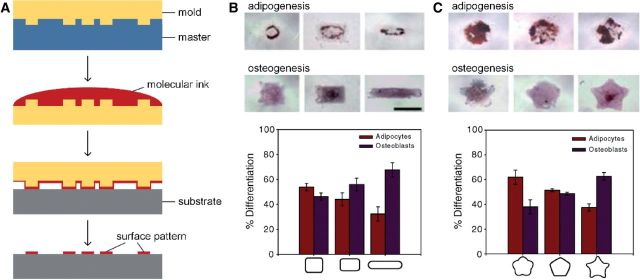
Patterning with master uses a template with patterns to replicate patterns on a substrate, and the process is sometimes called ‘microcontact printing’ (µCP). Typically, as shown in Fig. 7A, a mold, typically made of elastomeric polymer (such as polydimethylsiloxane (PDMS)), is created according to the master, and then used to print patterns on the substrate with molecular inks [152]. In this patterning technique, the mold contacts with the substrate to transfer the patterns onto the surface by the molecular inks. As early as 1990s, Singhvi et al. [153] created patterns on a golden substrate with a PDMS stamp to control cell distribution and shape, and demonstrated such spatial restriction is helpful to maintain albumin secretion, which is an important physiological function of hepatocytes. Another example is the regulation of cell fate by patterning with master: Kilian et al. [154] created patterns with different shapes on a glass substrate coated by gold via µCP, after a period of culturing mesenchymal stem cells (MSCs) on the surface, the cells displayed different adipogenesis and osteogenesis profiles, indicated that modulation of cell shape was able to direct cell differentiation (Fig. 7B and C). Patterning with master has also been used to patterning proteins [155, 156], DNA [157] and cells [158, 159] for biomedical applications.
Figure 7.
Operating steps of µCP and regulation effects of the patterns on cell fate (A: operating steps of µCP, B: regulation effects of the patterns on cell fate by varying aspect ratio [154] (Copyright 2010 National Academy of Sciences), C: regulation effects of different pattern shapes on cell fate [154] (Copyright 2010 National Academy of Sciences)).
Another process is imprinting with master, which uses a template made of hard material for creating patterns on the substrate [160]. Imprinting with master makes mechanically deformation on material surface to form patterns. Therefore, the master for imprinting must be hard enough to fabricate patterns on a relatively soft substrate.
By combining imprinting with master and lithography, nanoimprint lithography (NIL) creates nano-patterns on a substrate by imprinting a mold with the nano-patterns into a resist coated on the substrate and subsequent etching process. NIL was firstly reported in 1996 by Prof. S. Chou and his coworkers [161]. They prepared a mold of nano-patterned silicon dioxide by EBL and etching, and then transferred the pattern to a PMMA resist coated on a silicon substrate by indenting to form patterned resist with different thickness, followed by anisotropic etching to finally transfer the pattern onto the substrate. Such NIL process used a nano-template to create pattern, rather than by using a stylus, brought about relatively higher throughput. NIL has been used to create nano-patterns on various substrates for biomedical applications [162–164], or fabricate polymers [165, 166], proteins [167–169] and DNA [170] patterns on the substrates.
Self-assemble of molecules or nano-particles
Self-assemble is an energy-saving process to prepare patterns. During the self-assemble, intermolecular or inter-particle forces make molecules or nano-particles arranged in a regular pattern, in order to minimize total free energy of the entire surface. Molecules or nano-particles can self-assemble in an area ranging from nano-scale to micro-scale. Many types of materials were employed in self-assemble fabrication of patterns, such as block copolymers [171–173], nano-spheres [174, 175], nano-particles [176], biomolecules [177, 178] and so on.
3D patterning
Since the cells live and act in a 3D physiological environment in natural tissues and organs, 3D patterns on biomaterial surfaces would provide spatial architectures closed to physiological conditions and beneficial for tissue reconstruction and repair. As a result, 3D patterning technique is becoming an attractive research hotspot in the field of surface modification. In the present review paper, 3D patterning does not refer to a specific patterning techniques, many of those described in previous sections could be used to create 3D patterns via minor modifications or by combining with other techniques.
Two-photon lithography (TPL) and multiphoton lithography (MPL) are direct-write technique that is capable of creating 3D patterns on polymeric surfaces by laser beam. During a TPL or MPL process, two-photon or multiphoton absorption occurs at a photosensitive surface by attaining energy from the laser beam, thereby chemical reactions (usually polymerization) take place to form 3D patterns at the laser spot. For example, Nielson et al. [179] fabricated 3D patterns with bovine serum albumin by photocrosslinking using MPL on a coverglass according to high-resolution X-ray computed tomographic data. The 3D patterns were exactly replicated by using a dynamic mask and the resolution was as high as submicron (∼0.5 μm). Besides replicated patterns, crosslinked protein with unstrained structures in micro-scale could be fabricated using MPL [180]. For biomedical applications, MPL can be used to fabricate micro-patterns or 3D structures with proteins [179–181], hydrogels [182–184], bioabsorbable polymers [185, 186], gelatin [187, 188] and so on.
Interference lithography (IL), which is also called holographic lithography or interference holography, uses interference patterns formed by two coherent laser beams to build periodic 3D patterns. By adjusting parameters (e.g. phase, amplitude and polarization) of the coherent laser beams, features of the interference patterns could be modified. During an IL process, the interfering laser beams can be used to induce polymerization reactions to create 3D patterns on a substrate. The advantage of IL is that the patterning process is simple without using masks, and the throughput is relatively higher than other patterning processes. However, the alignment of the coherent beams is complex, and any changes for the patterns need to simultaneously adjust both beams. Although IL was developed in recent decade and relatively widely used in the field of microelectronics or optoelectronics [189–191], this technique has also been employed to fabricate patterns on materials for biomedical applications. For example, Prodanov et al. [163] produced nano-grooved surfaces with different features on titanium by laser IL, reactive ion etching and NIL techniques, animal implantation experiment demonstrated that the pattern with alternate 75 nm ridge and 225 nm groove achieved best early (4 weeks) osteointegration among all those patterned surfaces.
Summary
Various surface modification techniques commonly used for bone-related implants are reviewed in this article. In practical terms, one approach would be chosen from various feasible candidates for surface modification according to the target effect and physiochemical properties of the substrate, also cost as an important factor. As comprehensive utilization of multiple methods is often required to fulfill the needs, it is necessary to fully understand the principles and effects of such many modification techniques, and their latest advances prior to the processing.
Acknowledgements
This work was partly supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Korea (2012R1A1A2040717), the National Basic Research Program of China funded by the Ministry of Science and Technology (MOST) of China (2011CB606205), the National Science and Technology Supporting Program of China funded by the MOST of China (2012BAI17B02), and the National Natural Science Fund funded by the National Natural Science Foundation of China (21371106).
References
- 1.Zhao G, Schwartz Z, Wieland M, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A 2005;74:49–58. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki O, Kamakura S, Katagiri T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J Biomed Mater Res B Appl Biomater 2006;77:201–12. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater 2009;8:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponche A, Bigerelle M, Anselme K. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 1: physico-chemical effects. Proc Inst Mech Eng H 2010;224:1471–86. [DOI] [PubMed] [Google Scholar]
- 6.Galetz MC, Fleischmann EW, Konrad CH, et al. Abrasion resistance of oxidized zirconium in comparison with CoCrMo and titanium nitride coatings for artificial knee joints. J Biomed Mater Res B Appl Biomater 2010;93:244–51. [DOI] [PubMed] [Google Scholar]
- 7.Guo W-G, Qiu Z-Y, Cui H, et al. Strength and fatigue properties of three-step sintered dense nanocrystal hydroxyapatite bioceramics. Front Mater Sci 2013;7:190–5. [Google Scholar]
- 8.Shikinami Y, Okuno M. Mechanical evaluation of novel spinal interbody fusion cages made of bioactive, resorbable composites. Biomaterials 2003;24:3161–70. [DOI] [PubMed] [Google Scholar]
- 9.Tschernitschek H, Borchers L, Geurtsen W. Nonalloyed titanium as a bioinert metal—a review. J Prosthet Dent 2006;96:12. [PubMed] [Google Scholar]
- 10.Morks MF, Kobayashi A. Development of ZrO2/SiO2 bioinert ceramic coatings for biomedical application. J Mech Behav Biomed Mater 2008;1:165–71. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, An M, Wang Q, et al. Quantitative proteomic analysis of human osteoblast-like MG-63 cells in response to bioinert implant material titanium and polyetheretherketone. J Proteomics 2012;75:3560–73. [DOI] [PubMed] [Google Scholar]
- 12.Utzschneider S, Becker F, Grupp TM, et al. Inflammatory response against different carbon fiber-reinforced PEEK wear particles compared with UHMWPE in vivo. Acta Biomater 2010;6:4296–304. [DOI] [PubMed] [Google Scholar]
- 13.Nine M, Choudhury D, Hee A, et al. Wear debris characterization and corresponding biological response: artificial hip and knee joints. Materials 2014;7:980–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rytlewski P, Żenkiewicz M. Laser-induced surface modification of polystyrene. Appl Surf Sci 2009; 256:857–61. [Google Scholar]
- 15.Ortiz R, Moreno-Flores S, Quintana I, et al. Ultra-fast laser microprocessing of medical polymers for cell engineering applications. Mater Sci Eng C Mater Biol Appl 2014;37:241–50. [DOI] [PubMed] [Google Scholar]
- 16.Balla VK, Banerjee S, Bose S, et al. Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta Biomater 2010;6:2329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandyopadhyay A, Krishna BV, Xue W, et al. Application of laser engineered net shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants. J Mater Sci Mater Med 2009;20(Suppl. 1):S29–34. [DOI] [PubMed] [Google Scholar]
- 18.Roy M, Vamsi Krishna B, Bandyopadhyay A, et al. Laser processing of bioactive tricalcium phosphate coating on titanium for load-bearing implants. Acta Biomater 2008;4:324–33. [DOI] [PubMed] [Google Scholar]
- 19.Lo WJ, Grant DM, Ball MD, et al. Physical, chemical, and biological characterization of pulsed laser deposited and plasma sputtered hydroxyapatite thin films on titanium alloy. J Biomed Mater Res 2000;50:536–45. [DOI] [PubMed] [Google Scholar]
- 20.Mroz W, Budner B, Syroka R, et al. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser deposition. J Biomed Mater Res B Appl Biomater 2014, doi: 10.1002/jbm.b.33170. [DOI] [PubMed] [Google Scholar]
- 21.Rajesh P, Muraleedharan CV, Komath M, et al. Laser surface modification of titanium substrate for pulsed laser deposition of highly adherent hydroxyapatite. J Mater Sci Mater Med 2011;22:1671–9. [DOI] [PubMed] [Google Scholar]
- 22.Prosecka E, Buzgo M, Rampichova M, et al. Thin-layer hydroxyapatite deposition on a nanofiber surface stimulates mesenchymal stem cell proliferation and their differentiation into osteoblasts. J Biomed Biotechnol 2012;2012:428503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Zhai D, Ma H, et al. Stimulation of osteogenic and angiogenic ability of cells on polymers by pulsed laser deposition of uniform akermanite-glass nanolayer. Acta Biomater 2014;10:3295–306. [DOI] [PubMed] [Google Scholar]
- 24.Oechsner H. Ion and plasma beam assisted thin film deposition. Thin Solid Films 1989;175:119–27. [Google Scholar]
- 25.Reyntjens S, Puers R. Focused ion beam induced deposition: fabrication of three-dimensional microstructures and Young’s modulus of the deposited material. J Micromech Microeng 2000;10:181. [Google Scholar]
- 26.Ong JL, Lucas LC, Lacefield WR, et al. Structure, solubility and bond strength of thin calcium phosphate coatings produced by ion beam sputter deposition. Biomaterials 1992;13:249–54. [DOI] [PubMed] [Google Scholar]
- 27.Cui FZ, Luo ZS, Feng QL. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J Mater Sci Mater Med 1997;8:403–5. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Lee I-S, Zhang S-M, et al. Biomimetic apatite formation on calcium phosphate-coated titanium in Dulbecco’s phosphate-buffered saline solution containing CaCl2 with and without fibronectin. Acta Biomater 2010;6:2274–81. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Qiu ZY, Zhang SM, et al. Biomimetic fibronectin/mineral and osteogenic growth peptide/mineral composites synthesized on calcium phosphate thin films. Chem Commun 2011;47:11056–8. [DOI] [PubMed] [Google Scholar]
- 30.Cui FZ, Luo ZS. Biomaterials modification by ion-beam processing. Surf Coat Technol 1999;112:278–85. [Google Scholar]
- 31.Lee IS, Whang CN, Park JC, et al. Biocompatibility and charge injection property of iridium film formed by ion beam assisted deposition. Biomaterials 2003;24:2225–31. [DOI] [PubMed] [Google Scholar]
- 32.Feng QL, Chen QH, Cui FZ. Highly adhesive calcium phosphate layer on UHMWPE prepared by IBAD. Curr Appl Phys 2001;1:213–7. [Google Scholar]
- 33.Song JS, Lee S, Cha GC, et al. Surface modification of silicone rubber by ion beam assisted deposition (IBAD) for improved biocompatibility. J Appl Polym Sci 2005;96:1095–101. [Google Scholar]
- 34.Bogaerts A, Neyts E, Gijbels R, et al. Gas discharge plasmas and their applications. Spectrochim Acta B 2002;57:609–58. [Google Scholar]
- 35.Desmet T, Morent R, Geyter ND, et al. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromolecules 2009;10:2351–78. [DOI] [PubMed] [Google Scholar]
- 36.Chu PK, Chen JY, Wang LP, et al. Plasma-surface modification of biomaterials. Mater Sci Eng R Rep 2002;36:143–206. [Google Scholar]
- 37.Demnati I, Grossin D, Combes C, et al. A comparative physico-chemical study of chlorapatite and hydroxyapatite: from powders to plasma sprayed thin coatings. Biomed Mater 2012;7:054101. [DOI] [PubMed] [Google Scholar]
- 38.Roy M, Bandyopadhyay A, Bose S. Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant. J Biomed Mater Res B Appl Biomater 2011;99:258–65. [DOI] [PubMed] [Google Scholar]
- 39.Karamian E, Khandan A, Motamedi MR, et al. Surface characteristics and bioactivity of a novel natural HA/zircon nanocomposite coated on dental implants. Biomed Res Int 2014;2014:410627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Li K, Zheng X, et al. In vitro and in vivo evaluation of zinc-modified ca-si-based ceramic coating for bone implants. PLoS One 2013;8:e57564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue W, Liu X, Zheng X, et al. In vivo evaluation of plasma-sprayed wollastonite coating. Biomaterials 2005;26:3455–60. [DOI] [PubMed] [Google Scholar]
- 42.Goller G. The effect of bond coat on mechanical properties of plasma sprayed bioglass-titanium coatings. Ceram Int 2004;30:351–5. [Google Scholar]
- 43.Miola M, Ferraris S, Di Nunzio S, et al. Surface silver-doping of biocompatible glasses to induce antibacterial properties. Part II: Plasma sprayed glass-coatings. J Mater Sci Mater Med 2009;20:741–9. [DOI] [PubMed] [Google Scholar]
- 44.Yang F, Xie Y, Li H, et al. Human bone marrow-derived stromal cells cultured with a plasma sprayed CaO–ZrO2–SiO2 coating. J Biomed Mater Res B Appl Biomater 2010;95:192–201. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Ong JL, Tian J. Deposition of highly adhesive ZrO(2) coating on Ti and CoCrMo implant materials using plasma spraying. Biomaterials 2003;24:619–27. [DOI] [PubMed] [Google Scholar]
- 46.Reclaru L, Eschler PY, Lerf R, et al. Electrochemical corrosion and metal ion release from Co–Cr–Mo prosthesis with titanium plasma spray coating. Biomaterials 2005;26:4747–56. [DOI] [PubMed] [Google Scholar]
- 47.Khor KA, Gu YW, Pan D, et al. Microstructure and mechanical properties of plasma sprayed HA/YSZ/Ti-6Al-4V composite coatings. Biomaterials 2004;25:4009–17. [DOI] [PubMed] [Google Scholar]
- 48.Cattini A, Bellucci D, Sola A, et al. Microstructural design of functionally graded coatings composed of suspension plasma sprayed hydroxyapatite and bioactive glass. J Biomed Mater Res B Appl Biomater 2014;102:551–60. [DOI] [PubMed] [Google Scholar]
- 49.Auclair-Daigle C, Bureau MN, Legoux JG, et al. Bioactive hydroxyapatite coatings on polymer composites for orthopedic implants. J Biomed Mater Res A 2005;73:398–408. [DOI] [PubMed] [Google Scholar]
- 50.Wu GM, Hsiao WD, Kung SF. Investigation of hydroxyapatite coated polyether ether ketone composites by gas plasma sprays. Surf Coat Technol 2009;203:2755–8. [Google Scholar]
- 51.Devine DM, Hahn J, Richards RG, et al. Coating of carbon fiber-reinforced polyetheretherketone implants with titanium to improve bone apposition. J Biomed Mater Res B Appl Biomater 2013;101:591–8. [DOI] [PubMed] [Google Scholar]
- 52.Balani K, Anderson R, Laha T, et al. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007;28:618–24. [DOI] [PubMed] [Google Scholar]
- 53.Monsalve M, Ageorges H, Lopez E, et al. Bioactivity and mechanical properties of plasma-sprayed coatings of bioglass powders. Surf Coat Technol 2013;220:60–6. [Google Scholar]
- 54.Lu YP, Li MS, Li ST, et al. Plasma-sprayed hydroxyapatite + titania composite bond coat for hydroxyapatite coating on titanium substrate. Biomaterials 2004;25:4393–403. [DOI] [PubMed] [Google Scholar]
- 55.Lewis F, Cloutier M, Chevallier P, et al. Influence of the 316 L stainless steel interface on the stability and barrier properties of plasma fluorocarbon films. ACS Appl Mater Interfaces 2011;3:2323–31. [DOI] [PubMed] [Google Scholar]
- 56.Testrich H, Rebl H, Finke B, et al. Aging effects of plasma polymerized ethylenediamine (PPEDA) thin films on cell-adhesive implant coatings. Mater Sci Eng C Mater Biol Appl 2013;33:3875–80. [DOI] [PubMed] [Google Scholar]
- 57.Gabler C, Zietz C, Gohler R, et al. Evaluation of osseointegration of titanium alloyed implants modified by plasma polymerization. Int J Mol Sci 2014;15:2454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Feng Q, Bachhuka A, et al. Surface modification by allylamine plasma polymerization promotes osteogenic differentiation of human adipose-derived stem cells. ACS Appl Mater Interfaces 2014;6:9733–41. [DOI] [PubMed] [Google Scholar]
- 59.Yuan Zhang YY, Changsheng LIU. Fluorescent labeling of nanometer hydroxyapatite. J Mater Sci Technol 2008;24:187–91. [Google Scholar]
- 60.Yuan Y, Liu C, Qian J, et al. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010;31:730–40. [DOI] [PubMed] [Google Scholar]
- 61.Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials 2002;23:2079–87. [DOI] [PubMed] [Google Scholar]
- 62.Becker M, Lorenz S, Strand D, et al. Covalent grafting of the RGD-peptide onto polyetheretherketone surfaces via Schiff base formation. ScientificWorldJournal 2013;2013:616535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasserman SR, Tao YT, Whitesides GM. Structure and reactivity of alkylsiloxane monolayers formed by reaction of alkyltrichlorosilanes on silicon substrates. Langmuir 1989;5:1074–87. [Google Scholar]
- 64.Deng J, Wang L, Liu L, et al. Developments and new applications of UV-induced surface graft polymerizations. Prog Polym Sci 2009;34:156–93. [Google Scholar]
- 65.Ul Ahad I, Bartnik A, Fiedorowicz H, et al. Surface modification of polymers for biocompatibility via exposure to extreme ultraviolet radiation. J Biomed Mater Res A 2014;102:3298–310. [DOI] [PubMed] [Google Scholar]
- 66.Wang N, Trunfio-Sfarghiu AM, Portinha D, et al. Nanomechanical and tribological characterization of the MPC phospholipid polymer photografted onto rough polyethylene implants. Colloids Surf B Biointerfaces 2013;108:285–94. [DOI] [PubMed] [Google Scholar]
- 67.CHEN Rui-chao SH, Ang LI, Guo-zhi XU, CHEN Rui-chao SH, Ang LI, et al. UV-induced self-initiated graft polymerization of acrylamide onto poly(ether ether ketone). Chem Res Chin Univ 2012;28:162–5. [Google Scholar]
- 68.Kallrot M, Edlund U, Albertsson AC. Surface functionalization of degradable polymers by covalent grafting. Biomaterials 2006;27:1788–96. [DOI] [PubMed] [Google Scholar]
- 69.Kallrot M, Edlund U, Albertsson AC. Covalent grafting of poly(l-lactide) to tune the in vitro degradation rate. Biomacromolecules 2007;8:2492–6. [DOI] [PubMed] [Google Scholar]
- 70.Biazar E, Zeinali R, Montazeri N, et al. Cell engineering: nanometric grafting of poly-N-isopropylacrylamide onto polystyrene film by different doses of gamma radiation. Int J Nanomedicine 2010;5:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho EH, Lee SG, Kim JK. Surface modification of UHMWPE with γ-ray radiation for improving interfacial bonding strength with bone cement (II). Curr Appl Phys 2005;5:475–9. [Google Scholar]
- 72.Kim S-C, Huh P. E-beam graft polymerization of hydrophilic PEG-methacrylate on the surface of PMMA. J Surf Eng Mater Adv Tech 2012;2:264–70. [Google Scholar]
- 73.Schulze A, Maitz MF, Zimmermann R, et al. Permanent surface modification by electron-beam-induced grafting of hydrophilic polymers to PVDF membranes. R Soc Chem Adv 2013;3:22518–26. [Google Scholar]
- 74.Ha SW, Hauert R, Ernst KH, et al. Surface analysis of chemically-etched and plasma-treated polyetheretherketone (PEEK) for biomedical applications. Surf Coat Technol 1997;96:293–9. [Google Scholar]
- 75.de Rancourt Y, Couturaud B, Mas A, et al. Synthesis of antibacterial surfaces by plasma grafting of zinc oxide based nanocomposites onto polypropylene. J Colloid Interface Sci 2013;402:320–6. [DOI] [PubMed] [Google Scholar]
- 76.Buchanan RA, Lee IS, Williams JM. Surface modification of biomaterials through noble metal ion implantation. J Biomed Mater Res 1990;24:309–18. [DOI] [PubMed] [Google Scholar]
- 77.Boampong DK, Green SM, Unsworth A. N+ ion implantation of Ti6Al4V alloy and UHMWPE for total joint replacement application. J Appl Biomater Biomech 2003;1:164–71. [PubMed] [Google Scholar]
- 78.Wan YZ, Raman S, He F, et al. Surface modification of medical metals by ion implantation of silver and copper. Vacuum 2007;81:1114–8. [Google Scholar]
- 79.Guo M, Li M, Liu X, et al. N-containing functional groups induced superior cytocompatible and hemocompatible graphene by NH(2) ion implantation. J Mater Sci Mater Med 2013;24:2741–8. [DOI] [PubMed] [Google Scholar]
- 80.Bačáková L, Mareš V, Lisá V, et al. Molecular mechanisms of improved adhesion and growth of an endothelial cell line cultured on polystyrene implanted with fluorine ions. Biomaterials 2000;21:1173–9. [DOI] [PubMed] [Google Scholar]
- 81.Inoue Y, Yoshimura Y, Ikeda Y, et al. Ultra-hydrophobic fluorine polymer by Ar-ion bombardment. Colloids Surf B Biointerfaces 2000;19:257–61. [DOI] [PubMed] [Google Scholar]
- 82.Ensinger W, Höchbauer T, Rauschenbach B. Treatment uniformity of plasma immersion ion implantation studied with three-dimensional model systems. Surf Coat Technol 1998;103–104:218–21. [Google Scholar]
- 83.Conrad JR, Radtke JL, Dodd RA, et al. Plasma source ion-implantation technique for surface modification of materials. J Appl Phys 1987;62:4591–6. [Google Scholar]
- 84.Ueda M, Oliveira RM, Rossi JO, et al. Improvements of plasma immersion ion implantation (PIII) and deposition (PIII&D) processing for materials surface modification. Surf Coat Technol 2013;229:97–104. [Google Scholar]
- 85.Sun M, Yang SZ, Li B. New plasma source ion-implantation technique for inner surface modification of materials. Nucl Instrum Methods Phys Res B 1996;111:187–9. [Google Scholar]
- 86.Thomae RW. Plasma-immersion ion implantation. Nucl Instrum Methods Phys Res B 1998;139:37–42. [Google Scholar]
- 87.Brown IG, Godechot X, Yu KM. Novel metal ion surface modification technique. Appl Phys Lett 1991;58:1392–4. [Google Scholar]
- 88.Brown IG, Anders A, Anders S, et al. Plasma synthesis of metallic and composite thin films with atomically mixed substrate bonding. Nucl Instrum Methods Phys Res B 1993;80–81(Part 2):1281–7. [Google Scholar]
- 89.Lu T, Qiao Y, Liu X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus 2012;2:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang N, Yang P, Leng YX, et al. Surface modification of biomaterials by plasma immersion ion implantation. Surf Coat Technol 2004;186:218–26. [Google Scholar]
- 91.Wan GJ, Huang N, Leng YX, et al. TiN and Ti–O/TiN films fabricated by PIII-D for enhancement of corrosion and wear resistance of Ti–6Al–4V. Surf Coat Technol 2004;186:136–40. [Google Scholar]
- 92.Volz K, Enders B, Ensinger W. Nitrogen plasma immersion ion implantation and silicon sputter deposition combined with methane implantation as an in-line process for improving corrosion and wear performance of stainless steels. Surf Coat Technol 2000;128–129:479–83. [Google Scholar]
- 93.Shi W, Li XY, Dong H. Improved wear resistance of ultra-high molecular weight polyethylene by plasma immersion ion implantation. Wear 2001;250:544–52. [Google Scholar]
- 94.Powles RC, McKenzie DR, Fujisawa N, et al. Production of amorphous carbon by plasma immersion ion implantation of polymers. Diamond Relat Mater 2005;14:1577–82. [Google Scholar]
- 95.Poon RWY, Ho JPY, Liu X, et al. Anti-corrosion performance of oxidized and oxygen plasma-implanted NiTi alloys. Mater Sci Eng A 2005;390:444–51. [Google Scholar]
- 96.Poon RWY, Ho JPY, Liu X, et al. Improvements of anti-corrosion and mechanical properties of NiTi orthopedic materials by acetylene, nitrogen and oxygen plasma immersion ion implantation. Nucl Instrum Methods Phys Res B 2005;237:411–6. [Google Scholar]
- 97.Shanaghi A, Chu PK, Sabour Rouhaghdam AR, et al. Structure and corrosion resistance of Ti/TiC coatings fabricated by plasma immersion ion implantation and deposition on nickel–titanium. Surf Coat Technol 2013;229:151–5. [Google Scholar]
- 98.Poon RWY, Yeung KWK, Liu XY, et al. Carbon plasma immersion ion implantation of nickel–titanium shape memory alloys. Biomaterials 2005;26:2265–72. [DOI] [PubMed] [Google Scholar]
- 99.Xie Y, Liu X, Huang A, et al. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials 2005;26:6129–35. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, Kwok DTK, Wang W, et al. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials 2010;31:413–9. [DOI] [PubMed] [Google Scholar]
- 101.Wang H, Lu T, Meng F, et al. Enhanced osteoblast responses to poly ether ether ketone surface modified by water plasma immersion ion implantation. Colloids Surf B Biointerfaces 2014;117:89–97. [DOI] [PubMed] [Google Scholar]
- 102.Liu X, Poon RWY, Kwok SCH, et al. Structure and properties of Ca-plasma-implanted titanium. Surf Coat Technol 2005;191:43–8. [Google Scholar]
- 103.Qiao Y, Zhang W, Tian P, et al. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 2014;35:6882–97. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W, Chu PK. Enhancement of antibacterial properties and biocompatibility of polyethylene by silver and copper plasma immersion ion implantation. Surf Coat Technol 2008;203:909–12. [Google Scholar]
- 105.Jin G, Qin H, Cao H, et al. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014;35:7699–713. [DOI] [PubMed] [Google Scholar]
- 106.Mei S, Wang H, Wang W, et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014;35:4255–65. [DOI] [PubMed] [Google Scholar]
- 107.Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009;5:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater 2014;10:3815–26. [DOI] [PubMed] [Google Scholar]
- 109.Harrison RG. On the stereotropism of embryonic cells. Science 1911;34:279–81. [DOI] [PubMed] [Google Scholar]
- 110.Whitesides GM, Ostuni E, Takayama S, et al. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 2001;3:335–73. [DOI] [PubMed] [Google Scholar]
- 111.Wójciak-Stothard B, Curtis A, Monaghan W, et al. Guidance and activation of murine macrophages by nanometric scale topography. Exp Cell Res 1996;223:426–35. [DOI] [PubMed] [Google Scholar]
- 112.Schena M, Shalon D, Davis RW, et al. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995;270:467–70. [DOI] [PubMed] [Google Scholar]
- 113.Negrete OD, Cerrina F. Step-and-scan maskless lithography for ultra large scale DNA chips. Microelectron Eng 2008;85:834–7. [Google Scholar]
- 114.Kirstein S. Scanning near-field optical microscopy. Curr Opin Colloid Interface Sci 1999;4:256–64. [Google Scholar]
- 115.Guijt RM, Breadmore MC. Maskless photolithography using UV LEDs. Lab on a Chip 2008;8:1402–4. [DOI] [PubMed] [Google Scholar]
- 116.Shin D-S, Lee K-N, Yoo B-W, et al. Automated maskless photolithography system for peptide microarray synthesis on a chip. J Comb Chem 2010;12:463–71. [DOI] [PubMed] [Google Scholar]
- 117.Ibrahim S, Higgins DA, Ito T. Direct-write multiphoton photolithography: a systematic study of the etching behaviors in various commercial polymers. Langmuir 2007;23:12406–12. [DOI] [PubMed] [Google Scholar]
- 118.Holden MA, Jung S-Y, Cremer PS. Patterning enzymes inside microfluidic channels via photoattachment chemistry. Anal Chem 2004;76:1838–43. [DOI] [PubMed] [Google Scholar]
- 119.Nielson R, Shear JB. Parallel chemical dosing of subcellular targets. Anal Chem 2006;78:5987–93. [DOI] [PubMed] [Google Scholar]
- 120.Odawara A, Gotoh M, Suzuki I. Control of neural network patterning using collagen gel photothermal etching. Lab on a Chip 2013;13:2040–6. [DOI] [PubMed] [Google Scholar]
- 121.Pfleging W, Torge M, Bruns M, et al. Laser- and UV-assisted modification of polystyrene surfaces for control of protein adsorption and cell adhesion. Appl Surf Sci 2009;255:5453–7. [Google Scholar]
- 122.Pfleging W, Bruns M, Welle A, et al. Laser-assisted modification of polystyrene surfaces for cell culture applications. Appl Surf Sci 2007;253:9177–84. [Google Scholar]
- 123.Rebollar E, Frischauf I, Olbrich M, et al. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials 2008;29:1796–806. [DOI] [PubMed] [Google Scholar]
- 124.Ahrem H, Pretzel D, Endres M, et al. Laser-structured bacterial nanocellulose hydrogels support ingrowth and differentiation of chondrocytes and show potential as cartilage implants. Acta Biomater 2014;10:1341–53. [DOI] [PubMed] [Google Scholar]
- 125.Ducker R, Garcia A, Zhang J, et al. Polymeric and biomacromolecular brush nanostructures: progress in synthesis, patterning and characterization. Soft Matter 2008;4:1774–86. [Google Scholar]
- 126.Brough B, Christman KL, Wong TS, et al. Surface initiated actin polymerization from top-down manufactured nanopatterns. Soft Matter 2007;3:541–6. [DOI] [PubMed] [Google Scholar]
- 127.Christman KL, Schopf E, Broyer RM, et al. Positioning multiple proteins at the nanoscale with electron beam cross-linked functional polymers. J Am Chem Soc 2009;131:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmelmer U, Paul A, Küller A, et al. Nanostructured polymer brushes. Small 2007;3:459–65. [DOI] [PubMed] [Google Scholar]
- 129.Steenackers M, Küller A, Ballav N, et al. Morphology control of structured polymer brushes. Small 2007;3:1764–73. [DOI] [PubMed] [Google Scholar]
- 130.Idota N, Tsukahara T, Sato K, et al. The use of electron beam lithographic graft-polymerization on thermoresponsive polymers for regulating the directionality of cell attachment and detachment. Biomaterials 2009;30:2095–101. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Subbiahdoss G, Swartjes J, et al. Length-scale mediated differential adhesion of mammalian cells and microbes. Adv Funct Mater 2011;21:3916–23. [Google Scholar]
- 132.Muszanska AK, Rochford ETJ, Gruszka A, et al. Antiadhesive polymer brush coating functionalized with antimicrobial and RGD peptides to reduce biofilm formation and enhance tissue integration. Biomacromolecules 2014;15:2019–26. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, da Silva Domingues JF, Subbiahdoss G, et al. Conditions of lateral surface confinement that promote tissue-cell integration and inhibit biofilm growth. Biomaterials 2014;35:5446–52. [DOI] [PubMed] [Google Scholar]
- 134.Stiles PL. Direct deposition of micro- and nanoscale hydrogels using Dip Pen Nanolithography (DPN). Nat Methods 2010;7. [Google Scholar]
- 135.Rakickas T, Ericsson EM, Ruželė Ž, et al. Functional hydrogel density patterns fabricated by dip-pen nanolithography and photografting. Small 2011;7:2153–7. [DOI] [PubMed] [Google Scholar]
- 136.Zheng Z, Jang J-W, Zheng G, et al. Topographically flat, chemically patterned PDMS stamps made by dip-pen nanolithography. Angew Chem Int Ed 2008;47:9951–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim JH, Ginger DS, Lee KB, et al. Direct-write dip-pen nanolithography of proteins on modified silicon oxide surfaces. Angew Chem Int Ed Engl 2003;42:2309–12. [DOI] [PubMed] [Google Scholar]
- 138.Sistiabudi R, Ivanisevic A. Dip-pen nanolithography of bioactive peptides on collagen-terminated retinal membrane. Adv Mater 2008;20:3678–81. [Google Scholar]
- 139.Hirtz M, Oikonomou A, Georgiou T, et al. Multiplexed biomimetic lipid membranes on graphene by dip-pen nanolithography. Nat Commun 2013;4:2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sekula S, Fuchs J, Weg-Remers S, et al. Multiplexed lipid dip-pen nanolithography on subcellular scales for the templating of functional proteins and cell culture. Small 2008;4:1785–93. [DOI] [PubMed] [Google Scholar]
- 141.Li H, He Q, Wang X, et al. Nanoscale-controlled enzymatic degradation of poly(l-lactic acid) films using dip-pen nanolithography. Small 2011; 7:226–9. [DOI] [PubMed] [Google Scholar]
- 142.Arrabito G, Reisewitz S, Dehmelt L, et al. Biochips for cell biology by combined dip-pen nanolithography and DNA-directed protein immobilization. Small 2013;9:4243–9. [DOI] [PubMed] [Google Scholar]
- 143.Roy D, Munz M, Colombi P, et al. Directly writing with nanoparticles at the nanoscale using dip-pen nanolithography. Appl Surf Sci 2007;254:1394–8. [Google Scholar]
- 144.Wang WM, Stoltenberg RM, Liu S, et al. Direct patterning of gold nanoparticles using dip-pen nanolithography. ACS Nano 2008;2:2135–42. [DOI] [PubMed] [Google Scholar]
- 145.Wang H-T, Nafday OA, Haaheim JR, et al. Toward conductive traces: dip pen nanolithography® of silver nanoparticle-based inks. Appl Phys Lett 2008;93:143105. [Google Scholar]
- 146.Dorjderem N, Sergey R, Richard CH. Immobilization of motile bacterial cells via dip-pen nanolithography. Nanotechnology 2010;21:235105. [DOI] [PubMed] [Google Scholar]
- 147.Kim J, Shin Y-H, Yun S-H, et al. Direct-write patterning of bacterial cells by dip-pen nanolithography. J Am Chem Soc 2012;134:16500–3. [DOI] [PubMed] [Google Scholar]
- 148.Salaita K, Wang Y, Fragala J, et al. Massively parallel dip–pen nanolithography with 55 000-pen two-dimensional arrays. Angew Chem Int Ed 2006;45:7220–3. [DOI] [PubMed] [Google Scholar]
- 149.El Zubir O, Barlow I, Leggett GJ, et al. Fabrication of molecular nanopatterns at aluminium oxide surfaces by nanoshaving of self-assembled monolayers of alkylphosphonates. Nanoscale 2013;5:11125–31. [DOI] [PubMed] [Google Scholar]
- 150.Hu Y, Das A, Hecht MH, et al. Nanografting de novo proteins onto gold surfaces. Langmuir 2005;21:9103–9. [DOI] [PubMed] [Google Scholar]
- 151.Bano F, Fruk L, Sanavio B, et al. Toward multiprotein nanoarrays using nanografting and DNA directed immobilization of proteins. Nano Lett 2009;9:2614–8. [DOI] [PubMed] [Google Scholar]
- 152.Wang Z, Zhang P, Kirkland B, et al. Microcontact printing of polyelectrolytes on PEG using an unmodified PDMS stamp for micropatterning nanoparticles, DNA, proteins and cells. Soft Matter 2012;8:7630–7. [Google Scholar]
- 153.Singhvi R, Kumar A, Lopez G, et al. Engineering cell shape and function. Science 1994;264:696–8. [DOI] [PubMed] [Google Scholar]
- 154.Kilian KA, Bugarija B, Lahn BT, et al. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA 2010;107:4872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rozkiewicz DI, Kraan Y, Werten MW, et al. Covalent microcontact printing of proteins for cell patterning. Chemistry (Easton) 2006;12:6290–7. [DOI] [PubMed] [Google Scholar]
- 156.Offenhausser A, Bocker-Meffert S, Decker T, et al. Microcontact printing of proteins for neuronal cell guidance. Soft Matter 2007;3:290–8. [DOI] [PubMed] [Google Scholar]
- 157.Lange SA, Benes V, Kern DP, et al. Microcontact printing of DNA molecules. Anal Chem 2004;76:1641–7. [DOI] [PubMed] [Google Scholar]
- 158.Xu L, Robert L, Ouyang Q, et al. Microcontact printing of living bacteria arrays with cellular resolution. Nano Lett 2007;7:2068–72. [DOI] [PubMed] [Google Scholar]
- 159.Zhang K, Chou CK, Xia X, et al. Block-cell-printing for live single-cell printing. Proc Natl Acad Sci USA 2014;111:2948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Johansson F, Carlberg P, Danielsen N, et al. Axonal outgrowth on nano-imprinted patterns. Biomaterials 2006;27:1251–8. [DOI] [PubMed] [Google Scholar]
- 161.Chou SY, Krauss PR, Renstrom PJ. Imprint lithography with 25-nanometer resolution. Science 1996;272:85–7. [Google Scholar]
- 162.Ferrari A, Cecchini M, Serresi M, et al. Neuronal polarity selection by topography-induced focal adhesion control. Biomaterials 2010;31:4682–94. [DOI] [PubMed] [Google Scholar]
- 163.Prodanov L, Lamers E, Domanski M, et al. The effect of nanometric surface texture on bone contact to titanium implants in rabbit tibia. Biomaterials 2013;34:2920–7. [DOI] [PubMed] [Google Scholar]
- 164.Nandakumar A, Truckenmüller R, Ahmed M, et al. A fast process for imprinting micro and nano patterns on electrospun fiber meshes at physiological temperatures. Small 2013;9:3405–9. [DOI] [PubMed] [Google Scholar]
- 165.Yabu H, Saito Y, Shimomura M, et al. Thermal nanoimprint lithography of polymer films on non-adhesive substrates by using mussel-inspired adhesive polymer layers. J Mater Chem C 2013;1:1558–61. [Google Scholar]
- 166.Subramani C, Cengiz N, Saha K, et al. Direct fabrication of functional and biofunctional nanostructures through reactive imprinting. Adv Mater 2011;23:3165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Merino S, Retolaza A, Trabadelo V, et al. Protein patterning on the micro- and nanoscale by thermal nanoimprint lithography on a new functionalized copolymer. J Vac Sci Technol B 2009;27:2439–43. [Google Scholar]
- 168.Falconnet D, Pasqui D, Park S, et al. A novel approach to produce protein nanopatterns by combining nanoimprint lithography and molecular self-assembly. Nano Lett 2004;4:1909–14. [Google Scholar]
- 169.Trabadelo V, Retolaza A, Merino S, et al. Protein patterning by thermal nanoimprint lithography and NH3-plasma functionalization of polystyrene. J Vac Sci Technol B 2009;27:1060–2. [Google Scholar]
- 170.Fehrman Cory EM, Aga JRS, Lombardi IIIJP, et al. Nanoimprint lithography of deoxyribonucleic acid biopolymer films. J Micro-Nanolith MEM 2013;12:040501. [Google Scholar]
- 171.Luo M, Epps TH. Directed block copolymer thin film self-assembly: emerging trends in nanopattern fabrication. Macromolecules 2013;46:7567–79. [Google Scholar]
- 172.Jeong S-J, Kim JY, Kim BH, et al. Directed self-assembly of block copolymers for next generation nanolithography. Mater Today 2013;16:468–76. [Google Scholar]
- 173.Smart AG. A self-assembled nanopattern exhibits near-perfect order. Phys Today 2012;65:14–5. [Google Scholar]
- 174.Hirotaka O, Hiroaki T, Mitsuo T, et al. Nanopattern transfer from high-density self-assembled nanosphere arrays on prepatterned substrates. Nanotechnology 2009; 20:455303. [DOI] [PubMed] [Google Scholar]
- 175.Chen K, Taflove A, Kim YL, et al. Self-assembled patterns of nanospheres with symmetries from submicrons to centimeters. Appl Phys Lett 2005;86:033101. [Google Scholar]
- 176.Banerjee R, Hazra S, Banerjee S, et al. Nanopattern formation in self-assembled monolayers of thiol-capped Au nanocrystals. Phys Rev E Stat Nonlin Soft Matter Phys 2009;80:056204. [DOI] [PubMed] [Google Scholar]
- 177.Dinca V, Kasotakis E, Catherine J, et al. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett 2007;8:538–43. [DOI] [PubMed] [Google Scholar]
- 178.Busuttil K, Rotaru A, Dong M, et al. Transfer of a protein pattern from self-assembled DNA origami to a functionalized substrate. Chem Commun 2013;49:1927–9. [DOI] [PubMed] [Google Scholar]
- 179.Nielson R, Kaehr B, Shear JB. Microreplication and design of biological architectures using dynamic-mask multiphoton lithography. Small 2009;5:120–5. [DOI] [PubMed] [Google Scholar]
- 180.Spivey EC, Ritschdorff ET, Connell JL, et al. Multiphoton lithography of unconstrained three-dimensional protein microstructures. Adv Funct Mater 2013;23:333–9. [Google Scholar]
- 181.Kaehr B, Shear JB. Multiphoton fabrication of chemically responsive protein hydrogels for microactuation. Proc Natl Acad Sci USA 2008;105:8850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Seidlits SK, Schmidt CE, Shear JB. High-resolution patterning of hydrogels in three dimensions using direct-write photofabrication for cell guidance. Adv Funct Mater 2009;19:3543–51. [Google Scholar]
- 183.Jhaveri SJ, McMullen JD, Sijbesma R, et al. Direct three-dimensional microfabrication of hydrogels via two-photon lithography in aqueous solution. Chem Mater 2009;21:2003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials 2008;29:2962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Melissinaki V, Gill AA, Ortega I, et al. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 2011;3:045005. [DOI] [PubMed] [Google Scholar]
- 186.Claeyssens F, Hasan EA, Gaidukeviciute A, et al. Three-dimensional biodegradable structures fabricated by two-photon polymerization. Langmuir 2009;25:3219–23. [DOI] [PubMed] [Google Scholar]
- 187.Ovsianikov A, Deiwick A, Van Vlierberghe S, et al. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011;12:851–8. [DOI] [PubMed] [Google Scholar]
- 188.Ovsianikov A, Deiwick A, Van Vlierberghe S, et al. Laser fabrication of 3D gelatin scaffolds for the generation of bioartificial tissues. Materials 2011;4:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lasagni A, Mücklich F, Nejati MR, et al. Periodical surface structuring of metals by laser interference metallurgy as a new fabrication method of textured solar selective absorbers. Adv Eng Mater 2006;8:580–4. [Google Scholar]
- 190.Goldin SY, Cashingad A, Perez A, et al. Simulations of and experiments on the holographic lithography fabrication of photonic crystals. Opt Mater 2008;30:1759–65. [Google Scholar]
- 191.Leibovici MCR, Burrow GM, Gaylord TK. Pattern-integrated interference lithography: prospects for nano- and microelectronics. Opt Express 2012;20:23643–52. [DOI] [PubMed] [Google Scholar]