Abstract
Heart valve and blood vessel replacement using artificial prostheses is an effective strategy for the treatment of cardiovascular disease at terminal stage. Natural extracellular matrix (ECM)-derived materials (decellularized allogeneic or xenogenic tissues) have received extensive attention as the cardiovascular scaffold. However, the bioprosthetic grafts usually far less durable and undergo calcification and progressive structural deterioration. Glutaraldehyde (GA) is a commonly used crosslinking agent for improving biocompatibility and durability of the natural scaffold materials. However, the nature ECM and GA-crosslinked materials may result in calcification and eventually lead to the transplant failure. Therefore, studies have been conducted to explore new crosslinking agents. In this review, we mainly focused on research progress of ECM-derived cardiovascular scaffolds and their crosslinking strategies.
Keywords: extracellular matrix, tissue engineering, scaffold, cross-linking agent, calcification
Introduction
Cardiovascular disease such as heart valve disease, coronary heart disease and heart failure is the main cause of morbidity and mortality. Current valve substitutes for replacing the diseased heart valves mainly include mechanical prostheses and bioprosthetic valves, such as allograft valves and xenograft valves [1, 2]. Mechanical valves are associated with significant risk of thromboembolic complications and need lifelong anticoagulation therapy [3], and they also lack the ability to grow, repair and remodel, which limits their long-term application in human body [4–6]. The reduced availability of allografts due to donor scarcity remains significant challenge for cardiovascular disease treatment. Both the porcine aortic valves and bovine pericardial tissues as a part of xenograft valves do not require the treatment of anticoagulation, which could enhance survival and quality of life patients, especially the pediatric patients with congenital [2, 7]. However, these valves are usually far less durable and frequently undergo calcification associated with progressive structural deterioration [2, 8]. The blood vessels used for transplantation mainly come from the patient’s vein, internal mammary and radial artery, etc [9]. But the available autologous blood vessels from patients themselves are always limited. In order to solve the problem of insufficient source of autologous vein grafts, synthetic grafts and xenogenic tubular tissues are widely used in constructing tissue engineering blood vessel. The synthetic materials included polyethylene terephthalate (Dacron®), expanded polytetrafluoroethylene and polyurethane [10]. These materials are successfully applied for large-diameter arteries replacement (>6 mm), and they possess the advantages of good biocompatibility, easy formation of desired shapes and ready availability. However, these synthetic materials are not degradable, and not suitable for small-diameter blood vessel replacement (<6 mm) due to thrombogenicity [11]. Other synthetic materials, which are widely used for constructing porous scaffolds, are biodegradable polymers, including polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone, polyhydroxyalkanoates and polyhydroxybutyrate [12]. PGA and PLA are commonly used in cardiovascular tissue engineering because their degradation products are metabolized and eliminated easily. However, PGA tends to lose its mechanical strength within 4 weeks, and needs long time to be completely absorbed.
The xenogenic valve and tubular tissues such as porcine heart valves, bovine pericardium and carotid arteries, have the advantage of being readily available. However, the raw tissues may induce immunogenicity after implantation in vivo, so decellularized materials, which mainly are extracellular matrix (ECM), are often used as cardiovascular scaffolds [13–15]. The decellularization could remove the cellular components and retain the structure of ECM, but it also lead to the loss of ECM integrity and degenerative structural failure, and the ECM-derived scaffolds may be calcified after implantation [7, 13]. In order to avoid these limitations, ECM-derived scaffolds are crosslinked using crosslinking agents to prevent degeneration, enhance mechanical strength and reduce calcification [16]. In recent years, significant progress has been make in the development of ECM-derived scaffolds for cardiovascular tissue replacement and tissue engineering, especially in the development of new crosslinking strategies. Therefore, the aim of this review is to discuss the development of crosslinking strategies for preparation of ECM-derived cardiovascular scaffolds.
Crosslinking Strategies
An ideal biomaterial crosslinking agent should be no cytotoxicity and have low cost. It could improve the mechanical performance of the materials and inhibit calcification. There are many crosslinking agents for fixing ECM-derived scaffolds, which may be classified as (i) chemical crosslinking agents and (ii) natural crosslinking agents. The chemical crosslinking agents include glutaraldehyde (GA), carbodiimide (1-ethyl-3-(3-dimethyl aminopropyl)-carbodiimide (EDC)), epoxy compounds, six methylene diisocyanate, glycerin and alginate, etc. [17–21]; and the natural crosslinking agents include genipin (GP), nordihydroguaiaretic acid (NDGA), tannic acid and procyanidins (PC).
Chemical crosslinking agents
Glutaraldehyde
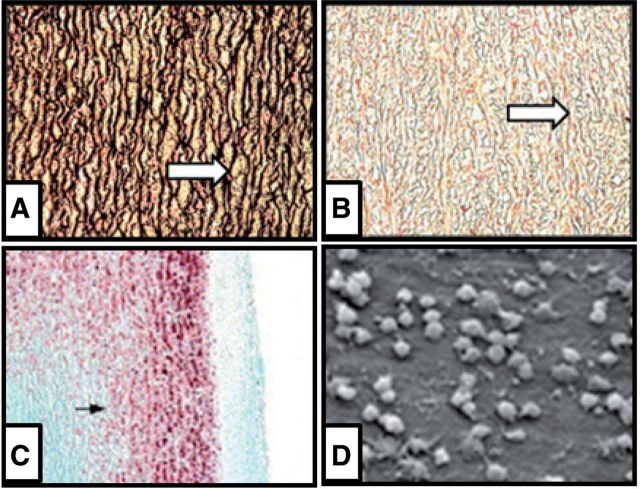
GA has been extensively used as a crosslinking agent to fix bioprosthetic valves and bovine pericardium, and it can significantly improve mechanical strength and durability of the ECM-derived scaffolds [22, 23]. GA reacts with amino groups available in protein molecules, which helps to form a more tightly crosslinked network between many protein molecules [23]. The ECM-derived scaffolds fixed with GA could significantly improve tensile strength and pliability and reduce antigenicity [22]. GA crosslinking can make scaffolds non-resorbable and non-amenable for its ability to resist to matrix metalloproteinase, but it cannot resist to elastase (Fig. 1A and B). In addition, the toxicity of GA (Fig. 1C) and GA-induced calcification (Fig. 1D) limits the long-term implantation, and results in final failure of the implant [23].
Figure 1.
Disadavantage of GA-crosslinked ECM. Histology of GA-treated aortic samples before (A) and after (B) elastase. Calcium deposition in subdermally implanted GA-treated aorta explanted at 21 days (C). Scanning electron microscopy of human endothelial cells on GA-treated bovine pericardium shows sporadic cell cadavers (D). (Reprinted with permission from Refs. [22, 50].)
Detoxifying strategies have been proposed to increase the biocompatibility and durability of the GA-fixed ECM-derived scaffolds, and many methods were used to improve cellular adhesion and proliferation on GA-fixed scaffolds, including pretreatment with citric acid or amino acid solutions to remove free aldehyde groups [24–26]. Although these methods aim to develop a detoxifying treatment for GA-fixed ECM-derived scaffolds and have positive effects on the improvement of biocompatibility, they do not have obvious effect on calcification of GA-fixed materials [26].
The mechanism of calcification of GA-fixed ECM-derived scaffolds is complex. It is considered that there are many factors such as phospholipids, free aldehyde groups, and residual antigenicity play important roles in inducing calcification [27]. There are many methods for inhibition of calcification of GA-crosslinked scaffolds. Since the phospholipids and cholesterol are two substances which are thought to induce calcification, ethanol or its solutions have been used to extract these molecules from the tissue to inhibit the calcification [28, 29]. Some studies have shown that elastin may play a key role in tissue calcification and some calcification-related diseases such as atherosclerosis and heart valves calcification have been found associated with elastin degradation [30]. Therefore, trivalent metal ions (Fe3+ or Al3+) are used, in which stable crosslinking forms between Al3+/Fe3+ and elastic proteins in order to stabilize the microstructure of elastic protein [30].
1-Ethyl-3-(3-dimethyl aminopropyl)-carbodiimide
EDC is a kind of compounds that contain a double bond which can react with many groups, such as carboxyl, hydroxyl and sulfydryl groups [31]. EDC fixation of ECM-derived scaffolds mainly involves the activation of carboxyl groups of glutamic and aspartic acid residues in the peptide chain and formation of O-acylisourea intermediate which can be nucleophilic attacked by free amino groups of lysine or hydroxyl lysine residues [32]. In addition, EDC crosslinking forms a network crosslinking which effectively increases the mechanical stability of collagen material and prevents the movement of macromolecules and infiltration of water molecules [20]. N-hydroxysuccinimide, an affinity reagent, added to EDC solution will effectively increase the number of crosslinks [21]. EDC-crosslinked ECM-derived scaffolds are soft and similar to natural tissue materials, and reveal low residual toxicity, which is in favor of the recellularization of scaffolds [23, 32]. Olde Damink et al. [21] have shown that the EDC-crosslinked collagen can resist enzymatic degradation of collagen matrix in vitro. However, EDC crosslinking may not be able to inhibit calcification, which limits its potential application in preparation of cardiovascular grafts [19].
Epoxy compounds
Epoxy compounds have multiple epoxy functional groups which can react with amino, carboxyl and hydroxyl groups. Epoxy compounds have been used to preserve biological tissue materials and this new fixation technique has recently been employed to crosslink biological heart valves and vascular grafts [17, 18]. Epoxy compounds crosslinked ECM-derived scaffolds are white, soft and no shrink, in which the collagen maintains loose and natural state [33]. However, epoxy compounds crosslinking is linear and has low crosslinking degree, and it cannot resist enzymatic degradation of collagen and has poor stability [33]. In addition, epoxy compounds in crosslinked ECM-derived scaffolds have shown certain toxicity and will cause immune response and calcification, which is similar to GA [18, 23, 34].
Natural crosslinking agents
Natural substances as crosslinking agents show superiority in many aspects, especially in terms of cytotoxicity and anti-calcification ability. Many different kind of natural crosslinking agents such as GP, NDGA, tannic acid and PC have been studied for crosslinking cardiovascular scaffolds.
Genipin
GP is obtained from the natural compound geniposide which extracted from the fruit of Gardenia jasminoides Ellis, and it is one of the active ingredients of the traditional Chinese medicine extraction. It belongs to the iridoid compounds, which have multiple active groups, such as hydroxyl and carboxyl [35]. The applications of GP crosslinking acellular ECM in tissue engineering have been reported [36]. GP can spontaneously react with the free amino groups of lysine, hydroxyl lysine and arginine within some biomaterials and generate iridoid nitrides, and then form intramolecularly and intermolecularly crosslinking by the polymerization process with a heterocyclic structure [36, 37]. GP prefers to form annular crosslinking, which is more stable than the reticular crosslinking formed by GA and the linear crosslinking formed by pEPC. GP-crosslinked ECM-derived scaffolds, which are dark blue pigments, have ability of resistance to enzymatic degradation in vitro. Compared with GA, GP-crosslinked ECM-derived scaffolds have lower inflammatory response, and would not release the GP in the process of preservation [36, 38, 39]. However, the dark blue appearance of GP-crosslinked ECM-derived scaffolds, together with the exorbitant price and the complex extraction process, will greatly limit its application for treatment of cardiovascular tissues.
Nordihydroguaiaretic acid
NDGA, which is isolated from the creosote bush, is natural plant polyphenol compounds containing two functional ortho-catechols at the ends of a short alkane [40–42]. It has antioxidative and anticancer activity, and has the ability to crosslink collagen fibers, which results in an increase of the mechanical properties of the tissue matrix [42]. NDGA crosslinks collagen fibers by forming bisquinone crosslinking between NDGA molecules first, which further form a crosslinked NDGA network, and collagen fibers are then firmly embedded into this network to form a stable matrix [43]. The crosslinked collagen fibers appear brown, which is similar to natural collagen fibers. NDGA crosslinking concentration will directly affect the mechanical tensile strength and hardness of collagen [40]. However, NDGA at the concentration above 100 µM was cytotoxic to cells [41].
Tannin acid
Tannin acid (TA) is d-glucose gallic acid ester containing multiple phenolic hydroxyl groups and aromatic rings. It is widely found in fruits, seeds of leguminous plant, grain and a variety of drinks (such as wine, tea, cocoa and apple juice) [44, 45]. TA has a relatively high molecular weight, and can interact with carbohydrate, proteins and other biological macromolecules [44]. The term ‘tannin’ was first proposed in 1796. It was originally known because it can react with collagen protein and transform the animal’s skin into leather, which is therefore the original leather tanning method [46]. The mechanism of interaction between TA and collagen is mainly through hydrogen bonding and hydrophobic effects, which is influenced by the molecular weight and three-dimensional space structure [47, 48]. TA also has obvious crosslinking effect on elastin and improves its stability and enzyme degradation resistance [49]. TA can significantly reduce the calcification of GA-crosslinked aortic elastin after in vivo transplantation [50].
Procyanidins
Flavonoids are plant secondary metabolites widely distributed in fruits and vegetables [51]. Recently, the researchers realized that flavonoids can crosslink the cardiovascular scaffold materials, which could solve the disadvantages of GA, such as residual toxicity and calcification. PC, one of the most important flavonoids, is widely researched currently [52–54].
PC is an oligomer or polymer composed of flavan-3-ol (e.g. epicatechin or catechin), and it is naturally occurring plant metabolites widely available in fruits, vegetables, nuts, seeds, flowers and barks. The main characteristic of PC is that it can produce anthocyanin after heating in the acid medium, so it is named PC [45]. Most foods contain exclusively B-type PC which is linked by C4–C8 and/or C4–C6 bonds, and a small number of foods contain A-type PC which contain an additional ether bond between C2 and O7. Polymeric forms of PC are the predominant existence in many foods that contain PC [55]. High-pressure liquid chromatography analyses demonstrated that grape seed PC extract contains approximately 75–80% oligomeric PC and 3–5% monomeric PC [56].
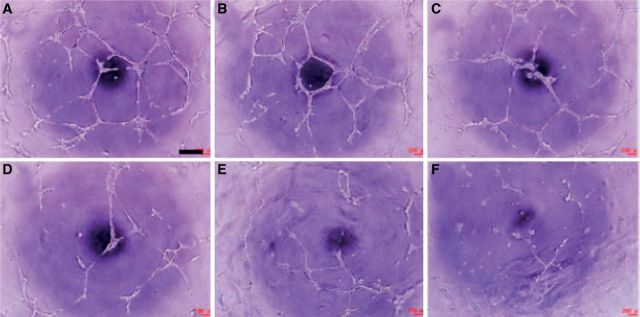
PC displays a variety of biological activities, such as antioxidant, anti-inflammatory, anti-bacterial, anti-tumor, anti-calcification and cardiovascular protection effects. PC can reduce the expression and secretion of MMP-2 which have been demonstrated as an angiogenic factor. In addition, PC can also inhibit the activity of MMP-2 [57]. In addition, many studies have shown that PC could effectively inhibit tumor angiogenesis (Fig. 2), and angiogenesis-mediated tumor growth (Fig. 3) [58, 59]. Teissedre et al. [60] reported that PC extracted from grapes and red wine have remarkable scavenging activities and could inhibit oxidative modification of LDL in vitro. Therefore, dietary containing PC is important for maintaining health and reducing the incidence of atherosclerosis by their antioxidant activity, and decrease the risk of cardiovascular disease [56, 61].
Figure 2.
In vitro angiogenesis in the presence of PC at 0 (A), 0.1 (B), 0.5 (C), 1.0 (D), 1.5 (E) and 100 (F) µg/ml. Scale bar is 400 µm. (Reprinted with permission from Ref. [59].)
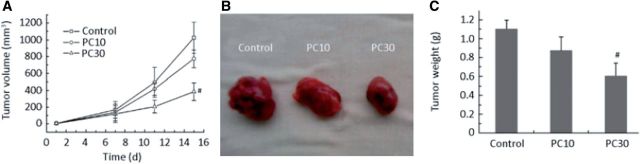
Figure 3.
Anti-tumor effect of PC on tumor volume (A), tumor morphology (B) and tumor weight (C). PC 10 and PC 30 are 10 and 30 mg PC/kg bodyweight, respectively. Number sign indicates P < 0.05 compared with control. (Reprinted with permission from Ref. [59].)
PC Crosslinking of ECM-Derived Scaffolds
Stability and biocompatibility of PC-crosslinked scaffolds
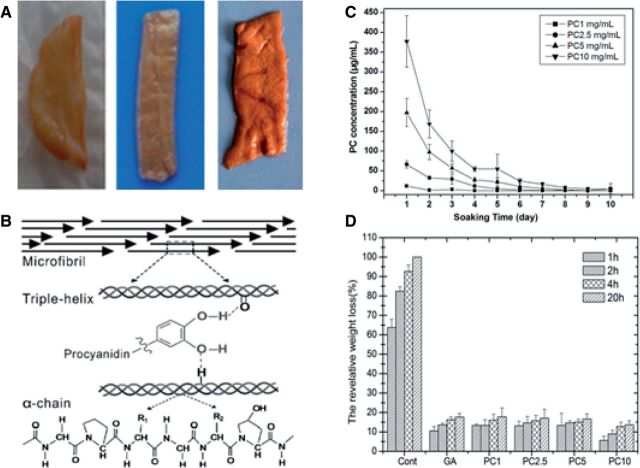
PC-crosslinked decellularized cardiovascular scaffold materials (Fig. 4A), including decellularized porcine aortic heart valves, bovine pericardiums and blood vessels have been successfully prepared. PC-crosslinked ECM-derived scaffolds show palm red, soft and stretch, and do not shrink [52, 62]. Han et al. [63] have reported that PC could crosslink collagen (Fig. 4B) and no calcification appears in PC-crosslinked collagen after implanting in rat subcutis. Through the treatment of detergent or hydrogen bonding weaken agent, the interaction between PC and collagen could be damaged, which suggests that PC crosslinking mechanism might be related to the hydrogen bonding interaction which is mainly formed between protein amide carbonyl and PC phenolic hydroxyl groups. Proline molecule, which is a good hydrogen bond receptor, has a carbonyl oxygen adjacent to the amino groups. Collagen is rich in proline, so it can form stable hydrogen bonds with PC [64].
Figure 4.
PC-crosslinked decellularized tissue scaffolds and the crosslinking mechanism and their stability. (Reprinted with permission from Ma Bing et al. Journal of East China Normal University 2013;5:61–79. Ref. [62]. L. He et al. International Journal of Biological Macromolecules 2011;48:354–359. W.Y. Zhai et al. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014;102:1190–8.)
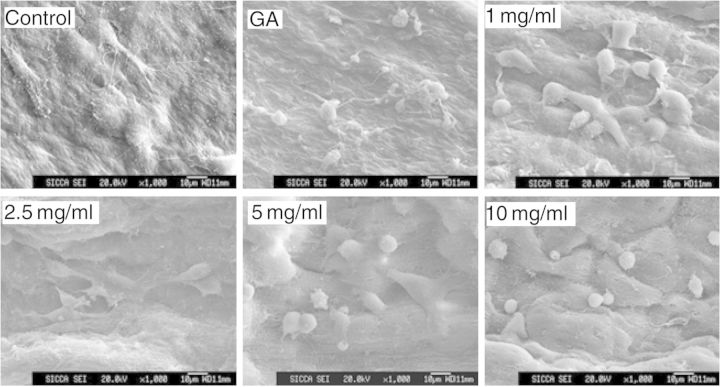
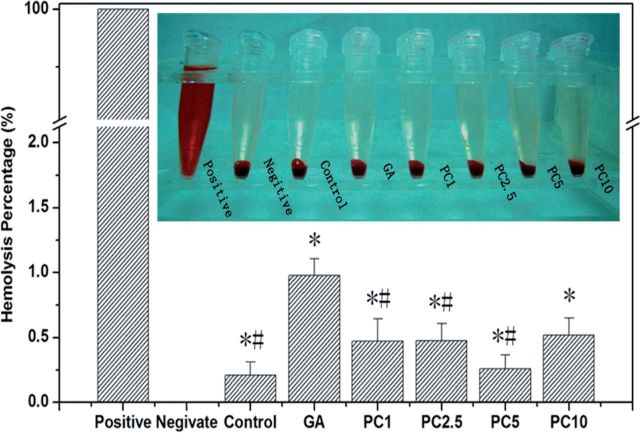
PC can crosslink the decellularized ECM to produce soft matrixes and the stability, mechanical properties and in vitro enzyme degradation resistance are significantly enhanced (Fig. 4C and D). In addition, PC has no inhibition for the cell proliferation (Fig. 5), and PC-crosslinked scaffold materials have good biocompatibility (Fig. 6) and hemolysis (Fig. 7) [52, 62].
Figure 5.
The proliferation effect of PC on bovine aortic heart valve interstitial cells (HVICs), HUVECs and A549 cells. IR is 1.5 µg/ml irinotecan. (Reprinted with permission from Refs. [59, 62].)
Figure 6.
SEM images of the biocompatibility of PC-crosslinked decellularized arotic scaffold materials seeding with HUVECs. (Reprinted with permission from W.Y. Zhai et al. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014;102:1190–8.)
Figure 7.
The hemolysis and hemolytic rate of the PC-crosslinked decellularized bovine pericardium ECM. (Reprinted with permission from Ma Bing et al. Journal of East China Normal University 2013;5:61–79.)
Anti-calcification effect of PC
Calcification in human valves occurs commonly, and is enhanced in damaged valves with congenital anomalies or rheumatic valvulitis [65]. The calcification mechanism is incompletely understood and some hypothesis theories have been proposed. One of the main roles that have been implicated in calcification is organic matrix compositions, including collagen, elastin and other noncollagenous proteins [66–68]. In recent years, researchers have found that elastin, an important component of cardiovascular prostheses, is one of the main causes of calcification, which plays a critical role in the long-term implantation of the prostheses [69]. Calcification that occurs in arteries includes intimal calcification mainly associated with cells and collagen and medial calcification associated with elastin [69, 70]. Although GA could adequately crosslink the collagen component to resist collagenase, it is unable to protect elastin against enzymatic degeneration [50], and the degeneration of elastin may lead to the loss of elastic recoil and calcification [30]. PC can specifically bind to the hydrophobic regions in proline-rich collagen and elastin, form multiple hydrogen bonds and effectively protect elastin from enzyme degradation and inhibit the elastin-associated calcification (Fig. 8) [71].
Figure 8.
Mineralization of decellularized porcine aortic valves soaked in SBF. (A) Decellularized valve matrix before soaking, (B) decellularized valves after soaking for 20 days. (C) Glutaraldehyde-crosslinked decellularized valves after soaking for 20 days. (D) PC-crosslinked decellularized valves after soaking for 20 days. (Reprinted with permission from Ref. [52].)
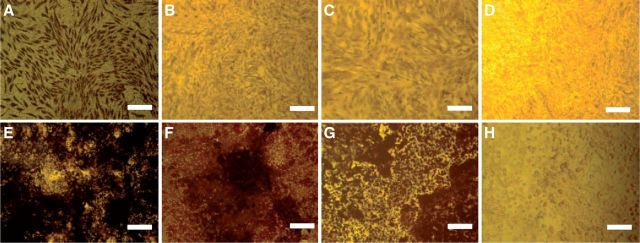
Cell injury also plays an important role in calcification, which may increase Ca2+ and cytosolic phosphate in cells [72]. Studies have shown that bone-marrow-derived mesenchymal stem cells used in tissue engineering and cardiovascular-derived cells, such as valvular interstitial cells (VICs) and vascular smooth muscle cells (VSMCs), could differentiate into osteoblast-like cells which express bone formation biomarkers such as alkaline phosphatase (ALPase) and osteocalcine [73, 74]. In our study, we have proved that PC could inhibit ALPase (Fig. 9) activity and mineral deposition (Fig. 10) of VICs and MSCs. It also can decrease the content of Ca2+ and in cells. It is shown that PC-crosslinked decellularized materials can block calcium phosphate nucleation and suppress mineral deposition, and effectively inhibit calcification [62].
Figure 9.
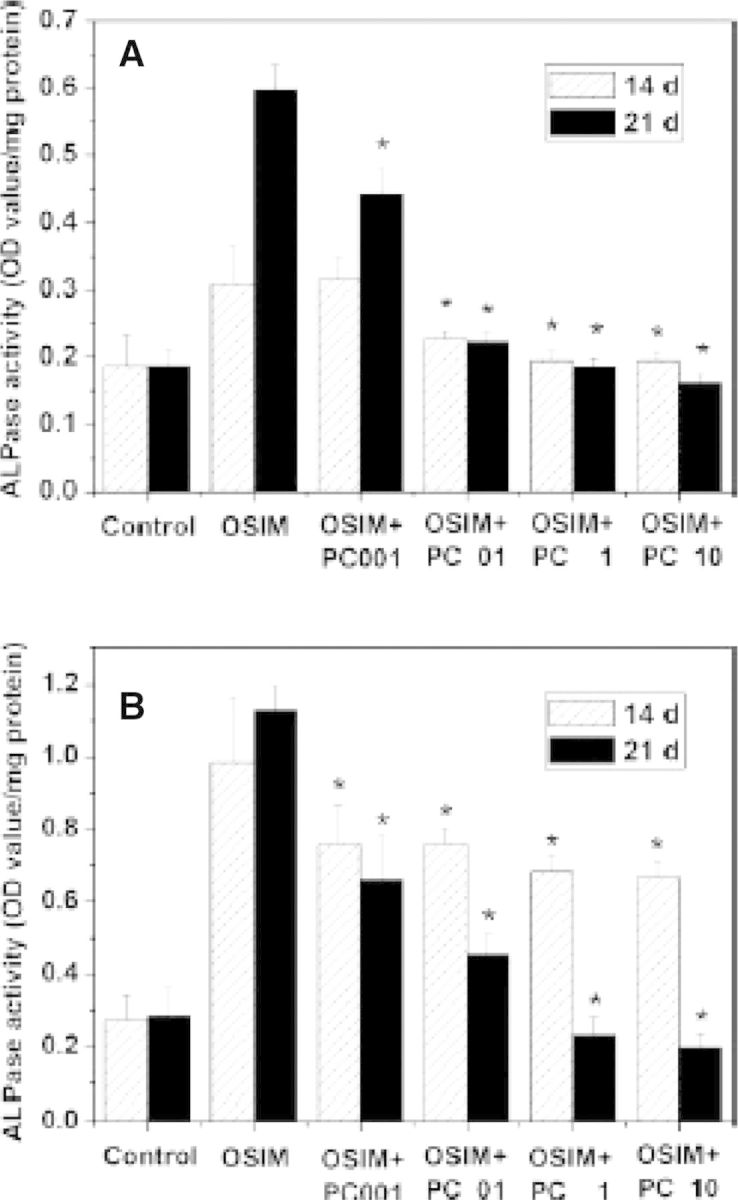
Inhibition effect of PC on ALPase activity of valvular-related cells (n 5 4). (A) VICs; (B) MSCs. OSIM, osteosynthesis inducing medium; PC, procyanidins. PC001, PC01, PC1 and PC10 represent 0.01, 0.1, 1 and 10 µg/ml PC, respectively. *P < 0.01 compared with OSIM group. (Reprinted with permission from Ref. [52].)
Figure 10.
The von Kossa staining of VICs (A–D) and MSCs (E–H) after osteoinduction (n = 4). (A) OSIM; (B) OSIM + 1 µg/ml PC; (C) OSIM + 10 µg/ml PC; (D) control; (E) OSIM; (F) OSIM + 1 µg/ml PC; (G) OSIM + 10 µg/ml PC; (H) control. OSIM, osteosynthesis-inducing medium. Bars = 150 µm. (Reprinted with permission from Ref. [52].)
Conclusions and Future Perspective
In this review, we summarized the research progress of ECM-derived scaffolds and crosslinking strategies for preparation of cardiovascular scaffolds. ECM retains intact organized structure in favors of cell adhesion and proliferation, and they are the promising materials for fabricating cardiovascular scaffolds. Moreover, crosslinking is one of the most important strategies to improve the durability of ECM-derived scaffolds, prevent the degeneration of the materials and enhance the mechanical strength of the scaffolds. The most commonly studied crosslinking agents including chemical crosslinking agents and natural crosslinking agents have been reviewed. The chemical crosslinking agents, such as GA, EDC and pEPC, may have toxicity and result in immune response and calcification. Natural crosslinking agents, such as GP, NDGA, TA and PC, are obtained from plants and have no toxic effect on the human body. Furthermore, PC has the ability to completely inhibit calcification in vitro and in vivo, and shown promising potential for crosslinking the ECM-derived scaffolds due to its good biocompatibility and anti-calcification activity. Future studies should focus on the optimization of the PC crosslinking method, and detailed evaluation of the performance of PC-crosslinked ECM-derived scaffolds, such as the mechanical strength, long-term durability, degradation rate and biocompatibility, which need future experimental verification in vitro and in vivo, and further explore the mechanisms of crosslinking and anti-calcification effect of PC.
Acknowledgement
This work was supported by a grant from the National Natural Science Foundation (Grant No.: 31070870).
References
- 1.Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol 2003;41:893–904. [DOI] [PubMed] [Google Scholar]
- 2.Julie RF, Boris AN, Joseph PV. Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg 2001;72:577–91. [DOI] [PubMed] [Google Scholar]
- 3.Cannegieter SC, Rosendaal FR, Briet E. Thrombembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994;89:635–41. [DOI] [PubMed] [Google Scholar]
- 4.Ralf S, Simon PH, Jason SS, et al. Tissue engineering of heart valves: in vitro experiences. Ann Thorac Surg 2000;70:140–4. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Smith D, Novick W, et al. Long-term function of cryopreserved aortic homografts. J Thorac Cardiovasc Surg 1993;106:154–66. [PubMed] [Google Scholar]
- 6.Mitchell RN, Jonas RA, Schoen FJ. Pathology of explanted cryopreserved allograft heart valves: comparison with aortic valves from orthotopic heart transplants. J Thorac Cardiovasc Surg 1998;115:118–27. [DOI] [PubMed] [Google Scholar]
- 7.Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 2003;23:1002–6. [DOI] [PubMed] [Google Scholar]
- 8.Karen M, Frederick JS. Heart valve tissue engineering concepts, approaches, progress, and challenges. Ann Biomed Eng 2006;34:1799–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberto JM, Joshua MU, Shawn MG, et al. Tissue-engineered vascular grafts: autologous off-the-shelf vascular access? Semin Nephrol 2012;32:582–91. [DOI] [PubMed] [Google Scholar]
- 10.Avione YL, Nathan M, Cameron B, et al. Regenerative implants for cardiovascular tissue engineering. Transl Res 2014;163:3221–41. [DOI] [PubMed] [Google Scholar]
- 11.Thomas LV, Lekshmi V, Nair PD. Tissue engineered vascular grafts—preclinical aspects. Int J Cardiol 2013;167:1091–100. [DOI] [PubMed] [Google Scholar]
- 12.Swathi R, Elliot LC. Biomaterials for vascular tissue engineering. Regen Med 2010;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 2005;79:1072–80. [DOI] [PubMed] [Google Scholar]
- 14.Dahl SL, Koh J, Prabhakar V, et al. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant 2003;12:659–66. [PubMed] [Google Scholar]
- 15.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000;21:2215–31. [DOI] [PubMed] [Google Scholar]
- 16.Courtman DW, Errett BF, Wilson GJ. The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices. J Biomed Mater Res 2001;55:576–86. [DOI] [PubMed] [Google Scholar]
- 17.Sung HW, Hsu CS, Lee YS. Physical properties of a porcine internal thoracic artery fixed with an epoxy compound. Biomaterials 1996;17:2357–65. [DOI] [PubMed] [Google Scholar]
- 18.Sung HW, Hsu HL, Shih CC, et al. Cross-linking characteristics of biological tissues fixed with monofunctional or multifunctional epoxy compounds. Biomaterials 1996;17:1405–10. [DOI] [PubMed] [Google Scholar]
- 19.Jorge-Herrero E, Fernández P, Turnay J, et al. Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials 1999;20:539–45. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Xu SY. EDC/NHS-crosslinked type II collagen-chondroitin sulfate scaffold: characterization and in vitro evaluation. J Mater Sci Mater Med 2008;19:567–75. [DOI] [PubMed] [Google Scholar]
- 21.Olde Damink LH, Dijkstra PJ, van Luyn MJ, et al. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials 1996;17:679–84. [DOI] [PubMed] [Google Scholar]
- 22.Norbert WG, Inka J, Hanngörg Z, et al. Detoxification and endothelialization of glutaraldehyde-fixed bovine pericardium with titanium coating a new technology for cardiovascular tissue engineering. Circulation 2009;119:1653–60. [DOI] [PubMed] [Google Scholar]
- 23.Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 1996;17:471–84. [DOI] [PubMed] [Google Scholar]
- 24.Sang SK, Sang HL, Seung WC, et al. Tissue engineering of heart valves by recellularization of glutaraldehyde-fixed porcine valves using bone marrow-derived cells. Exp Mol Med 2006;38:273–83. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Schoen FJ, Levy RJ. Mechanism of efficacy of 2-amino oleic acid for inhibition of calcification of glutaraldehyde-pretreated porcine bioprosthetic heart valves. Circulation 1994;90:323–9. [DOI] [PubMed] [Google Scholar]
- 26.Jorge-Herrero E, Ferna´ndez P, Escudero C, et al. Calcification of pericardial tissue pretreated with different amino acids. Biomaterials 1996;17:571–5. [DOI] [PubMed] [Google Scholar]
- 27.Cheul L, Soo HK, Seung HC, et al. High-concentration glutaraldehyde fixation of bovine pericardium in organic solvent and post-fixation glycine treatment: in vitro material assessment and in vivo anticalcification effect. Eur J Cardio Thorac Surg 2011;39:381–7. [DOI] [PubMed] [Google Scholar]
- 28.Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation 1997;95:479–88. [DOI] [PubMed] [Google Scholar]
- 29.Pathak CP, Adams AK, Simpson T, et al. Treatment of bioprosthetic heart valve tissue with long chain alcohol solution to lower calcification potential. J Biomed Mater Res A 2004;69:140–4. [DOI] [PubMed] [Google Scholar]
- 30.Vyavahare NR, Ogle M, Schoen FJ, et al. Elastin calcification and its prevention with aluminum chloride pretreatment. Am J Pathol 1999;155:973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung HW, Chang WH, Ma CY, et al. Crosslinking of biological tissues using genipin and carbodiimide. J Biomed Mater Res A 2003;64:427–38. [DOI] [PubMed] [Google Scholar]
- 32.Olde Damink LH, Dijkstra PJ, van Luyn MJ, et al. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996;17:765–73. [DOI] [PubMed] [Google Scholar]
- 33.Sung HW, Chang Y, Chiu CT, et al. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res 1999;47:116–26. [DOI] [PubMed] [Google Scholar]
- 34.Van Wachem PB, Brouwer LA, Zeeman R, et al. In vivo behavior of epoxy-crosslinked porcine heart valve cusps and walls. J Biomed Mater Res 2000;53:18–27. [DOI] [PubMed] [Google Scholar]
- 35.Chiono V, Pulieri E, Vozzi G, et al. Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J Mater Sci Mater Med 2008;19:889–98. [DOI] [PubMed] [Google Scholar]
- 36.Sung HW, Liang IL, Chen CN, et al. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J Biomed Mater Res 2001;55:538–46. [DOI] [PubMed] [Google Scholar]
- 37.Liang HC, Chang WH, Lin KJ, et al. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A 2003;65:271–82. [DOI] [PubMed] [Google Scholar]
- 38.Chang Y, Tsai CC, Liang HC, et al. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials 2002;23:2447–57. [DOI] [PubMed] [Google Scholar]
- 39.Sung HW, Huang RN, Huang LL, et al. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res 1998;42:560–7. [DOI] [PubMed] [Google Scholar]
- 40.Koob TJ, Hernandez DJ. Mechanical and thermal properties of novel polymerized NDGA gelatin hydrogels. Biomaterials 2003;24:1285–92. [DOI] [PubMed] [Google Scholar]
- 41.Koob TJ, Willis TA, Hernandez DJ. Biocompatibility of NDGA-polymerized collagen fibers. I. Evaluation of cytotoxicity with tendon fibroblasts in vitro. J Biomed Mater Res 2001;56:31–9. [DOI] [PubMed] [Google Scholar]
- 42.Fujimoto N, Kohta R, Kitamura S, et al. Estrogenic activity of an antioxidant, nordihydro-guaiaretic acid (NDGA). Life Sci 2004;74:1417–25. [DOI] [PubMed] [Google Scholar]
- 43.Ju YM, Yu B, Koob TJ, et al. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J Biomed Mater Res A 2008;87:136–46. [DOI] [PubMed] [Google Scholar]
- 44.Rivero S, Garcia MA, Pinotti A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr polym 2010;82:270–6. [Google Scholar]
- 45.Santos BC, Scalbert A. Proanthocyanidins and tannin-like compounds: nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric 2000;80:1094–117. [Google Scholar]
- 46.Serrano J, Pimi RP, Dauer A, et al. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 2009;53(Suppl. 2):S310–29. [DOI] [PubMed] [Google Scholar]
- 47.Barbehenn RV, Peter Constabel C. Tannins in plant–herbivore interactions. Phytochemistry 2011;72:1551–65. [DOI] [PubMed] [Google Scholar]
- 48.Frazier RA, Deaville ER, Green RJ, et al. Interactions of tea tannins and condensed tannins with proteins. J Pharm Biomed Anal 2010;51:490–5. [DOI] [PubMed] [Google Scholar]
- 49.Isenburg JC, Karamchandani NV, Simionescu DT, et al. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006;27:3645–51. [DOI] [PubMed] [Google Scholar]
- 50.Isenburg JC, Simionescu DT, Vyavahare NR. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials 2005;26:1237–45. [DOI] [PubMed] [Google Scholar]
- 51.Guyot S, Marnet N, Sanoner P, et al. Variability of the polyphenolic composition of cider apple (Malus domestica) fruits and juices. J Agric Food Chem 2003;51:6240–7. [DOI] [PubMed] [Google Scholar]
- 52.Zhai W, Chang J, Lü X, et al. Procyanidins cross linked heart valve matrix: anticalcification effect. J Biomed Mater Res B 2009;90:913–21. [DOI] [PubMed] [Google Scholar]
- 53.Schoen FJ. Heart valve tissue engineering-quo vadi? Curr Opin Biotechnol 2011;22:698–705. [DOI] [PubMed] [Google Scholar]
- 54.Han B, Nimni ME. Transdermal delivery of amino acids and antioxidants enhance collagen synthesis: in vivo and in vitro studies. Connect Tissue Res 2005;46:251–7. [DOI] [PubMed] [Google Scholar]
- 55.Keqin Ou, Liwei Gu. Absorption and metabolism of proanthocyanidins. J Funct Foods 2014;7:43–53. [Google Scholar]
- 56.Debasis B, Chandan KS, Sidhartha DR, et al. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res 2003;523–524:87–97. [DOI] [PubMed] [Google Scholar]
- 57.Strek M, Gorlach S, Podsedek A, et al. Procyanidin oligomers from Japanese quince (Chaenomeles japonica) fruit inhibit activity of MMP-2 and MMP-9 metalloproteinases. J Agric Food Chem 2007;55:6447–52. [DOI] [PubMed] [Google Scholar]
- 58.Nomoto H, Iigo M, Hamada H, et al. Chemoprevention of colorectal cancer by grape seed proanthocyanidin is accompanied by a decrease in proliferation and increase in apoptosis. Nutr Cancer 2004;49:81–8. [DOI] [PubMed] [Google Scholar]
- 59.Wanyin Z, Chunping J, Hui Z, et al. Procyanidins inhibit tumor angiogenesis by crosslinking extracellular matrix. Chin J Cancer Res 2011;23:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teissedre PL, Frankel EN, Waterhouse AL, et al. Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wines. J Sci Food Agric 1996;70:55–61. [Google Scholar]
- 61.Singh RP, Tyagi AK, Dhanalakshmi S, et al. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer 2004;108:733–40. [DOI] [PubMed] [Google Scholar]
- 62.Zhai W, Chang J, Lin K, et al. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials 2006;27:3684–90. [DOI] [PubMed] [Google Scholar]
- 63.Han B, Jaurequi J, Tang WB, et al. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrics. J Biomed Mater Res A 2003;65:118–24. [DOI] [PubMed] [Google Scholar]
- 64.Hagerman AE, Butler LG. The specificity of proanthocyanidin–protein interactions. J Biol Chem 1981;256:4494–7. [PubMed] [Google Scholar]
- 65.Kookmin MK, Guillermo AH, Harold DB. Role of glutaraldehyde in calcification of porcine aortic valve fibroblasts. Am J Pathol 1999;154:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fartasch M, Haneke E, Hornstein OP. Mineralization of collagen and elastic fibers in superficial dystrophic cutaneous calcification: an ultrastructural study. Dermatologica 1990;181:187–92. [DOI] [PubMed] [Google Scholar]
- 67.Gura TA, Wright KL, Veis A, et al. Identification of specific calcium-binding non-collagenous proteins associated with glutaraldehyde-preserved bovine pericardium in the rat subdermal model. J Biomed Mater Res 1997;35:483–95. [DOI] [PubMed] [Google Scholar]
- 68.Handford PA. Fibrillin-1, a calcium binding protein of extracellular matrix. Biochim Biophys Acta 2000;1498:84–90. [DOI] [PubMed] [Google Scholar]
- 69.Paule WJ, Bernick S, Strates B, et al. Calcification of implanted vascular tissues associated with elastin in an experimental animal model. J Biomed Mater Res 1992;26:1169–77. [DOI] [PubMed] [Google Scholar]
- 70.Bobryshev YV, Lord RS, Warren BA. Calcified deposit formation in intimal thickenings of the human aorta. Atherosclerosis 1995;118:9–21. [DOI] [PubMed] [Google Scholar]
- 71.Jason CI, Dan TS, Naren RV. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials 2004;25:3293–302. [DOI] [PubMed] [Google Scholar]
- 72.Kim KM. Apoptosis and calcification. Scanning Microsc 1995;9:1137–78. [PubMed] [Google Scholar]
- 73.Rajamannan NM, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003;107:2181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajamannan NM, Nealis TB, Subramaniam M, et al. Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation 2005;111:3296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]