Abstract
Background
Pilocytic astrocytoma is a rare tumor in adults. This report is of a prospective clinical trial with long-term follow-up.
Methods
Between 1986 and 1994, 20 eligible adults with supratentorial pilocytic astrocytomas were enrolled in a prospective intergroup trial of radiotherapy (RT) after biopsy (3 patients) or observation after gross (11 patients) or subtotal (6 patients) resection.
Results
At the time of analysis (median follow-up, 20.8 years), 2 patients (10%) have died and 18 patients (90%) are alive. Neurologic and cognitive function were stable or improved over time for the majority of patients. No toxic effects of treatment or malignant transformations have been recorded at last follow-up. For the entire cohort the 20-year time to progression and overall survival rates are 95% and 90% respectively. The cause of death (2.2 and 16.1 years after enrollment) in both patients was unrelated to tumor although both were biopsy-only patients. One subtotally resected tumor progressed 1 month after enrollment requiring P32 injection into an enlarging cyst. Because of further progression this patient required RT 18 months later. This patient is alive without evidence of progression 18 years after RT.
Conclusion
The long-term follow-up results of this prospective trial confirm that adults with pilocytic astrocytomas have a favorable prognosis with regard to survival and neurologic function. Close observation is recommended for adults with pilocytic astrocytomas, reserving RT for salvage, as the majority remain stable after gross or subtotal resection and no adjuvant therapy.
Keywords: pilocytic astrocytoma, prospective, radiotherapy, resection
Pilocytic astrocytomas, World Health Organization grade I tumors,1 are well-circumscribed, enhancing lesions typically located in the cerebellum of children. In children, surgical resection alone results in a favorable outcome, with a 10-year cancer-specific survival >95%.2 Although pilocytic astrocytoma is the most common glioma in children,3 it is very uncommon in adults. Due to the rarity of these tumors in adults little is known regarding the natural history of these tumors or the effect of treatment in adult patients. In 2004 we reported initial results of a prospective intergroup trial that enrolled adults with supratentorial pilocytic astrocytomas, and to our knowledge this is the only published trial composed solely of adult patients with pilocytic astrocytomas.4 With a doubling of follow-up (from 10 years to 20.8 years) since the initial publication, we report the long-term results of this prospective trial.
Materials and Methods
In 1986, the North Central Cancer Treatment Group (NCCTG, now the Alliance for Clinical Trials in Oncology) opened prospective clinical trial 86-72-51 of observation after a subtotal or gross total resection for adults with supratentorial pilocytic astrocytomas. In 1991, the trial became an intergroup study when the Radiation Therapy Oncology Group (RTOG) joined the trial, and a new goal was added to investigate the effects of radiation therapy (RT) in adults with unresected pilocytic astrocytomas (biopsy only). The trial was closed after the accrual goal was met for the separate low-grade glioma study arms of the trial in 1994.5
Patients
Adult patients (age >18 years) with completely or incompletely resected supratentorial pilocytic astrocytomas were enrolled. All patients provided informed consent before enrolling in the trial. The institutional review boards for all sites that participated in the trial approved the study. Central pathologic review was performed by the trial neuropathologist (Bernd W. Scheithauer, MD, Mayo Clinic). Clinical and outcome data were collected prospectively by the NCCTG (Alliance) Statistics and Data Center, according to the protocol specifications.4 Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Treatment
After subtotal or gross total resection, patients were registered and observed. Patients with radiographic evidence of progression were treated off protocol. Patients in whom only biopsy had been performed received RT with localized radiation fields that included the tumor volume and edema (as defined by MRI or CT) with a 2-cm margin, treated to a total dose of 50.4 Gy in 28 fractions. After RT completion, each patient's RT chart, simulator and port films, RT plan, and preoperative imaging studies were reviewed for quality assurance by one of the authors (E.G.S.).
Evaluation
Patients underwent physical examination at study entry (baseline), every 4 months for 2 years, every 6 months for 3 years, and annually until year 25. The examination included a neurologic examination, determination of the neurologic function score (NFS; Table 1), a Folstein Mini-Mental State Examination (MMSE),6 assessment of toxicity in patients who received RT, and CT or MRI. Progression was defined on imaging if an increase in tumor size was plainly apparent or the tumor size had increased by at least 25% as determined by the product of the perpendicular diameters. A change of more than three MMSE points was considered clinically significant.7
Table 1.
Neurologic function scores and definitions
| Score | Definition |
|---|---|
| 0 | No neurologic symptoms; fully active at home/work without assistance |
| 1 | Minor neurologic symptoms; fully active at home/work without assistance |
| 2 | Moderate neurologic symptoms; fully active at home/work but requires assistance |
| 3 | Moderate neurologic symptoms; less than fully active at home/work and requires assistance |
| 4 | Severe neurologic symptoms; totally inactive requiring complete assistance at home or in an institution; unable to work |
Statistical Analysis
Date of data lock for this manuscript was October 21, 2014. Frequency distributions and summary statistics were calculated for all clinical and histologic variables. The time-to-event distributions (eg, overall survival, time to progression, and progression-free survival) and confidence intervals were estimated using Kaplan-Meier curves.8 Statistical analyses were conducted by the Alliance Statistics and Data Center.
Results
Patients and Clinical Features
Twenty-one patients (18 resected, 3 biopsied) were accrued between 1986 and 1994. One resected patient was found to be ineligible after additional pathologic review, and the patient's diagnosis was changed to ganglioglioma (the patient was alive without evidence of disease 11.1 years after gross total resection). Therefore, data on 20 eligible patients are available for analysis with a median follow-up of 20.8 years for the living patients (range, 17.1–27.0 years). Patient characteristics are listed in Table 2. The median age at enrollment was 32 years (range, 20–47 years) and the majority of patients (55%) underwent gross total resection. The tumors in the three patients who only underwent biopsy were located in the pineal region, left frontal lobe, and left thalamus.
Table 2.
Patient characteristics at study entry
| Characteristics | Patient Group |
||
|---|---|---|---|
| Observation (n = 17) | Radiation (n = 3) | Total (n = 20) | |
| Age (y) | |||
| <40 | 12 (71) | 2 (67) | 14 (70) |
| ≥40 | 5 (29) | 1 (33) | 6 (30) |
| Sex | |||
| Male | 6 (35) | 1 (33) | 7 (35) |
| Female | 11 (65) | 2 (67) | 13 (65) |
| NFS | |||
| 0 | 6 (35) | 1 (33) | 7 (35) |
| 1 | 6 (35) | 1 (33) | 7 (35) |
| 2 | 0 (0) | 1 (33) | 1 (5) |
| Unknown | 5 (29) | 0 (0) | 5 (25) |
| MMSE score | |||
| 0–26 | 1 (6) | 0 (0) | 1 (5) |
| 27–30 | 9 (53) | 3 (100) | 12 (60) |
| Unknown | 7 (41) | 0 (0) | 7 (35) |
| Preoperative tumor diameter (cm) | |||
| <5 | 16 (94) | 2 (67) | 18 (90) |
| ≥5 | 1 (6) | 1 (33) | 2 (10) |
| Tumor location | |||
| Temporal lobe | 8 (47) | 0 (0) | 8 (40) |
| Other site | 9 (53) | 3 (100) | 12 (60) |
| Extent of operation | |||
| Gross total resection | 11 (65) | 0 (0) | 11 (55) |
| Subtotal resection | 6 (35) | 0 (0) | 6 (30) |
| Biopsy | 0 (0) | 3 (100) | 3 (15) |
NFS, neurologic function score; MMSE, Mini-Mental Status Examination. Numbers in parentheses are percentages.
Overall Survival, Time to Progression, and Progression-Free Survival
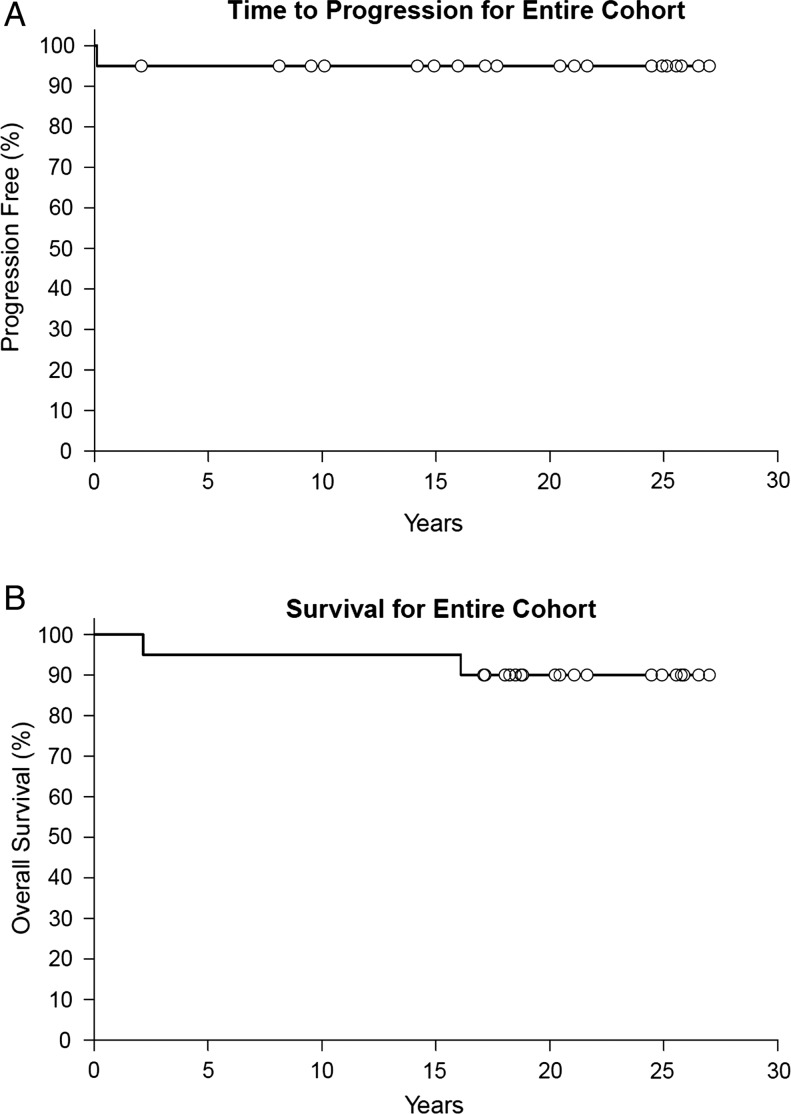
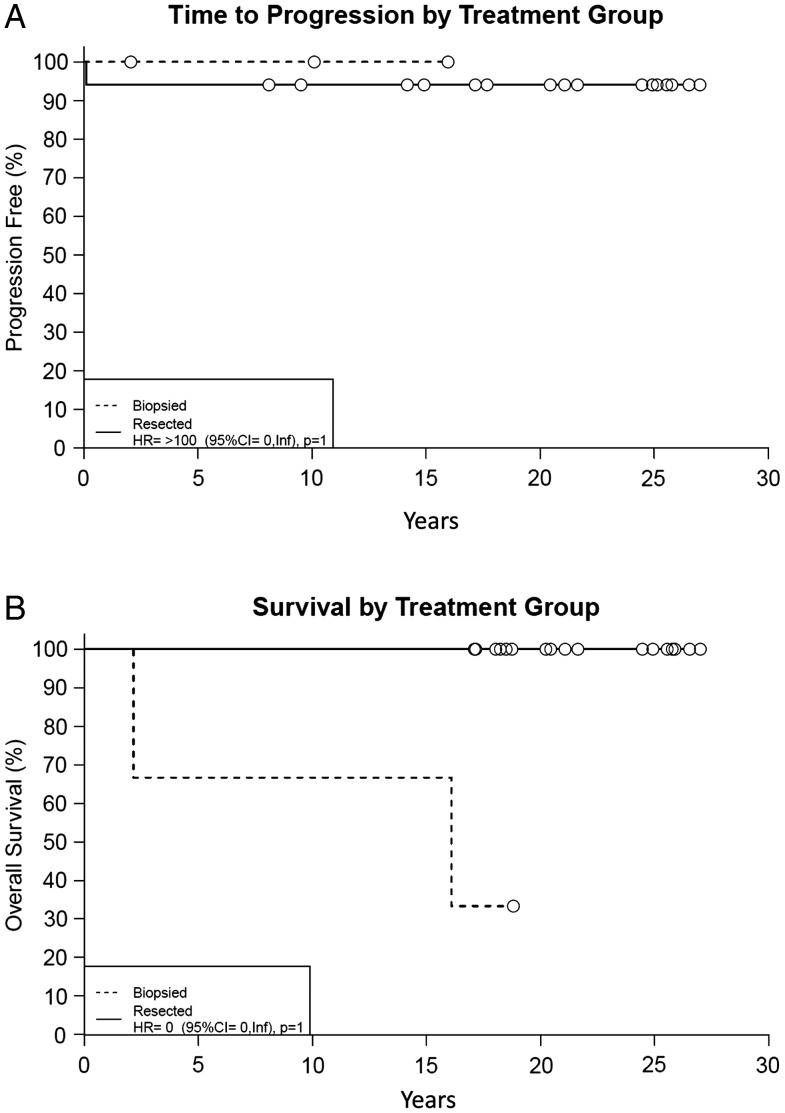
Table 3 lists the key characteristics and outcome data for all 20 patients. Time to progression, overall survival, and progression-free survival for the entire cohort, by treatment group, and extent of resection are outlined in Table 4 and Figs 1 and 2. One biopsied patient (Patient 17) died ∼2 years after completing RT. The cause of death is unknown, with no evidence of progression at evaluation 1 month before the patient's death. Another biopsied patient (Patient 20) died due to respiratory failure 16.1 years after enrollment with no evidence of progression prior to death. One resected patient (Patient 9) had disease progression after minimal subtotal resection (ie, <25% of tumor resected). One month after surgery the patient had growth of a cyst and was treated with P32, a radioactive isotope. Approximately 18 months later, the patient had clinical and radiographic evidence of progression. The patient was subsequently treated with 55.8 Gy of RT and has no evidence of progression 18 years later. The remaining 17 patients (85%) are alive at last follow-up with no evidence of progression.
Table 3.
Treatment and patient outcome
| Patient No. | Age, y | Sex | Tumor Location | Extent of Resection | Radiation Dose (Gy) | Tumor Progression, y | Survival Status, ya |
|---|---|---|---|---|---|---|---|
| 1 | 20 | M | R Temporal | GTR(Lob) | None | No, 20.4 | Alive, 20.4 |
| 2 | 47 | F | Third Ventricle | GTR | None | No, 27.0 | Alive, 27.0 |
| 3 | 32 | M | L Temp/Occ | GTR | None | No, 26.5 | Alive, 26.5 |
| 4 | 21 | F | L Temporal | GTR | None | No, 25.8 | Alive, 25.8 |
| 5 | 46 | M | L Front/Temp | GTR | None | No, 25.1 | Alive, 25.9 |
| 6 | 21 | F | L Thalamus | GTR | None | No, 25.6 | Alive, 25.6 |
| 7 | 40 | F | L Temporal | GTR(Lob) | None | No, 24.5 | Alive, 24.5 |
| 8 | 22 | M | L Frontal | STR | None | No, 24.9 | Alive, 24.9 |
| 9 | 40 | F | R Thalamus | STR | None | Yes, 0.1 | Alive, 20.2 |
| 10 | 35 | F | L Occipital | GTR(Lob) | None | No, 21.6 | Alive, 21.6 |
| 11 | 31 | F | L Frontal | Bx | 50.4 Gy | No, 10.1 | Alive, 18.8 |
| 12 | 22 | M | R Temporal | GTR | None | No, 21.1 | Alive, 21.1 |
| 13 | 41 | F | R Frontal | GTR | None | No, 8.1 | Alive, 18.7 |
| 14 | 37 | F | L Thalamus | STR | None | No, 17.7 | Alive, 18.5 |
| 15 | 32 | F | R Temporal | GTR | None | No, 14.2 | Alive, 18.2 |
| 16 | 31 | F | R Parietal | STR | None | No, 14.9 | Alive, 18.3 |
| 17 | 21 | F | L Thalamus | Bx | 50.4 Gy | No, 2.1 | Dead, 2.2 |
| 18 | 37 | M | L Brainstem | STR | None | No, 17.1 | Alive, 17.1 |
| 19 | 32 | F | L Front/Temp | STR | None | No, 9.5 | Alive, 17.1 |
| 20 | 41 | M | Third Ventricle/Pineal | Bx | 50.4 Gy | No, 15.9 | Dead, 16.1 |
Patient No., patient number; M, male; R, right; GTR, gross total resection; lob, lobectomy performed at operation; F, female; L, left; STR, subtotal resection; Bx, biopsy.
aYears since study entry.
Table 4.
Progression-free, overall survival, and progression-free survival rates
| Treatment | Progression-free Rates, % (95% CI) |
Overall Survival Rates, % (95% CI) |
Progression-free Survival Rates, % (95% CI) |
|||
|---|---|---|---|---|---|---|
| At 5, 10, 15 Years | At 20 Years | At 5, 10, 15 Years | At 20 Years | At 5, 10, 15 Years | At 20 Years | |
| All (20 pts) | 95 (86–100) | 95 (86–100) | 95 (86–100) | 90 (78–100) | 90 (78–100) | 83 (67–100) |
| Resected (17 pts) | 94 (84–100) | 94 (84–100) | 100 | 100 | 94 (84–100) | 94 (84–100) |
| GTR (11 pts) | 100 | 100 | 100 | 100 | 100 | 100 |
| STR (6 pts) | 83 (58–100) | 83 (58–100) | 100 | 100 | 83 (58–100) | 83 (58–100) |
| Biopsy only (3 pts) | 100 | NA | 67 (30–100) | NA | 67 (30–100) | NA |
CI, confidence interval; pts, patients; GTR, gross total resection; STR, subtotal resection; NA, Not Available.
Fig. 1.
Time to progression (A) and survival (B) for entire cohort.
Fig. 2.
Time to progression (A) and survival (B) by treatment group. Biopsied patients (n = 3) received RT. Resected patients (n = 17) underwent either subtotal or gross total resection.
Mini-Mental State Examination
Thirteen patients (10 resected, 3 biopsied) underwent a baseline MMSE (Table 2) and the median score was 30 (range, 25–30). Thirteen patients (11 resected, 2 biopsied) underwent two or more MMSEs (median score, 8; range, 2–13), and the median interval between the earliest and latest MMSE was 8.1 years (range, 2.1–14.9 years). No clinically significant changes were seen in 10 of 11 resected patients; one resected patient (Patient 4) had a significant gain in score compared with baseline 6 months after enrollment, an improvement that was maintained 4.1 years later. Two biopsied patients had declines in at least one MMSE score. Patient 17 had a significant decrease in MMSE score 1 year after enrollment and the MMSE score did not return to baseline even by 2.1 years after enrollment (shortly before the patient's death). For Patient 20 only one score (1.9 years after enrollment) was significantly below baseline, and all other scores were close or equal to baseline out to 14.2 years after enrollment.
Neurologic Function Score
Fifteen patients (12 resected, 3 biopsied) had a baseline NFS (Table 2) and the median score was 1 (range, 0–2). NFS was obtained at two or more evaluations in 18 patients (15 resected, 3 biopsied); of these, 5 patients (3 resected, 2 biopsied) had worse NFS's while 13 had improved or stable NFS's at follow-up. The NFS of Patient 9 changed from 1 to 2 at the time of progression. A biopsied patient (Patient 17) had an NFS of 2 a month before death. Excluding the 2 patients without serial NFS evaluations and the 3 patients with progression or who died, at the most recent evaluation (median interval between enrollment and most recent NFS evaluation 8.1 years; range, 1.3–16.3 years) the NFS was 0 (12 patients; 80%) or 1 (3 patients; 20%).
Toxicity
No toxicity had been reported in the RT (biopsied) arm at the latest follow-up examination.
Discussion
With a median follow-up of over 20 years, the results of this prospective trial confirm that adults with pilocytic astrocytomas have a favorable prognosis. However, these favorable results contradict the results of some other adult series. A series of 44 adult patients from the University of Bonn reported 10-year progression-free survival and overall survival rates of 67% and 77%, respectively.9 Investigators from Princess Margaret Hospital identified 30 adult patients diagnosed from 1971–2007 and noted 10-year progression-free survival and overall survival rates of 35% and 85%, respectively.10 The improved outcome in this prospective trial compared with the two retrospective trials is likely due to the younger patient population enrolled in the prospective trial (with no patients over 47 years old) and the high rate of gross total resection. A review of adult pilocytic astrocytoma patients identified in the Surveillance, Epidemiology, and End Results (SEER) Program confirmed younger age and greater extent of resection to be positive prognostic factors.2
The two patients in the biopsy-only group had worse overall survival than the surgically resected group in this prospective trial, although neither patient died as a result of tumor progression. The biopsy-only patients were treated with RT, which has been associated with worse survival in adult patients with pilocytic astrocytomas, although this is likely due to patient selection.2 Other studies have shown that while adjuvant RT significantly improves tumor control it has no impact on survival (due to efficacy of salvage therapies).10
In the current prospective trial, a treatment paradigm of resection followed by close observation, reserving RT for salvage or for biopsy-only patients, resulted in good patient function over time. Neurologic function, prospectively measured and quantified with the NFS, was stable or improved for the majority of patients. For the vast majority of patients cognitive function as measured by the MMSE remained stable over time, recognizing more discriminating neurocognitive assessment tools may have identified more subtle cognitive changes.11
Gross total resection of pilocytic astrocytoma has been deemed by some to be curative, resulting in superior outcomes when compared with subtotal resection, and therefore complete resection is often strongly advocated in this patient population.12,13 In the current prospective trial, overall survival, time to progression, and progression-free survival outcomes after subtotal resection and gross total resection were similar. In contrast, retrospective studies have typically found better outcomes after gross total resection,9,12,13 although this finding has not been consistent for all series14 and all these retrospective studies are of similar or smaller size compared with the current prospective trial. A large review of adult pilocytic astrocytoma patients identified in the SEER Program found gross total resection to be strongly associated with better survival outcomes, recognizing that changes in the site-specific coding system concerning extent of resection made it difficult to differentiate extent of resection in some cases in the registry.2
To date, no cases of malignant transformation have occurred in our prospective trial, likely due to the relatively small size of the study population, as malignant transformation in adult patients is a rare event.15 The cause of malignant transformation remains unknown. Some have implicated RT as the primary cause of anaplastic change based on limited anecdotal evidence; however, it is important to note there are as many reports of spontaneous malignant transformation in adult patients9,15–19 as there are after RT.14 A large review of over 2000 adult and pediatric pilocytic astrocytomas identified 34 (1.7%) tumors with anaplastic features, the vast majority being in adult patients.20 Interestingly only a small minority (12%) had a history of prior RT.
Conclusion
With long-term follow-up the results of this prospective trial support the treatment paradigm of resection followed by observation for this patient population based on the favorable tumor control and survival. In addition, most patients maintained their neurologic function over time. Since so few patients on this trial received RT it is difficult to comment on the role of RT for adult patients with pilocytic astrocytoma although a reasonable approach is to reserve RT for salvage or for unresectable disease.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), CA25224, CA37404, CA15083, and CA35415 and the Linse Bock Foundation (to the legacy North Central Cancer Treatment Group and Mayo Clinic), and U10CA21661 to the legacy Radiation Therapy Oncology Group (now known as NRG Oncology).
Conflict of interest statement. None declared.
References
- 1. Louis DN, Ohgaki H, Wiestler OD et al. . The 2007 WHO classification of tumours of the central nervous system. [erratum appears in Acta Neuropathol. 2007 Nov;114(5):547] Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson DR, Brown PD, Galanis E, Hammack JE. Pilocytic astrocytoma survival in adults: analysis of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurooncol. 2012;108(1):187–193. [DOI] [PubMed] [Google Scholar]
- 3. Rosemberg S, Fujiwara D. Epidemiology of pediatric tumors of the nervous system according to the WHO 2000 classification: a report of 1,195 cases from a single institution. Childs Nervous System. 2005;21(11):940–944. [DOI] [PubMed] [Google Scholar]
- 4. Brown PD, Buckner JC, O'Fallon JR et al. . Adult patients with supratentorial pilocytic astrocytomas: a prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys. 2004;58(4):1153–1160. [DOI] [PubMed] [Google Scholar]
- 5. Shaw E, Arusell R, Scheithauer B et al. . Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20(9):2267–2276. [DOI] [PubMed] [Google Scholar]
- 6. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 7. Brown PD, Jensen AW, Felten SJ et al. . Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 8. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9. Stuer C, Vilz B, Majores M, Becker A, Schramm J, Simon M. Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer. 2007;110(12):2799–2808. [DOI] [PubMed] [Google Scholar]
- 10. Ishkanian A, Laperriere NJ, Xu W et al. . Upfront observation versus radiation for adult pilocytic astrocytoma. Cancer. 2011;117(17):4070–4079. [DOI] [PubMed] [Google Scholar]
- 11. Brown PD, Buckner JC, O'Fallon JR et al. . Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination examination. J Clin Oncol. 2003;21(13):2519–2524. [DOI] [PubMed] [Google Scholar]
- 12. Ye JM, Ye MJ, Kranz S, Lo P. A 10 year retrospective study of surgical outcomes of adult intracranial pilocytic astrocytoma. J Clin Neurosci. 2014;21(12):2160–2164. [DOI] [PubMed] [Google Scholar]
- 13. Wade A, Hayhurst C, Amato-Watkins A, Lammie A, Leach P. Cerebellar pilocytic astrocytoma in adults: a management paradigm for a rare tumour. Acta Neurochir (Wien). 2013;155(8):1431–1435. [DOI] [PubMed] [Google Scholar]
- 14. Ellis JA, Waziri A, Balmaceda C, Canoll P, Bruce JN, Sisti MB. Rapid recurrence and malignant transformation of pilocytic astrocytoma in adult patients. J Neurooncol. 2009;95(3):377–382. [DOI] [PubMed] [Google Scholar]
- 15. Tomlinson F, Scheithauer B, Hayostek C. The significance of atypia and histologic malignancy in pilocytic astrocytoma of the cerebellum. J Child Neurol. 1993;9:301–310. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki T, Saito R, Kumabe T et al. . Transformation of adult cerebellar pilocytic astrocytoma to glioblastoma. Brain Tumor Pathol. 2014;31(2):108–112. [DOI] [PubMed] [Google Scholar]
- 17. Claus D, Sieber E, Engelhardt A, Rechlin T, Neubauer U, Volk B. Ascending central nervous spreading of a spinal astrocytoma. J Neurooncol. 1995;25(3):245–250. [DOI] [PubMed] [Google Scholar]
- 18. Otero-Rodriguez A, Sarabia-Herrero R, Garcia-Tejeiro M, Zamora-Martinez T. Spontaneous malignant transformation of a supratentorial pilocytic astrocytoma. Neurocirugia (Astur). 2010;21(3):245–252. [DOI] [PubMed] [Google Scholar]
- 19. Steinberg GK, Shuer LM, Conley FK, Hanbery JW. Evolution and outcome in malignant astroglial neoplasms of the cerebellum. J Neurosurg. 1985;62(1):9–17. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez FJ, Scheithauer BW, Burger PC, Jenkins S, Giannini C. Anaplasia in pilocytic astrocytoma predicts aggressive behavior. Am J Surg Pathol. 2010;34(2):147–160. [DOI] [PubMed] [Google Scholar]