Abstract
Despite being perhaps the most studied form of aphasia, the critical lesion location for Broca's aphasia has long been debated, and in chronic patients, cortical damage often extends far beyond Broca's area. In a group of 70 patients, we examined brain damage associated with Broca's aphasia using voxel-wise lesion-symptom mapping (VLSM). We found that damage to the posterior portion of Broca's area, the pars opercularis, is associated with Broca's aphasia. However, several individuals with other aphasic patterns had considerable damage to pars opercularis, suggesting that involvement of this region is not sufficient to cause Broca's aphasia. When examining only individuals with pars opercularis damage, we found that patients with Broca's aphasia had greater damage in the left superior temporal gyrus (STG; roughly Wernicke's area) than those with other aphasia types. Using discriminant function analysis and logistic regression, based on proportional damage to the pars opercularis and Wernicke's area, to predict whether individuals had Broca's or another types of aphasia, over 95% were classified correctly. Our findings suggest that persons with Broca's aphasia have damage to both Broca's and Wernicke's areas, a conclusion that is incongruent with classical neuropsychology, which has rarely considered the effects of damage to both areas.
Keywords: Broca's aphasia, lesion-symptom mapping, pars opercularis, stroke, superior temporal gyrus
Introduction
Broca's aphasia is one of the most common and, perhaps, iconic types of aphasia. Individuals with Broca's aphasia typically have impaired speech production, relatively spared, though not necessarily normal, auditory comprehension, and, in most cases, agrammatism (Goodglass 1993). It is a form of aphasia that is easily distinguished by experienced clinicians from other types of aphasia such as conduction, global, or Wernicke's aphasia. Although the type of speech impairment that characterized Paul Broca's most famous case, Leborgne (1861) has been disputed (LaPointe 2012; Code 2013), it seems clear that he did not have what would be considered typical Broca's aphasia. Leborgne was almost completely mute and mostly spoke only one word, “tan,” the name by which he was later identified. Following the examination of several others whose damage included the left frontal lobe, Broca (1865) concluded that the third frontal convolution, an area commonly referred to today as Broca's area, is the seat of articulate language. This claim was disputed by many of Broca's contemporaries (e.g., Jackson 1866; Freud 1891; see Lorch 2008 for a more detailed discussion), but perhaps the most fervent challenge was posed by one of Broca's former interns, Pierre Marie. In a seminal paper that focused on a large number of case studies in aphasia, Marie (1906a) asserted that the third frontal convolution plays no role in language, a claim that shocked the neurological society establishment in France as well as abroad. Marie next reviewed Broca's writings on aphasia and challenged many of Broca's assumptions, especially in regard to the seat of articulate language (Marie 1906c). Marie suggested that aphasia required damage to Wernicke's area and that “Broca's aphasia” was a combination of aphasia, caused by damage to Wernicke's area, and anarthria, a motor speech problem caused by damage to what he referred to as the “lenticular zone,” a collection of subcortical structures such as the putamen and pallidum (Marie 1906a). Jules Dejerine, a prominent academic neurologist and a rival of Marie, defended Broca's original position, arguing that damage to Broca's area was necessary for Broca's aphasia, although he acknowledged the important contribution of other brain structures such as the insula, parietal regions, and underlying white matter (Dejerine 1906a,b, 1914, as discussed in Paciaroni and Bogousslavsky 2011; Krestel et al. 2013). However, Marie countered that Dejerine (and others) failed to observe a consistent relationship between damage to the third frontal convolution and Broca's aphasia: that is, some individuals presented with symptoms of Broca's aphasia and yet had the third frontal convolution completely intact, while other patients who had damage to the third frontal convolution did not have the speech symptoms of Broca's aphasia (Marie 1906b,d). The idea that Broca's aphasia could not be attributed to localized brain damage was later promoted by proponents of more holistic views of aphasia such as Goldstein (1910) and Head (1926).
A century after Broca's initial description of Leborgne, the localizationist view of aphasia was resurrected, primarily by Geschwind (1965, 1970). Geschwind asserted that Broca's aphasia is caused by damage to Broca's area. As before, this view was challenged on the grounds that (1) damage confined to Broca's area often does not lead to Broca's aphasia and (2) those with Broca's aphasia typically have damage that extends beyond Broca's area and involves structures such as the left insula and regions in the parietal lobe (Mohr et al. 1978). This observation was underscored by Dronkers et al. (2007) who used high-resolution MRI to re-examine Leborgne's brain and found that his cortical lesion extended far beyond inferior frontal gyrus and included structures such as the insula and the inferior parietal lobe. In some cases, the damage that causes Broca's aphasia actually spares Broca's area (e.g., Fridriksson et al. 2007). Although the debates about the region(s) causing Broca's aphasia have continued for well over a century, the current consensus is that the damage probably includes parts of Broca's area and some other adjacent structures (Dejerine 1906a,b; Geschwind 1970; Mohr et al. 1978; Lazar and Mohr 2011). It remains unknown, however, what exactly those other adjacent structures are.
Broca's aphasia has received disproportionate attention in the literature compared with other aphasia types over the past few decades, perhaps because individuals with Broca's aphasia have grammatical processing problems that manifest in both speech production and comprehension and display a distinctive non-fluent speech pattern. One reason for this interest may be based on the premise that grammatical problems displayed by such patients can tell us something about the neural architecture that supports syntax (Caramazza and Zurif 1976; Grodzinsky and Friederici 2006). However, since the lesion location that causes Broca's aphasia still eludes us, it is difficult to see what we can assume in this matter or, for example, if the symptoms of Broca's aphasia can be related to contemporary neuroanatomical models of speech processing (Hickok and Poeppel 2007; Hickok 2012). To identify the pattern of cortical damage that gives rise to Broca's aphasia, we examined a group of 70 participants of whom 20 had Broca's aphasia. Each of the 70 participants underwent clinical examination as well as high-resolution MRI. Voxel-wise lesion-symptom mapping (VLSM) was utilized to compare patients with Broca's aphasia with patients with aphasia of other kinds.
Methods
Participants
The study sample was selected from a database that includes 76 patients with chronic left hemispheric stroke. All patients were at least 6 months post-stroke and the mean time since stroke onset was 36.7 months (SD = 45.51; range = 6–276). Six persons with global aphasia were excluded from the study as these patients have severe speech and language deficits in both production and comprehension. Aphasia types were classified according to the Western Aphasia Battery (WAB; Kertesz 1982). Of the 70 participants whose data comprised the final study sample, 20 had Broca's aphasia, 29 anomic aphasia, 10 conduction aphasia, 6 Wernicke's aphasia, and 1 transcortical motor aphasia. An additional 4 participants were diagnosed by 2 speech–language pathologists as having apraxia of speech with mild anomia. The mean sample age was 61.04 (SD = 12.14; range = 36–83), and 30 were women. There was no significant difference between the individuals with Broca's aphasia and the rest of sample concerning time post-stroke, t = 0.93, P = 0.35. However, participants with Broca's aphasia (mean age = 55.7; SD = 10.76) tended to be younger than their counterparts with other types of aphasia (mean age = 63.2; SD = 12.1), t = 2.43, P = 0.02. Figure 1 shows lesion overlay maps for the individuals included in the final sample (N = 70). A lesion map that included all participants revealed the greatest lesion overlap in the white matter underlying the left superior, anterior insula (MNI: −30,2,18), where 48 participants had damage. The greatest lesion overlap for individuals with Broca's aphasia was in the anterior insula (MNI: −42,0,15) and in the white matter deep to the temporal–parietal junction for participants who had other types of aphasia (MNI: −38,−40,24).
Figure 1.
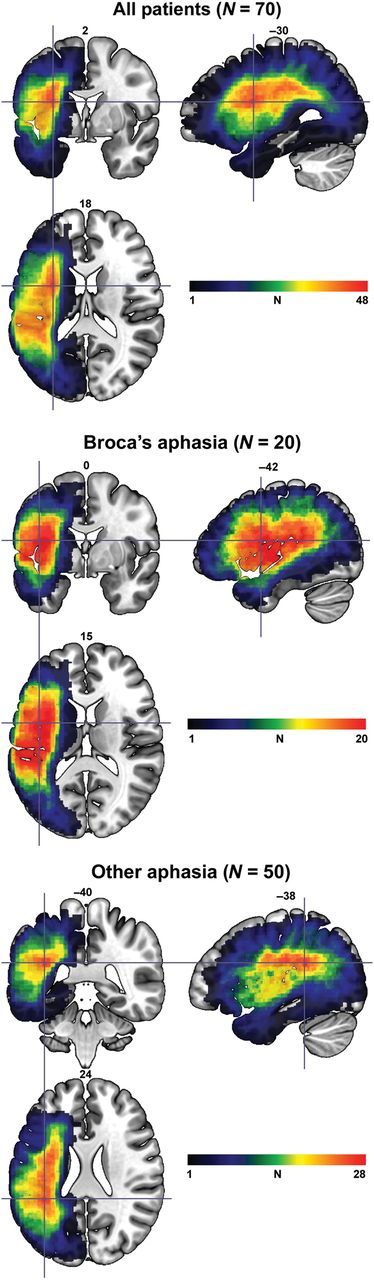
Lesion overlay maps for all 70 individuals included in the study (top panel), individuals with Broca's aphasia (middle panel), and individuals with forms other than Broca's aphasia (bottom panel). The crosshairs denote the voxel where the highest lesion overlap occurred. The color bars represent the degree of overlap in each lesion map. Note that the upper limits for the color bars are based on the maximum lesion overlap for each lesion overlay map. Slice numbers in standard space are denoted above each image.
Image Acquisition and Image Preprocessing
For the purpose of lesion detection and demarcation, each participant underwent MRI using a Siemens 3-T system equipped with a 12-element head-coil. The following imaging sequences were utilized: T2-MRI with a 3D SPACE (Sampling Perfection with Application optimized Contrasts by using different flip angle Evolutions) protocol, field of view (FOV) = 256 × 256 mm, 160 sagittal slices, variable flip angle, repetition time (TR) = 3200 ms, echo time (TE) = 352 ms, using the same slice positioning and angulation as the T1 sequence; T1-MRI using a magnetization prepared rapid gradient echo - turbo field echo sequence, FOV = 256 × 256 mm, 160 sagittal slices, a 9-degree flip angle, TR = 2250 ms, inversion time = 900 ms, TE = 4.2 ms. Using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/), lesions were demarcated on individually on T2-MRI, using the T1-MRI for guidance, in native space by the first author (J.F.) or post-doctoral fellows and graduate students who were closely supervised by him. We typically demarcate lesions on T2 images to appreciate the extent of gliosis, which is not visible on T1 images. The following data pipeline was used to transform the lesions into standard space: first, the T1-MRI images, T2-MRI images, and lesions were co-registered. Then, the T1-MRI images were segmented and normalized to the 2-mm3 ICBM standard brain template using cost function masking of the lesion with the following parameters: [2 2 2 4] Gaussians per class, 60 mm bias full-width at half maximum, very light regularization, 3 mm sampling distance, and trilinear interpolation. The lesions, as demarcated on the T2-MRI, were yoked into the standard space with the T1-MRI images.
VLSM Analyses
Lesion comparisons between participants with or without Broca's aphasia were implemented using non-parametric mapping (NPM; Rorden et al. 2007) with which independent group t-tests compared the presence or absence of a lesion in a given cortical area on a voxel-by-voxel basis between the 2 groups. Permutation thresholding with 1000 iterations was used to apply corrections for multiple comparisons (using family-wise error) in the whole-brain analyses. Importantly, only results that survived correction for multiple comparisons are presented herein. To verify the specific anatomical location of our results, MRIcron (www.mricro.com) was used to overlay regions of significance onto the Automatic Anatomical Labeling standard brain map (Tzourio-Mazoyer et al. 2002).
To further validate the NPM results, a leave-one-out cross-validation (LOOCV) method was used to classify individuals as having either Broca's or some other types of aphasia based on the extent of damage to the cortical areas identified in the VLSM analyses. For a sample size of 70, the LOOCV approach uses data from 69 participants to predict the aphasia type of the 70th participant. More specifically, 70 linear regression analyses were run where data from 69 participants were used to predict the aphasia type of the 70th participant. Once the analysis had been run 70 times, the average accuracy of the prediction is reported (i.e., how often the aphasia type of the 70th participant was predicted correctly). This analysis relied on the discriminant analysis function in SPSS, version 21 (IBM Corp., Armonk, NY, USA). In addition, a logistic regression approach was utilized to predict aphasia type.
Results
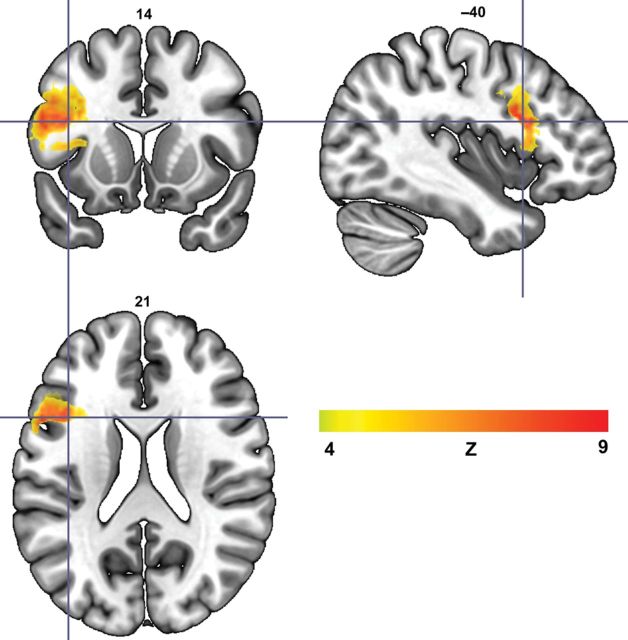
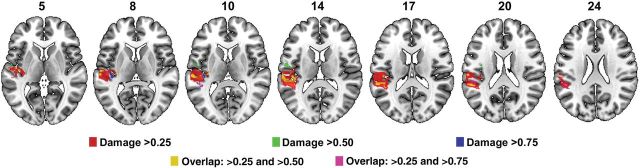
A whole-brain NPM analysis revealed that individuals with Broca's aphasia are more likely to have damage to the left inferior frontal gyrus compared with those who have other kinds of aphasia (Fig. 2). Specifically, most of the damage that predicts Broca's aphasia is located in the posterior portion of Broca's area, the pars opercularis. For the purpose of clarity, this area is henceforth referred to as the “Broca's area cluster” (BAC). The threshold for statistical significance was Z = 4.79 (corrected, P < 0.01) and the maximum voxel value was Z = 8.67. No statistically significant result was found where individuals with other types of aphasia were more likely to have damage than their Broca's aphasia counterparts. Although damage to the BAC was strongly related to having Broca's aphasia, several individuals who did not have Broca's aphasia had considerable damage to this region (4 individuals without Broca's aphasia had >75% damage; 6 had >50% damage, and 12 had at least 25% damage). Accordingly, damage to the BAC is a strong but not a perfect predictor of Broca's aphasia. In a follow-up VLSM analysis that included only those individuals who had considerable involvement in the BAC, we again compared participants with and without Broca's aphasia. This analysis was run in 3 parts: The first analysis included those who had at least 75% damage to the BAC (12 with Broca's aphasia; 4 who did not have Broca's aphasia); the second analysis included individuals who had at least 50% damage to the BAC (18 with Broca's aphasia; 6 who did not have Broca's aphasia); and the third analysis included individuals whose lesion included at least 25% of the BAC (18 with Broca's aphasia; 12 who did not have Broca's aphasia). These analyses revealed that individuals with Broca's aphasia are more likely to have damage in an area that is mostly confined within the superior temporal gyrus (STG) and, to a lesser extent, inferior parietal cortex than participants who also have considerable damage to the portion of the pars opercularis seen in Figure 2, but do not have Broca's aphasia (Fig. 3). For the purpose of clarity, this area is henceforth referred to as the “Wernicke's area cluster” (WAC). For each of the 3 analyses, the statistical significance values were as follows (Z threshold, P-value, and maximum voxel Z-score): “Damage >75%” Zthresh = 4.23, P < 0.05, Zmax = 4.28; “Damage >50%” Zthresh = 5.97, P < 0.01, Zmax = 10.18; “Damage >25%” Zthresh = 5.26, P < 0.01, Zmax = 7.48. Based on these whole-brain analyses, it appears that participants with Broca's aphasia have considerable damage to portions of the pars opercularis and regions that are classically considered part of Wernicke's area.
Figure 2.
VLSM analysis that compared brain damage in individuals with Broca's aphasia (N = 20) with individuals with other types of aphasia (N = 50). The lesion cluster that predicts Broca's aphasia is mostly confined within the posterior portion of Broca's area, the pars opercularis. The color bar represents Z-scores in the voxels that comprise the statistically significant cluster and the crosshairs denote the voxel with the highest Z-score.
Figure 3.
VLSM analyses that included only individuals with different degrees of damage to the pars opercularis region shown in this figure (referred to as area BAC in the text) and compared individuals with and without Broca's aphasia. Results from individuals with at least 25% BAC damage are shown in red, with at least 50% damage in green (overlap with >25% damage shown in yellow), and at least 75% damage in blue (overlap with >25% damage shown in violet).
To corroborate the whole-brain VLSM results, 3 separate LOOCV classification analyses were conducted whereby participants were classified as having Broca's or some other types of aphasia based on proportional damage to the BAC and WAC. For this analysis, the WAC is defined based on the results from the analysis that included only participants whose damage included at least 50% of the BAC (cluster shown in green and yellow in Fig. 3). This analysis splits the study group in 2 based on the extent of damage to both BAC and WAC; patients had to have at least the required extent of damage to both regions to be grouped together. Proportions examined were 25%, 50%, and 75%. Then, proportional damage was used to classify patients' aphasia type (Broca's vs. other types of aphasia) using the leave-one-out method. For example, the first classification analysis grouped participants together who had at least 25% damage to both BAC and WAC versus those who had <25% damage to either BAC, WAC, or both.
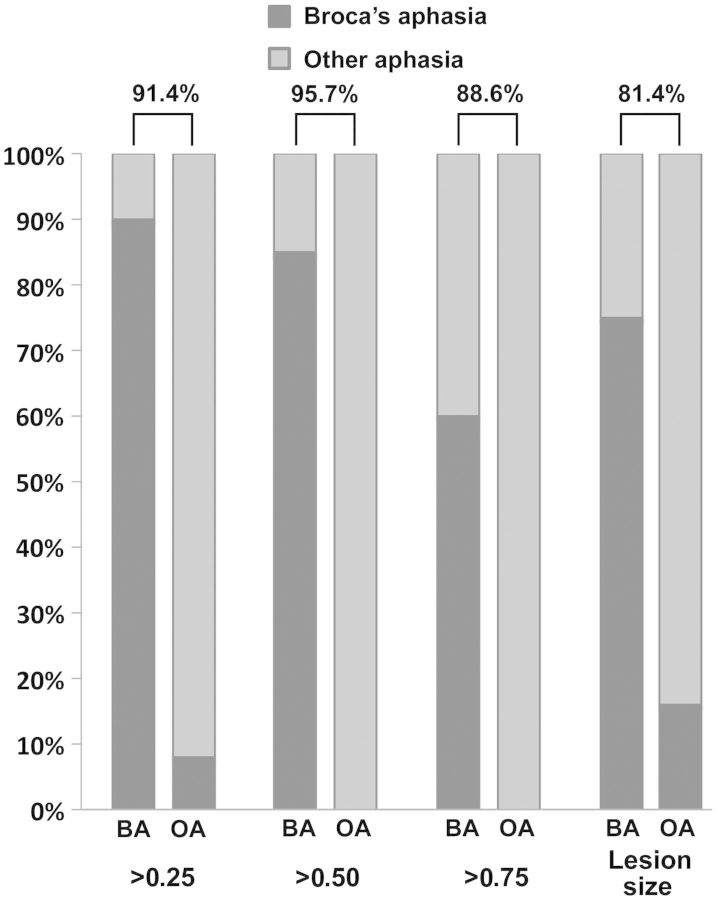
For the “damage >25%” analysis, the overall classification accuracy was 91.4% (Fig. 4). Broca's aphasia was classified with 90% accuracy (18/20 correctly classified) and those with other types of aphasia with 92% accuracy (46/50 correctly classified). For the “damage >50%” analysis, the overall classification accuracy was 95.7%. This analysis classified individuals with Broca's aphasia with 85% accuracy (17/20 classified correctly) and those with other types of aphasia with 100% accuracy (50/50 classified correctly). The overall accuracy of the ‘Damage >75%’ analysis was 88.6%. Here, those with Broca's aphasia were correctly classified with 60% accuracy (12/20 classified correctly) and those with other types of aphasia with 100% accuracy (50/50 patients classified correctly). In this context, it is important to note that numerical differences in prediction accuracy across different predictors does not necessarily mean one predictor is significantly better than another. Although the extent of damage to both BAC and WAC seems to be important for classifying Broca's aphasia, it could be the case that larger lesions are more likely to hit both areas and, consequently, result in Broca's aphasia. To examine this possibility, a fourth analysis included lesion size as classifier. For the purpose of this analysis, the number of voxels that comprised the lesion in each participant was entered as a classifier using the LOOCV approach. The overall accuracy of this analysis was 81.4%; Broca's aphasia was classified with 75% accuracy (15/20 classified correctly), and other aphasia classified with 84% accuracy (42/50 classified correctly).
Figure 4.
Percent accuracy (Y-axis) for discriminant function analyses that included different proportions of damage (<0.25, 0.50, and 0.75) to BAC and WAC or lesion size as predictors of the presence of Broca's aphasia. For each pair of columns, classification accuracy for Broca's aphasia is shown on the left and for other types of aphasia on the right. For 100% classification accuracy, the left column would be completely dark gray and the right column completely light gray. The overall accuracy for each classification analysis is shown above each column pair.
We also conducted a logistic regression using the proportion of injury to both the BAC and WAC as our 2 independent variables to predict the presence or absence of Broca's aphasia. The logistic regression found that both BAC (P < 0.0006) and WAC (P < 0.0011) improved a predictive value after the other was known, and the combined model was statistically significant (χ2 = 56.4, df = 2, P < 0.0001) correctly categorizing 66 of the 70 participants (94.3% accuracy). This model used the coefficients 6.0335 and 4.9948 (with odds ratios of 417.2 and 147.7) for the proportion of injury to BAC and WAC, respectively, with an intercept of −3.8. In a subsequent analysis, we also examined the interaction between BAC damage and WAC damage. The accuracy of the model was the same (94.3%) and the significance of the individual factors remained (P < 0.006 and <0.022) with the interaction also reaching significance (P = 0.049). Finally, we conducted a logistic regression that included the proportional injury to BAC, proportional injury to WAC, and overall lesion volume as predictors (we modeled this as a proportion of the largest injury simply to make the scale of the coefficients similar to the other predictors). This model was able to classify 95.7% of the participants with coefficients (with odds ratios and P-values) of 6.7449 (849, P < 0.0005), 5.9346 (378, P < 0.0008), and 5.1564 (174, P < 0.03) for BAC, WAC, and lesion volume, respectively, with an intercept of −5.56. Note that in each of these analyses, the extent of WAC injury was a reliable and positive predictor for Broca's aphasia once variability associated with BAC injury had been removed (furthermore, when considered as single factors in isolation both WAC [χ2 = 6.3, df = 1, P < 0.0123] and lesion volume [χ2 = 7.1, df = 1, P < 0.0079] are reliable predictors of Broca's aphasia using logistic regression).
Discussion
The current study demonstrates that although damage to the posterior portion of Broca's area is a strong predictor of Broca's aphasia, it is insufficient as an absolute predictor. Rather, individuals with Broca's aphasia also tend to have damage to the left STG, Wernicke's area. When only one of these regions is damaged, some other form of speech or language impairment is likely to occur. These findings were affirmed in 3 separate analyses. First, VLSM that compared binary lesions among persons with and without Broca's aphasia confirmed that damage in the pars opercularis was more likely to occur in individuals with Broca's aphasia than in those with other aphasic symptomatology. A second VLSM analysis that included only participants whose cortical damage involved the pars opercularis region identified in the first analysis, compared those with and without Broca's aphasia. This analysis revealed that damage to a portion of Wernicke's area was more likely to occur in individuals with Broca's aphasia than their counterparts who had damage to the pars opercularis but did not have Broca's aphasia. Finally, to further strengthen the VLSM results, we used discriminant function analyses and logistic regression with proportional damage to the pars opercularis and Wernicke's area as independent factors to predict whether individuals did or did not have Broca's aphasia. The discriminant function analyses and logistic regression predicted Broca's aphasia with 95.7% and 94.1% accuracy, respectively.
Although it is commonly accepted that the damage in Broca's aphasia is likely to involve several cortical structures, what exactly those structures are remained unclear, until now. Our findings suggest that chronic Broca's aphasia is not typically caused by damage to a single region. This damage is likely to include 2 structures: the pars opercularis and Wernicke's area. From a historical perspective, it is tempting to suggest that these findings support Marie's ideas about Broca's aphasia. Indeed, Marie argued that Broca's aphasia had to include damage to Wernicke's area (which he suggested was comprised of the STG and the inferior parietal lobule; Marie 1906a,c). However, he also argued that Broca's aphasia would not involve Broca's area damage, a claim that is not supported by the current data. In contrast, Dejerine, and several others, argued that the damage that causes Broca's aphasia involves Broca's area in addition to other structures such as the insula and inferior parietal lobule (Dejerine 1906a,b; Geschwind 1970; Mohr et al. 1978; Lazar and Mohr 2011). More than a century after their debates, the current study suggests that both Marie and Dejarine were partially correct. In this context, it is important to point out that neither Marie or Dejarine benefited from the use of standardized aphasia tests or modern VLSM approaches. Their intuitions were based solely on the study of case series of patients. Given these constraints, it is quite remarkable that their conclusions were so close to what we have discovered relying on advanced neuroimaging methods and standardized aphasia testing.
Broca's aphasia is not characterized by a singular impairment, and our results highlight its multifaceted nature: symptoms include decreased speech fluency, mild-to-moderate impairment of speech comprehension, and, in some cases, agrammatism (Goodglass 1993). Two recent reports suggest that speech fluency is primarily affected in cases that involve damage to the anterior segment of the arcuate fasciculus (Fridriksson et al. 2013) or the aslant tract, a white matter structure that connects the pars opercularis and the supplementary motor region (Catani et al. 2013). By most accounts, auditory comprehension in aphasia is most affected by temporal lobe involvement (e.g., Hillis et al. 2001; Dronkers et al. 2004; Turken and Dronkers 2011; Fridriksson et al. 2013). Additionally, impaired grammatical processing has been associated with damage to several areas, including Broca's area (Caramazza and Zurif 1976; Grodzinsky and Friederici 2006; Newhart et al. 2012), the temporal lobe (Dronkers et al. 2004; Magnusdottir et al. 2013), and the arcuate fasciculus (Wilson et al. 2011). Accordingly, it would seem straighforward to suggest that each of the typical symptoms associated with Broca's aphasia could be attributed to damage in different cortical regions, but this may be an oversimplification. Recent work in our laboratory (Fridriksson et al. 2013) examining the factors influencing speech fluency found that though univariate analyses highlighted inferior frontal regions related to speech production and posterior temporal regions related to speech comprehension, when considered together, it was rather the white matter connections between areas that appeared most important for overall fluency. Thus, as normal language function is an interplay between factors in both comprehension and production, it is likely that the overall pattern of damage described in the current study contributes to a specific language impairment profile, a condition that is typically referred to as Broca's aphasia.
Among the 20 patients with Broca's aphasia included in the current study, not a single one presented with spared BAC but damaged WAC. Nevertheless, a question that remains unaddressed here is why some persons who do not appear to have any damage to Broca's area nevertheless have Broca's aphasia. This issue was strongly argued by Marie and, for example, was demonstrated in a case study published by the current authors (Fridriksson et al. 2007). The patient described in our 2007 report has damage to Marie's lenticular zone—the basal ganglia, thalamus, arcuate fasciculus, and the external and extreme capsules—and was included in the current study sample. In each of the classification analyses conducted here, this person was (incorrectly) classified as not having Broca's aphasia. Almost a century before our case report, during 1 of 3 oral debates on aphasia between Marie, Dejerine, and others in 1908 (Klippel 1908), Dejerine-Klumpke argued that focusing only on cortical destruction was insufficient for explaining clinical symptoms in patients, including those with Broca's aphasia. What she primarily emphasized was that damage that disconnects Broca's area and the posterior language regions could also result in Broca's aphasia. Most importantly, damage in the lenticular zone that disconnects the anterior and posterior speech and language regions can cause Broca's aphasia, even though this is not the most typical pattern of damage in patients with Broca's aphasia. Klippel (1908) reported that many considered Dejerine-Klumpke the winner of the Marie-Dejerine debates as her point regarding disconnection was not easily countered by Marie, who continued to argue that many patients with Broca's aphasia do not have Broca's area damage and that the crucial damage is in the lenticular zone. Soon after Marie's seminal publication in 1906, Jakob (1906, as discussed by Tsapkini et al. 2008) implied that the number of these kinds of patients who have Broca's aphasia and only damage to the lenticular zone is fairly small. This view is supported by our new data that suggest that Broca's aphasia is most commonly associated with cortical injury.
Given that we have identified damage to 2 specific areas that gives rise to Broca's aphasia, it should be straightforward for others to challenge our findings (BAC and WAC are available as ‘regions of interest’ online—URL TBD). If our conclusions are correct, then damage in most chronic patients with Broca's aphasia should involve the BAC and WAC. However, we do not claim that the same results would be true for acute Broca's aphasia, as the acute functional disruption may be much more extensive than the anatomical injury (due to factors such as diaschisis). In fact, it may be that lesions involving only Broca's area (and the surrounding tissue) cause Broca's aphasia initially, but only patients who also have damage to Wernicke's area fail to recover. That is, perhaps patients with damage to Broca's area and Wernicke's area initially have global aphasia, but then recover into Broca's aphasia as their comprehension improves over the first few months following stroke. Indeed, there is quite a bit of evidence for this hypothesis (Kertesz 1984; Ochfeld et al. 2010).
Viewing these finding from the perspective of the dual stream model (Hickok and Poeppel 2007), it is clear that the damage that causes Broca's aphasia involves both the dorsal and ventral streams. Accordingly, it is difficult to see how the classification of ‘Broca's aphasia’ can be helpful in studying the neurobiology of language. This is because Broca's aphasia represents a syndrome rather than a single impairment. Although we believe that VLSM is quite useful and can inform the brain–language relationship, focusing on syndromes, rather than specific symptoms, is less useful for understanding how language is organized in the brain. This issue has been discussed amply elsewhere (Caramazza 1984; Caramazza and Badecker 1989; Willmes and Poeck 1993). Nevertheless, Broca's aphasia appears to be a distinct aphasic syndrome arising from a specific pattern of cortical damage. If this were not the case, then it is difficult to see how a data-driven classification analysis that predicts aphasia type solely based on damage to 2 cortical regions could adjudicate between participants with and without Broca's aphasia with greater than 95% accuracy. Similarly, other types of aphasia should also be associated with specific patterns of brain damage. Most cases of aphasia are caused by ischemic stroke that typically involves the left middle cerebral artery (MCA). The pattern of cortical damage following left MCA stroke depends on the extent and which of the 2 major MCA divisions were involved, regardless of the role of individual cortical regions in speech and language processing. Specifically, ischemic stroke follows vascular, not functional, boundaries of the cortex (Damasio and Geschwind 1984). Therefore, a given pattern of language impairment in a given aphasia type probably reflects typical locations of cortical damage in ischemic stroke, whereas unusual cases would be more likely to occur as a consequence of mechanisms where the pattern of brain damage is less predictable (e.g., as a result of hemorrhagic stroke, gunshot wounds, or tumors).
In this study, aphasia type was classified based on the WAB. Other test batteries or clinical impression can also be used to classify aphasia; yet, no gold standard exists for diagnosing aphasia and distinguishing between aphasia types. Although the WAB subtests do not provide in-depth data regarding different aspects of language and speech, it is a test that is widely used in clinical practice as well as in research and is commonly used to designate aphasia types in published reports (Yang et al. 2008; Kim et al. 2011; Robson et al. 2014). It is a caveat that the WAB does not specifically test for grammatical processing problems, a feature that some, but not all, might consider central to the diagnosis of Broca's aphasia (e.g., see Goodglass 1993). Therefore, the current results do not reflect the presence or absence of agrammatism. Perhaps a more problematic aspect of the WAB in relation to aphasia classification pertains to its speech fluency scale. As pointed out by Trupe (1984), the fluency rating scale on the WAB suffers from somewhat poor interrater reliability. Moreover, a difference in one point on this scale could, for example, distinguish between a given patient being classified as having Broca's aphasia as opposed to conduction aphasia, 2 syndromes that are vastly different in clinical presentation. To tackle this potential caveat, a prospective study might seek a different approach by excluding all patients whose fluency ratings fall near the WAB marker of fluent versus non-fluent speech (e.g., a rating of 4 and 5). Another approach might seek to classify aphasia type solely based on clinical judgment with or without relying on the WAB or another aphasia test. AphasiaBank (Forbes et al. 2012), which includes a data from over 300 aphasic individuals, has collected WAB data along with clinical impressions as to aphasia type. Among 257 patients whose aphasia type was classified according to the WAB, 85% were classified as having the same aphasia type based on clinicians' impressions. Consistent with these data, it could be surmised that 10 of the 70 patients (15%) in the current sample would have been classified differently based on the WAB versus clinical judgment. Accordingly, it is crucial to emphasize that the current results should be interpreted in relation to WAB limitations. It is certainly possible that more in-depth behavioral analysis of each participant could have influenced our group sizes and, accordingly, the results.
In regard to traditional VLSM studies, our findings raise some important issues. Typically, VLSM studies only focus on mass univariate analyses, which sometimes fail to show secondary damage as contributing to a given symptom (Smith et al. 2013). Consistent with the initial VLSM analysis carried out here, we could have determined that the damage that causes Broca's aphasia only needs to involve Broca's area, a conclusion that is wrong. Rather, while the univariate voxel-wise analyses demonstrate that damage to Broca's area is a reliable predictor of Broca's aphasia, they are unable to identify how information from other regions can refine this model. The second analysis that identified damage in Wernicke's area gives a far more comprehensive picture of the pattern of damage that gives rise to Broca's aphasia. Other studies that focus on specific symptoms could also make a similar mistake of relying on the initial univariate analysis alone. As a follow-up analysis, it might be valuable to verify VLSM results by comparing patterns of brain damage among individuals who have a given behavioral impairment, or certain severity of impairment. Another potentially useful approach would be to implement multivariate analyses (Smith et al. 2013). However, sufficient sample size to yield statistically significant results would, in most cases, have to be increased considerably.
In conclusion, our findings suggest that Broca's aphasia is caused by damage to Broca's and Wernicke's areas. Although the lesion pattern among Broca's aphasic individuals may vary considerably, it appears that both of these structures are highly likely to be damaged. Regarding Marie's postulations on Broca's aphasia, we can only conclude that he was onto something in regard to the involvement of Wernicke's area. However, his conclusions regarding Broca's area damage, or lack thereof, are not supported by the current study.
Notes
Conflict of Interest: None declared.
References
- Broca P. 1861. Remarques Sur le Siége de la Faculté Du Langage Articulé, Suivies D'une Observation D'aphémie (Perte de la Parole). Bull Soc Anat. 6:330–357. [Google Scholar]
- Broca P. 1865. Sur le siège de la facultlé du language articulé. Bull Soc Anthropol. 6:337–393. [Google Scholar]
- Caramazza A. 1984. The logic of neuropsychological research and the problem of patient classification in aphasia. Brain Lang. 21(1):9–20. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Badecker W. 1989. Patient classification in neuropsychological research. Brain Cogn. 10(2):256–295. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. 1976. Dissociation of algorithmic and heuristic processes in language comprehension: evidence from aphasia. Brain Lang. 3(4):572–582. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, Thompson CK, Rogalski E. 2013. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 136(8):2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code C. 2013. Did Leborgne have one or two speech automatisms? J Hist Neurosci. 22(3):319–320. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Geschwind N. 1984. The neural basis of language. Annu Rev Neurosci. 7:127–147. [DOI] [PubMed] [Google Scholar]
- Dejerine J. 1906a. L'aphasie sensorielle: sa localisation et sa physiologie pathologique. Presse Med. 55:437–439. [Google Scholar]
- Dejerine J. 1906b. L'aphasie motrice: sa localisation et sa physiologie pathologique. Presse Med. 57:453–457. [Google Scholar]
- Dejerine J. 1914. Sémiologie des affections du système nerveux. Paris: Masson; p. 6. [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. 2007. Paul Broca's historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 130(5):1432–1441. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. 2004. Lesion analysis of the brain areas involved in language comprehension. Cognition. 92(1–2):145–177. [DOI] [PubMed] [Google Scholar]
- Forbes MM, Fromm D, Macwhinney B. 2012. AphasiaBank: a resource for clinicians. Semin Speech Lang. 33(3):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. 1891. Zur Auffassung der Aphasien. Eine kritische Studie. Leipzig und Wien: Deuticke. [Google Scholar]
- Fridriksson J, Bonilha L, Rorden C. 2007. Case report severe Broca's aphasia without Broca's area damage. Behav Neurol. 18(4):237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Guo D, Fillmore P, Holland A, Rorden C. 2013. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain. 136(11):3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. 1965. Disconnexion syndromes in animals and man. I. Brain 88(2):237–294. [DOI] [PubMed] [Google Scholar]
- Geschwind N. 1970. The organization of language and the brain. Science. 170(3961):940–944. [DOI] [PubMed] [Google Scholar]
- Goodglass H. 1993. Understanding aphasia. San Diego: (CA: ): Academic press. [Google Scholar]
- Goldstein K. 1910. Ueber Aphasie. Beihefte zur Med. Klinik. 1:1–32. [Google Scholar]
- Grodzinsky Y, Friederici AD. 2006. Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol. 16(2):240–246. [DOI] [PubMed] [Google Scholar]
- Head H. 1926. Aphasia and kindred disorders of speech. Cambridge: (MA: ): Cambridge University Press. [Google Scholar]
- Hickok G. 2012. Computational neuroanatomy of speech production. Nat Rev Neurosci. 13(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. 2007. The cortical organization of speech processing. Nat Rev Neurosci. 8:393–402. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. 2001. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol. 50(5):561–566. [DOI] [PubMed] [Google Scholar]
- Jackson JH. 1866. Notes on the physiology and pathology of language. Med Times Gazette. 1:659. [Google Scholar]
- Kertesz A. 1984. Neurobiological aspects of recovery from aphasia in stroke. Int Rehabil Med. 6(3):122–127. [DOI] [PubMed] [Google Scholar]
- Kertesz A. 1982. The Western Aphasia Battery. New York: Grune & Stratton. [Google Scholar]
- Kim SH, Lee DG, You H, Son SM, Cho YW, Chang MC, Lee J, Jang SH. 2011. The clinical application of the arcuate fasciculus for stroke patients with aphasia: a diffusion tensor tractography study. NeuroRehabilitation. 29(3):305–310. [DOI] [PubMed] [Google Scholar]
- Klippel M. 1908. Deuxieme discussion sur l'aphasie. Rev Neurol. 16:974–1024. [Google Scholar]
- Krestel H, Annoni J-M, Jagella C. 2013. White matter in aphasia: a historical review of the Dejerines’ studies. Brain Lang. 127(3):526–532. [DOI] [PubMed] [Google Scholar]
- LaPointe LL. 2012. Paul Broca and the Origins of Language in the Brain. San Diego: (CA: ): Plural Publishing Inc. [Google Scholar]
- Lazar RM, Mohr JP. 2011. Revisiting the contributions of Paul Broca to the study of aphasia. Neuropsychol Rev. 21(3):236–239. [DOI] [PubMed] [Google Scholar]
- Lorch MP. 2008. The merest Logomachy: The 1868 Norwich discussion of aphasia by Hughlings Jackson and Broca. Brain. 131(6):1658–1670. [DOI] [PubMed] [Google Scholar]
- Magnusdottir S, Fillmore P, den Ouden DB, Hjaltason H, Rorden C, Kjartansson O, Bonilha L, Fridriksson J. 2013. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: a lesion-symptom mapping study. Hum Brain Mapp. 34(10):2715–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie P. 1906c. Aphasia from 1861 to 1866. Essay of historical criticism on the genesis of the doctrine of aphasia. Sem Méd. 26:565–571. [Google Scholar]
- Marie P. 1906b. New case of aphasia of Broca without lesion of the third left frontal. Bull Mém Soc Méd Hôp Paris. 3(23):1180–1183. [Google Scholar]
- Marie P. 1906d. New case of cortical lesion of the foot of the third left frontal in a right-handed man without language disorders. Bull Mém Soc Méd Hôp Paris. 3(23):1295–1298. [Google Scholar]
- Marie P. 1906a. The third frontal convolution plays no special role in the function of language. Sem Méd. 26:241–247. [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. 1978. Broca aphasia: pathologic and clinical. Neurology. 28(4):311–324. [DOI] [PubMed] [Google Scholar]
- Newhart M, Trupe LA, Gomez Y, Cloutman L, Molitoris JJ, Davis C, Leigh R, Gottesman RF, Race D, Hillis AE. 2012. Asyntactic comprehension, working memory, and acute ischemia in Broca's area versus angular gyrus. Cortex. 48(10):1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochfeld EN, Newhart M, Molitoris JJ, Leigh R, Davis C, Cloutman L, Crinion J, Hillis AE. 2010. Ischemia in Broca's area is associated with Broca's aphasia more reliably in acute than chronic stroke. Stroke. 41:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciaroni M, Bogousslavsky J. 2011. Jules Joseph Dejerine versus Pierre Marie. Front Neurol Neurosci. 29:162–169. [DOI] [PubMed] [Google Scholar]
- Robson H, Zahn R, Keidel JL, Binney RJ, Sage K, Lambon Ralph MA. 2014. The anterior temporal lobes support residual comprehension in Wernicke's aphasia. Brain. 137(3):931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. 2007. Improving lesion-symptom mapping. J Cogn Neurosci. 19(7):1081–1088. [DOI] [PubMed] [Google Scholar]
- Smith DV, Clithero JA, Rorden C, Karnath H-O. 2013. Decoding the anatomical network of spatial attention. Proc Natl Acad Sci USA. 110(4):1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupe AE. 1984. Reliability of rating spontaneous speech in the Western Aphasia Battery: implications for classification. In: Brookshire RH, editors. Clinical aphasiology. Minneapolis: BRK Publishers; p. 55–69. [Google Scholar]
- Tsapkini K, Vivas AB, Triarhou LC. 2008. “Does Broca’s area exist?” Christofredo Jakob's 1906 response to Pierre Marie's holistic stance. Brain Lang. 105(3):211–219. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. 2011. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 15(1):273–289. [DOI] [PubMed] [Google Scholar]
- Willmes K, Poeck K. 1993. To what extent can aphasic syndromes be localized? Brain. 116(6):1527–1540. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Patterson DK, Henry ML, Ogar JM, Gorno-tempini ML. 2011. Syntactic processing depends on dorsal language tracts. Neuron. 72(2):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZH, Zhao XQ, Wang CX, Chen HY, Zhang YM. 2008. Neuroanatomic correlation of the post-stroke aphasias studied with imaging. Neurol Res. 30(4):356–360. [DOI] [PubMed] [Google Scholar]